Abstract
We previously showed that the cell–cell junction protein plakoglobin (PG) not only suppresses motility of keratinocytes in contact with each other, but also, unexpectedly, of single cells. Here we show that PG deficiency results in extracellular matrix (ECM)-dependent disruption of mature focal adhesions and cortical actin organization. Plating PG−/− cells onto ECM deposited by PG+/− cells partially restored normal cell morphology and inhibited PG−/− cell motility. In over 70 adhesion molecules whose expression we previously showed to be altered in PG−/− cells, a substantial decrease in fibronectin (FN) in PG−/− cells stood out. Re-introduction of PG into PG−/− cells restored FN expression, and keratinocyte motility was reversed by plating PG−/− cells onto FN. Somewhat surprisingly, based on previously reported roles for PG in regulating gene transcription, PG-null cells exhibited an increase, not a decrease, in FN promoter activity. Instead, PG was required for maintenance of FN mRNA stability. PG−/− cells exhibited an increase in activated Src, one of the kinases controlled by FN, a phenotype reversed by plating PG−/− cells on ECM deposited by PG+/− keratinocytes. PG−/− cells also exhibited Src-independent activation of the small GTPases Rac1 and RhoA. Both Src and RhoA inhibition attenuated PG−/− keratinocyte motility. We propose a novel role for PG in regulating cell motility through distinct ECM–Src and RhoGTPase-dependent pathways, influenced in part by PG-dependent regulation of FN mRNA stability.
Keywords: Armadillo protein, Desmosome, Extracellular matrix, Keratinocyte
Introduction
Epithelial tissues derive positional and functional cues from their surroundings, in part through cell–cell and cell–substrate interactions involving cadherins and integrins, respectively (Hynes, 2002; Wheelock and Johnson, 2003). Adhesion-dependent signaling pathways are critical for normal development and differentiation in adult tissues; defects in both types of adhesive contact contribute to disease pathogenesis, including inherited disease and cancer progression (Dusek et al., 2007; Janes and Watt, 2006; Jeanes et al., 2008; Watt, 2002).
In addition to their independent roles in tissue morphogenesis and homeostasis, cadherin- and integrin-based cell–substrate adhesive contacts engage in ‘cross-talk’ mechanisms, in which one junction type affects the expression, assembly, turnover and/or function of the other junctions or junction components (Hodivala and Watt, 1994; Levenberg et al., 1998; Marsden and DeSimone, 2003; Monier-Gavelle and Duband, 1997; Ojakian et al., 2001). A variety of signaling mediators contribute to communication between cadherins and integrins. These include cell surface adhesion receptors such as nectins (Ogita and Takai, 2008), receptor-mediated growth-factor-dependent pathways such as TGFβ/SMAD (Kim et al., 2009), non-receptor kinases and such as Src family members (Arregui et al., 2000; Avizienyte and Frame, 2005), small GTPases (Balzac et al., 2005; Fukuhara et al., 2004; Takai et al., 2008), and mechanotransduction pathways (Chen and Gumbiner, 2006; Tsai and Kam, 2009).
Desmosomes are a type of cadherin-based intercellular junction crucial for structural stability of tissues during embryonic development and adult tissue morphogenesis (Cheng and Koch, 2004; Garrod and Chidgey, 2008; Green and Gaudry, 2000). Defects in desmosome structure and function, resulting from mutation of its individual components or inhibition of desmosomal cadherin adhesive functions by bacterial toxins and autoimmune antibodies, lead to skin and/or heart disease (Bazzi and Christiano, 2007; Getsios et al., 2004b; McGrath and Wessagowit, 2005). Moreover, desmosomal molecules are aberrantly expressed in many types of cancer (Chidgey and Dawson, 2007). In addition to its roles in maintaining tissue integrity, evidence is emerging that desmosomes also participate in signal transduction and transcriptional regulation (Garrod and Chidgey, 2008; Green and Simpson, 2007). Despite the growing body of literature indicating the importance of these pathways in tissue development and homeostasis, much less is known about the potential roles of desmosome molecules as molecular intermediates that regulate cell–substrate adhesion.
Plakoglobin (PG; also known as junction plakoglobin, JUP) is a member of the Armadillo family of proteins, and a close relative of β-catenin. Although it is able to associate with the cytoplasmic tails of both classic and desmosomal cadherins (Cowin et al., 1986), PG is thought to be present predominantly in desmosomes of epithelial cells with mature intercellular contacts (Hinck et al., 1994). In desmosomes, PG participates in linking the cytoplasmic tail of desmosomal cadherins to intermediate filaments (North et al., 1999; Yin and Green, 2004). Accordingly, PG plays a critical role in desmosomal integrity, as demonstrated by reduced expression and membrane incorporation of other desmosomal proteins (Yin et al., 2005a), as well as impaired cell–cell adhesion (Caldelari et al., 2001) and adhesive strength (Acehan et al., 2008) in keratinocytes lacking PG. Moreover, PG-null mice and patients with pathogenic homozygous PG mutations have impaired tissue integrity associated with skin and heart defects (Aberle et al., 1995; Bierkamp et al., 1996; McKoy et al., 2000; Ruiz et al., 1996). PG is also found in the cytoplasm and nucleus (Green and Simpson, 2007; Schmidt and Koch, 2007), where it is able to act independently of its function in intercellular adhesion. Its adhesion-independent functions are still not well defined, but the data suggest that PG can regulate gene expression and protein stability (Aktary et al., 2010; Hakimelahi et al., 2000; Shimizu et al., 2008) in both a β-catenin-dependent and -independent manner (Raurell et al., 2006; Teuliere et al., 2004; Yin and Green, 2004; Zhurinsky et al., 2000).
Recently we demonstrated that PG not only inhibits motility of keratinocytes in contact, but also inhibits Src-dependent single cell motility (Yin et al., 2005b). The observed changes in motility and altered cell morphology of PG−/− keratinocytes suggested to us that PG could be regulating cell–substrate interactions by modulating components of the extracellular matrix (ECM), its integrin receptors and/or the molecules involved in ECM-triggered motility cues. Using a combination of live cell imaging and cross plating, we show here that PG expression has a potent impact on the organization of actin, its associated membrane protrusions, focal adhesions and Src-dependent motility, in large part through regulation of the expression levels of the underlying ECM components. In particular, the ability of PG to regulate fibronectin (FN; also know as Fn1) mRNA stability was identified as a novel mechanism contributing to PG-dependent suppression of keratinocyte motility. Further analysis indicated that PG-dependent alterations in activity of the small GTPases Rac1 and RhoA act in parallel with FN/Src-dependent regulation of cortical actin structures to fine tune the motile behavior of keratinocytes. Collectively, these results indicate that a desmosomal molecule, PG, is capable of regulating single cell motility through matrix deposition in concert with Rho GTPases, independently of its role as a cell–cell adhesion molecule.
Results
PG regulates keratinocyte cell polarity and single-cell motility
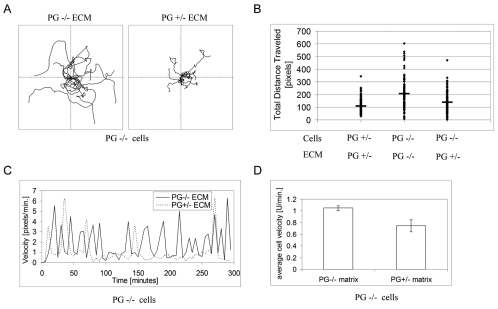
To begin to address the mechanism by which PG regulates single cell motility, we first carried out live cell imaging analysis to identify individual components of motility behavior (Fig. 1A; supplementary material Movie 1). Analysis of motility of individual cells revealed that both net displacement and total distance traveled was approximately twofold greater in PG−/− keratinocytes compared with PG+/− cells. The resulting directed migration index (net displacement/total distance traveled) was not significantly higher in PG-null cells (Fig. 1B,C) indicating that PG deficiency increases random rather than directional motility. Reconstituting PG expression by adenoviral transduction reduced the motility of PG−/− cells, suggesting that single cell keratinocyte motility is controlled by PG (see below). The average cell velocity of PG−/− cells was elevated over twofold (Fig. 1D). However, when velocity was calculated between each pair of time points analyzed, to create a ‘velocity map’, it became evident that PG−/− and PG+/− cells exhibited distinctively different motility signatures. PG+/− cells had a slower but more constant motility; by contrast, PG−/− cells exhibited periods of very rapid movement interrupted by periods of slower or even no movement (Fig. 1E).
Fig. 1.
Plakoglobin regulates keratinocyte motility by increasing cell velocity. (A) Representative tracks of ten randomly chosen PG+/− and PG−/− cells from five 5-hour trials involving a minimum of 50 cells per trial. The intersection of the x- and y-axes was taken as the starting point of each cell path. Each tick mark on the axes represents 50 pixels. (B) Graph showing the net displacement (left) and total distance traveled (right) of PG+/− and PG−/− cells (mean ± s.d.; n≥3 trials, with at least 50 cells per trial). (C) Directed migration index (ratio of the ‘net displacement’ and ‘total distance traveled’) of PG+/− and PG−/− cells. (D) The average velocity of ≥50 PG+/− and PG−/− cells, from three independent experiments (mean ± s.d.; n≥3 trials, with at least 50 cells per trial). (E) Velocity maps of a representative PG+/− (solid line) and PG−/− (dotted line) keratinocyte. Cells were followed for 5 hours, with velocity measured at 5-minute intervals.
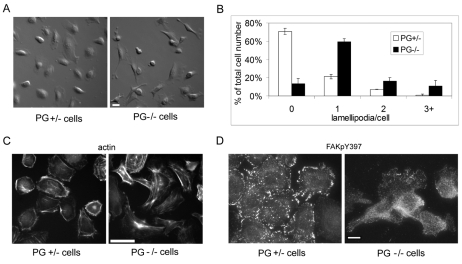
Because keratinocyte motility is largely regulated by the ability of cells to polarize and form lamellipodia, we next determined the differences in cell morphology and number of lamellipodia between PG−/− and PG+/− cells. The organization of actin and associated focal adhesions was also assessed. Over 70% of PG+/− cells exhibited an epithelioid morphology characterized by a lack of lamellipodia and prominent cortical actin (Fig. 2A-C). In addition, focal contacts were more numerous and prominent in PG+/− cells, as illustrated by staining for FAK(Tyr397-P) [phospho-tyrosine 397 is a focal adhesion kinase (FAK) autophosphorylation site dependent upon focal adhesion formation; Fig. 2D]. In contrast to PG+/− cells, 80% of PG−/− cells had at least one lamellipodium present (Fig. 2B), consistent with an increased propensity for motile behavior. A third of the PG−/− cells had more than one lamellipodium accompanied by a more elongated, fibroblast-like morphology (Fig. 2A,B). The presence of more than one lamellipodium has been related to frequent switches in the direction of motion (Sehgal et al., 2006) (supplementary material Movie 1), consistent with an increase in random, rather than directional, motility of PG−/− cells.
Fig. 2.
Plakoglobin regulates actin cytoskeleton organization in mouse keratinocytes. (A) DIC images of PG+/− and PG−/− cells. Scale bar: 20 μm. (B) Number of lamellipodia per cell (PG+/− white bars; PG−/− black bars). (C) Alexa-Fluor-350-conjugated phalloidin-actin staining of PG+/− and PG−/− cells. Scale bar: 20 μm. (D) PG+/− and PG−/− keratinocytes stained for FAK(Tyr397-P) (FAKpY397) to show the focal contacts. Scale bar: 20 μm.
PG regulates the expression of molecules involved in cell–substrate interactions
Because cell–substrate interactions play a crucial role in the regulation of cell motility and actin cytoskeleton remodeling (DeMali et al., 2003; Ridley et al., 2003), we proceeded to determine whether PG regulates the expression of ECM components, integrins and other adhesion-related molecules.
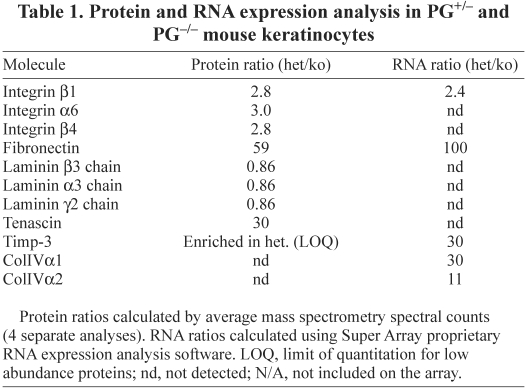
We used a previously described method of cell ‘de-roofing’ (removing cell cytoplasm and nuclei) by mildly basic hypotonic solution (Langhofer et al., 1993; Sehgal et al., 2006) to obtain samples enriched in ECM and cell–ECM-adhesion-related molecules. The samples were trypsinized and analyzed by mass spectrometry (MS). Details of this novel method for mass spectrometry sample preparation and analysis are discussed elsewhere (Todorovic et al., 2010). Differences in protein levels were observed between integrin receptors, ECM and cytoskeletal molecules, as well as between membrane glycoproteins and matrix proteolysis regulators (Table 1). Using a microarray specifically targeting ECM and adhesion molecules, we further expanded the list of molecules regulated by PG (Table 1) and have shown that at least some of them (including FN) are regulated at the mRNA level. From the mass spectrometry and array data, we selected several well-characterized molecules involved in keratinocyte adhesion and motility that were predicted to be significantly differentially expressed in PG+/− and PG−/− cells, and tested their protein levels by immunoblotting. Integrin β1 essential for motility and adhesion to LN-332 and FN (Mizushima et al., 1997; van der Flier and Sonnenberg, 2001), but also found to be inhibitory to keratinocyte motility on LN-332 during wound re-epithelialization (Margadant et al., 2009), FN and Col IV (provisional matrix and basement membrane components, respectively) (Clark, 1990; Sado et al., 1998), were all lower in PG−/− cells, consistent with expression patterns established by MS and microarray analysis (supplementary material Fig. S1A). Consistent with the enrichment of basal cell components, de-roofed cells retained FN and integrin α6 while losing their nuclei (supplementary material Fig. S1B). Not all ECM molecules relevant for keratinocyte adhesion and motility are differentially regulated by PG: a major basement membrane component laminin-332 was not affected (Table 1) (Todorovic et al., 2010).
Table 1.
Protein and RNA expression analysis in PG+/− and PG−/− mouse keratinocytes
PG-dependent changes in extracellular matrix regulate the adhesive and motile behavior of mouse keratinocytes
In order to test whether PG controls keratinocyte motility by regulating the deposition of the underlying substrate, we allowed PG+/− and PG−/− cells to deposit their own matrix, and then removed the cells by ‘de-roofing’ them while retaining the basal cell components (supplementary material Fig. S1B). PG-deficient keratinocytes were plated onto the pre-deposited matrices and their motility was analyzed. In contrast to the behavior on their own matrix, on a population basis, PG−/− cells exhibited significantly diminished motility when plated on matrix deposited by PG+/− keratinocytes (Fig. 3A,B; supplementary material Movie 2). The velocity map revealed some interesting sub-features of PG−/− cell behavior on PG+/−-deposited matrix. These cells still exhibited periods of rapid migration, but they were fewer in number and interspersed with periods of greatly reduced velocity (Fig. 3C; supplementary material Movie 2). This behavior resulted in an overall decrease in average velocity of the PG−/− cells (Fig. 3D).
Fig. 3.
Plakoglobin-dependent ECM deposition regulates the motile behavior of mouse keratinocytes. (A) Representative tracks showing motile behavior of 10 randomly chosen PG−/− cells on the ECM deposited by either PG−/− or PG+/− keratinocytes. (B) Graph showing the distribution of total distances traveled for individual PG+/− and PG−/− keratinocytes on pre-deposited matrices (n=110, horizontal black bars indicate the median value). (C) Velocity maps of a representative PG−/− keratinocyte on PG−/− ECM (solid line) and PG+/− ECM (dotted line). (D) Average velocity of PG−/− cells on PG−/−- and PG+/−-deposited ECMs (n≥50; error bars represent ± s.d.).
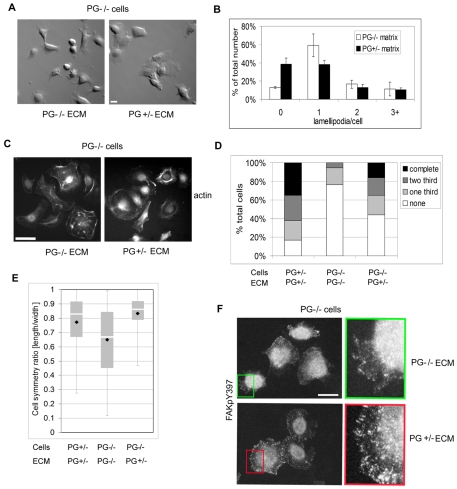
Along with the restoration of more normal motility behavior, the altered morphology and actin-associated structures observed in PG-deficient keratinocytes were also partially restored when plated on the substrate deposited by PG+/− keratinocytes (Fig. 4A,C). These changes included an increase in the number of cells with no lamellipodia from 10% to 40% and an increase in the number of cuboidal and round cells with more pronounced cortical actin, and partial normalization of focal contact structures (Fig. 4B–F).
Fig. 4.
Plakoglobin-dependent ECM deposition regulates actin cytoskeleton organization. (A) DIC images of PG−/− on PG−/−- and PG+/−-deposited ECM. Scale bar: 20 μm. (B) Number of lamellipodia per PG−/− cell (PG−/− ECM, white bars; PG+/− ECM, black bars). (C) Alexa-Fluor-350-conjugated phalloidin-actin staining of PG−/− keratinocytes on PG+/− ECM and PG−/− ECM. (D) Diagram showing the percentage of PG+/−- and PG−/− cells (n≥50) on PG+/−- and PG−/−-deposited matrices with cortical actin staining absent (no ring), or present over one third (one third), two thirds (two thirds) or whole cell border (complete ring). (E) Bar and whisker graph representing the radial symmetry of PG+/− and PG−/− cells on predeposited matrices (n>100; y-axis represents the ratio between the two perpendicular cell diameters, with higher value always being used as a numerator; the closer the ratio is to 1, the more round or cuboidal the cell; black diamonds indicate the mean value; white bar is 50th percentile; gray box delineates 25th and 75th percentile, whiskers represent minimum and maximum values). (F) PG−/− keratinocytes stained for FAK(Tyr397-P) to show the focal contacts on PG+/− and PG−/− ECM. Scale bars: 20 μm.
PG-induced FN expression regulates keratinocyte motility
The ability of PG+/− matrix to attenuate the increased motility exhibited by PG−/− keratinocytes led us to further examine differentially expressed ECM molecules for their ability to regulate PG-dependent cell motility. From the analysis of the expression patterns of the ECM components (Table 1; supplementary material Fig. S1A,B), a 50-fold decrease in expression of fibronectin (FN) in PG−/− cells stood out, whereas the level of laminin-332 (LN-332) remained unchanged (Table 1) (Todorovic et al., 2010).
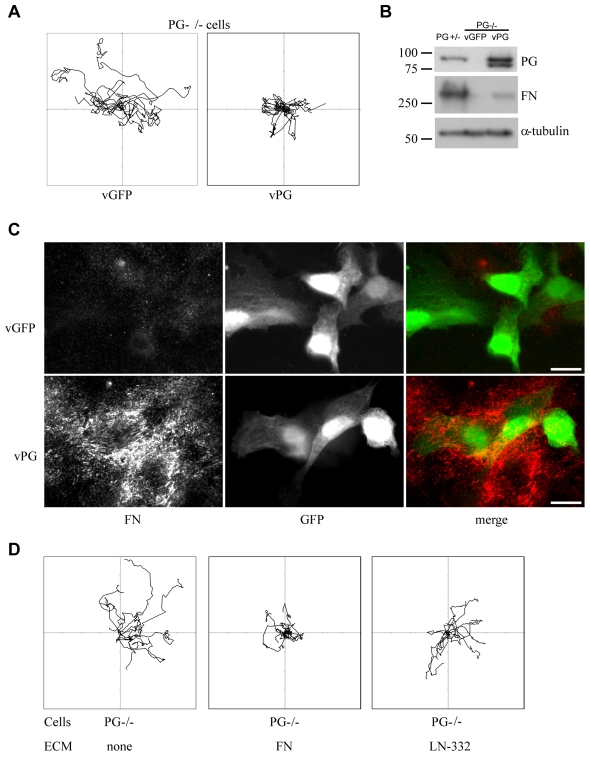
To confirm that PG was responsible for this change in FN expression, we re-established PG expression in PG-null cells by adenoviral transduction. Ectopically expressed PG effectively reduced keratinocyte migration as assessed by live cell imaging and tracking analysis (Fig. 5A). Furthermore, PG−/− keratinocytes showed an increase in the overall level of FN upon PG transduction (Fig. 5B). Infected PG-expressing cells that were re-plated to allow for the de novo deposition of matrix also showed increased deposition of FN as compared with adjacent non-transduced, or control GFP-transduced cells (Fig. 5C).
Fig. 5.
Fibronectin contributes to plakoglobin-dependent inhibition of cell motility. (A) Representative tracks (n=10) showing the decrease in motility of PG−/− cells upon introduction of PG by adenovirus as compared to GFP-only control. (B) Western blot analysis of FN expression in the whole cell lysates of PG+/−, control (GFP) and PG-transduced PG−/− cells. (C) Immunofluorescence staining showing restoration of FN expression and deposition in PG−/− cells transduced with adenovirus expressing PG (green) compared with GFP-expressing control cells. Scale bar: 20 μm. (D) Representative tracks (n=10) showing the motile behavior of PG−/− keratinocytes plated onto plastic- (no predeposited matrix), fibronectin (FN)- and laminin-332 (LN-332)-coated dishes.
To test how FN deposition would influence motility of PG−/− cells, we plated cells on pre-deposited recombinant FN and recorded their motility with live cell imaging. PG−/− keratinocytes show significantly reduced motility on pre-deposited FN as compared with their own matrix whereas pre-deposited matrix enriched in LN-332 did not appear to have a dramatic inhibitory effect (Fig. 5D). These results suggest that FN is one of the matrix molecules specifically involved in PG-dependent inhibition of cell motility. The data demonstrate that PG can control the motility of mouse keratinocytes through regulation of FN expression and deposition.
PG regulates FN mRNA stability
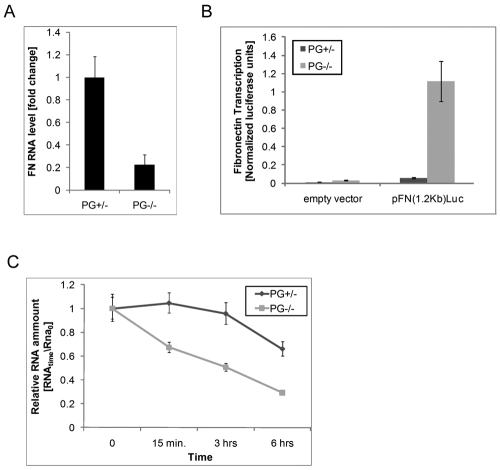
The RNA array results (Table 1) suggested that the decrease in FN protein expression correlates with a decrease in FN mRNA in PG−/− cells. To test this directly, we conducted quantitative reverse transcriptase PCR (qRT-PCR) and confirmed that PG−/− cells contained at least fivefold less FN mRNA than the control cells (Fig. 6A). Because both PG and its closest family member β-catenin are well established as regulators of transcription (Maeda et al., 2004; McCrea et al., 2009), and because we observed upregulation of both FN protein and mRNA, we next addressed whether FN promoter activity depends on PG. Surprisingly, using a luciferase reporter downstream of a 1.2 kb FN promoter sequence (Michaelson et al., 2002) we showed that FN promoter activity was increased, not reduced as might have been predicted based on the observed reduction in FN mRNA levels (Fig. 6B). As FN levels can be controlled through stabilization of its mRNA (Mimura et al., 2004), we assessed the stability of FN mRNA in PG+/− and PG−/− cells by inhibiting de novo RNA synthesis with actinomycin D and measuring the relative levels of mRNA by qRT-PCR. Whereas the level of FN mRNA was relatively stable in PG+/− cells until the later 6-hour time point, a rapid decline in FN mRNA occurred over time, dropping to ~60% of its initial value as early as 15 minutes after actinomycin D treatment (Fig. 6C). These results suggest that PG regulates FN levels at least in part through maintenance of mRNA stability in mouse keratinocytes.
Fig. 6.
Plakoglobin increases fibronectin RNA levels through increasing its stability. (A) qRT-PCR analysis of the FN RNA, which is significantly decreased in PG−/− cells as compared with controls (experiment done in triplicate with two sets of primers and normalized to Gapdh; error bars represent ± s.d.). (B) Luciferase activity analysis shows an increase in FN promoter activity in PG−/− cells, suggesting that PG does not increase FN levels through promoter activation (Firefly luciferase activity normalized to Renilla activity used as a transfection efficiency control; experiment done in quadruplicate, error bars represent ± s.d.). (C) FN RNA stability analysis in PG+/− and PG−/− cells treated with actinomycin D (2 μg/ml) for the specified times. Samples from treated cells were normalized to their matching PG+/− and PG−/− controls (time 0), both of which were given a relative value of 1 (experiment done in triplicate with two different sets of primers; error bars represent ± s.d.).
PG regulates Src activity in a matrix-dependent manner
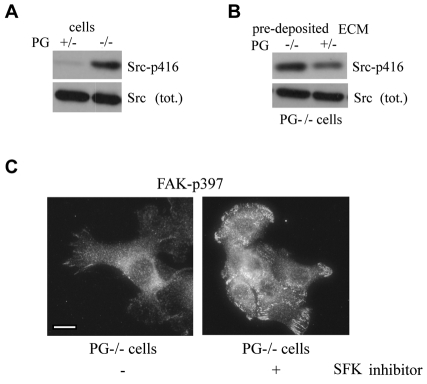
Previously we demonstrated that the increase in motility of PG-deficient cells is dependent upon the activity of Src kinase (Yin et al., 2005b), leading us to hypothesize that PG can regulate Src activity. In order to test this, we compared the levels of activated Src in PG+/− and PG−/− cells by immunoblotting using an antibody recognizing the activated form of Src [Src(Tyr416-P)]. The results indicate an increase in the levels of activated Src in PG−/− cells (Fig. 7A).
Fig. 7.
Plakoglobin regulates Src activity through ECM deposition. (A) Western blot analysis of the levels of activated Src [Src(Tyr416-P); Scr-p416] in PG+/− and PG−/− keratinocytes in steady state. (B) Western blot showing a decrease in activated Src in PG−/− cells on the ECM deposited by PG+/− cells 5 hours after replating. (C) Immunofluorescence showing more robust focal adhesions in PG−/− cells treated with the Src inhibitor PP2. Scale bar: 20 μm.
As Src activity is regulated by ECM interactions with integrins (Playford and Schaller, 2004), we investigated whether the PG-dependent changes in ECM composition were responsible for the observed difference in Src activity between PG−/− and PG+/− cells. When plated on the matrix deposited by PG+/− cells, Src activity in PG−/− cells was significantly reduced (Fig. 7A,B). This finding supports the idea that PG modulates Src activity at least in part through the regulation of ECM expression and deposition.
One of the major motility pathways regulated by cell–ECM interactions is mediated through Src-dependent phosphorylation of FAK and subsequent changes in focal adhesions. Changes in the interaction of the cells with the underlying matrix leading to Src-dependent translocation of FAK from robust focal adhesions to smaller focal complexes have been correlated with increased cell motility (Beningo et al., 2001; Beningo and Wang, 2002). Consistent with this, less motile PG+/− keratinocytes exhibited more prominent FAK staining corresponding to zyxin-containing mature focal adhesions (Fig. 2D and not shown). However, PG−/− cells exhibited more punctate and immature focal complexes. To test whether the formation of robust focal adhesions in PG+/− keratinocytes was dependent upon decreased Src activity in these cells (Fig. 7A), we used a chemical inhibitor of Src kinase (PP2) to treat PG−/− cells and assessed its ability to rescue focal adhesion formation. Treatment of PG−/− cells with PP2 led to a significant increase in robust focal adhesions, as demonstrated by FAK(Tyr397-P) staining (Fig. 7C), corresponding to decreased motility of the treated cells (Fig. 8A) (Yin et al., 2005b).
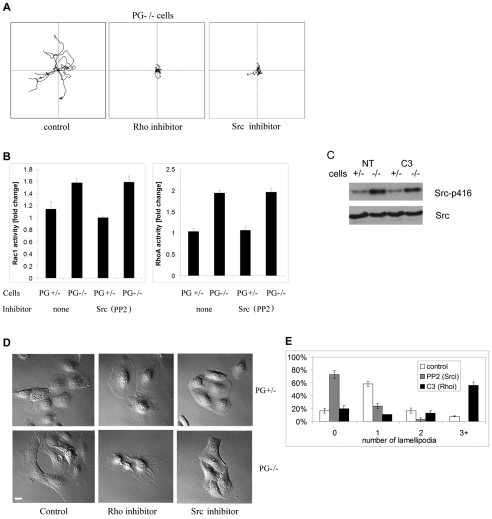
Fig. 8.
Plakoglobin regulates motility through independent regulation of RhoGTPase and Src activity. (A) Representative tracks (n=10) showing the effects of inhibition of Rho (C3 transferase, 2 μg/ml) and Src (PP2, 10 μM) on PG−/− keratinocyte motility. (B) Rho and Rac1 G-LISA showing that increase in their activity in PG−/− cells is independent from Src activity. (C) Western blot demonstrating that the increased Src activity in PG−/− cells is independent from Rho activity. (D) DIC images of PG+/− and PG−/− keratinocytes, either untreated or treated with the Rho and Src inhibitors. Scale bar: 20 μm. (E) Number of lamellipodia in PG−/− cells (control, white bars; Src-inhibitor-treated, gray bars; Rho-inhibitor-treated, black bars).
Taken together, these results suggest that PG inhibits cell motility by regulating the expression levels of ECM molecules (including FN). These PG-dependent changes in ECM composition in turn inhibit motility through the downregulation of Src activation and increase in mature focal adhesion formation.
PG regulates RhoGTPase-dependent motility in a Src-independent manner
FN-rich PG+/− matrix was largely effective at restoring PG−/− cell morphology and motile behavior. However, in live cell imaging experiments it appeared that a proportion of cells with fibroblastic morphology continued to move rapidly, if they were able to break away from the matrix. This behavior may have led to the incomplete reversion of the velocity maps (Fig. 3C). Moreover, the restoration of PG−/− cell morphology and cortical actin symmetry on PG+/− deposited ECM was incomplete as well (Fig. 4A-E). To explore additional factors that might contribute to PG-dependent regulation of motility, we carried out analysis of the Rho small GTPases, known to be important regulators of the actin cytoskeleton and motility, often downstream from Src kinase (Arthur and Burridge, 2001; Arthur et al., 2000). To assess whether Rho activity was required for the increased motility of PG−/− cells, we treated them with the cell-penetrating C3 transferase, a potent and specific inhibitor of RhoA. The inhibition of RhoA led to a significant reduction in PG−/− motility (Fig. 8A). Rho G-LISA assays revealed that both RhoA and Rac1 activity were elevated in PG−/− cells (Fig. 8B). Surprisingly, the Src inhibitor PP2 was unable to reduce the activity of either RhoA or Rac1 (Fig. 8B). Likewise, the Rho inhibitor C3 failed to significantly lower the level of activated Src in PG−/− cells (Fig. 8C), suggesting that PG regulates keratinocyte motility through at least two independent signaling branches, ECM–Src on the one hand and RhoGTPase on the other. In addition, whereas the inhibition of Src leads to significant decrease in the number of lamellipodia, the selective inhibition of RhoA has an opposite effect, with cells extending many protrusions but not able to move (Fig. 8D,E).
Discussion
The desmosomal armadillo protein plakoglobin links desmosomal cadherins, desmocollins and desmogleins, to the plakin family of molecules and the associated intermediate filament cytoskeleton (Cowin, 1994). Compromising these linkages by ablation of PG results in severe loss of tissue integrity in vivo (Ruiz et al., 1996; Bierkamp et al., 1996) and cell–cell adhesion strength in vitro (Yin et al., 2005a). However, recently PG has also been shown to be involved in cell–cell adhesion-independent activities, including inhibition of motility of cells with disrupted cell–cell contacts (Yin et al., 2005b), and regulation of transcription (Green and Simpson, 2007).
Here we demonstrate that PG regulates cell–cell adhesion-independent motility through modulating expression and deposition of the extracellular matrix. Using a novel approach for preparation and mass spectrometric analysis of cell-adhesion-related molecules (Todorovic et al., 2010), we show that PG affects the expression levels of basal cell components involved in cell–ECM interactions, which in turn leads to changes in cell–substrate adhesion, actin cytoskeleton organization, focal adhesion maturation and cell polarity. Moreover, PG regulates Src activity in an ECM-dependent manner, as well as RhoGTPase activity, both of which are instrumental in its regulation of keratinocyte motility. These findings establish a regulatory role for a desmosomal molecule in controlling the expression of, and cellular interactions with, ECM components.
Among ECM molecules a role for both FN and LN-332 in the regulation of keratinocyte motility has been well established. Whereas LN-332 is the main component of skin basement membrane and is required for both activation and inhibition of keratinocyte motility through distinct pathways (Litjens et al., 2006; Mercurio and Rabinovitz, 2001; Rabinovitz and Mercurio, 1997), FN is important in regulating cell migration over the provisional matrix in skin wound healing, disappearing after wound closure (Santoro and Gaudino, 2005). Here, we show that although LN-332 is not affected by PG expression, FN mRNA levels are significantly reduced in PG−/− cells, with protein levels being by 50-fold higher in control.
In recent years PG has been established as a transcriptional regulator both independently and through Wnt/β-catenin pathway regulation (Maeda et al., 2004; McCrea et al., 2009). Therefore, we expected to see PG directly regulating transcription of FN mRNA especially because β-catenin has already been shown to regulate FN expression in Xenopus fibroblasts (Gradl et al., 1999). Surprisingly, we show here that PG regulates FN mRNA level by enhancing its stability, rather than enhancing its promoter activity (Fig. 6). Whereas the increase in FN promoter activity might be explained as a compensatory attempt of the cells to synthesize the missing protein, the mechanism by which PG regulates the mRNA stability of fibronectin is an unexplored area of Armadillo protein biology. Recent data show the ability of plakophilins to bind to and regulate mRNA transcription (Borrmann et al., 2006; Wolf et al., 2010). Moreover, the Wnt/β-catenin pathway has been implicated recently in regulating the turnover of labile mRNAs through adenylate and/or uridylate-rich elements and their binding proteins (Briata et al., 2003). It remains to be seen whether PG stabilizes FN mRNAs directly or indirectly; however, this finding brings to light a new subset of PG cell–cell adhesion-independent activities.
A PG-dependent increase in FN expression was associated with attenuated migration of single keratinocytes in vitro. That FN decreased, rather than increased, motility might be explained by previous observations that the process of FN deposition and polymerization requires tight control to properly titrate cell behavior. Indeed, in certain cases, cells have been shown to respond in a biphasic manner to increasing concentrations of FN, and at high concentrations, FN has been shown to decrease the rate of migration, as in the case of airway epithelial cells (Hocking and Chang, 2003); or decrease protrusiveness, as in the case of CHO cells (Cox et al., 2001).
In tissue culture mouse keratinocytes produce large amounts of FN and LN-332, whereas human keratinocytes predominantly produce LN-332 (J.C.R.J. unpublished observations). In addition, human keratinocytes are more motile than mouse keratinocytes in culture. Thus, it seems possible that PG relieves a FN-dependent inhibition of mouse keratinocyte motility, allowing them to move freely on the underlying LN-332 (Hamill et al., 2009; Sehgal et al., 2006). Although the mechanism of such inhibition is unclear, it is tempting to speculate that FN and LN-332 might be competing for the integrin receptors required for motility on LN-332. Furthermore, the decrease in FN observed in PG−/− cells is accompanied by a decrease in β1 integrin expression (supplementary material Fig. S1; Table 1) (Todorovic et al., 2010), which may be due to the loss of FN as a primary α5β1 integrin ligand. Thus, because α3β1integrin has been recently implicated in the inhibition of keratinocyte motility on newly deposited LN-332 (Margadant et al., 2009), the decrease of β1 integrin could lead to attenuation of this inhibitory effect. Mass spectrometry also revealed an increase in Syndecan-1 in the PG−/− keratinocytes (M.J.S.P. and V.T. unpublished observations). Because Syndecan-1 enhances keratinocyte motility through regulation of ECM deposition and integrin surface expression (Stepp et al., 2007) it is possible that this glycoprotein contributes to PG-dependent regulation of keratinocyte motility.
Increased activity of Src and its phosphorylation of FAK have been shown to accompany weakening of cell–cell adhesions and de-regulation of E-cadherin (Avizienyte et al., 2002; Frame, 2004). This Src/FAK signaling axis causes a switch in adhesion type predominance in epithelial cells from cadherin based to integrin based adhesions, promoting a more motile phenotype (Avizienyte and Frame, 2005). Here we demonstrate that elevated motility in PG−/− keratinocytes relies on alterations to the underlying matrix and maturation state of cell–substrate adhesive structures or focal contacts. However, the observed elevation of RhoA and Rac1 activity were not sensitive to the inhibition of Src and vice versa, suggesting the existence of multiple parallel pathways for PG in controlling keratinocyte motility behavior. Supporting this idea, the morphological responses of the PG−/− cells treated with Src and RhoA inhibitors were divergent. On the one hand, the inhibition of Src led to formation of strong focal adhesions (Fig. 7C), accompanied by a lack of lamellipodial protrusions (Fig. 8E). On the other hand, selective inhibition of RhoA inhibited cell motility (Fig. 8A) (Ridley et al., 2003) but left the cells with many protrusions (Fig. 8D,E), most probably as a result of active Rac1 (Fig. 8B) (Rottner et al., 1999), which is responsible for lamellipodia formation. This is further supported by the observation that PG+/− cells, whose Rac1 activation levels are substantially lower (Fig. 8B), show less prominent protrusions upon Rho inhibition (Fig. 8D).
Taken together with previous findings, our data are consistent with a model in which desmosomal cadherins use PG as a molecular ‘switch’ to modulate cell–cell adhesion-dependent and -independent functions, through regulation of its subcellular localization. In normal cells, partial or complete detachment from cell–cell contacts would lead to a rise in the amount of free cytoplasmic and nuclear PG, which would inhibit motility and survival mechanisms, preventing the inappropriate spread of cells. Under conditions where physiological tissue remodeling and cell migration occur, this regulatory mechanism may be attenuated by recruitment of PG into intercellular junctions present in the coherent migrating sheet of cells, thus allowing controlled migration in activated cells at the leading edge of a wound. In tumors, this regulatory mechanism might be completely lost leading to uncontrolled migration and increased survival of single tumor cells lacking PG. In addition to providing insight into mechanisms of epithelial remodeling in wound healing and cancer, further investigation of the cell–cell adhesion-independent role of PG in regulating matrix deposition and cell–matrix interactions may ultimately shed light on how PG dysfunction contributes to inherited diseases of the skin and heart.
Materials and Methods
Cell culture
Keratinocyte cultures established from PG knockout (PG−/−) or heterozygous control (PG+/−) mouse skin (Caldelari et al., 2001) were cultured in defined keratinocyte serum-free medium (Invitrogen), supplemented with 10 ng/ml EGF and 10−10 M choleratoxin. Calcium level was adjusted to 0.07 mM using 1 M CaCl2.
ECM preparation
Keratinocyte matrix was prepared as described previously (Langhofer et al., 1993). Briefly, cells were plated on tissue culture dishes or glass coverslips and allowed to reach confluency. The culture medium was removed and the cells were washed in sterile phosphate-buffered saline (PBS). The cells were ruptured by treating them for 5 minutes in sterile 20 mM NH4OH, followed by three rapid washes in sterile distilled water. The remaining ECM and cell membranes were then washed several times in sterile PBS, followed by water, and then immediately analyzed or used for cell plating. Laminin-332-enriched matrix was prepared from 804G cells as described before (Langhofer et al., 1993). Recombinant FN was purchased from Sigma.
Western blotting
Whole and ‘de-roofed’ cell samples were prepared using urea sample buffer. The amount of protein in whole cell lysates was measured using the Amido Black Assay (Sheffield et al., 1987). Equal amounts of protein were fractionated on a 7.5% SDS-polyacrylamide gel, and immunoblotting was performed as described previously (Kowalczyk et al., 1997). Primary antibodies against FN (Sigma), PG (mouse mAb clone 11E4), α-tubulin (12G10), Src(Tyr416-P) and total Src (Cell Signaling Technology) were used. The following secondary antibodies were used: HRP-conjugated goat anti-mouse and anti-rabbit (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
Indirect immunofluorescence
PG+/− and PG−/− mouse keratinocytes were fixed with 4% paraformaldehyde and stained for integrin α6 and fibronectin, using GoH3 (Chemicon) and anti-FN (Sigma) antibody, respectively. Alexa Fluor 488 goat anti-rat and Alexa Fluor 568 goat anti-rabbit (Molecular Probes, Eugene, OR; 1:400 dilution) were used as secondary antibodies. For visualization of the actin cytoskeleton and focal adhesions, cells were fixed by formal saline (3.7% formaldehyde in PBS) followed by 2 minutes in ice-cold acetone. Cells were then stained using a monoclonal anti-FAK(Tyr397-P) antibody (BD Transduction Labs) together with Alexa Fluor 488 goat anti-mouse antibody and Rhodamine-conjugated phalloidin for F-actin staining.
Mass spectrometry and data analysis
Protein sample preparation, mass spectrometry and data analysis were conducted as previously described (Todorovic et al., 2010). Even though mouse keratinocyte cell culture medium does not contain serum, proteins present in the medium were identified using control samples (glass surfaces without cells). The contaminating proteins could then be deleted from the protein lists generated for the de-roofed cells, leaving only monolayer-isolated proteins for comparison of unique spectra as the metric for relative quantification.
Live cell imaging
Keratinocytes were plated at high density on four-chamber borosilicate glass coverslips overnight to deposit their own matrix. The next day cells were de-roofed and fresh PG−/− cells were plated at 10–20% density. After cells had attached and spread (2 hours), medium was changed to eliminate debris, and live-cell imaging was conducted using a Leica DMI 6000 microscope (×20, DIC), and a Hamamatsu digital camera. Cells were imaged for 5 hours in 5-minute intervals. Images were processed using Simple PCI (Hamamatsu), and individual cell movement was analyzed with Metamorph (Molecular Devices). Motility assays were performed a minimum of three times, with at least 50 cells analyzed in each trial.
Adenoviral constructs and transduction
The pAdEasy adenovirus packaging system kindly provided by Warren G. Tourtellote (Northwestern University Feinberg School of Medicine) was used to generate previously characterized myc-tagged, full-length human PG (Palka and Green, 1997) as described previously (Yin et al., 2005a). Infection rates were monitored using GFP expressed in tandem with PG; at least 30% of replated cells still retained GFP expression. In order to allow the PG and GFP control transduced cells to deposit fresh extracellular matrix, the cells were re-plated for matrix deposition 24 hours after infection and left overnight. The next day cells were either fixed and stained for fibronectin deposition or de-roofed as described above. Control GFP-transduced cells were plated upon the pre-deposited matrices and live-cell images were taken as described.
RNA array
The levels of expression of ECM and membrane related molecules in PG+/− and PG−/− cells was determined by using Oligo GEArray Mouse Extracellular Matrix and Adhesion Molecules MicroArray (SABiosciences, Frederick, MD) according to the manufacturer's directions. The data was analyzed using GEArray Expression Analysis Suite.
G-LISA
The levels of active RhoA and Rac1 were determined using RhoA- and Rac1-specific colorimetric activation assays (G-LISA; Cytoskeleton) according to the manufacturer's directions. A specific Rho inhibitor, cell permeable C3 transferase, was obtained from Cytoskeleton. The absorbances were read at 490 nm on a Synergy2 plate reader (BioTek).
Quantitative RT-PCR
RNA was isolated and purified from PG+/− and PG−/− keratinocytes and was treated as described in the text using an RNeasy mini kit (Qiagen) according to manufacturer's recommendations. First strand synthesis was performed on normalized RNA samples using a SuperScript III First-Strand Synthesis SuperMix for qRT-PCR kit (Invitrogen). qRT-PCR was performed using SBYR Green PCR Master Mix (Applied Biosystems) with the following primers: 5′-CTTTGTCAAGCTCATTTCCTGG-3′ (mouse Gadph – forward 1), 5′-TCTTGCTCAGTGTCCTTGC-3′ (mouse Gadph – reverse 1), 5′-CCTCTGCGCCCTTGAGCTAGGA-3′ (mouse Gadph – forward 2), 5′-CACAAGAAGATGCGGCCGTCTC-3′ (mouse Gadph – reverse 2), 5′-CTTTGGCAGTGGTCATTTCAG-3′ (mouse FN – forward 1), 5′-ATTCTCCCTTTCCATTCCCG-3′ (mouse FN – reverse 1), 5′-TGCCTTCAACTTCTCCTGTG-3′ (mouse FN – forward 2), 5′-CACTAACCACGTACTCCACAG-3′ (mouse FN – reverse 2).
FN promoter luciferase assay
PG+/− and PG−/− keratinocytes were transiently transfected with a mixture of 0.1 μg pRL-TK transfection efficiency control vector (Promega) and 2 μg of pGL3 (empty vector) or pFN(1.2Kb)Luc (generous gift from Jesse Roman) reporter vectors using the polyethylenimine method as previously described (Stiehl et al., 2006). Transfection efficiency was established at 20–40% using pLZRS-pBMN-EGFP (Getsios et al., 2004a) and determining the proportion of green cells. Cell lysates were prepared and luciferase assays conducted using Dual Luciferase Reporter Assay System (Promega) according to manufacturer's recommendations. Luminescence was measured using a Synergy2 plate reader luminescence module (BioTek).
Supplementary Material
Acknowledgments
The authors thank Eliane Mueller for the PG+/− and PG−/− keratinocytes, and colleagues for helpful discussions. We also thank Jesse Roman for the pFN(1.2KB)Luc and control plasmids, as well as Jeffrey D. Ritzenthaler for providing the protocols on how to use the constructs. In addition, we thank Kimberly Smith for help with setting up the luciferase assays. This work was supported by R01AR43380, R01AR041836-17S1 (American Recovery and Rehabilitation Act), R01CA122151 and the J. L. Mayberry endowment (to K.J.G.) and NIH training grant T32 CA070085 and F32 AR055444 to V.T. V.T. and B.V.D. performed most of the work. T.Y. performed the initial study. A.D.D. conducted RhoA and Rac1 G-LISA assays, as well as FN qPCR analysis. M.J.S.P. analyzed differential protein expression by mass spectrometry. E.A. performed live cell imaging. V.T., K.J.G., J.C.R.J. wrote the manuscript. K.J.G. and J.C.R.J. provided guidance for the project. The authors declare that they have no conflict of interest. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/20/3576/DC1
References
- Aberle H., Bierkamp C., Torchard D., Serova O., Wagner T., Natt E., Wirsching J., Heidkamper C., Montagna M., Lynch H. T. (1995). The human plakoglobin gene localizes on chromosome 17q21 and is subjected to loss of heterozygosity in breast and ovarian cancers. Proc. Natl. Acad. Sci. USA 92, 6384-6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acehan D., Petzold C., Gumper I., Sabatini D. D., Muller E. J., Cowin P., Stokes D. L. (2008). Plakoglobin is required for effective intermediate filament anchorage to desmosomes. J. Invest. Dermatol. 128, 2665-2675 [DOI] [PubMed] [Google Scholar]
- Aktary Z., Chapman K., Lam L., Lo A., Ji C., Graham K., Cook L., Li L., Mackey J. R., Pasdar M. (2010). Plakoglobin interacts with and increases the protein levels of metastasis suppressor Nm23-H2 and regulates the expression of Nm23-H1. Oncogene 29, 2118-2129 [DOI] [PubMed] [Google Scholar]
- Arregui C., Pathre P., Lilien J., Balsamo J. (2000). The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. J. Cell Biol. 149, 1263-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur W. T., Burridge K. (2001). RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 12, 2711-2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur W. T., Petch L. A., Burridge K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719-722 [DOI] [PubMed] [Google Scholar]
- Avizienyte E., Frame M. C. (2005). Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 17, 542-547 [DOI] [PubMed] [Google Scholar]
- Avizienyte E., Wyke A. W., Jones R. J., McLean G. W., Westhoff M. A., Brunton V. G., Frame M. C. (2002). Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 4, 632-638 [DOI] [PubMed] [Google Scholar]
- Balzac F., Avolio M., Degani S., Kaverina I., Torti M., Silengo L., Small J. V., Retta S. F. (2005). E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J. Cell Sci. 118, 4765-4783 [DOI] [PubMed] [Google Scholar]
- Bazzi H., Christiano A. M. (2007). Broken hearts, woolly hair, and tattered skin: when desmosomal adhesion goes awry. Curr. Opin. Cell Biol. 19, 515-520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo K. A., Wang Y. L. (2002). Flexible substrata for the detection of cellular traction forces. Trends Cell. Biol. 12, 79-84 [DOI] [PubMed] [Google Scholar]
- Beningo K. A., Dembo M., Kaverina I., Small J. V., Wang Y. L. (2001). Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153, 881-888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierkamp C., McLaughlin K. J., Schwarz H., Huber O., Kemler R. (1996). Embryonic heart and skin defects in mice lacking plakoglobin. Dev. Biol. 180, 780-785 [DOI] [PubMed] [Google Scholar]
- Borrmann C. M., Grund C., Kuhn C., Hofmann I., Pieperhoff S., Franke W. W. (2006). The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur. J. Cell Biol. 85, 469-485 [DOI] [PubMed] [Google Scholar]
- Briata P., Ilengo C., Corte G., Moroni C., Rosenfeld M. G., Chen C. Y., Gherzi R. (2003). The Wnt/beta-catenin→Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol. Cell 12, 1201-1211 [DOI] [PubMed] [Google Scholar]
- Caldelari R., de Bruin A., Baumann D., Suter M. M., Bierkamp C., Balmer V., Muller E. (2001). A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J. Cell Biol. 153, 823-834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Gumbiner B. M. (2006). Crosstalk between different adhesion molecules. Curr. Opin. Cell Biol. 18, 572-578 [DOI] [PubMed] [Google Scholar]
- Cheng X., Koch P. J. (2004). In vivo function of desmosomes. J. Dermatol. 31, 171-187 [DOI] [PubMed] [Google Scholar]
- Chidgey M., Dawson C. (2007). Desmosomes: a role in cancer? Br. J. Cancer. 96, 1783-1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. (1990). Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J. Invest. Dermatol. 94, 128S-134S [DOI] [PubMed] [Google Scholar]
- Cowin P. (1994). Plakoglobin. In Molecular Biology of Desmosomes and Hemidesmosomes (ed. Collins J. E., Garrod D. R.), pp. 1-131 Austin: R.G. Landes Co. [Google Scholar]
- Cowin P., Kapprell H.-P., Franke W. W., Tamkun J., Hynes R. O. (1986). Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 46, 1063-1073 [DOI] [PubMed] [Google Scholar]
- Cox E. A., Sastry S. K., Huttenlocher A. (2001). Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol. Biol. Cell 12, 265-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali K. A., Wennerberg K., Burridge K. (2003). Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15, 572-582 [DOI] [PubMed] [Google Scholar]
- Dusek R. L., Godsel L. M., Green K. J. (2007). Discriminating roles of desmosomal cadherins: beyond desmosomal adhesion. J. Dermatol. Sci. 45, 7-21 [DOI] [PubMed] [Google Scholar]
- Frame M. C. (2004). Newest findings on the oldest oncogene; how activated src does it. J. Cell Sci. 117, 989-998 [DOI] [PubMed] [Google Scholar]
- Fukuhara T., Shimizu K., Kawakatsu T., Fukuyama T., Minami Y., Honda T., Hoshino T., Yamada T., Ogita H., Okada M., et al. (2004). Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J. Cell Biol. 166, 393-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D., Chidgey M. (2008). Desmosome structure, composition and function. Biochim. Biophys. Acta 1778, 572-587 [DOI] [PubMed] [Google Scholar]
- Getsios S., Amargo E. V., Dusek R. L., Ishii K., Sheu L., Godsel L. M., Green K. J. (2004a). Coordinated expression of desmoglein 1 and desmocollin 1 regulates intercellular adhesion. Differentiation 72, 419-433 [DOI] [PubMed] [Google Scholar]
- Getsios S., Huen A. C., Green K. J. (2004b). Working out the strength and flexibility of desmosomes. Nat. Rev. Mol. Cell Biol. 5, 271-281 [DOI] [PubMed] [Google Scholar]
- Gradl D., Kuhl M., Wedlich D. (1999). The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol. Cell. Biol. 19, 5576-5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. J., Gaudry C. A. (2000). Are desmosomes more than tethers for intermediate filaments? Nat. Rev. Mol. Cell Biol. 1, 208-216 [DOI] [PubMed] [Google Scholar]
- Green K. J., Simpson C. L. (2007). Desmosomes: new perspectives on a classic. J. Invest. Dermatol. 127, 2499-2515 [DOI] [PubMed] [Google Scholar]
- Hakimelahi S., Parker H. R., Gilchrist A. J., Barry M., Li Z., Bleackley R. C., Pasdar M. (2000). Plakoglobin regulates the expression of the anti-apoptotic protein BCL-2. J. Biol. Chem. 275, 10905-10911 [DOI] [PubMed] [Google Scholar]
- Hamill K. J., Kligys K., Hopkinson S. B., Jones J. C. (2009). Laminin deposition in the extracellular matrix: a complex picture emerges. J. Cell Sci. 122, 4409-4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L., Nathke I. S., Papkoff J., Nelson W. J. (1994). Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J. Cell Biol. 125, 1327-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking D. C., Chang C. H. (2003). Fibronectin matrix polymerization regulates small airway epithelial cell migration. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L169-L179 [DOI] [PubMed] [Google Scholar]
- Hodivala K. J., Watt F. M. (1994). Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol. 124, 589-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687 [DOI] [PubMed] [Google Scholar]
- Janes S. M., Watt F. M. (2006). New roles for integrins in squamous-cell carcinoma. Nat. Rev. Cancer 6, 175-183 [DOI] [PubMed] [Google Scholar]
- Jeanes A., Gottardi C. J., Yap A. S. (2008). Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 27, 6920-6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Kugler M. C., Wei Y., Kim K. K., Li X., Brumwell A. N., Chapman H. A. (2009). Integrin alpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J. Cell Biol. 184, 309-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A. P., Bornslaeger E. A., Borgwardt J. E., Palka H. L., Dhaliwal A. S., Corcoran C. M., Denning M. F., Green K. J. (1997). The amino-terminal domain of desmoplakin binds to plakoglobin and clusters desmosomal cadherin-plakoglobin complexes. J. Cell Biol. 139, 773-784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhofer M., Hopkinson S. B., Jones J. C. R. (1993). The matrix secreted by 804G cells contains laminin related components that participate in hemidesmosome assembly in vitro. J. Cell Sci. 105, 753-764 [DOI] [PubMed] [Google Scholar]
- Levenberg S., Katz B.-Z., Yamada K. M., Geiger B. (1998). Long-range and selective autoregulation of cell-cell or cell-matrix adhesion by cadherin or integrin ligands. J. Cell Sci. 111, 347-357 [DOI] [PubMed] [Google Scholar]
- Litjens S. H., de Pereda J. M., Sonnenberg A. (2006). Current insights into the formation and breakdown of hemidesmosomes. Trends Cell. Biol. 16, 376-383 [DOI] [PubMed] [Google Scholar]
- Maeda O., Usami N., Kondo M., Takahashi M., Goto H., Shimokata K., Kusugami K., Sekido Y. (2004). Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene 23, 964-972 [DOI] [PubMed] [Google Scholar]
- Margadant C., Raymond K., Kreft M., Sachs N., Janssen H., Sonnenberg A. (2009). Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J. Cell Sci. 122, 278-288 [DOI] [PubMed] [Google Scholar]
- Marsden M., DeSimone D. W. (2003). Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr. Biol. 13, 1182-1191 [DOI] [PubMed] [Google Scholar]
- McCrea P. D., Gu D., Balda M. S. (2009). Junctional music that the nucleus hears: cell-cell contact signaling and the modulation of gene activity. Cold Spring Harbor Perspect. Biol. 1, a002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. A., Wessagowit V. (2005). Human hair abnormalities resulting from inherited desmosome gene mutations. Keio J. Med. 54, 72-79 [DOI] [PubMed] [Google Scholar]
- McKoy G., Protonotarios N., Crosby A., Tsatsopoulou A., Anastasakis A., Coonar A., Norman M., Baboonian C., Jeffery S., McKenna W. J. (2000). Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355, 2119-2124 [DOI] [PubMed] [Google Scholar]
- Mercurio A. M., Rabinovitz I. (2001). Towards a mechanistic understanding of tumor invasion-lessons from the alpha6beta 4 integrin. Semin. Cancer Biol. 11, 129-141 [DOI] [PubMed] [Google Scholar]
- Michaelson J. E., Ritzenthaler J. D., Roman J. (2002). Regulation of serum-induced fibronectin expression by protein kinases, cytoskeletal integrity, and CREB. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L291-L301 [DOI] [PubMed] [Google Scholar]
- Mimura Y., Ihn H., Jinnin M., Asano Y., Yamane K., Tamaki K. (2004). Epidermal growth factor induces fibronectin expression in human dermal fibroblasts via protein kinase C delta signaling pathway. J. Invest. Dermatol. 122, 1390-1398 [DOI] [PubMed] [Google Scholar]
- Mizushima H., Takamura H., Miyagi Y., Kikkawa Y., Yamanaka N., Yasumitsu H., Misugi K., Miyazaki K. (1997). Identification of integrin-dependent and -independent cell adhesion domains in COOH-terminal globular region of laminin-5 alpha 3 chain. Cell Growth Differ. 8, 979-987 [PubMed] [Google Scholar]
- Monier-Gavelle F., Duband J.-L. (1997). Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by β1 and β3 integrins in migrating neural crest cells. J. Cell Biol. 137, 1663-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North A. J., Bardsley W. G., Hyam J., Bornslaeger E. A., Cordingley H. C., Trinnaman B., Hatzfeld M., Green K. J., Magee A. I., Garrod D. R. (1999). Molecular map of the desmosomal plaque. J. Cell Sci. 112, 4325-4336 [DOI] [PubMed] [Google Scholar]
- Ogita H., Takai Y. (2008). Cross-talk among integrin, cadherin, and growth factor receptor: roles of nectin and nectin-like molecule. Int. Rev. Cytol. 265, 1-54 [DOI] [PubMed] [Google Scholar]
- Ojakian G., Ratcliffe D., Schwimmer R. (2001). Integrin regulation of cell-cell adhesion during epithelial tubule formation. J. Cell Sci. 114, 941-952 [DOI] [PubMed] [Google Scholar]
- Palka H. L., Green K. J. (1997). Roles of plakoglobin end domains in desmosome assembly. J. Cell Sci. 110, 2359-2371 [DOI] [PubMed] [Google Scholar]
- Playford M. P., Schaller M. D. (2004). The interplay between Src and integrins in normal and tumor biology. Oncogene 23, 7928-7946 [DOI] [PubMed] [Google Scholar]
- Rabinovitz I., Mercurio A. M. (1997). The integrin α6β4 functions in carcinoma cel migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 139, 1873-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raurell I., Castano J., Franci C., Garcia de Herreros A., Dunach M. (2006). Presenilin-1 interacts with plakoglobin and enhances plakoglobin-Tcf-4 association. Implications for the regulation of beta-catenin/Tcf-4-dependent transcription. J. Biol. Chem. 281, 1401-1411 [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003). Cell migration: integrating signals from front to back. Science 302, 1704-1709 [DOI] [PubMed] [Google Scholar]
- Rottner K., Hall A., Small J. V. (1999). Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9, 640-648 [DOI] [PubMed] [Google Scholar]
- Ruiz P., Brinkmann V., Ledermann B., Behrend M., Grund C., Thalhammer C., Vogel F., Birchmeier C., Gunthert U., Franke W. W., et al. (1996). Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J. Cell Biol. 135, 215-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sado Y., Kagawa M., Naito I., Ueki Y., Seki T., Momota R., Oohashi T., Ninomiya Y. (1998). Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J. Biochem. 123, 767-776 [DOI] [PubMed] [Google Scholar]
- Santoro M. M., Gaudino G. (2005). Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 304, 274-286 [DOI] [PubMed] [Google Scholar]
- Schmidt A., Koch P. J. (2007). Desmosomes: just cell adhesion or is there more? Cell Adh. Migr. 1, 28-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal B. U., DeBiase P. J., Matzno S., Chew T. L., Claiborne J. N., Hopkinson S. B., Russell A., Marinkovich M. P., Jones J. C. (2006). Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J. Biol. Chem. 281, 35487-35498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J. B., Graff D., Li H. P. (1987). A solid-phase method for the quantitation of protein in the presence of sodium dodecyl sulfate and other interfering substances. Anal. Biochem. 166, 49-54 [DOI] [PubMed] [Google Scholar]
- Shimizu M., Fukunaga Y., Ikenouchi J., Nagafuchi A. (2008). Defining the roles of beta-catenin and plakoglobin in LEF/T-cell factor-dependent transcription using beta-catenin/plakoglobin-null F9 cells. Mol. Cell. Biol. 28, 825-835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp M. A., Liu Y., Pal-Ghosh S., Jurjus R. A., Tadvalkar G., Sekaran A., Losicco K., Jiang L., Larsen M., Li L., et al. (2007). Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. J. Cell Sci. 120, 2851-2863 [DOI] [PubMed] [Google Scholar]
- Stiehl D. P., Wirthner R., Koditz J., Spielmann P., Camenisch G., Wenger R. H. (2006). Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J. Biol. Chem. 281, 23482-23491 [DOI] [PubMed] [Google Scholar]
- Takai Y., Ikeda W., Ogita H., Rikitake Y. (2008). The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 24, 309-342 [DOI] [PubMed] [Google Scholar]
- Teuliere J., Faraldo M. M., Shtutman M., Birchmeier W., Huelsken J., Thiery J. P., Glukhova M. A. (2004). beta-catenin-dependent and -independent effects of DeltaN-plakoglobin on epidermal growth and differentiation. Mol. Cell. Biol. 24, 8649-8661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic V., Desai B. V., Eigenheer R. A., Yin T., Amargo E. V., Mrksich M., Green K. J., Patterson M. J. (2010). Detection of differentially expressed Basal cell proteins by mass spectrometry. Mol. Cell. Proteomics 9, 351-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J., Kam L. (2009). Rigidity-dependent cross talk between integrin and cadherin signaling. Biophys. J. 96, L39-L41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier A., Sonnenberg A. (2001). Function and interactions of integrins. Cell Tissue Res. 305, 285-298 [DOI] [PubMed] [Google Scholar]
- Watt F. M. (2002). Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919-3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M. J., Johnson K. R. (2003). Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 19, 207-235 [DOI] [PubMed] [Google Scholar]
- Wolf A., Krause-Gruszczynska M., Birkenmeier O., Ostareck-Lederer A., Huttelmaier S., Hatzfeld M. (2010). Plakophilin 1 stimulates translation by promoting eIF4A1 activity. J. Cell Biol. 188, 463-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T., Green K. J. (2004). Regulation of desmosome assembly and adhesion. Semin. Cell Dev. Biol. 15, 665-677 [DOI] [PubMed] [Google Scholar]
- Yin T., Getsios S., Caldelari R., Godsel L. M., Kowalczyk A. P., Muller E. J., Green K. J. (2005a). Mechanisms of plakoglobin-dependent adhesion: desmosome-specific functions in assembly and regulation by epidermal growth factor receptor. J. Biol. Chem. 280, 40355-40363 [DOI] [PubMed] [Google Scholar]
- Yin T., Getsios S., Caldelari R., Kowalczyk A. P., Muller E. J., Jones J. C., Green K. J. (2005b). Plakoglobin suppresses keratinocyte motility through both cell-cell adhesion-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA 102, 5420-5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurinsky J., Shtutman M., Ben-Ze'ev A. (2000). Plakoglobin and β-catenin: protein interactions, regulation and biological roles. J. Cell Sci. 113, 3127-3139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.