Abstract
Appropriate regulation of signal transduction pathways is essential for normal development and is often disrupted in disease. Therefore, many regulatory mechanisms and feedback loops have evolved to ensure appropriate signalling. One mechanism previously suggested to modulate a range of signal transduction pathways involves the internalisation and destruction of transmembrane receptors by the endocytic trafficking machinery. Strikingly, a recent report has suggested that the endocytic trafficking of the Drosophila JAK–STAT pathway receptor Domeless (Dome) does not act to downregulate pathway activity, but rather is necessary for in vivo signalling. Here, we examine this relationship to address the interaction of Drosophila JAK–STAT pathway signalling and endocytic trafficking. We show that Dome is trafficked through clathrin-mediated endocytosis and a directed RNAi screen identified several components of the endocytic machinery as negative regulators of pathway signalling. We demonstrate that Dome signals both from the plasma membrane and internalised vesicles and show, using knockdown experiments, that endocytic components negatively regulate JAK–STAT signalling in vivo. As such, disruption in endocytic trafficking represents a potent negative regulator of the disease relevant JAK–STAT signalling cascade.
Keywords: Drosophila, JAK, STAT, Endocytosis
Introduction
The exchange of information between cells, and the appropriate, regulated response to those signals are essential processes required for development, cell differentiation and homeostasis. An example of such information transfer is mediated by signal transduction cascades activated by the binding of ligands to transmembrane receptors. This ligand–receptor interaction activates a cascade of downstream molecules resulting in a specific coordinated response, including morphological changes, cellular proliferation, differentiation and cell death (Calo et al., 2003; Sadowski et al., 2009).
The appropriate and timely regulation of signalling cascades to produce appropriate cellular responses is crucial, and their breakdown has been implicated in a wide array of disorders. Regulation of the JAK–STAT signal transduction cascade represents one such disease-relevant pathway and misregulation of pathway signalling has been shown to lead to immune disorders (Minegishi et al., 2006), gigantism (Metcalf et al., 2000) and erythrocytosis (Gouilleux et al., 1995), as well as a range of blood malignancies and solid neoplasias (Calo et al., 2003; Igaz et al., 2001; Rawlings et al., 2004).
The JAK–STAT transduction cascade is stimulated by interferons, cytokines and growth factors, which activate a large family of single-pass, non-tyrosine-kinase receptors (Leonard and Lin, 2000), whose cytoplasmic domains are constitutively associated with Janus kinases (JAKs) (Behrmann et al., 2004). In the canonical model, binding of the extracellular ligand to the receptor induces conformational changes in its cytoplasmic domain that activate the associated JAKs (Muller-Newen et al., 2000). Predimerised signal transducers and activators of transcription (STATs) are tyrosine phosphorylated by the JAKs, translocate to the nucleus, bind to DNA sequences present in the promoters of pathway target genes and activate transcription. This gene expression then drives a wide range of developmental, haematological and immune-related responses in many tissues (Arbouzova and Zeidler, 2006; Brown et al., 2003).
Endocytosis is the process by which cells internalise material from the extracellular environment. Much of our current knowledge comes from study of clathrin-mediated endocytosis, although other routes of entry specific for particular cargoes are also used (Conner and Schmid, 2003; Grant and Donaldson, 2009; Traub, 2009). Material to be internalised, including ligand–receptor complexes, is enclosed in vesicles that bud from the interior face of the plasma membrane into the cell. In the case of clathrin-mediated uptake, cargo is concentrated into specialised areas of the cell surface, termed coated pits, where the major coat protein is clathrin. Cargo is selected by interaction of the cytoplasmic tails of receptors with endocytic adaptors, the best characterised of which is AP2. Following scission of the endocytic vesicle from the plasma membrane, the endocytic vesicle fuses with the early endosome from where cargo is sorted, either for recycling via a Rab4- or Rab11-dependent pathway or for degradation in lysosomes via Rab7-positive late endosomes (Pryor and Luzio, 2009).
Previous studies have suggested a link between endocytic trafficking and JAK–STAT signalling (Devergne et al., 2007; Marchetti et al., 2006). In addition, research centred on interferon (IFN) signalling in vertebrate systems has demonstrated that IFN receptors are internalised by both clathrin-dependent and -independent mechanisms (Marchetti et al., 2006; Sadir et al., 2001). Furthermore, this endocytic processing does not simply attenuate receptor activity by degradation, but can also act as a modulator of signalling, such that qualitative differences in signalling result from endocytosis via different routes (Claudinon et al., 2007; Marchetti et al., 2006). By contrast, one study in Drosophila has suggested that JAK–STAT signalling is inhibited in vivo following a block in endocytic trafficking caused by mutations in a range of endocytic components (Devergne et al., 2007).
Both the JAK–STAT pathway and the endocytic trafficking machinery have been maintained throughout evolution and have been studied in both vertebrate and invertebrate model systems (reviewed by Arbouzova and Zeidler, 2006; Grant and Donaldson, 2009; Narayanan and Ramaswami, 2001; Traub, 2009). Drosophila in particular represents a powerful experimental model for the study of these processes, combining low levels of molecular complexity, reduced redundancy and the availability of genetic tools including both cell-based and in vivo RNAi (Kuttenkeuler and Boutros, 2004).
Here, we describe our analysis of the interplay between Drosophila JAK–STAT signalling and the endocytic pathway. We show that ligand–receptor complexes are trafficked through the endocytic pathway and report on a focused, high-resolution RNAi-based screen, which identified several endocytic pathway components as negative regulators of JAK–STAT signalling. Furthermore, and in results at odds with previously published work (Devergne et al., 2007), we find that receptor–ligand complexes trapped both on the plasma membrane and within the endocytic trafficking pathway are capable of inducing an enhanced STAT92E-mediated transcriptional response. Finally, we translated these cell-based findings in vivo and show that knockdown of endocytic components is sufficient to increase JAK–STAT signalling in a range of tissues and assays. As such, and in contrast to the results of Devergne and colleagues (Devergne et al., 2007), we suggest that endocytic trafficking acts as a negative regulator of Drosophila JAK–STAT pathway signalling.
Results
Trafficking of Upd2 in Kc167 cells
Genome-wide RNAi screens undertaken to identify regulators of Drosophila JAK–STAT signalling have previously identified the endocytic regulators Rab5 and TSG101 as negative regulators of STAT-mediated transcriptional activation (Müller et al., 2005) whereas in vivo studies in the Drosophila ovary have suggested that trafficking into early endosomes is a prerequisite for JAK–STAT signalling in vivo (Devergne et al., 2007).
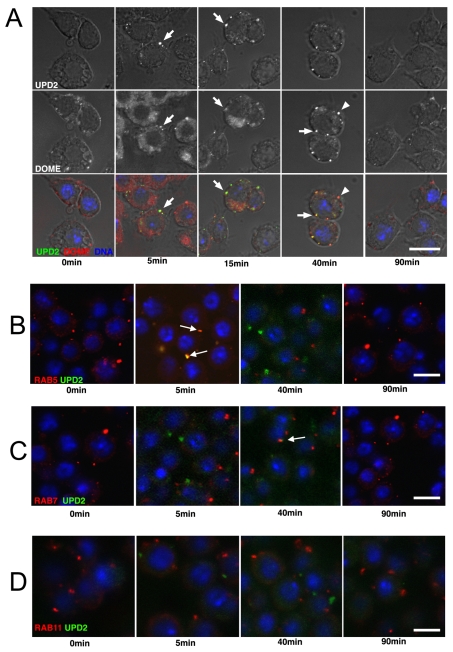
To clarify the role of endocytic trafficking in JAK–STAT signalling, we used the Drosophila Kc167 cell line previously described as a model for JAK–STAT signalling (Müller et al., 2005). We visualised the subcellular distribution of the Drosophila Domeless (Dome) receptor (Brown et al., 2001; Chen et al., 2002) in untreated cells (Fig. 1A, 0 minutes) having first established the fidelity of the Dome antibody (supplementary material Fig. 1A) and following stimulation with medium conditioned with an Unpaired2-GFP (Upd2-GFP) fusion (Hombria et al., 2005), although it should be noted that the activity of the GFP fusion in comparison with the untagged protein has not been addressed. We first undertook pulse–chase experiments to visualise endocytic uptake of ligand and both 5 and 15 minutes after stimulation, bright puncta of Upd2-GFP colocalised with Dome in all cells (Fig. 1A, 5 minutes), whereas after 40 minutes, additional puncta containing only Dome were also detected. By 90 minutes after Upd2-GFP stimulation, ligand was no longer detectable and only Dome was present (Fig. 1A, 90 minutes).
Fig. 1.
Upd2–Dome is trafficked through the endocytic pathway. (A) Cells treated with Upd2-GFP for 15 minutes at 4°C (top row or green) before a chase of the indicated time, acid washing and fixation. Staining of endogenous Domeless (Dome; middle row or red) shows a punctuate pattern that colocalises with Upd2-GFP at 5 and 15 minute time points (white arrows). By 40 minutes, puncta of Dome not colocalised with Upd2-GFP are visible (arrowhead), whereas by 90 minutes, ligand is no longer detected. (B–D) Colocalisation of Upd2-GFP (green) with vesicles labelled by antibodies specific to the early endosomal marker Rab5 (arrows in B) and the late endosomal marker Rab7 (arrow in C). Similar colocalisation is not observed with the marker of recycling endosomes Rab11 (D). Scale bars: 10 μm.
Consistent with a model in which Upd2 is trafficked through canonical clathrin-mediated endocytosis, double labelling for the early endosomal marker Rab5 (Gorvel et al., 1991) showed colocalisation of intracellular Upd2-GFP with Rab5 labelled early endosomes 5 minutes after stimulation (Fig. 1B), which, when quantified, showed that 77% (n=63) of GFP-positive vesicles colocalised with Rab5 at this time point. At the later 40 minute time point, colocalisation was principally observed in late endosomes (Fig. 1C) with 79% (n=102) of GFP-positive vesicles colocalising with Rab7 (Lebrand et al., 2002). Colocalisation with Rab11-labelled recycling endosomes (Tanaka and Nakamura, 2008) was not observed (Fig. 1D). Similar results were also obtained using fluorescently tagged Rab5, Rab7 and Rab11 (supplementary material Fig. S1B–D) (Marois et al., 2006) whereas colocalisation of Upd2-GFP with the late-endosome- or lysosome-specific dye lysotracker in live cells was also observed at 40 minutes (supplementary material Fig. S1E).
Overall, these findings suggest that Upd2–Dome complexes traffic through the canonical clathrin-mediated endocytic pathway in Kc167 cells; a result that is consistent with previous in vivo studies (Devergne et al., 2007).
JAK–STAT transcriptional output is modulated by endocytic-related genes
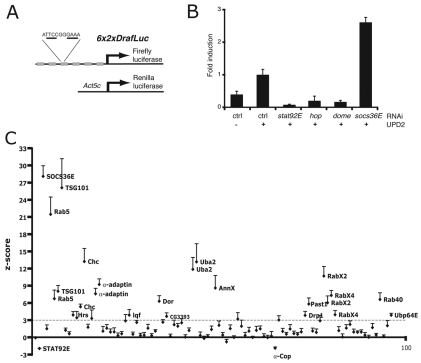
To determine the potential effect of endocytic trafficking on the transcriptional output of JAK–STAT signalling, we used the established 6x2xDrafLuc reporter assay (Müller et al., 2005) (Fig. 2A). Kc167 cells transfected with 6x2xDrafLuc and the constitutively expressed Act5c-RL control expressing Renilla luciferase were treated with double stranded (ds) RNA to trigger RNAi-mediated destruction of the mRNAs encoding the canonical pathway components dome, hop and stat92E. Upd2-GFP or mock-conditioned medium was then added to stimulate the JAK–STAT pathway and the resultant luciferase activity measured after 18 hours. For control RNAi-treated cells, addition of Upd2-GFP significantly increased pathway activity over background levels, whereas knockdown of positively acting pathway components was sufficient to reduce reporter activity to below background levels (Fig. 2B). Conversely, knockdown of the negative regulator socs36E (Callus and Mathey-Prevot, 2002; Müller et al., 2005) was sufficient to significantly increase reporter activity (Fig. 2B).
Fig. 2.
RNAi screen for endocytic regulators of JAK–STAT signalling. (A) Schematic representation of the 6x2DrafLuc reporter showing the six groups of paired STAT92E binding sites and the pAct5c-Renilla Luciferase plasmid used as a viability control. The palindromic DNA sequence recognised by STAT92E is underlined. (B) Stimulation of the 6x2xDrafLuc reporter in cells previously treated with dsRNA targeting the indicated JAK–STAT pathway components and following treatment with ligand as indicated. Values are normalised to activity elicited by Upd2 in cells treated with control dsRNA. Error bars show s.d. of four samples. (C) Screen results plotted as z-scores (y-axis) calculated for each dsRNA (x-axis) compared with the controls present on each assay plate for each of four independent experiments. Genes that exceed a threshold of 3 s.d. on average from the mean of controls are considered to be genuine interactors and are labelled. A full list of genes tested and dsRNAs used is provided in supplementary material Table S1.
We next drew up a list of putative Drosophila endocytic genes based on gene ontological and literature searches and designed independent, in-silico-optimised (Arziman et al., 2005) dsRNAs to target the mRNA encoding each factor (supplementary material Table S1). In total, 93 dsRNAs targeting both controls and 67 endocytic genes were tested in quadruplicate as part of a medium-throughput screen (Fig. 2C). Results are shown as z-scores calculated with respect to controls present in every assay plate and repeated in four independent experimental replicates (see the Materials and Methods). As expected, dsRNAs targeting rab5, tsg101 and socs36E were re-identified as negative regulators of JAK–STAT signalling (Fig. 1C) (Müller et al., 2005), confirming the technical robustness of the assay. A number of additional endocytic related genes were also identified as significant JAK–STAT pathway modulators. These include additional loci involved in the formation of the early endosomes including: clathrin heavy chain (chc), the epsin homologue liquid facets (lqf) and the AP2 adaptor α-adaptin, as well as additional small GTPases including RabX2 and RabX4 (Zhang et al., 2007). In addition, a number of genes are also identified whose functions reflected aspects of ubiquitylation and/or protein sorting, including deep orange, the Drosophila homologue of the VPS18 ubiquitin ligase (Yogosawa et al., 2005), Hrs, a factor involved in the sorting of ubiquitylated membrane proteins within the early endosome (Lloyd et al., 2002; Raiborg et al., 2002) and the SUMO-E1 subunit Uba2 (Johnson et al., 1997). Genes identified as significant interactors and their associated gene ontology are listed in Table 1. It should, however, be noted that the z-scores plotted represent a measure of statistical significance and do not directly indicate the strength of interaction (i.e. socs36E is not necessarily 10× ‘better’ than RabX4).
Table 1.
Genes identified as significant interactors and their associated gene ontology
Although a failure to interact in this assay cannot be taken as proof that a gene does not modulate JAK–STAT signalling, it is intriguing that although 8 of 21 early endosomal genes (38%) interact, only 2 of 28 sorting or recycling-related genes (7%) show significant effects. Although fewer in number, 3 of 5 (60%) of ubiquitylation-related genes were also identified. It remains to be determined whether these differences reflect the underlying biology of Upd2–Dome trafficking in vivo.
Trapping of JAK–STAT components within the endocytic pathway
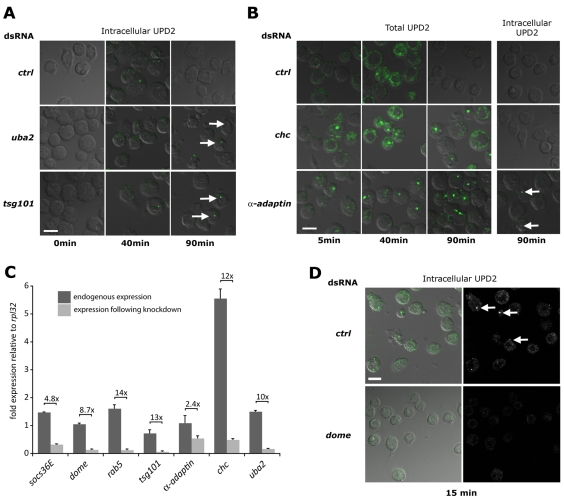
Given the identification of endocytic genes as negative regulators of STAT92E-mediated transcription, we next set out to directly visualise Upd2–Dome throughout the endocytic system in these genetic backgrounds. We therefore treated cells with dsRNA before pulsing with Upd2-GFP for 15 minutes at 4°C and chasing with unconditioned medium for the indicated times. After 0, 40 and 90 minutes, cells were washed with low-pH buffer to remove extracellular Upd2-GFP, fixed and visualised. Using this approach, discrete intracellular puncta of Upd2-GFP were clearly visible in all genotypes after 40 minutes' chase, but were no longer apparent by 90 minutes in the control (Fig. 3A). By contrast, intracellular puncta were still visible at 90 minutes in cells treated with dsRNA targeting uba2 and tsg101 (arrows in Fig. 3A), suggesting a block in the normal trafficking of Upd2–Dome through the endocytic pathway under these knockdown conditions. This is consistent with a model in which the duration and/or intensity of signalling is increased upon knockdown of uba2 and tsg101. Although not unexpected for well-characterised endocytic components such as TSG101, the trapping of Upd2-GFP following the knockdown of the SUMO-E1 conjugation enzyme subunit Uba2 suggests that SUMOylation has a role in intracellular trafficking or destruction of ligands following normal JAK–STAT pathway signalling.
Fig. 3.
Endocytic gene knockdown and trapping of Upd2–Dome. Kc167 cells treated with the indicated dsRNAs before stimulation with Upd2-GFP (green) and incubation for the indicated times. Cells were either fixed directly (Total UPD2) or acid washed to remove external ligand (Intracellular UPD2). Cells are visualised by transmitted DIC illumination (grey). (A) Intracellular Upd2-GFP is clearly detectable within cells treated with control dsRNA following a 40 minute chase but cleared by 90 minutes. By contrast, cells treated with dsRNA targeting uba2 or tsg101 retain Upd2-GFP-labelled vesicles at 90 minutes (arrows). (B) Cells stained to visualise both cell surface and internalised Upd2-GFP are still associated with ligand following a 40 minute chase. Whereas wild-type cells treated with control dsRNA have cleared Upd2 after 90 minutes, cells treated with dsRNA targeting chc and α-adaptin retain Upd2-GFP (column 3). However, acid washing to remove extracellular Upd2-GFP reveals that no ligand is present within chc-knockdown cells, and only a few vesicles are present following knockdown of α-adaptin (arrows in column 4). (C) Quantitative-PCR of the indicated genes expressed in Kc167 cells showing fold expression relative to the ribosomal protein L32 gene (rpl32). Endogenous levels (dark bars) and expression following knockdown with dsRNA (light bars) are shown, as is the level of knockdown. All knockdowns are significant (P<0.001), except that of α-adaptin. (D) Intracellular Upd2-GFP is detected in cells treated with control dsRNA (arrows) but not in cells in which the domeless (dome) receptor is knocked down. Scale bars: 10 μm.
This apparent trapping is also consistent with the increase in transcriptional pathway output caused by knockdown of these loci (Fig. 2C), suggesting a link between endocytic trafficking and the downregulation of JAK–STAT signalling in the wild-type scenario.
Domeless signals from the plasma membrane
In addition to enabling visualisation of Upd2–GFP trapped within intracellular compartments, microscopy analysis also allowed us to address previous reports suggesting that the Drosophila JAK–STAT receptor Dome does not signal from the plasma membrane (Devergne et al., 2007). This is particularly unexpected given previous results in HeLa cells following siRNA knockdown of human CHC (Marchetti et al., 2006) and our own transcriptional assays, which indicate an increase in pathway activity following knockdown of chc and α-adaptin (Fig. 2C) – treatments that are predicted to block the endocytosis of Upd2–Dome complexes from the plasma membrane. We therefore tested the behaviour of Upd2-GFP in control, chc and α-adaptin dsRNA knockdown conditions. After 40 minutes' chase, Upd2 was still associated with cells treated with all dsRNAs (Fig. 3B). By 90 minutes, control cells had cleared detectable Upd2-GFP both from the plasma membranes and internally. However, significant levels of extracellular ligand were still associated with cells in which chc and α-adaptin mRNA had been knocked down (Fig. 3B). Furthermore, acid washing of these cells (Knisely et al., 2008) to remove Upd2-GFP on the plasma membrane completely removed all signal from the Chc-depleted cells at 90 minutes, indicating that bound Upd2 was located on the plasma membrane (Fig. 3B). As such, the increased STAT92E transcriptional output elicited by knockdown of Chc and α-adaptin (Fig. 2C) and the trapping of Upd2 on the plasma membrane (Fig. 3B) suggests that Dome can signal from the plasma membrane.
As observed for Chc, knockdown of α-adaptin also trapped a large proportion of Upd2-GFP on the plasma membrane (Fig. 3B). However, in this case, a small proportion of Upd2 was also internalised and subsequently trapped in intracellular vesicles (arrows in Fig. 3B). Given that this residual internalisation might occur because of incomplete knockdown, we undertook Q-PCR to determine the expression levels of targeted genes both before and after dsRNA treatment. Generally, dsRNA-mediated knockdown after 4 days was robust, and ranged from 2.4- to 14-times lower than endogenous levels (Fig. 3C). However the reduction in α-adaptin mRNA represented the least-effective knockdown, and the expression of residual transcript might explain the presence of intracellular puncta in Fig. 3B (arrows).
Finally, to ensure that the observed uptake and trapping of Upd2-GFP was not a consequence of fluid-phase uptake of conditioned medium, we also undertook experiments in which we knocked down either the JAK–STAT pathway receptor domeless (Arbouzova and Zeidler, 2006; Brown et al., 2003) or a control dsRNA not expressed in Kc167 cells. Although intracellular Upd2-GFP was present in controls after 15 minutes, no GFP-labelled vesicles were found after knockdown of dome, suggesting that uptake is indeed receptor dependent (Fig. 3D).
JAK–STAT and endocytic interactions in vivo
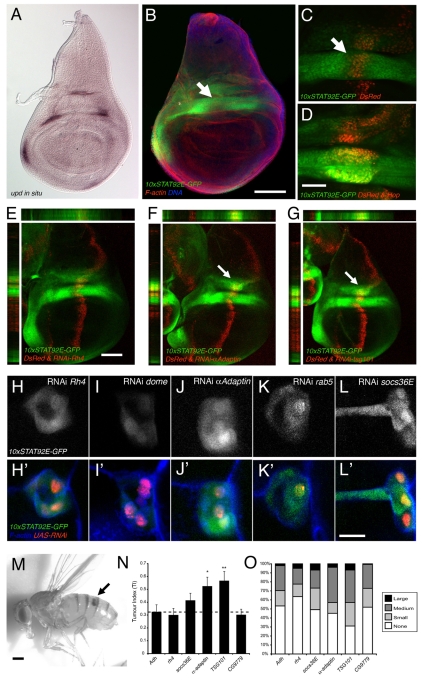
Having shown that knockdown of endocytic components is sufficient to modulate the JAK–STAT cascade in Kc167 cells, we set out to examine whether this was also the case in vivo. The 10xSTAT92E-GFP reporter has been shown to be dynamically expressed throughout embryogenesis, larval and adult life, in patterns consistent with known Upd expression and so represents an in vivo reporter of JAK–STAT pathway activity (Bach et al., 2007). This reporter is expressed within the third instar wing imaginal disc in a pattern that mirrors upd expression (Fig. 4A,B) and is sensitive to modulation of pathway activity by the ectopic expression of Hop (Fig. 4C,D).
Fig. 4.
Modulation of in vivo JAK–STAT signalling by endocytic genes. (A–G) Third instar larval wing imaginal discs showing either the pattern of Upd expression [A; reproduced from Mukherjee et al. (Mukherjee et al., 2005)] or the resulting pattern of 10xSTAT-GFP reporter expression (B–G). The expression domain of patched-Gal4 (red cells in C–G) bisects the central hinge domain of JAK–STAT pathway activity (arrows in B and C) with co-expression of UAS-hop, producing overactivation of the reporter in this area (D). Misexpression of UAS-RNAi transgenes targeting the indicated genes shows a clear increase in GFP in the dorsal hinge following knock down of a-adaptin and tsg101 (arrows in F and G). (H–L′) Migrating border cells showing expression of the 10xSTAT-GFP reporter (white or green) in cells expressing the indicated UAS-RNAi transgenes. Bottom row includes phalloidin to indicate cellular morphology (blue) and the nuclear DsRed (red) as a reporter of Gal4 expression. All samples were captured under identical microscope settings. (M) Adult female Drosophila heterozygous for the dominant gain-of-function hopTuml allele with a melanised haematological tumour in the abdomen (arrow). (N) Graph showing the average tumour index of adult flies heterozygous for hopTuml and expressing the indicated the UAS-RNAi transgenes within the larval haematopoietic organ. Knockdown of alcohol dehydrogenase (Adh) and rhodopsin 4 (rh4) were used as controls. 0.05>*P>0.01, 0.01>**P>0.001. (O) Distribution of tumour sizes within individuals counted, showing the qualitative increase in tumour sizes following knockdown of the indicated genes. Scale bars: 100 μm (A,B), 20 μm (C,D and H–L′), 100 μm (E–G), 0.36 mm (M).
To test the potential interaction of endocytic gene knockdown within the developing wing, we generated a triple recombinant carrying ptc-Gal4 expressed in a stripe through the middle of the wing (red in Fig. 4E–G), UAS-DsRed to mark the Gal4 expression domain and the 10xSTAT-GFP reporter (Fig. 4E). In this way, ptc-Gal4 can be used to express in vivo inducible shRNA constructs (Dietzl et al., 2007) (henceforth termed UAS-RNAi) to knockdown genes of interest. This knockdown is induced both in regions lacking JAK–STAT pathway activity and through the area of pathway activity induced by Upd expression within the future wing hinge (arrows in Fig. 4B,C). We therefore crossed the ptc-Gal4, UAS-DsRed, 10xSTAT92E-GFP recombinant to UAS-RNAi transgenes that knocked down a control gene, α-adaptin or tsg101 (Fig. 4E–G). Although not sufficient to activate signalling in regions not already stimulated by Upd, knockdown of endocytic trafficking machinery components upregulated the 10xSTAT92E-GFP reporter activity within the medial hinge region of Upd expression as seen in x-y, x-z and y-z sections (arrows in Fig. 4F,G). This reporter upregulation occurred specifically within cells exposed to high levels of Upd and suggests that endocytic trafficking normally acts as a negative regulator of Upd-stimulated JAK–STAT pathway signalling in imaginal disc tissue.
We next examined the influence of endocytic trafficking on JAK–STAT pathway activity during oogenesis; a developmental process that requires JAK–STAT signalling for patterning of the follicular epithelium surrounding the developing oocyte (Xi et al., 2003) and the specification and migration of border cells (Beccari et al., 2002; Silver et al., 2005). Using the same 10xSTAT92E-GFP reporter in combination with DsRed-labelled clones of Gal4-expressing cells we knocked down control genes, components of the endocytic trafficking machinery and socs36E within the migrating border cells of stage-10 oocytes (Fig. 4H,L). Using identical settings to visualise all samples, it was clear that knockdown of Dome was sufficient to reduce reporter expression (compare Fig. 4H with 4I), whereas knockdown of α-adaptin, rab5 and socs36E increased levels of 10xSTAT92E-GFP reporter expression; a finding that is consistent with previous reports at earlier stages of border cell migration (Silver et al., 2005). As previously shown in imaginal disc tissue, knockdown of endocytic trafficking components in Upd-expressing border cells resulted in an increase in JAK–STAT signalling.
Finally, we tested the effects of UAS-RNAi knockdown of components of the endocytic machinery in an independent whole-organism assay for tumour development. As is the case in human myeloproliferative disease (Kralovics et al., 2005; Vainchenker and Constantinescu, 2005), constitutive gain-of-function mutations in the Drosophila JAK homologue Hop (termed hopTuml) (Luo et al., 1995) result in the over-proliferation of haemocytes within the larval lymph gland, giving rise to black melanotic tumours in both larvae and adults (Fig. 4M). We expressed UAS-RNAi constructs within the lymph gland during larval stages in an attempt to modulate the frequency and severity of the resulting tumour phenotypes as assessed by a count of tumour sizes in adult flies, which was quantified as a tumour index (TI) (Shi et al., 2006; Bina et al., 2010). Using both the TI and the distribution of tumour sizes within individual flies following knockdown of the Adh and rh4 loci as controls, we compared knockdown of endocytic genes originally identified as negative regulators of STAT92E activity. Although knockdown of both Chc and Rab5 in vivo cause lethality (not shown), UAS-RNAi constructs targeting α-adaptin and tsg101 resulted in a statistically significant increase in the TI observed, and a qualitative increase in the severity of tumours (Fig. 4N,O).
We therefore show, both in Kc167 cells and in three independent in vivo experiments undertaken in several cell types, that knockdown of components of the endocytic machinery results in an increase in the strength of JAK–STAT pathway signalling. This result is consistent with a model in which activated ligand–receptor complex is trapped on the plasma membrane or within endocytic vesicles in a conformation that is competent to signal and activate STAT92E-mediated transcription.
Discussion
Modulation of the JAK–STAT signal transduction pathway by endocytic trafficking of receptor–ligand complexes represents a comparatively little-studied regulatory mechanism. Here, we present data from the low-redundancy Drosophila experimental system showing that the pathway ligand Upd2 and receptor Domeless are endocytosed via a clathrin-dependent process before colocalising with markers characteristic of early and late endosomes. Furthermore, we have undertaken a directed RNAi-based screen for components of the endocytic machinery that has identified several components as negative regulators of Drosophila JAK–STAT pathway transcriptional regulation. Knockdown of a number of these components leads to trapping or retention of Upd2 within cells suggesting that transcriptional upregulation of STAT92E is a consequence of prolonged receptor activation and/or accumulation within endocytic compartments. Finally, we undertook a series of in vivo experiments to validate the cell-based findings and obtained data from several cell types indicating that disruption of endocytic trafficking results in an increase in pathway signalling.
Endocytic trafficking and signalling
The regulation of receptor tyrosine kinases involves a close link between endocytosis and signalling (Di Fiore and De Camilli, 2001; Disanza et al., 2009; Sorkin and von Zastrow, 2009) and regulation of Notch signalling by endocytosis in Drosophila has also been extensively studied (Moberg et al., 2005; Vaccari and Bilder, 2005). It is thus clear that endocytic trafficking represents a significant factor in the regulation of numerous signal transduction pathways. However, relatively little is understood regarding the precise interaction of JAK–STAT signalling and the endocytic pathway, although one report has suggested that activation of STAT1 by IFNγ receptors is differentially regulated by clathrin-mediated endocytosis (Marchetti et al., 2006).
In the low-complexity Drosophila model system, knockdown of chc results in both trapping of Upd2 on the plasma membrane (Fig. 3B) and an increase in the transcriptional activity of STAT92E (Fig. 2C). Similarly, the knockdown of tsg101 appears to prevent degradation of Upd2–Dome complexes (Fig. 3A), as well as leading to increased activation of the 6x2xDrafLuc reporter (Fig. 2C). We therefore propose that, at steady state, Dome is present on the plasma membrane of unstimulated cells where it is able to interact with extracellular ligands. Following stimulation by Upd2, Dome–ligand complexes begin to signal to the nucleus to induce gene expression and simultaneously stimulate their own clathrin-dependent endocytosis. Signalling continues as the Dome–Upd2 complex traverses the endocytic pathway through Rab5- and Rab7-positive endosomes until complexes are ultimately sorted into MVBs and destroyed in the lysosome. When endocytosis is disrupted by RNAi-mediated knockdown of chc or tsg101, the activated Dome receptor continues to signal and stimulate transcription. However, inhibition of endocytic trafficking now leads to a build up of active Dome–ligand complexes on the plasma membrane or in endosomal compartments, which cannot be further processed, thus allowing activated receptors to increase downstream transcriptional STAT activity.
Endocytic trafficking as a negative regulator of JAK–STAT pathway activity
A previous study of Drosophila JAK–STAT pathway signalling and endocytosis used STAT92E accumulation, as well as a tissue-specific enhancer trap line sensitive to JAK–STAT signalling in posterior follicle cells as readouts for pathway activity (Devergne et al., 2007). Using these tools, Devergne and colleagues suggest that Dome is incapable of signalling from the plasma membrane, a result based on loss-of-function clones generated by mitotic recombination to genetically remove chc and deep orange. They therefore suggest that activated Dome is only able to trigger a transcriptional response to Upd from within endocytic compartments. Therefore, mutations blocking endocytosis result in a decrease in signalling-competent intracellular Dome and a concomitant decrease in transcriptional output. This conclusion is, however, inconsistent with the data we show here, which indicate an increase in pathway signalling following the removal of endocytosis pathway components (Fig. 2).
Although yet to be definitively established, one potential explanation for the contradiction between our data and that of Devergne and co-workers might lie in the different approaches used to modulate endocytosis. Although our RNAi-based approach occurs over a relatively short time frame, the longer-term removal of genes encoding endocytic components might inadvertently result in cellular mis-specification. As such, it is possible that the pointed-lacZ reporter might not be expressed because of a change in cell fate. Indeed, the STAT92E-binding sites that regulate expression of pointed-lacZ have not been identified, and although its expression is dependent on the JAK–STAT pathway in posterior follicle cells (Xi et al., 2003), its expression within other tissues, such as the developing eye, is not. Indeed, normal pointed-dependent (O'Neill et al., 1994) photoreceptor recruitment occurs within STAT92E mutant eye clones (Zeidler et al., 1999).
JAK–STAT pathway signalling, endocytosis and Notch
Previous reports have shown that removal of tsg101 and components that mediate sorting of ubiquitylated transmembrane proteins to late endosomes (Roxrud et al., 2010) within the developing eye imaginal disc result in the activation of Notch pathway signalling and non-autonomous overgrowth of surrounding tissue (Moberg et al., 2005; Vaccari and Bilder, 2005). Strikingly, this non-autonomous overgrowth is mediated by the ectopic expression of Upd, which is a target gene of Notch in the developing eye (Chao et al., 2004; Flaherty et al., 2009). Given this link, it is possible that the increase in JAK–STAT pathway activity we observe could be the indirect consequence of Notch activation. Although we cannot exclude this possibility completely for each of the systems tested, data from the wing disc suggest that the increase in JAK–STAT pathway signalling observed is direct. First, the pattern of Notch activity within the third instar wing disc (Cooper et al., 2000) does not reproduce the Upd expression pattern (Fig. 4A), suggesting that Upd is not a target of Notch signalling within the wing. Second, although ptc-Gal4/UAS-RNAi-tsg101 knocks down TSG101 levels throughout the stripe of red cells shown in Fig. 4G, only the area that intersects with Upd-expressing cells (arrow in Fig. 4G) upregulates the 10xSTAT92E-GFP reporter. This suggests that the other regions of TSG101 knockdown in the wing pouch and presumptive notum do not ectopically express Upd.
When considered together with the visual evidence indicating a trapping of Upd2–Dome within Kc167 cells following disruption of endocytic trafficking (Fig. 3) and the consistent interactions seen in multiple in vivo assays, we are confident that a direct interaction between JAK–STAT pathway signalling and endocytic trafficking represents the most plausible explanation for the effects observed.
Materials and Methods
Cell culture
Drosophila Kc167 cells (Cherbas et al., 1988) were maintained at 25°C in Schneider's Drosophila medium supplemented with 10% foetal calf serum, 1% penicillin and streptomycin (Invitrogen). Cells were maintained for a maximum of ten passages. Medium conditioned with Upd2 was generated by transfecting Kc167 cells (5×105/well in a six-well plate) with pAc5-Upd2-GFP (Hombria et al., 2005) or pAc5-Upd2-HA. After 4 days, the supernatant was centrifuged and aliquotted at − 80°C. To increase the sensitivity of dsRNA experiments to negative regulators, we titrated the concentration of Upd2 conditioned medium to increase the ‘headroom’ available within the assay design. A 1:99 dilution ratio of conditioned to unconditioned medium gave maximal socs36E-induced fold activation (see supplementary material Fig. S2).
Directed RNAi screen
Genes with Gene Ontology terms linked to endocytosis were identified using Flybase search tools (http://flybase.org/) and a library of two independent dsRNAs targeting each gene, with an average length of 300 nucleotides, was designed using the E-RNAi tool (Arziman et al., 2005). Primers flanked by T7-promotor binding sites were used to amplify regions from genomic DNA and the resulting product in vitro transcribed using MEGAscript® T7 Kit (Ambion). Primer and amplicon sequence information is available in supplementary material Table S1.
For screening, Drosophila Kc167 cells (5×106/well in a six-well plate) were transfected with 500 ng 6x2xDrafLuc reporter, 250 ng pAc5.1-Sid-1, 25 ng pAct-RL (Müller et al., 2005) and pAc5.1 (empty vector) to a total of 2 μg plasmid DNA. After 8 hours, transfected cells were resuspended in serum-free medium and counted on a haemocytometer. 4×104 cells were transferred in 100 μl volume into 96-well screening plates containing 1.5 μg/well dsRNA in serum-free medium and incubated for 60 minutes before addition of 100 μl serum-containing medium. After 4 days to allow knockdown of targeted genes, 50 μl of a 1:99 dilution of Upd2 conditioned medium in normal medium was added. After 18 hours, cells were then lysed and Firefly and Renilla luciferase activities were determined using a Mithras LB940 luminometer (Berthold Instruments). Controls present in each plate were used to calculate the z-score of all wells with dsRNAs producing a change greater than three standard deviations from the mean of all controls classed as hits. The screen was repeated as four independent replicates and results shown represent the average of the scores obtained.
Quantitative PCR
Quantitative PCR (qPCR) used 2.5×106 Kc167 cells seeded in a 12-well plate and treated with dsRNA as outlined above. After 4 days, RNA was isolated using RNeasy (Qiagen) and reverse transcribed using Verso cDNA kit (Ambion). qPCR was performed utilising SYBR Green JumpStart Taq Ready Mix for Quantitative PCR (Sigma) and the primers listed in supplementary material Table S2 on a MyIQ detection system (Bio-Rad). Results were normalised relative to levels of Ribosomal protein L32 (RpL32) mRNA.
Cell-based endocytic assays
For the visualisation of Upd endocytosis, 1×105 Kc167 cells were seeded in serum-free medium into eight-well chambers (Lab-Tek II coverglass system, nunc™) and allowed to settle for 2 hours. Cells were then stimulated with an equal volume of Upd2-GFP conditioned medium at 4°C for 15 minutes. Bound Upd2-GFP was then ‘chased’ by replacing the overlying medium with Schneider's medium at 26°C. Trafficking of Upd2-GFP was visualised over a time course by sequentially fixing at the indicated time points before antibody staining and microscopy, as described above. For experiments involving gene knockdown 1×106 Kc167 cells were resuspended in 250 μl serum-free medium and added to 2 μg/well dsRNA in a 24-well plate. After 45 minutes, an equal volume of normal Schneider's medium (including 10% FCS) was added. After 4 days, cells were counted and seeded in eight-well chambers as above.
Low pH ‘acid washes’ were performed by treating cells for 2 minutes with ice-cold acid-wash buffer (0.5 M glacial acetic acid, 150 mM NaCl, pH 2.5) followed by recovery in ice-cold PBS for 2 minutes. Both steps were repeated three times (Knisely et al., 2008).
Antibodies to detect GFP and HA were purchased from Abcam, Dome antibody was a kind gift from Stephen Brown (University of Sheffield, Sheffield, UK), anti-SOCS36E was a kind gift from Isabella Serras (Universitat de Barcelona, Barcelona, Spain), antibodies for Rab5, Rab7 and Rab11 were a kind gift from Akira Nakamura (Tanaka and Nakamura, 2008), whereas constructs for Rab5-CFP, Rab7-YFP, Rab11-YFP (Marois et al., 2006) were a gift from Suzanne Eaton (MPI for Molecular Cell Biology and Genetics, Dresden, Germany). Secondary antibodies were purchased from Invitrogen. LysoTracker Red (Invitrogen) was used at a final concentration of 50 nM on live cells. Colocalisation of Upd-GFP with Rab5 and Rab7 was scored manually from several fields of cells using Photoshop to compare the signal in separate fluorescence channels.
Histology and imaging
Wing imaginal discs were dissected from late wandering third instar larvae and fixed in PBS, 0.2% Triton-X100, 6% formaldehyde for 10 minutes. Ovaries were dissected and fixed with 0.8% formaldehyde in heptane for 18 minutes. Following fixation, tissue was washed in PBS, 0.2% Triton X-100 (PT), blocked in 5% BSA in PT; ovaries were incubated in primary and secondary antibody dilutions overnight at 4°C. They were then incubated in 30% and 50% glycerol overnight at 4°C before mounting.
Immunostaining of both ‘acid-washed’ and untreated cells was undertaken following fixation in PBS, 0.1% Triton X-100, 4% formaldehyde. The cells were then blocked with 1% BSA in PT. Primary antibodies were incubated overnight at 4°C. All images were obtained on a Zeiss 512 confocal microscope using a 63× objective and were analysed using Volocity 4 Quantification (PerkinElmer).
Fly stocks and culture
All fly stocks were kept at 25°C unless stated otherwise. The recombinant fly stocks w1118; P{w+,10xSTAT-GFP}, ptc-Gal4 / Cyo and w1118; P{w+,10xSTAT-GFP}, ptc-Gal4, P{w+,UAS-DsRed} / Cyo were generated using the second chromosomal insertion of the 10xSTAT92E-GFP reporter (Bach et al., 2007), the Gal4 expressing enhancer trap present in the patched locus P{GawB}ptcNP3253 (Brand et al., 1994) and P{w+, UAS-DsRed}B (Bloomington Stock 6281). For mosaic clone analysis, recombinant fly stocks consisting of the P{Act>y+>Gal4}25 FLP-out cassette (Ito et al., 1997), P{w+,UAS-RedStinger}4 (Bloomington Stock 8546) and the P{w+,10xSTAT-GFP} reporter were generated by standard techniques. Resulting females of the genotype y,w; P{Act>y+>Gal4}25, P{w+,UAS-RedStinger}4, P{w+,10xSTAT-GFP} / Cyo were crossed to y,w,P{hs-FLP}1; P{w+, UAS-RNAi} / Cyo, P{w+,hs-Hid} males. The resulting offspring were heat shocked as early pupae for 15 minutes at 37°C and returned to 25°C. Ovaries of flies lacking Cyo balancers were dissected for immunostaining.
Analysis of tumour index used a recombinant between domeP[G5] (Bourbon et al., 2002) and hopTum-l. The w, domeP[G5], hopTum-l / FM7 females (Bina et al., 2010) were crossed to males of the inducible UAS-RNAi lines listed below. Eggs were collected at 19°C for 24 hours, aged at 19°C for a further 24 hours and then transferred to 25°C for 11 days. Adults were scored according to published methods (Shi et al., 2006). Inducible UAS-RNAi lines used were: rhodopsin4 (46919), domeless (19717), socs36E (51821), α-adaptin (4267), rab5 (34096) and tsg10 (23944) (Dietzl et al., 2007) and were purchased from the Vienna Drosophila RNAi Centre. VDRC Transformant IDs are given in brackets for each line used.
Supplementary Material
Acknowledgments
We thank Suzanne Eaton for Rab-CYP and -YFP constructs, Akira Nakamura and Stephen Brown for antibodies, Alex Whitworth for access to a qPCR machine, Jeremy Sanderson and Kirsty Johnstone for technical support. Confocal imaging was undertaken in the Wellcome Trust supported Sheffield Light Microscopy Facility (GR077544AIA). O.M.V. was supported by a project grant of the Deutsche Forschungsgemeinschaft (DFG). M.P.Z. is a Cancer Research UK Senior Cancer Research Fellow and member of the MRC Centre for Developmental and Biomedical Genetics. Work in the E.S. lab was supported by MRC program grant G0300452. Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/20/3457/DC1
References
- Arbouzova N. I., Zeidler M. P. (2006). JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133, 2605-2616 [DOI] [PubMed] [Google Scholar]
- Arziman Z., Horn T., Boutros M. (2005). E-RNAi: a web application to design optimized RNAi constructs. Nucleic Acids Res. 33, W582-W588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach E. A., Ekas L. A., Ayala-Camargo A., Flaherty M. S., Lee H., Perrimon N., Baeg G. H. (2007). GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 7, 323-331 [DOI] [PubMed] [Google Scholar]
- Beccari S., Teixeira L., Rorth P. (2002). The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech. Dev. 111, 115-123 [DOI] [PubMed] [Google Scholar]
- Behrmann I., Smyczek T., Heinrich P. C., Schmitz-Van de Leur H., Komyod W., Giese B., Muller-Newen G., Haan S., Haan C. (2004). Janus kinase (Jak) subcellular localization revisited: the exclusive membrane localization of endogenous Janus kinase 1 by cytokine receptor interaction uncovers the Jak.receptor complex to be equivalent to a receptor tyrosine kinase. J. Biol. Chem. 279, 35486-35493 [DOI] [PubMed] [Google Scholar]
- Bina S., Wright V., Fisher K., Milo M., Zeidler M. (2010). Transcriptional targets of Drosophila JAK/STAT pathway signalling as effectors of haematopoietic tumour formation. EMBO Rep. 11, 201-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon H. M., Gonzy-Treboul G., Peronnet F., Alin M. F., Ardourel C., Benassayag C., Cribbs D., Deutsch J., Ferrer P., Haenlin M., et al. (2002). A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech. Dev. 110, 71-83 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Manoukian A. S., Perrimon N. (1994). Ectopic expression in Drosophila. Methods Cell Biol. 44, 635-654 [DOI] [PubMed] [Google Scholar]
- Brown S., Hu N., Hombria J. C. (2001). Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr. Biol. 11, 1700-1705 [DOI] [PubMed] [Google Scholar]
- Brown S., Hu N., Hombria J. C. (2003). Novel level of signalling control in the JAK/STAT pathway revealed by in situ visualisation of protein-protein interaction during Drosophila development. Development 130, 3077-3084 [DOI] [PubMed] [Google Scholar]
- Callus B. A., Mathey-Prevot B. (2002). SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene 21, 4812-4821 [DOI] [PubMed] [Google Scholar]
- Calo V., Migliavacca M., Bazan V., Macaluso M., Buscemi M., Gebbia N., Russo A. (2003). STAT proteins: from normal control of cellular events to tumorigenesis. J. Cell Physiol. 197, 157-168 [DOI] [PubMed] [Google Scholar]
- Chao J. L., Tsai Y. C., Chiu S. J., Sun Y. H. (2004). Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development 131, 3839-3847 [DOI] [PubMed] [Google Scholar]
- Chen H. W., Chen X., Oh S. W., Marinissen M. J., Gutkind J. S., Hou S. X. (2002). mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 16, 388-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas P., Cherbas L., Lee S. S., Nakanishi K. (1988). 26-[125I]iodoponasterone A is a potent ecdysone and a sensitive radioligand for ecdysone receptors. Proc. Natl. Acad. Sci. USA 85, 2096-2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinon J., Monier M. N., Lamaze C. (2007). Interfering with interferon receptor sorting and trafficking: impact on signaling. Biochimie 89, 735-743 [DOI] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. (2003). Regulated portals of entry into the cell. Nature 422, 37-44 [DOI] [PubMed] [Google Scholar]
- Cooper M. T., Tyler D. M., Furriols M., Chalkiadaki A., Delidakis C., Bray S. (2000). Spatially restricted factors cooperate with notch in the regulation of Enhancer of split genes. Dev. Biol. 221, 390-403 [DOI] [PubMed] [Google Scholar]
- Devergne O., Ghiglione C., Noselli S. (2007). The endocytic control of JAK/STAT signalling in Drosophila. J. Cell Sci. 120, 3457-3464 [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., De Camilli P. (2001). Endocytosis and signaling. an inseparable partnership. Cell 106, 1-4 [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156 [DOI] [PubMed] [Google Scholar]
- Disanza A., Frittoli E., Palamidessi A., Scita G. (2009). Endocytosis and spatial restriction of cell signaling. Mol. Oncol. 3, 280-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty M. S., Zavadil J., Ekas L. A., Bach E. A. (2009). Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of notch signaling by the JAK/STAT pathway. Dev. Dyn. 238, 2235-2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. (1991). rab5 controls early endosome fusion in vitro. Cell 64, 915-925 [DOI] [PubMed] [Google Scholar]
- Gouilleux F., Pallard C., Dusanter-Fourt I., Wakao H., Haldosen L. A., Norstedt G., Levy D., Groner B. (1995). Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 14, 2005-2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. D., Donaldson J. G. (2009). Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombria J. C., Brown S., Hader S., Zeidler M. P. (2005). Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 288, 420-433 [DOI] [PubMed] [Google Scholar]
- Igaz P., Toth S., Falus A. (2001). Biological and clinical significance of the JAK-STAT pathway; lessons from knockout mice. Inflamm. Res. 50, 435-441 [DOI] [PubMed] [Google Scholar]
- Ito K., Awano W., Suzuki K., Hiromi Y., Yamamoto D. (1997). The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761-771 [DOI] [PubMed] [Google Scholar]
- Johnson E. S., Schwienhorst I., Dohmen R. J., Blobel G. (1997). The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509-5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely J. M., Lee J., Bu G. (2008). Measurement of receptor endocytosis and recycling. Methods Mol. Biol. 457, 319-332 [DOI] [PubMed] [Google Scholar]
- Kralovics R., Passamonti F., Buser A. S., Teo S. S., Tiedt R., Passweg J. R., Tichelli A., Cazzola M., Skoda R. C. (2005). A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779-1790 [DOI] [PubMed] [Google Scholar]
- Kuttenkeuler D., Boutros M. (2004). Genome-wide RNAi as a route to gene function in Drosophila. Brief. Funct. Genomic. Proteomic. 3, 168-176 [DOI] [PubMed] [Google Scholar]
- Lebrand C., Corti M., Goodson H., Cosson P., Cavalli V., Mayran N., Faure J., Gruenberg J. (2002). Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Lin J. X. (2000). Cytokine receptor signaling pathways. J. Allergy Clin. Immunol. 105, 877-888 [DOI] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. (2002). Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261-269 [DOI] [PubMed] [Google Scholar]
- Luo H., Hanratty W. P., Dearolf C. R. (1995). An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 14, 1412-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M., Monier M. N., Fradagrada A., Mitchell K., Baychelier F., Eid P., Johannes L., Lamaze C. (2006). Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors. Mol. Biol. Cell 17, 2896-2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois E., Mahmoud A., Eaton S. (2006). The endocytic pathway and formation of the Wingless morphogen gradient. Development 133, 307-317 [DOI] [PubMed] [Google Scholar]
- Metcalf D., Greenhalgh C. J., Viney E., Willson T. A., Starr R., Nicola N. A., Hilton D. J., Alexander W. S. (2000). Gigantism in mice lacking suppressor of cytokine signalling-2. Nature 405, 1069-1073 [DOI] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Morio T., Watanabe K., Agematsu K., Tsuchiya S., Takada H., Hara T., Kawamura N., Ariga T., et al. (2006). Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25, 745-755 [DOI] [PubMed] [Google Scholar]
- Moberg K. H., Schelble S., Burdick S. K., Hariharan I. K. (2005). Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell 9, 699-710 [DOI] [PubMed] [Google Scholar]
- Mukherjee T., Hombría J. C., Zeidler M. P. (2005). Opposing roles for Drosophila JAK/STAT signalling during cellular proliferation. Oncogene 24, 2503-2511 [DOI] [PubMed] [Google Scholar]
- Müller P., Kuttenkeuler D., Gesellchen V., Zeidler M. P., Boutros M. (2005). Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436, 871-875 [DOI] [PubMed] [Google Scholar]
- Muller-Newen G., Kuster A., Wijdenes J., Schaper F., Heinrich P. C. (2000). Studies on the interleukin-6-type cytokine signal transducer gp130 reveal a novel mechanism of receptor activation by monoclonal antibodies. J. Biol. Chem. 275, 4579-4586 [DOI] [PubMed] [Google Scholar]
- Narayanan R., Ramaswami M. (2001). Endocytosis in Drosophila: progress, possibilities, prognostications. Exp. Cell Res. 271, 28-35 [DOI] [PubMed] [Google Scholar]
- O'Neill E. M., Rebay I., Tjian R., Rubin G. M. (1994). The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78, 137-147 [DOI] [PubMed] [Google Scholar]
- Pryor P. R., Luzio J. P. (2009). Delivery of endocytosed membrane proteins to the lysosome. Biochim. Biophys. Acta 1793, 615-624 [DOI] [PubMed] [Google Scholar]
- Raiborg C., Bache K. G., Gillooly D. J., Madshus I. H., Stang E., Stenmark H. (2002). Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4, 394-398 [DOI] [PubMed] [Google Scholar]
- Rawlings J. S., Rosler K. M., Harrison D. A. (2004). The JAK/STAT signaling pathway. J. Cell Sci. 117, 1281-1283 [DOI] [PubMed] [Google Scholar]
- Roxrud I., Stenmark H., Malerod L. (2010). ESCRT & Co. Biol. Cell 102, 293-318 [DOI] [PubMed] [Google Scholar]
- Sadir R., Lambert A., Lortat-Jacob H., Morel G. (2001). Caveolae and clathrin-coated vesicles: two possible internalization pathways for IFN-gamma and IFN-gamma receptor. Cytokine 14, 19-26 [DOI] [PubMed] [Google Scholar]
- Sadowski L., Pilecka I., Miaczynska M. (2009). Signaling from endosomes: location makes a difference. Exp. Cell Res. 315, 1601-1609 [DOI] [PubMed] [Google Scholar]
- Shi S., Calhoun H. C., Xia F., Li J., Le L., Li W. X. (2006). JAK signaling globally counteracts heterochromatic gene silencing. Nat. Genet. 38, 1071-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D. L., Geisbrecht E. R., Montell D. J. (2005). Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 132, 3483-3492 [DOI] [PubMed] [Google Scholar]
- Sorkin A., von Zastrow M. (2009). Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Nakamura A. (2008). The endocytic pathway acts downstream of Oskar in Drosophila germ plasm assembly. Development 135, 1107-1117 [DOI] [PubMed] [Google Scholar]
- Traub L. M. (2009). Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583-596 [DOI] [PubMed] [Google Scholar]
- Vaccari T., Bilder D. (2005). The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell 9, 687-698 [DOI] [PubMed] [Google Scholar]
- Vainchenker W., Constantinescu S. N. (2005). A unique activating mutation in JAK2 (V617F) is at the origin of polycythemia vera and allows a new classification of myeloproliferative diseases. Hematology Am. Soc. Hematol. Educ. Program 2005, 195-200 [DOI] [PubMed] [Google Scholar]
- Xi R., McGregor J. R., Harrison D. A. (2003). A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev. Cell 4, 167-177 [DOI] [PubMed] [Google Scholar]
- Yogosawa S., Hatakeyama S., Nakayama K. I., Miyoshi H., Kohsaka S., Akazawa C. (2005). Ubiquitylation and degradation of serum-inducible kinase by hVPS18, a RING-H2 type ubiquitin ligase. J. Biol. Chem. 280, 41619-41627 [DOI] [PubMed] [Google Scholar]
- Zeidler M. P., Perrimon N., Strutt D. I. (1999). Polarity determination in the Drosophila eye: a novel role for unpaired and JAK/STAT signaling. Genes Dev. 13, 1342-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Schulze K. L., Hiesinger P. R., Suyama K., Wang S., Fish M., Acar M., Hoskins R. A., Bellen H. J., Scott M. P. (2007). Thirty-one flavors of Drosophila rab proteins. Genetics 176, 1307-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.