Abstract
Microtubules – polymers of tubulin – perform essential functions, including regulation of cell shape, intracellular transport and cell motility. How microtubules are adapted to perform multiple diverse functions is not well understood. Post-translational modifications of tubulin subunits diversify the outer and luminal surfaces of microtubules and provide a potential mechanism for their functional specialization. Recent identification of a number of tubulin-modifying and -demodifying enzymes has revealed key roles of tubulin modifications in the regulation of motors and factors that affect the organization and dynamics of microtubules.
Keywords: Cilia, Modification, Tubulin
Introduction
The α- and β-tubulin heterodimer – the building block of microtubules – undergoes multiple post-translational modifications (PTMs) (Table 1). The modified tubulin subunits are non-uniformly distributed along microtubules. Analogous to the model of the ‘histone code’ on chromatin, diverse PTMs are proposed to form a biochemical ‘tubulin code’ that can be ‘read’ by factors that interact with microtubules (Verhey and Gaertig, 2007). In this Commentary, we will discuss recent advances in understanding the mechanism and functions of tubulin PTMs. Recent data provide strong support to a model that tubulin PTMs regulate microtubule effectors.
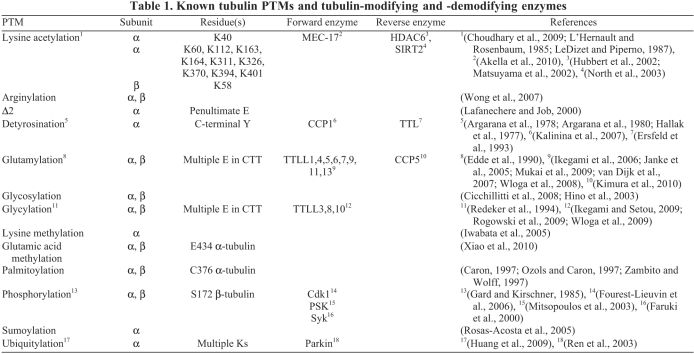
Table 1.
Known tubulin PTMs and tubulin-modifying and -demodifying enzymes
Conserved tubulin PTMs that occur on microtubules
Most PTMs appear on tubulin subunits after polymerization into microtubules. One exception is phosphorylation on serine residue S172 of β-tubulin by the Cdk1 kinase that occurs on the unpolymerized tubulin dimer and inhibits its incorporation into assembling microtubules (Fourest-Lieuvin et al., 2006). Tubulin is also phosphorylated inside microtubules (Faruki et al., 2000; Gard and Kirschner, 1985; Laurent et al., 2004; Ma and Sayeski, 2007; Matten et al., 1990; Wandosell et al., 1987), presumably on tyrosine (Y) or S residues that are different from S172 on the β-subunit, but the sites and significance of the post-assembly phosphorylation events are not known. Tubulin PTMs that occur on microtubules and have been well characterized include acetylation of lysine (K) residues, detyrosination, glycylation and glutamylation (see below).
Acetylation of K40 on α-tubulin
Highly conserved acetylation of the ε-amino group of residue K40 of α-tubulin (L'Hernault and Rosenbaum, 1985; LeDizet and Piperno, 1987) is the only PTM that occurs on an amino acid moiety that extends to the microtubule lumen (Nogales et al., 1998) (Fig. 1). A recent mass spectrometry study in mammalian cells detected acetylation of K residues on multiple additional sites on α- and β-tubulin (Choudhary et al., 2009). However, K40 is the main – if not the only – site of acetylation on tubulin in Tetrahymena thermophila, on the basis of the observation that antibodies against acetylated K residues do not crossreact with the K40R point mutant of α-tubulin that cannot be acetylated (Akella et al., 2010). Acetylated of K40 residue (acetyl-K40) on α-tubulin occurs on diverse microtubules including cytoplasmic, spindle, centriolar and axonemal microtubules (Piperno and Fuller, 1985). The acetyl-K40 mark appears with a delay after microtubule assembly and is seen as a marker of polymer age (Gundersen et al., 1984; Piperno et al., 1987; Schulze et al., 1987). Recently, MEC-17, a protein related to GCN5 histone acetyltransferases (Steczkiewicz et al., 2006) and required for the function of touch receptor neurons in Caenorhabditis elegans (Chalfie and Au, 1989; Zhang et al., 2002), was identified as an important α-tubulin acetyltransferase (αTAT) (Akella et al., 2010). MEC-17 deficiencies result in the loss of acetyl-K40 α-tubulin in Tetrahymena, C. elegans, zebrafish embryos and HeLa cells and – in vitro – recombinant mouse MEC-17 exclusively acetylates K40 on α-tubulin (Akella et al., 2010). However, the genome of Chlamydomonas reinhardtii, an organism in which α-tubulin acetylation was discovered and the αTAT activity characterized (Greer et al., 1985; L'Hernault and Rosenbaum, 1985; LeDizet and Piperno, 1987; Maruta et al., 1986), lacks a MEC-17 homolog (Akella et al., 2010). Thus, another ciliary αTAT is likely to exist. In animal cells, several acetyltransferases colocalize with acetylated microtubules and some regulate the level of acetyl-K40 α-tubulin, including N-acetyltransferase 1 (NAT) 1 (Ohkawa et al., 2008), NAT10 (Shen et al., 2009) and elongator protein 3 (ELP3) (Creppe et al., 2009; Solinger et al., 2010), but it is not known whether any of these enzymes have direct activity on α-tubulin. Deacetylation of acetyl-K40 on α-tubulin is catalyzed by HDAC6 (Hubbert et al., 2002; Matsuyama et al., 2002) and sirtuin 2 (SIRT2) (North et al., 2003) deacetylases, enzymes that are related to histone deacetylases.
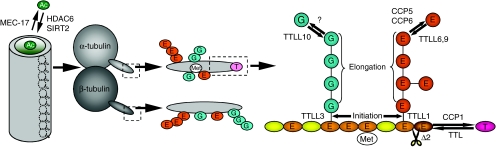
Fig. 1.
Tubulin PTM types and sites. Shown are the well-characterized post-translational modifications that act on tubulin and the enzymes that are responsible for these modifications. Most of the modifications occur at the C-terminal tail (CTT) domains of α- and β-tubulin. Ac, acetylated lysine; E, glutamic acid; G. glycine; Met, methylated glutamic acid; Δ2, proteolytic removal of the penultimate C-terminal amino acid.
C-terminal proteolytic events of α-tubulin – detyrosination and Δ2 PTM
Tubulin detyrosination is a proteolytic removal of the C-terminal Y residue on α-tubulin (Hallak et al., 1977). Detyrosination occurs after incorporation of tubulin subunits into the microtubule lattice (Kumar and Flavin, 1981) on diverse microtubules (Geuens et al., 1986; Gundersen and Bulinski, 1986; Gundersen et al., 1984; Johnson, 1998; Webster et al., 1987). Detyrosination is likely to be generated by the cytosolic carboxypeptidase 1 (CCP1, also known as NNA1), a finding based on the observation that a mutation in Ccp1 in mice greatly decreases the level of microtubule detyrosination (Kalinina et al., 2007). It remains to be determined whether CCP1 has α-tubulin detyrosination activity in vitro. Detyrosinated tubulin heterodimers that are released from microtubules can revert to an unmodified state by tyrosination (Barra et al., 1973) catalyzed by tubulin tyrosine ligase (TTL) (Ersfeld et al., 1993). Detyrosinated α-tubulin that remains in the microtubule lattice may undergo a second proteolysis, Δ2 PTM, that removes the now terminal glutamic acid (E) residue. The Δ2 α-tubulin that is released from microtubules during depolymerization cannot revert to an unmodified state (Lafanechere and Job, 2000) and, thus, this PTM can limit the amount of α-tubulin that undergoes recycling.
Tubulin polymodifications – glutamylation and glycylation
Glutamylation (Edde et al., 1990) and glycylation (Redeker et al., 1994) are two related PTMs that form as peptide side chains on the C-terminal tail (CTT) domain of tubulin (Fig. 1). Because of their polymeric nature, glycylation and glutamylation are referred to as ‘polymodifications’. The side chains branch off multiple glutamic acids within the CTT of α- and β-tubulin. Tubulin glutamylation is abundant on microtubules of axonemes, centrioles and basal bodies, and on some cytoplasmic and spindle microtubules (Bobinnec et al., 1998b; Bre et al., 1994; Wolff et al., 1992). The related PTM, tubulin glycylation, is restricted to ciliated cell types, and is enriched on axonemes and basal bodies (Bre et al., 1996). Polymodifications are catalyzed by TTL-like proteins (TTLLs) that have homology with TTL (Ikegami and Setou, 2009; Janke et al., 2005; Rogowski et al., 2009; van Dijk et al., 2007; Wloga et al., 2008; Wloga et al., 2009). A polymodification side chain is formed in two biochemically distinct steps: initiation and elongation. These two steps are often, but not always, mediated by distinct TTLLs. First, a E or glycine (G) residue is attached to tubulin through an isopeptide bond with the γ-carboxyl group of a primary sequence glutamic acid, by a TTLL with chain-initiating activity, such as TTLL1, TTLL4 and TTLL7 glutamic acid ligases (E-ligases) (Janke et al., 2005; Mukai et al., 2009; Wloga et al., 2008) and TTLL3 glycine ligase (G-ligase) (Rogowski et al., 2009; Wloga et al., 2009). The side chain is extended by TTLLs with elongase activity such as TTLL6 and TTLL9 E-ligases (Suryavanshi et al., 2010; van Dijk et al., 2007; Wloga et al., 2008) and TTLL10 G-ligase (Ikegami and Setou, 2009; Rogowski et al., 2009). Some TTLLs, such as mouse TTLL7 E-ligase (Mukai et al., 2009) or Drosophila melanogaster TTLL3 G-ligase (Rogowski et al., 2009) have both side-chain-initiating and -elongating activities. In vivo, the length of the side chain correlates with the microtubule type. For example, in ciliates, the side chains are longer in axonemes when compared with those present on microtubules in the cell body (Iftode et al., 2000; Wloga et al., 2008). The extent to which either of the two tubulin subunits is modified is spatially regulated. For example, in superior cervical ganglion neurons, tubulin glutamylation is abundant on β-tubulin in the somatodendritic compartment, but α-tubulin is glutamylated to a higher extent in the axon (Ikegami et al., 2006). Such patterns of tubulin polymodifications can be formed by selective targeting of G- and E-ligases to specific cellular locations such as neuronal projections or cilia (Ikegami et al., 2006; Suryavanshi et al., 2010; van Dijk et al., 2007; Wloga et al., 2009). The side-chain elongation occurs gradually after microtubule assembly. Consequently, microtubules in the newly forming organelles, such as the nascent basal bodies and axonemes, have short side chains that lengthen as the organelle matures (Iftode et al., 2000; Sharma et al., 2007). Thus, the length of polymodification side chains could have a role in distinguishing between microtubular organelles that are either in the assembly or maintenance state. Tubulin glutamylation is important for assembly and motility of cilia (see below). Although tubulin glycylation is a marker of ciliated cells, there are evolutionary lineages with cilia that lack this PTM (Fennell et al., 2008; Schneider et al., 1997). In vivo, there is competition between the two polymodifications, probably because the E- and G-ligases attach side chains to the same glutamic acids in the CTTs (Rogowski et al., 2009; Wloga et al., 2009). Thus, the primary role of tubulin glycylation might be to negatively regulate tubulin glutamylation. Organisms that lack tubulin glycylation could use other means to inhibit tubulin glutamylation, including tubulin deglutamylation (see below) or other competing PTMs. In Toxoplasma gondii, a potentially polymodifiable residue of α-tubulin, E434, undergoes carboxyl methylation (Xiao et al., 2010), a PTM of unknown effect, that could also compete with tubulin glutamylation (Plessmann et al., 2004).
Biochemical studies have detected activities that shorten polymodification side chains: deglutamylation (Audebert et al., 1993) and deglycylation (Bre et al., 1998). A recent study identifies the carboxypeptidase CCP5 as the tubulin deglutamylase (Kimura et al., 2010). CCP5 has an amino acid sequence related to the putative detyrosination enzyme CCP1 (Kalinina et al., 2007). A mutation of CCP5 in C. elegans increases the level of tubulin glutamylation in cilia of sensory neurons, and purified mammalian CCP5 has tubulin deglutamylase activity in vitro (Kimura et al., 2010). To our knowledge, it has not yet been reported how deregulation of CCP5 affects microtubules. It can be speculated that, among the CCP5-related carboxypeptidases that lack an assigned enzymatic activity (Rodriguez de la Vega et al., 2007), there are tubulin deglycylases.
Tubulin PTMs affect the organization and dynamics of microtubules
The use of antibodies that specifically recognize post-translationally modified tubulins reveals differences in the level of PTMs among distinct microtubules, or even along the length of the same microtubule. Microtubules with a slow turnover and, in particular, ‘superstable’ centriolar basal body and axonemal microtubules have high levels of diverse tubulin PTMs (Bobinnec et al., 1998b; Bre et al., 1998; Khawaja et al., 1988; Piperno et al., 1987). In migrating mammalian cells, a subset of microtubules that orient towards the leading edge are more stable and enriched in acetylation and detyrosination (Palazzo et al., 2004). The question arises whether tubulin PTMs have a role in stabilizing microtubules or whether they simply accumulate on microtubules that are stabilized by other mechanisms such as plus-end capping (Infante et al., 2000). In mammalian cells, experimental stabilization of microtubules with paclitaxel increases the levels of tubulin acetylation and detyrosination (Gundersen et al., 1987; Hammond et al., 2010; Piperno et al., 1987; Witte et al., 2008). Thus, it is possible that at least some PTMs accumulate on long-lived microtubules as a consequence of increased time of exposure to modifying enzymes. However, recent studies indicate that some tubulin PTMs can have a stabilizing effect on microtubules.
The α-tubulin detyrosination and tyrosination cycle is important in vivo. Mice that lack TTL have increased levels of detyrosinated α-tubulin, defects in the differentiation of neurons and die within hours of birth (Erck et al., 2005). The Purkinje cell degeneration (pcd) mice that carry a mutation in the gene encoding CCP1 (Kalinina et al., 2007) have increased levels of tyrosinated α-tubulin, display adult-onset degeneration of Purkinje and retinal neurons, and have structurally defective sperm (Fernandez-Gonzalez et al., 2002; Handel and Dawson, 1981; Mullen et al., 1976; Wang and Morgan, 2007). It seems that the phenotypes observed in mice deficient in those enzymes involved in the detyrosination–tyrosination cycle result from impaired microtubule dynamics. In vitro, fibroblasts isolated from Ttl-null mice have a less-polarized shape and defective motility (Peris et al., 2006). These fibroblasts also have excessively detyrosinated microtubules that are abnormally long and hyperstable, and this phenotype is rescued by overexpression of kinesin-13, a microtubule-end depolymerizer (Peris et al., 2009). Consistently, microtubules enriched in detyrosinated tubulin show increased resistance to kinesin-13-mediated end depolymerization in vitro (Peris et al., 2009). In mice, the knockout phenotypes for genes encoding TTL and kinesin superfamily protein 2A (KIF2A, a kinesin-13-family protein expressed in the nervous system) are similar (Homma et al., 2003; Peris et al., 2009). Thus, detyrosinated α-tubulin at or near the microtubule end might protect against depolymerization by kinesin-13. Tubulin detyrosination also affects the dynamics of the plus ends of microtubules by inhibiting binding of the plus-end tracking proteins (+TIPs) that have a CAP-Gly domain: CLIP170, CLIP115 and p150Glued (Peris et al., 2006).
Tubulin glutamylation also strongly influences the dynamics of microtubules. Overexpression of the TTLL6-type E-ligase (β-tubulin elongase) in Tetrahymena thermophila leads to the accumulation of hyperglutamylated nocodazole-resistant cytoplasmic microtubules (Wloga et al., 2010). The extent to which tubulin is glutamylated might affect the binding affinity of neuronal stabilizing microtubule-associated proteins (MAPs) such as MAP2 and Tau (Bonnet et al., 2001; Boucher et al., 1994). Consistent with this, depletion of the TTLL7 E-ligase in differentiating PC-12 cells by RNA interference (RNAi) inhibits the emergence of MAP2-enriched neurites (Ikegami et al., 2006).
However, tubulin glutamylation can also have a microtubule-destabilizing effect through the regulation of microtubule-severing factors katanin (McNally and Vale, 1993) and spastin (Hazan et al., 1999). In Tetrahymena, a mutation of multiple adjacent polymodifiable glutamic acid residues on the CTT of β-tubulin (Thazhath et al., 2002) phenocopies a loss of katanin (Sharma et al., 2007) (see next section). In C. elegans, substitution of a glutamic acid residue in the CTT of β-tubulin that is likely to serve in glutamylation rescues the embryonic lethality caused by overproduction of katanin (Lu et al., 2004). Overexpression of the TTLL11 E-ligase in HeLa cells causes fragmentation of microtubules that can be rescued by knockdown of spastin using small interfering RNA (siRNA); moreover, hyperglutamylated microtubules have increased sensitivity to spastin-mediated severing in vitro (Lacroix et al., 2010). Spastin (and probably katanin) are predicted to function as a ring complex of six subunits, with a centrally located pore that incorporates the tubulin CTT (Roll-Mecak and Vale, 2008). Consistently, antibodies that recognize a terminal E-residue (Rudiger et al., 1999) reduce spastin-mediated microtubule severing activity in vitro (Roll-Mecak and Vale, 2008). To reconcile the microtubule stabilizing and destabilizing activities of tubulin glutamylation observed in various contexts, we propose that the outcomes of this PTM depend on the specific structure of the glutamyl side chain (length and positions) and the availability of microtubule effectors (either stabilizing MAPs or severing factors).
The conserved α-tubulin acetylation at K40 has only a subtle effect on microtubule dynamics. Tetrahymena mutants assemble diverse microtubules from α-tubulin that contains the K40R mutation and cannot be acetylated (Gaertig et al., 1995), and mice with excessively highly acetylated α-tubulin (after deletion of HDAC6 deacetylase) appear almost normal except for subtle increases in bone mineral content and reduced immune responses (Zhang et al., 2008). However, in cultured mammalian cells, broad inhibition of HDACs with trichostatin A increases the level of acetyl-K40 α-tubulin and causes stabilization of microtubules because of their increased resistance to nocodazole (Matsuyama et al., 2002). Fibroblasts isolated from Hdac6-null mice have stabilized microtubules that show shorter depolymerization events and increased resistance to nocodazole (Tran et al., 2007). However, the potential microtubule-stabilizing effect of acetyl-K40 in mammalian cells must be subtle because other studies have failed to detect a change in the parameters of microtubule dynamics in response to manipulations of HDAC6 levels (Haggarty et al., 2003; Palazzo et al., 2003). In one study, inhibition of HDAC6 with tubacin (Haggarty et al., 2003), reduced the rate of microtubule growth, but no such effect was observed after depletion of HDAC6 when using RNAi (Zilberman et al., 2009).
In Tetrahymena cells that lack the K40-specific αTAT, MEC-17, most if not all microtubules appear normal but cells are hyper-resistant to paclitaxel and hypersensitive to oryzalin, indicating that MEC-17 has microtubule-stabilizing activity (Akella et al., 2010). Although our earlier study failed to detect a tubulin phenotype in a K40R α-tubulin mutant that is drug resistant (Gaertig et al., 1995), re-examination of the same mutant strain by using longer drug exposure showed a drug-resistance phenotype similar to that seen in the MEC-17-knockout cells (Akella et al., 2010).
A recent study opens up the possibility that K40 acetylation regulates the levels of total tubulin (Solinger et al., 2010). In C. elegans nematodes with a genetic background that increases the level of MEC-12 proteins acetylated on K40, the total levels of transgenic MEC-12 decrease, and the effect is reversed by a K40Q substitution in MEC-12 (Solinger et al., 2010). Solinger and colleagues propose that the acetyl-K40 of α-tubulin is targeted for degradation through ubiquitylation, presumably following the disassembly of acetylated microtubules (Solinger et al., 2010). The acetyl-K40-mediated tubulin degradation could involve HDAC6, because this protein binds to acetyl-K40 α-tubulin and interacts with proteins that mediate ubiquitylation (Boyault et al., 2007). Moreover, α- but not β-tubulin undergoes ubiquitylation in the disassembling axonemes of Chlamydomonas reinhardtii (Huang et al., 2009). However, in Chlamydomonas, ubiquitylation was found on α-tubulin whose K-40 residues were acetylated, indicating that deacetylation of K40 is not a prerequisite of ubiquitylation (Huang et al., 2009). It is, however, possible that the acetyl-K40 mark facilitates ubiquitylation by promoting binding of HDAC6, a model that is consistent with the observation that HDAC6 preferentially deacetylates unpolymerized tubulin (Matsuyama et al., 2002).
Tubulin PTMs regulate the assembly of microtubular organelles
Centrioles, basal bodies and cilia contain exceptionally stable and extensively post-translationally modified microtubules. In most organisms, the microtubules in these organelles are compound polymers with tubules that have fused walls (triplets in basal bodies and centrioles, doublets in axonemes). Tubulin PTMs, and specifically detyrosination and polymodifications, have crucial roles in the assembly, maintenance and function of complex microtubule-based organelles. Male pcd mice are infertile and produce fewer and slowly moving sperm with variable axonemal defects (Fernandez-Gonzalez et al., 2002; Handel and Dawson, 1981). In Chlamydomonas, detyrosinated tubulin is primarily present on the outer doublets and the PTM levels gradually increase after axoneme assembly (Johnson, 1998), suggesting that the PTM is not needed for axoneme assembly but can stabilize axonemal microtubules.
Tubulin acetylation is also linked to ciliogenesis in mammals. In cultured human retinal pigment epithelial (RPE1) cells, loss of BBIP10, a Bardet-Biedl syndrome 4 (BBS4)-interacting protein, inhibits the assembly of primary cilia and, at the same time, strongly decreases the levels of acetylated cytoplasmic microtubules (Loktev et al., 2008). Also in RPE1 cells, inhibition of HDAC6 with tubacin blocks resorption of primary cilia (Pugacheva et al., 2007), pointing to a role for α-tubulin K40 deacetylation in the depolymerization of axonemal microtubules. However, it is not clear whether BBIP10 and HDAC6 affect cilia through the acetylation and/or deacetylation of K40 on α-tubulin or by other mechanisms. In particular, HDAC6 deacetylates or binds to several non-tubulin proteins (for a review, see Valenzuela-Fernandez et al., 2008). It has already been reported that, in C. elegans, HDAC6 affects cilia in the chemosensory neurons, despite the fact that this cell type does not detectably express the only isotype of α-tubulin with K40, MEC-12 (Fukushige et al., 1999; Li et al., 2010). Moreover, in protist models, α-tubulin acetylation is not needed for ciliogenesis (Akella et al., 2010; Gaertig et al., 1995; Kozminski et al., 1993). Thus, in mammals the acetylation and/or deacetylation regulators are important in the assembly and disassembly of cilia, but it is not clear whether these activities are mediated by the acetylation and/or deacetylation of α-tubulin.
Tubulin glutamylation has an increasingly well-documented role in the assembly and functions of complex microtubular organelles. Injection of antibodies that recognize glutamylation into mammalian cells induces disassembly of centrioles (Bobinnec et al., 1998a). In Tetrahymena, loss of TTLL1 and TTLL9 E-ligases causes defects in the docking of nascent basal bodies to the plasma membrane (Wloga et al., 2008). A morpholino-mediated depletion of the TTLL6 E-ligase in zebrafish is associated with shortening and loss of olfactory cilia (Pathak et al., 2007). In mice, mutations in either the TTLL1 E-ligase or associated protein PGs1 cause various defects in the sperm axoneme that are associated with male infertility (Campbell et al., 2002; Ikegami et al., 2010; Regnard et al., 2003; Vogel et al., 2010). Curiously, the Ttll1-deficient mice assemble structurally normal cilia in the trachea, despite low levels of tubulin glutamylation (but the motility of these cilia is strongly affected; see below) (Ikegami et al., 2010; Vogel et al., 2010). As discussed earlier for cytoplasmic microtubules, during axoneme assembly tubulin glutamylation can also act by regulating the microtubule-severing factors, specifically katanin. In Tetrahymena, substitution of aspartate for three glutamic acid residues that serve as polymodification sites on β-tubulin result in assembly of paralyzed cilia that have excessively short doublets and lack central microtubules. The same Tetrahymena mutants undergo an arrest in cytokinesis associated with an overgrowth of longitudinal microtubule bundles (that resemble the midbody microtubules in mammalian cells) (Thazhath et al., 2004; Thazhath et al., 2002). Importantly, knockouts of genes encoding subunits of the katanin complex produce a set of defects in cytokinesis and axoneme assembly that are strikingly similar to the phenotype observed in the β-tubulin polymodification-site mutants (Sharma et al., 2007). Consistently, a mutation in the katanin regulatory subunit p80 inhibits the assembly of central axonemal microtubules in Chlamydomonas reinhardtii (Dymek et al., 2004). Subunits of the katanin complex are present inside cilia and are enriched on doublet microtubules (Dymek et al., 2004; Sharma et al., 2007). Consistently, the axonemal doublets and, in particular, the B-subfiber are enriched in tubulin polymodifications (Kann et al., 1995; Kubo et al., 2010; Lechtreck and Geimer, 2000; Multigner et al., 1996; Suryavanshi et al., 2010). In the context of axoneme assembly, katanin most probably interacts with glutamylated and not with glycylated tubulin, because Tetrahymena Ttll3-null cells that lack almost all tubulin glycylation assemble shortened but structurally normal 9+2 axonemes (Wloga et al., 2009). It remains to be determined whether the function of katanin inside cilia involves destabilization of the microtubule lattice or a new activity.
In addition to the effects on katanin, tubulin glutamylation could affect complex microtubular organelles through its effects on motor proteins that transport precursors required for the assembly and maintenance of these organelles. For example, tubulin glutamylation could regulate motors proteins (e.g. kinesin-2 and DH1b dynein) that drive intraflagellar transport (IFT), a motility pathway required for the assembly and maintenance of cilia (for a review, see Pedersen and Rosenbaum, 2008). There is already evidence that tubulin PTMs, including glutamylation, affect diverse motor proteins (see next section).
Tubulin glycylation might also have a role in axoneme assembly. In Tetrahymena, a knockout of TTLL3, a chain-initiating G-ligase, causes shortening of axonemes (Wloga et al., 2009). The depletion of TTLL3 expression in zebrafish leads to shortening and complete loss of axonemes in the olfactory epithelium and Kupffer's vesicle (Wloga et al., 2009). siRNA-induced depletion of TTLL3 in Drosophila arrests the assembly of sperm axonemes at the individualization stage (Rogowski et al., 2009). It is probable that, at least to some extent, tubulin glycylation influences the process of axoneme assembly through competitive inhibition of tubulin glutamylation. Indeed, overproduction of the TTLL6 E-ligase in Tetrahymena inhibits axoneme assembly (Wloga et al., 2010). However, a complicating factor in the interpretation of these TTLL3 studies is that, on the basis of observations in Drosophila, several non-tubulin proteins are glycylated by TTLL3 (Rogowski et al., 2009).
In mammals and most other organisms that have ciliated lineages, TTLL10 catalyzes the elongation of glycyl side chains that are initiated by TTLL3 (Rogowski et al., 2009). Unexpectedly, in humans (but not in other mammals), the tubulin glycyl side chains are not elongated, a finding established because of the lack in reactivity of these side chains to an antibody specifically recognizing polyglycylation (Bre et al., 1996; Rogowski et al., 2009). Although humans have a TTLL10 gene (Rogowski et al., 2009), the protein is enzymatically inactive on tubulin, owing to the two amino acid substitutions that are human specific (Rogowski et al., 2009). Rogowski and colleagues propose that a non-elongated glycyl side chain is sufficient for axoneme assembly, probably because it fulfills the proposed main function of glycylation: competitive inhibition of glutamylation (Rogowski et al., 2009). The elongation of glycyl side chains that occurs in most ciliated lineages could have additional functions, such as the generation of specific patterns of sperm motility. There is already strong evidence that elongation of glutamyl side chains regulates ciliary motility (see below).
Tubulin PTMs influence navigation of motor proteins on microtubules
Growing evidence indicates that tubulin PTMs provide structural cues for motor proteins. In mammalian neurons, the motor protein KIF5 (a member of the kinesin-1 protein family) preferentially enters the axon (Jacobson et al., 2006; Nakata and Hirokawa, 2003). The axonal shaft is enriched in acetylated and detyrosinated microtubules, as compared with the dendrites (for a review, see Fukushima et al., 2009). In fibroblasts, kinesin-1 also associates preferentially with microtubules that are enriched in acetylation and detyrosination (Cai et al., 2009; Dunn et al., 2008). In vitro, the binding affinity of kinesin-1 to microtubules increases with increased quantities of K40-acetylated α-tubulin (Dompierre et al., 2007; Konishi and Setou, 2009; Reed et al., 2006). Stabilization of microtubules by paclitaxel in differentiating neurons elevates the levels of tubulin PTMs, including α-tubulin acetylation on K40 and detyrosination, and promotes the accumulation of kinesin-1 and other axonal markers in multiple neurites (Hammond et al., 2010; Witte and Bradke, 2008). However, the unequal distribution of the acetylated tubulin between the axon and dendrites might not be sufficient for the preferential accumulation of kinesin-1, because inhibition of HDAC6 activity induces excessive levels of K40-acetylated α-tubulin in multiple neurites, but does not affect the biased entry of kinesin-1 into the presumptive axon in differentiating neurons (Hammond et al., 2010; Witte and Bradke, 2008). However, overexpression of a non-acetylatable K40A mutant α-tubulin in cortical neurons inhibits the migration and branching of neurite projections (Creppe et al., 2009), and decreases the velocity of brain-derived neurotrophic factor (BDNF)-associated vesicles that move along microtubules (Dompierre et al., 2007). Loss of MEC-17 and its paralog W06B11.1 in C. elegans leads to a loss of acetyl-K40 MEC-12 α-tubulin and strong functional deficiency of touch receptor neurons (Akella et al., 2010). Adult C. elegans nematodes that express only a K40R or K40Q mutant MEC-12 α-tubulin have a reduced touch response, but the remaining level of touch response is higher when compared with animals that lack both αTATs MEC-17 or W06B11.1 (Akella et al., 2010). One possible explanation is that MEC-17 acetylates a K residue on α- or β-tubulin distinct from K40 (Choudhary et al., 2009). An antibody against acetylated K failed to react with microtubules that contain K40R α-tubulin in Tetrahymena (Akella et al., 2010). However, we cannot exclude the possibility that MEC-17 acetylates a K residue(s) distinct from K40 on animal tubulin in vivo. Alternatively, in animals, MEC-17 might acetylate a non-tubulin protein that is important in neurons. In zebrafish, depletion of MEC-17 expression using morpholinos decreases the levels of acetyl-K40 α-tubulin in a number of different neurons and is associated with developmental defects, including decreased head and eye size, a curved body axis and reduced mobility (Akella et al., 2010). It remains to be determined to what extent the significance of MEC-17 in vertebrates involves its function as an αTAT.
Tubulin detyrosination has an important role in regulating motors in the context of neural differentiation. Compared with dendrites, the axon is enriched in detyrosinated α-tubulin (Konishi and Setou, 2009). In vitro, kinesin-1 binds more strongly to microtubules enriched in detyrosinated α-tubulin (Konishi and Setou, 2009; Liao and Gundersen, 1998). In hippocampal neurons, depletion of TTL by using RNAi increases the levels of detyrosination in dendrites and leads to increased entry of kinesin-1 into dendrites (Konishi and Setou, 2009). Konishi and Setou propose that tyrosinated tubulin acts as a negative cue that excludes kinesin-1 from dendritic microtubules (Konishi and Setou, 2009). Interestingly, in vitro, kinesin-1 moves more slowly along microtubules that contain predominantly detyrosinated tubulin (obtained by carboxypeptidase treatment), as compared with untreated microtubules (Dunn et al., 2008).
Tubulin glutamylation also influences motors in the nervous system. Neurons of mice that carry a mutation in PGs1, a subunit of the α-tubulin-specific E-ligase complex TTLL1, have reduced levels of α-tubulin glutamylation and kinesin-3 KIF1A, and these changes are associated with abnormal synaptic transmission (Ikegami et al., 2007). Although the TTLL1-dependent tubulin glutamylation is not essential in mice, it could have a subtle role in the brain – a suggestion based on the observation that male Ttll1-null mice are less aggressive than male wild-type mice (Campbell et al., 2002).
Recent studies have uncovered a key function of tubulin glutamylation in ciliary motility. The beating of cilia is driven by coordinated activity of axonemal dynein-arm motors. In a typical 9+2 motile axoneme (Fig. 2), each of the nine doublets has a complete A-subfiber and an incomplete B-subfiber. Dynein arms are stably attached to the A-subfiber and cause axoneme bending by exerting transient forces on the B-tubule of an adjacent doublet (for a review, see Lindemann and Lesich, 2010). Tubulin glutamylation was first implicated in ciliary motility based on the observation that anti-glutamylated protein antibodies inhibit the motility of demembranated cilia exposed to ATP (Gagnon et al., 1996). Mutants deficient in either the TTLL9 E-ligase in Chlamydomonas or TTLL6 E-ligase in Tetrahymena have greatly reduced rates of cilia-dependent cell motility, but apparently normal axonemes. In both protists, loss of the E-ligase activity reduces the beat frequency and, in Tetrahymena, results in a grossly abnormal waveform (Kubo et al., 2010; Suryavanshi et al., 2010). TTLL9 and TTLL6 homologs are associated with activities that elongate glutamyl side chains (Suryavanshi et al., 2010; van Dijk et al., 2007; Wloga et al., 2008). Thus, in protists, non-elongated glutamyl side chains might be sufficient for axoneme assembly, but their elongation is needed for ciliary motility. In both protists, tubulin glutamylation is present mainly on the outer doublet microtubules; within the doublets, the PTM is mainly present on the B-subfiber (Kubo et al., 2010; Suryavanshi et al., 2010), the surface to which dynein-arm motor domains bind during the ATP-dependent cross-bridging cycle that underlies ciliary motility.
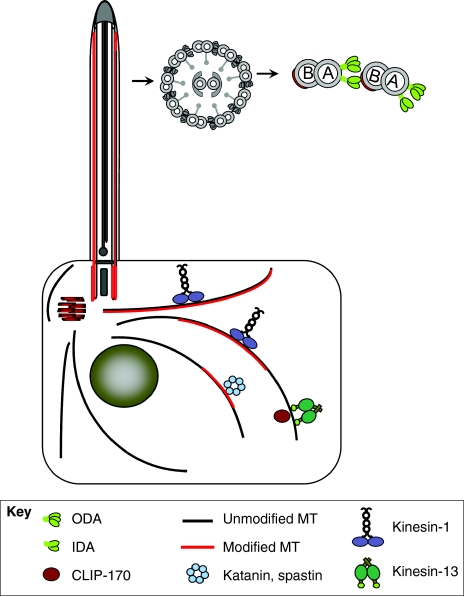
Fig. 2.
Tubulin PTMs regulate diverse activities by increasing the activity of microtubule effectors. Tubulin modifications regulate diverse activities including motor protein movement, microtubule severing, microtubule-end polymerization and activity of +TIPs. See text for further details.
There are two types of dynein arms: inner (IDA) and outer (ODA). ODAs strongly affect the beat frequency, whereas IDAs mainly affect the waveform (for a review, see Kamiya, 2002). Remarkably, both protist studies link tubulin glutamylation specifically to IDAs (Kubo et al., 2010; Suryavanshi et al., 2010). Chlamydomonas cells that lack TTLL9 and ODAs have paralyzed cilia (whereas either of the two single mutants move slowly), indicating that tubulin glutamylation is essential for productive IDA activity (Kubo et al., 2010). In both protist models, the velocity of microtubule sliding, which was induced in isolated (TTLL6- or TTLL9-deficient) mutant axonemes treated with ATP in vitro, was unaffected (Kubo et al., 2010; Suryavanshi et al., 2010). This is consistent with the lack of an important effect of tubulin glutamylation on ODAs that, in this assay, overrides IDAs (Kamiya, 2002). Remarkably, in both models the microtubule sliding velocity in axonemes treated with ATP greatly increases in double mutants that lack an E-ligase and ODAs, and in which microtubule sliding force is produced only by IDAs (Kubo et al., 2010; Suryavanshi et al., 2010).
Thus, tubulin glutamylation restrains the net force imposed on doublet microtubules by IDAs. Importantly, IDAs are processive motors (Kotani et al., 2007; Sakakibara et al., 1999). In the bending cilium, processive IDAs that act simultaneously with faster ODAs could act as a drag and, therefore, increase the axoneme curvature (Kotani et al., 2007). Tubulin glutamylation might be required for the processive force production that is generated specifically by IDAs. Either IDAs have unique properties that make them sensitive to the levels of tubulin glutamylation [such as unique features of the microtubule-binding domain (Carter et al., 2008)], or the pattern of tubulin glutamylation on the B-tubule is restricted spatially to only affect IDAs and not ODAs.
Recent studies report that mice with a TTLL1 E-ligase mutation suffer from primary ciliary dyskinesia, which is associated with reduced mucociliary clearance (Ikegami et al., 2010; Vogel et al., 2010). In the Ttll1 mutant mice, tracheal cilia beat faster and have an abnormal waveform, characterized by a lack of distinction between the power and recovery strokes, and decreased curvature (Ikegami et al., 2010). Although there are significant differences between studies on three models in which tubulin glutamylation has been linked to ciliary beating, in all studies tubulin glutamylation affects the beat frequency and, in at least two models (Tetrahymena and mouse), the waveform (Ikegami et al., 2010; Kubo et al., 2010; Suryavanshi et al., 2010). Differences between studies in specific ciliated models could be attributed to a distinct conserved TTLL E-ligase subtype being manipulated in each model, which could lead to differential effects on either the glutamyl chain initiation or elongation on either α- or β-tubulin. However, it is clear that tubulin glutamylation has a key role in the regulation of axonemal dynein. It is now important to determine whether tubulin glutamylation regulates non-axonemal (cytoplasmic) dynein motors that participate in the long-range retrograde transport along microtubules.
Concluding remarks and future perspectives
As we have seen, recent studies have established that PTMs on tubulin are crucial for the dynamics and organization of microtubules and their ability to serve as tracks for force production, either during long-range transport or bending of cilia. Although it is clear that tubulin PTMs strongly affect microtubule effectors, such as motor proteins or polymer-destabilizing factors (Fig. 2), the exact structural mechanisms remain to be investigated. Moreover, it remains to be explained how the activities of tubulin PTM enzymes are spatially regulated to create reproducible PTM patterns on microtubules.
Supplementary Material
Acknowledgments
Work in the laboratory of J.G. is currently supported by grants from the National Science Foundation (MBC-033965) and National Institutes of Health (R01GM089912). Deposited in PMC for release after 12 months.
Footnotes
This article is part of a Minifocus on microtubule dynamics. For further reading, please see related articles: ‘Microtubule plus-end tracking proteins (+TIPs)’ by Anna Akhmanova and Michel O. Steinmetz (J. Cell Sci. 123, 3415-3419), ‘Kinesins at a glance’ by Sharyn A. Endow et al., (J. Cell Sci. 123, 3420-3424), ‘Tubulin depolymerization may be an ancient biological motor process’ by J. Richard McIntosh et al. (J. Cell Sci. 123, 3425-3434) and ‘Towards a quantitative understanding of mitotic spindle assembly and mechanics’ by Alex Mogilner and Erin Craig (J. Cell Sci. 123, 3435-3445).
References
- Akella A. J., Wloga D., Kim J., Starostina N. G., Lyons-Abbott S., Morrissette N. S., Dougan S. T., Kipreos E. T., Gaertig J. (2010). MEC-17 is an alpha-tubulin acetyltransferase. Nature 467, 218-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argarana C. E., Barra H. S., Caputto R. (1978). Release of [14C]tyrosine from tubulinyl-[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol. Cell. Biochem 19, 17-21 [DOI] [PubMed] [Google Scholar]
- Argarana C. E., Barra H. S., Caputto R. (1980). Tubulinyl-tyrosine carboxypeptidase from chicken brain: properties and partial purification. J. Neurochem. 34, 114-118 [DOI] [PubMed] [Google Scholar]
- Audebert S., Desbruyeres E., Gruszczynski C., Koulakoff A., Gros F., Denoulet P., Edde B. (1993). Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons. Mol. Biol. Cell 4, 615-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra H. S., Rodriguez J. A., Arce C. A., Caputto R. (1973). A soluble preparation from rat brain that incorporates into its own proteins (14 C)arginine by a ribonuclease-sensitive system and (14 C)tyrosine by a ribonuclease-insensitive system. J. Neurochem. 20, 97-108 [DOI] [PubMed] [Google Scholar]
- Bobinnec Y., Khodjakov A., Mir L. M., Rieder C. L., Edde B., Bornens M. (1998a). Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 143, 1575-1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinnec Y., Moudjou M., Fouquet J. P., Desbruyeres E., Edde B., Bornens M. (1998b). Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil. Cytoskeleton 39, 223-232 [DOI] [PubMed] [Google Scholar]
- Bonnet C., Boucher D., Lazereg S., Pedrotti B., Islam K., Denoulet P., Larcher J. C. (2001). Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J. Biol. Chem. 276, 12839-12848 [DOI] [PubMed] [Google Scholar]
- Boucher D., Larcher J. C., Gros F., Denoulet P. (1994). Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein Tau and tubulin. Biochemistry 33, 12471-12477 [DOI] [PubMed] [Google Scholar]
- Boyault C., Sadoul K., Pabion M., Khochbin S. (2007). HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene 26, 5468-5476 [DOI] [PubMed] [Google Scholar]
- Bre M. H., de Nechaud B., Wolff A., Fleury A. (1994). Glutamylated tubulin probed in ciliates with the monoclonal antibody GT335. Cell Motil. Cytoskeleton 27, 337-349 [DOI] [PubMed] [Google Scholar]
- Bre M. H., Redeker V., Quibell M., Darmanaden-Delorme J., Bressac C., Cosson J., Huitorel P., Schmitter J. M., Rossler J., Johnson T., et al. (1996). Axonemal tubulin polyglycylation probed with two monoclonal antibodies: widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J. Cell Sci. 109, 727-738 [DOI] [PubMed] [Google Scholar]
- Bre M. H., Redeker V., Vinh J., Rossier J., Levilliers N. (1998). Tubulin polyglycylation: differential posttranslational modification of dynamic cytoplasmic and stable axonemal microtubules in paramecium. Mol. Biol. Cell 9, 2655-2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., McEwen D. P., Martens J. R., Meyhofer E., Verhey K. J. (2009). Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 7, e1000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. K., Waymire K. G., Heier R. L., Sharer C., Day D. E., Reimann H., Jaje J. M., Friedrich G. A., Burmeister M., Bartness T. J., et al. (2002). Mutation of a novel gene results in abnormal development of spermatid flagella, loss of intermale aggression and reduced body fat in mice. Genetics 162, 307-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J. M. (1997). Posttranslational modification of tubulin by palmitoylation: I. In vivo and cell-free studies. Mol. Biol. Cell 8, 621-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. P., Garbarino J. E., Wilson-Kubalek E. M., Shipley W. E., Cho C., Milligan R. A., Vale R. D., Gibbons I. R. (2008). Structure and functional role of dynein's microtubule-binding domain. Science 322, 1691-1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Au M. (1989). Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243, 1027-1033 [DOI] [PubMed] [Google Scholar]
- Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009). Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834-840 [DOI] [PubMed] [Google Scholar]
- Cicchillitti L., Penci R., Di Michele M., Filippetti F., Rotilio D., Donati M. B., Scambia G., Ferlini C. (2008). Proteomic characterization of cytoskeletal and mitochondrial class III beta-tubulin. Mol. Cancer Ther. 7, 2070-2079 [DOI] [PubMed] [Google Scholar]
- Creppe C., Malinouskaya L., Volvert M. L., Gillard M., Close P., Malaise O., Laguesse S., Cornez I., Rahmouni S., Ormenese S., et al. (2009). Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136, 551-564 [DOI] [PubMed] [Google Scholar]
- Dompierre J. P., Godin J. D., Charrin B. C., Cordelieres F. P., King S. J., Humbert S., Saudou F. (2007). Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 27, 3571-3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S., Morrison E. E., Liverpool T. B., Molina-Paris C., Cross R. A., Alonso M. C., Peckham M. (2008). Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J. Cell Sci. 121, 1085-1095 [DOI] [PubMed] [Google Scholar]
- Dymek E. E., Lefebvre P. A., Smith E. F. (2004). PF15p is the Chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryotic Cell 3, 870-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde B., Rossier J., Le Caer J. P., Desbruyeres E., Gros F., Denoulet P. (1990). Posttranslational glutamylation of alpha-tubulin. Science 247, 83-85 [DOI] [PubMed] [Google Scholar]
- Erck C., Peris L., Andrieux A., Meissirel C., Gruber A. D., Vernet M., Schweitzer A., Saoudi Y., Pointu H., Bosc C., et al. (2005). A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc. Natl. Acad. Sci. USA 102, 7853-7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersfeld K., Wehland J., Plessmann U., Dodemont H., Gerke V., Weber K. (1993). Characterization of the tubulin-tyrosine ligase. J. Cell Biol. 120, 725-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruki S., Geahlen R. L., Asai D. J. (2000). Syk-dependent phosphorylation of microtubules in activated B-lymphocytes. J. Cell Sci. 113, 2557-2565 [DOI] [PubMed] [Google Scholar]
- Fennell B. J., Al-shatr Z. A., Bell A. (2008). Isotype expression, post-translational modification and stage-dependent production of tubulins in erythrocytic Plasmodium falciparum. Int. J. Parasitol. 38, 527-539 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez A., La Spada A. R., Treadaway J., Higdon J. C., Harris B. S., Sidman R. L., Morgan J. I., Zuo J. (2002). Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science 295, 1904-1906 [DOI] [PubMed] [Google Scholar]
- Fourest-Lieuvin A., Peris L., Gache V., Garcia-Saez I., Juillan-Binard C., Lantez V., Job D. (2006). Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol. Biol. Cell 17, 1041-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T., Siddiqui Z. K., Chou M., Culotti J. G., Gogonea C. B., Siddiqui S. S., Hamelin M. (1999). MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J. Cell Sci. 112, 395-403 [DOI] [PubMed] [Google Scholar]
- Fukushima N., Furuta D., Hidaka Y., Moriyama R., Tsujiuchi T. (2009). Post-translational modifications of tubulin in the nervous system. J. Neurochem. 109, 683-693 [DOI] [PubMed] [Google Scholar]
- Gaertig J., Cruz M. A., Bowen J., Gu L., Pennock D. G., Gorovsky M. A. (1995). Acetylation of lysine 40 in alpha-tubulin is not essential in Tetrahymena thermophila. J. Cell Biol. 129, 1301-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon C., White D., Cosson J., Huitorel P., Edde B., Desbruyeres E., Paturle-Lafanechere L., Multigner L., Job D., Cibert C. (1996). The polyglutamylated lateral chain of alpha-tubulin plays a key role in flagellar motility. J. Cell Sci. 109, 1545-1553 [DOI] [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. (1985). A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J. Cell Biol. 100, 764-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuens G., Gundersen G. G., Nuydens R., Cornelissen F., Bulinski J. C., DeBrabander M. (1986). Ultrastructural colocalization of tyrosinated and detyrosinated alpha-tubulin in interphase and mitotic cells. J. Cell Biol. 103, 1883-1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer K., Maruta H., L'Hernault S. W., Rosenbaum J. L. (1985). Alpha-tubulin acetylase activity in isolated Chlamydomonas flagella. J. Cell Biol. 101, 2081-2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. (1986). Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur. J. Cell Biol. 42, 288-294 [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. (1984). Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell 38, 779-789 [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Khawaja S., Bulinski J. C. (1987). Postpolymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J. Cell Biol. 105, 251-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty S. J., Koeller K. M., Wong J. C., Grozinger C. M., Schreiber S. L. (2003). Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci. USA 100, 4389-4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. (1977). Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 73, 147-150 [DOI] [PubMed] [Google Scholar]
- Hammond J. W., Huang C. F., Kaech S., Jacobson C., Banker G., Verhey K. J. (2010). Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel M. A., Dawson M. (1981). Effects in spermiogenesis in the mouse of a male sterile neurological mutation, Purkinje cell degeneration. Gamete Res. 4, 185-192 [Google Scholar]
- Hazan J., Fonknechten N., Mavel D., Paternotte C., Samson D., Artiguenave F., Davoine C. S., Cruaud C., Durr A., Wincker P., et al. (1999). Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat. Genet. 23, 296-303 [DOI] [PubMed] [Google Scholar]
- Hino M., Kijima-Suda I., Nagai Y., Hosoya H. (2003). Glycosylation of the alpha and beta tubulin by sialyloligosaccharides. Zool. Sci. 20, 709-715 [DOI] [PubMed] [Google Scholar]
- Homma N., Takei Y., Tanaka Y., Nakata T., Terada S., Kikkawa M., Noda Y., Hirokawa N. (2003). Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 114, 229-239 [DOI] [PubMed] [Google Scholar]
- Huang K., Diener D. R., Rosenbaum J. L. (2009). The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J. Cell Biol. 186, 601-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X. F., Yao T. P. (2002). HDAC6 is a microtubule-associated deacetylase. Nature 417, 455-458 [DOI] [PubMed] [Google Scholar]
- Iftode F., Clerot J. C., Levilliers N., Bre M. H. (2000). Tubulin polyglycylation: a morphogenetic marker in ciliates. Biol. Cell 92, 615-628 [DOI] [PubMed] [Google Scholar]
- Ikegami K., Setou M. (2009). TTLL10 can perform tubulin glycylation when co-expressed with TTLL8. FEBS Lett. 583, 1957-1963 [DOI] [PubMed] [Google Scholar]
- Ikegami K., Mukai M., Tsuchida J., Heier R. L., Macgregor G. R., Setou M. (2006). TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J. Biol. Chem. 281, 30707-30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K., Heier R. L., Taruishi M., Takagi H., Mukai M., Shimma S., Taira S., Hatanaka K., Morone N., Yao I., et al. (2007). Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. USA 104, 3213-3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K., Sato S., Nakamura K., Ostrowski L. E., Setou M. (2010). Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc. Natl. Acad. Sci. USA 107, 10490-10495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. S., Stein M. S., Zhai Y., Borisy G. G., Gundersen G. G. (2000). Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J. Cell Sci. 113, 3907-3919 [DOI] [PubMed] [Google Scholar]
- Iwabata H., Yoshida M., Komatsu Y. (2005). Proteomic analysis of organ-specific post-translational lysine-acetylation and -methylation in mice by use of anti-acetyllysine and -methyllysine mouse monoclonal antibodies. Proteomics 5, 4653-4664 [DOI] [PubMed] [Google Scholar]
- Jacobson C., Schnapp B., Banker G. A. (2006). A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron 49, 797-804 [DOI] [PubMed] [Google Scholar]
- Janke C., Rogowski K., Wloga D., Regnard C., Kajava A. V., Strub J. M., Temurak N., van Dijk J., Boucher D., van Dorsselaer A., et al. (2005). Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308, 1758-1762 [DOI] [PubMed] [Google Scholar]
- Johnson K. A. (1998). The axonemal microtubules of the Chlamydomonas flagellum differ in tubulin isoform content. J. Cell Sci. 111, 313-320 [DOI] [PubMed] [Google Scholar]
- Kalinina E., Biswas R., Berezniuk I., Hermoso A., Aviles F. X., Fricker L. D. (2007). A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 21, 836-850 [DOI] [PubMed] [Google Scholar]
- Kamiya R. (2002). Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int. Rev. Cytol. 219, 115-155 [DOI] [PubMed] [Google Scholar]
- Kann M. L., Prigent Y., Fouquet J. P. (1995). Differential distribution of glutamylated tubulin in the flagellum of mouse spermatozoa. Tissue Cell 27, 323-329 [DOI] [PubMed] [Google Scholar]
- Khawaja S., Gundersen G. G., Bulinski J. C. (1988). Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J. Cell Biol. 106, 141-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Kurabe N., Ikegami K., Tsutsumi K., Konishi Y., Kaplan O. I., Kunitomo H., Iino Y., Blacque O. E., Setou M. (2010). Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs). J. Biol. Chem. 285, 22936-22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y., Setou M. (2009). Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 12, 559-567 [DOI] [PubMed] [Google Scholar]
- Kotani N., Sakakibara H., Burgess S. A., Kojima H., Oiwa K. (2007). Mechanical properties of inner-arm dynein-f (dynein I1) studied with in vitro motility assays. Biophys. J. 93, 886-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski K. G., Diener D. R., Rosenbaum J. L. (1993). High level expression of nonacetylatable alpha-tubulin in Chlamydomonas reinhardtii. Cell Motil. Cytoskeleton 25, 158-170 [DOI] [PubMed] [Google Scholar]
- Kubo T., Yanagisawa H. A., Yagi T., Hirono M., Kamiya R. (2010). Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr. Biol. 20, 441-445 [DOI] [PubMed] [Google Scholar]
- Kumar N., Flavin M. (1981). Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J. Biol. Chem. 256, 7678-7686 [PubMed] [Google Scholar]
- Lacroix B., van Dijk J., Gold N. D., Guizetti J., Aldrian-Herrada G., Rogowski K., Gerlich D. W., Janke C. (2010). Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 189, 945-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafanechere L., Job D. (2000). The third tubulin pool. Neurochem. Res. 25, 11-18 [DOI] [PubMed] [Google Scholar]
- Laurent C. E., Delfino F. J., Cheng H. Y., Smithgall T. E. (2004). The human c-Fes tyrosine kinase binds tubulin and microtubules through separate domains and promotes microtubule assembly. Mol. Cell. Biol. 24, 9351-9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck K. F., Geimer S. (2000). Distribution of polyglutamylated tubulin in the flagellar apparatus of green flagellates. Cell Motil. Cytoskeleton 47, 219-235 [DOI] [PubMed] [Google Scholar]
- LeDizet M., Piperno G. (1987). Identification of an acetylation site of Chlamydomonas alpha-tubulin. Proc. Natl. Acad. Sci. USA 84, 5720-5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. (1985). Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 24, 473-478 [DOI] [PubMed] [Google Scholar]
- Li Y., Wei Q., Zhang Y., Ling K., Hu J. (2010). The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J. Cell Biol. 189, 1039-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G., Gundersen G. G. (1998). Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J. Biol. Chem. 273, 9797-9803 [DOI] [PubMed] [Google Scholar]
- Lindemann C. B., Lesich K. A. (2010). Flagellar and ciliary beating: the proven and the possible. J. Cell Sci. 123, 519-528 [DOI] [PubMed] [Google Scholar]
- Loktev A. V., Zhang Q., Beck J. S., Searby C. C., Scheetz T. E., Bazan J. F., Slusarski D. C., Sheffield V. C., Jackson P. K., Nachury M. V. (2008). A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev. Cell 15, 854-865 [DOI] [PubMed] [Google Scholar]
- Lu C., Srayko M., Mains P. E. (2004). The Caenorhabditis elegans microtubule-severing complex MEI-1/MEI-2 katanin interacts differently with two superficially redundant beta-tubulin isotypes. Mol. Biol. Cell 15, 142-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Sayeski P. P. (2007). Identification of tubulin as a substrate of Jak2 tyrosine kinase and its role in Jak2-dependent signaling. Biochemistry 46, 7153-7162 [DOI] [PubMed] [Google Scholar]
- Maruta H., Greer K., Rosenbaum J. L. (1986). The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J. Cell Biol. 103, 571-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A., Shimazu T., Sumida Y., Saito A., Yoshimatsu Y., Seigneurin-Berny D., Osada H., Komatsu Y., Nishino N., Khochbin S., et al. (2002). In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 21, 6820-6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matten W. T., Aubry M., West J., Maness P. F. (1990). Tubulin is phosphorylated at tyrosine by pp60c-src in nerve growth cone membranes. J. Cell Biol. 111, 1959-1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally F. J., Vale R. D. (1993). Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 75, 419-429 [DOI] [PubMed] [Google Scholar]
- Mitsopoulos C., Zihni C., Garg R., Ridley A. J., Morris J. D. (2003). The prostate-derived sterile 20-like kinase (PSK) regulates microtubule organization and stability. J. Biol. Chem. 278, 18085-18091 [DOI] [PubMed] [Google Scholar]
- Mukai M., Ikegami K., Sugiura Y., Takeshita K., Nakagawa A., Setou M. (2009). Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on beta-tubulin through a random sequential pathway. Biochemistry 48, 1084-1093 [DOI] [PubMed] [Google Scholar]
- Mullen R. J., Eicher E. M., Sidman R. L. (1976). Purkinje cell degeneration, a new neurological mutation in the mouse. Proc. Natl. Acad. Sci. USA 73, 208-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multigner L., Pignot-Paintrand I., Saoudi Y., Job D., Plessmann U., Rudiger M., Weber K. (1996). The A and B tubules of the outer doublets of sea urchin sperm axonemes are composed of different tubulin variants. Biochemistry 35, 10862-10871 [DOI] [PubMed] [Google Scholar]
- Nakata T., Hirokawa N. (2003). Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162, 1045-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E., Wolf S. G., Downing K. H. (1998). Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391, 199-203 [DOI] [PubMed] [Google Scholar]
- North B. J., Marshall B. L., Borra M. T., Denu J. M., Verdin E. (2003). The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437-444 [DOI] [PubMed] [Google Scholar]
- Ohkawa N., Sugisaki S., Tokunaga E., Fujitani K., Hayasaka T., Setou M., Inokuchi K. (2008). N-acetyltransferase ARD1-NAT1 regulates neuronal dendritic development. Genes Cells 13, 1171-1183 [DOI] [PubMed] [Google Scholar]
- Ozols J., Caron J. M. (1997). Posttranslational modification of tubulin by palmitoylation: II. Identification of sites of palmitoylation. Mol. Biol. Cell 8, 637-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A., Ackerman B., Gundersen G. G. (2003). Cell biology: Tubulin acetylation and cell motility. Nature 421, 230 [DOI] [PubMed] [Google Scholar]
- Palazzo A. F., Eng C. H., Schlaepfer D. D., Marcantonio E. E., Gundersen G. G. (2004). Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303, 836-839 [DOI] [PubMed] [Google Scholar]
- Pathak N., Obara T., Mangos S., Liu Y., Drummond I. A. (2007). The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol. Biol. Cell 18, 4353-4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L. B., Rosenbaum J. L. (2008). Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85, 23-61 [DOI] [PubMed] [Google Scholar]
- Peris L., Thery M., Faure J., Saoudi Y., Lafanechere L., Chilton J. K., Gordon-Weeks P., Galjart N., Bornens M., Wordeman L., et al. (2006). Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J. Cell Biol. 174, 839-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris L., Wagenbach M., Lafanechere L., Brocard J., Moore A. T., Kozielski F., Job D., Wordeman L., Andrieux A. (2009). Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell Biol. 185, 1159-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. (1985). Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 101, 2085-2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. (1987). Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 104, 289-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessmann U., Reiter-Owona I., Lechtreck K. F. (2004). Posttranslational modifications of alpha-tubulin of Toxoplasma gondii. Parasitol. Res. 94, 386-389 [DOI] [PubMed] [Google Scholar]
- Pugacheva E. N., Jablonski S. A., Hartman T. R., Henske E. P., Golemis E. A. (2007). HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker V., Levilliers N., Schmitter J. M., Le Caer J. P., Rossier J., Adoutte A., Bre M. H. (1994). Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science 266, 1688-1691 [DOI] [PubMed] [Google Scholar]
- Reed N. A., Cai D., Blasius T. L., Jih G. T., Meyhofer E., Gaertig J., Verhey K. J. (2006). Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166-2172 [DOI] [PubMed] [Google Scholar]
- Regnard C., Fesquet D., Janke C., Boucher D., Desbruyeres E., Koulakoff A., Insina C., Travo P., Edde B. (2003). Characterisation of PGs1, a subunit of a protein complex co-purifying with tubulin polyglutamylase. J. Cell Sci. 116, 4181-4190 [DOI] [PubMed] [Google Scholar]
- Ren Y., Zhao J., Feng J. (2003). Parkin binds to alpha/beta tubulin and increases their ubiquitination and degradation. J. Neurosci. 23, 3316-3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de la Vega M., Sevilla R. G., Hermoso A., Lorenzo J., Tanco S., Diez A., Fricker L. D., Bautista J. M., Aviles F. X. (2007). Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 21, 851-865 [DOI] [PubMed] [Google Scholar]
- Rogowski K., Juge F., van Dijk J., Wloga D., Strub J. M., Levilliers N., Thomas D., Bre M. H., Van Dorsselaer A., Gaertig J., et al. (2009). Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 137, 1076-1087 [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A., Vale R. D. (2008). Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451, 363-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Acosta G., Russell W. K., Deyrieux A., Russell D. H., Wilson V. G. (2005). A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Mol. Cell Proteomics 4, 56-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger A. H., Rudiger M., Wehland J., Weber K. (1999). Monoclonal antibody ID5: epitope characterization and minimal requirements for the recognition of polyglutamylated alpha- and beta-tubulin. Eur. J. Cell Biol. 78, 15-20 [DOI] [PubMed] [Google Scholar]
- Sakakibara H., Kojima H., Sakai Y., Katayama E., Oiwa K. (1999). Inner-arm dynein c of Chlamydomonas flagella is a single-headed processive motor. Nature 400, 586-590 [DOI] [PubMed] [Google Scholar]
- Schneider A., Plessmann U., Weber K. (1997). Subpellicular and flagellar microtubules of Trypanosoma brucei are extensively glutamylated. J. Cell Sci. 110, 431-437 [DOI] [PubMed] [Google Scholar]
- Schulze E., Asai D. J., Bulinski J. C., Kirschner M. (1987). Posttranslational modification and microtubule stability. J. Cell Biol. 105, 2167-2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Bryant J., Wloga D., Donaldson R., Davis R. C., Jerka-Dziadosz M., Gaertig J. (2007). Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 178, 1065-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q., Zheng X., McNutt M. A., Guang L., Sun Y., Wang J., Gong Y., Hou L., Zhang B. (2009). NAT10, a nucleolar protein, localizes to the midbody and regulates cytokinesis and acetylation of microtubules. Exp. Cell Res. 315, 1653-1667 [DOI] [PubMed] [Google Scholar]
- Solinger J. A., Paolinelli R., Kloss H., Scorza F. B., Marchesi S., Sauder U., Mitsushima D., Capuani F., Sturzenbaum S. R., Cassata G. (2010). The Caenorhabditis elegans Elongator complex regulates neuronal alpha-tubulin acetylation. PLoS Genet. 6, e1000820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steczkiewicz K., Kinch L., Grishin N. V., Rychlewski L., Ginalski K. (2006). Eukaryotic domain of unknown function DUF738 belongs to Gcn5-related N-acetyltransferase superfamily. Cell Cycle 5, 2927-2930 [DOI] [PubMed] [Google Scholar]
- Suryavanshi S., Edde B., Fox L. A., Guerrero S., Hard R., Hennessey T., Kabi A., Malison D., Pennock D., Sale W. S., et al. (2010). Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr. Biol. 20, 435-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazhath R., Liu C., Gaertig J. (2002). Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat. Cell Biol. 4, 256-259 [DOI] [PubMed] [Google Scholar]
- Thazhath R., Jerka-Dziadosz M., Duan J., Wloga D., Gorovsky M. A., Frankel J., Gaertig J. (2004). Cell context-specific effects of the beta-tubulin glycylation domain on assembly and size of microtubular organelles. Mol. Biol. Cell 15, 4136-4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A. D., Marmo T. P., Salam A. A., Che S., Finkelstein E., Kabarriti R., Xenias H. S., Mazitschek R., Hubbert C., Kawaguchi Y., et al. (2007). HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J. Cell Sci. 120, 1469-1479 [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A., Cabrero J. R., Serrador J. M., Sanchez-Madrid F. (2008). HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell. Biol. 18, 291-297 [DOI] [PubMed] [Google Scholar]
- van Dijk J., Rogowski K., Miro J., Lacroix B., Edde B., Janke C. (2007). A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 26, 437-448 [DOI] [PubMed] [Google Scholar]
- Verhey K. J., Gaertig J. (2007). The tubulin code. Cell Cycle 6, 2152-2160 [DOI] [PubMed] [Google Scholar]
- Vogel P., Hansen G., Fontenot G., Read R. (2010). Tubulin tyrosine ligase-like 1 deficiency results in chronic rhinosinusitis and abnormal development of spermatid flagella in mice. Vet. Pathol. 47, 703-712 [DOI] [PubMed] [Google Scholar]
- Wandosell F., Serrano L., Avila J. (1987). Phosphorylation of alpha-tubulin carboxyl-terminal tyrosine prevents its incorporation into microtubules. J. Biol. Chem. 262, 8268-8273 [PubMed] [Google Scholar]
- Wang T., Morgan J. I. (2007). The Purkinje cell degeneration (pcd) mouse: an unexpected molecular link between neuronal degeneration and regeneration. Brain Res. 1140, 26-40 [DOI] [PubMed] [Google Scholar]
- Webster D. R., Gundersen G. G., Bulinski J. C., Borisy G. G. (1987). Assembly and turnover of detyrosinated tubulin in vivo. J. Cell Biol. 105, 265-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H., Bradke F. (2008). The role of the cytoskeleton during neuronal polarization. Curr. Opin. Neurobiol. 18, 479-487 [DOI] [PubMed] [Google Scholar]
- Witte H., Neukirchen D., Bradke F. (2008). Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D., Rogowski K., Sharma N., Van Dijk J., Janke C., Edde B., Bre M. H., Levilliers N., Redeker V., Duan J., et al. (2008). Glutamylation on alpha-tubulin is not essential but affects the assembly and functions of a subset of microtubules in Tetrahymena thermophila. Eukaryotic Cell 7, 1362-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D., Webster D. M., Rogowski K., Bre M. H., Levilliers N., Jerka-Dziadosz M., Janke C., Dougan S. T., Gaertig J. (2009). TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 16, 867-876 [DOI] [PubMed] [Google Scholar]
- Wloga D., Dave D., Meagley J., Rogowski K., Jerka-Dziadosz M., Gaertig J. (2010). Hyperglutamylation of tubulin can either stabilize or destabilize microtubules in the same cell. Eukaryotic Cell 9, 184-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A., de Nechaud B., Chillet D., Mazarguil H., Desbruyeres E., Audebert S., Edde B., Gros F., Denoulet P. (1992). Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur. J. Cell Biol. 59, 425-432 [PubMed] [Google Scholar]
- Wong C. C., Xu T., Rai R., Bailey A. O., Yates J. R., 3rd, Wolf Y. I., Zebroski H., Kashina A. (2007). Global analysis of posttranslational protein arginylation. PLoS Biol. 5, e258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., El Bissati K., Verdier-Pinard P., Burd B., Zhang H., Kim K., Fiser A., Angeletti R. H., Weiss L. M. (2010). Post-translational modifications to Toxoplasma gondii alpha- and beta-tubulins include novel C-terminal methylation. J. Proteome Res. 9, 359-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambito A. M., Wolff J. (1997). Palmitoylation of tubulin. Biochem. Biophys. Res. Commun. 239, 650-654 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ma C., Delohery T., Nasipak B., Foat B. C., Bounoutas A., Bussemaker H. J., Kim S. K., Chalfie M. (2002). Identification of genes expressed in C. elegans touch receptor neurons. Nature 418, 331-335 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kwon S., Yamaguchi T., Cubizolles F., Rousseaux S., Kneissel M., Cao C., Li N., Cheng H. L., Chua K., et al. (2008). Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol. Cell. Biol. 28, 1688-1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman Y., Ballestrem C., Carramusa L., Mazitschek R., Khochbin S., Bershadsky A. (2009). Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J. Cell Sci. 122, 3531-3541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.