Abstract
Cytoplasmic dynein in filamentous fungi accumulates at microtubule plus-ends near the hyphal tip, which is important for minus-end-directed transport of early endosomes. It was hypothesized that dynein is switched on at the plus-end by cargo association. Here, we show in Aspergillus nidulans that kinesin-1-dependent plus-end localization is not a prerequisite for dynein ATPase activation. First, the Walker A and Walker B mutations in the dynein heavy chain AAA1 domain implicated in blocking different steps of the ATPase cycle cause different effects on dynein localization to microtubules, arguing against the suggestion that ATPase is inactive before arriving at the plus-end. Second, dynein from ΔkinA (kinesin 1) mutant cells has normal ATPase activity despite the absence of dynein plus-end accumulation. In ΔkinA hyphae, dynein localizes along microtubules and does not colocalize with abnormally accumulated early endosomes at the hyphal tip. This is in contrast to the colocalization of dynein and early endosomes in the absence of NUDF/LIS1. However, the Walker B mutation allows dynein to colocalize with the hyphal-tip-accumulated early endosomes in the ΔkinA background. We suggest that the normal ability of dyenin to interact with microtubules as an active minus-end-directed motor demands kinesin-1-mediated plus-end accumulation for effective interactions with early endosomes.
Keywords: Dynein, Kinesin 1, LIS1, Aspergillus nidulans, Microtubule plus-end
Introduction
Cytoplasmic dynein is a multi-subunit microtubule motor that uses the ATPase activity of its heavy chain to move towards the minus-end of a microtubule. In vivo, cytoplasmic dynein is important for the positioning of nuclei and spindles and the retrograde transport of vesicles, signaling molecules and viruses (Karki and Holzbaur, 1999; Schroer, 2004; Soldati and Schliwa, 2006; Greber and Way, 2006; Abe and Cavalli, 2008). Interestingly, although cytoplasmic dynein is a minus-end-directed motor, it accumulates at the microtubule plus-end in many cell types (Vaughan et al., 1999; Han et al., 2001; Ma and Chisholm, 2002; Lee et al., 2003; Sheeman et al., 2003; Lenz et al., 2006; Vogel et al., 2009). This accumulation at the microtubule plus-end is important for a variety of functions. For example, it might facilitate interaction with cortical proteins for spindle positioning (Lee et al., 2003; Sheeman et al., 2003; Markus et al., 2009). In addition, because vesicles that need to undergo retrograde transport often start their journey at the plus-end, the accumulation of dynein at the plus-end is thought to facilitate loading of membranous cargoes (Vaughan et al., 2002; Lenz et al., 2006).
It has been found in several fungal organisms including Aspergillus nidulans, Saccharomyces cerevisiae and Ustilago maydis that the accumulation of dynein at the microtubule plus-end depends on plus-end-directed kinesins (Zhang et al., 2003; Carvalho et al., 2004; Lenz et al., 2006; Caudron et al., 2008). In filamentous fungi and in neurons, kinesin 1 is important for plus-end accumulation of dyenin (Zhang et al., 2003; Lenz et al., 2006; Yamada et al., 2009) and, importantly, as first discovered in U. maydis, dynein-mediated retrograde movement of early endosomes also depends on the function of kinesin 1 (Lenz et al., 2006; Abenza et al., 2009; Zekert and Fischer, 2009). These results strongly support the notion that plus-end accumulation of dynein is important for its function in vesicle trafficking. In filamentous fungi, the dynactin complex (Schroer, 2004) as well as several dynein subunits are also important for the accumulation of dynein at the microtubule plus-end (Zhang et al., 2002; Zhang et al., 2003; Zhang et al., 2008; Zhang et al., 2009; Liu et al., 2003; Lenz et al., 2006).
Whether and how dynein activity is regulated at the plus-end remain to be addressed. It is thought that dynein must remain inactive at the plus-end until it interacts with cargo and/or other protein factors (Lee et al., 2003; Sheeman et al., 2003; Zhang et al., 2003; Lenz et al., 2006; Steinberg, 2007). However, it is unclear whether dynein only gets activated at the plus-end or if it is already active before arriving at the plus-end. Here, we tested in A. nidulans whether dynein is an active ATPase before it arrives at the plus-end and whether plus-end localization is a prerequisite for activating the dynein ATPase. Our results suggest that dynein is an active ATPase before it arrives at the plus-end. The kinesin-1-mediated accumulation of dynein at the plus-ends might facilitate the interaction between early endosomes and the dynein motors but it is not a prerequisite for dynein ATPase activation.
Results
GFP-dynein with the AAA1-Walker B mutation highlights the plus-ends, whereas the AAA1-Walker A mutation causes dynein to bind along microtubules
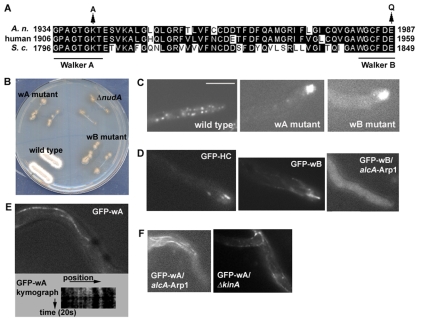
To test whether dynein has an active ATPase cycle before arriving at the plus-end, we introduced two dynein heavy chain (HC) mutations implicated in blocking ATP binding and hydrolysis, respectively, and observed the localization patterns of the mutant HCs. The dynein heavy chain belongs to the AAA+ (ATPase associated with a wide variety of cellular activities) class of ATPases (Neuwald et al., 1999; Iyer et al., 2004; Snider and Houry, 2008). Its motor domain forms a ring-like structure comprising six AAA domains (Samso and Koonce, 2004; Roberts et al., 2009). As indicated by studies on other AAA ATPases, an AAA domain responsible for ATP binding and hydrolysis contains a Walker A motif required for ATP binding and a Walker B motif required for ATP hydrolysis (Neuwald et al., 1999; Saraste et al., 1990; Pause and Sonenberg, 1992; Whiteheart et al., 1994; Babst et al., 1998; Reck-Peterson and Vale, 2004). In the dynein heavy chain, the first AAA1 domain is the major site of ATP hydrolysis (Gibbons et al., 1987; Gee et al., 1997) and is essential for dynein motor function (Reck-Peterson and Vale, 2004; Kon et al., 2004). In S. cerevisiae, both Walker A and Walker B mutations implicated in abolishing ATP-binding and hydrolysis, respectively, severely inhibit dynein-mediated spindle positioning when introduced into the genome by homologous recombination (Reck-Peterson and Vale, 2004). However, their effects on the microtubule plus-end localization of dynein have not yet been tested. In vitro kinetic studies indicate that ATP binding induces the detachment of dynein from the microtubule and subsequent ATP hydrolysis allows dynein to reattach to the microtubule (Hackney, 1996; Tsygankov et al., 2009; Hook, 2010). We reasoned that if dynein is a passive cargo of kinesin 1 and its ATPase only becomes active after arriving at the plus-end, both the Walker A and Walker B mutants (called wA and wB, respectively, for simplicity) should be transported to the plus-end and accumulate there without departure. Conversely, if dynein has an active ATPase cycle and is able to interact with microtubules prior to arriving at the plus-ends, the wA mutant should act as a rigor mutant and decorate microtubules along their entire length, rather than concentrating at the plus-ends. To test these possibilities, we replaced the endogenous dynein heavy chain nudA gene with alleles encoding a GFP-tagged mutant heavy chain containing either the wA mutation (NUDAK1940A) or the wB mutation (NUDAE1987Q) (Fig. 1A) and examined the consequences of these mutations on dynein localization in vivo. Both mutations produced a colony phenotype resembling that of a typical nud mutant, which indicates loss of dynein function in vivo (Fig. 1B). Consistent with this notion, RabA-labeled early endosomes, whose retrograde transport is driven by dynein (Abenza et al., 2009; Abenza et al., 2010), accumulated abnormally at the hyphal tip in both the wA and wB mutants (Fig. 1C), indicating that these mutations cause a block of dynein-mediated early endosome transport. Interestingly, these two mutations have different effects on the localization of GFP-labeled HC proteins. The wB mutant HC (GFP-wB) formed long comet-like structures representing microtubule plus-end localization (Fig. 1D). The GFP-wB comets appeared longer and less focused at the tip of the plus-end than the wild-type comets, suggesting that wB dynein localizes not only to the plus-end but also behind it [see Movie 1 (wild-type GFP-HC) and Movie 2 (GFP-wB) in the supplementary material]. This accumulation appears to be dynactin-dependent as it was not observed if the GFP-wB fusion was introduced into the conditional ARP1 knockdown mutant alcA-nudKArp1 (also called alcA-ARP1 for simplicity) background (Fig. 1D), in which both ARP1 and p150 levels are dramatically lowered by culturing cells on glucose (Zhang et al., 2008). By marked contrast, the wA mutant (GFP-wA) localizes along microtubules with no enrichment at the plus-ends [Fig. 1E; also see supplementary material Movie 3 (GFP-wA)]. Kymograph analyses demonstrated that the GFP-wA spots along the microtubules are static (Fig. 1E), consistent with an ATP-binding defect that causes a rigor phenotype. We further demonstrated that the localization of GFP-wA to microtubules is dynactin- and kinesin-1-independent as the microtubule decoration by GFP-wA was not affected by either knockdown of Arp1 (alcA-ARP1) or deletion of KINA (ΔkinA) (Fig. 1F), strongly supporting the conclusion that the wA mutant dynein heavy chain interacts with microtubules directly. The finding that the AAA1 mutation affecting ATP binding causes a different dynein localization pattern than the one affecting ATP hydrolysis indicates that the ATPase cycle of dynein is not turned off until it arrives at the microtubule plus-end.
Fig. 1.
An analysis of the AAA1 Walker A (wA) and Walker B (wB) mutants of the GFP-HC (dynein heavy chain). (A) An alignment of part of the dynein HC sequences from A. nidulans (A. n.), human and S. cerevisiae (S. c.) to show the positions of the wA and wB mutations in AAA1. The wA and wB motifs of AAA1 are underlined according to Iyer et al. (Iyer et al., 2004). The lysine residue (K) in wA and the glutamic acid (E) in wB are changed to alanine (A) and glutamine (Q), respectively, by mutagenesis (arrows). Accession numbers for A. nidulans HC, human cytoplasmic dynein 1 HC and S. cerevisiae HC are XP_657722, Q14204 and NP_012980, respectively. (B) Colony phenotype of the wA and wB mutants in comparison with wild type and the ΔnudA (HC) mutant. (C) Early endosomes labeled with mCherry-RABA accumulate at the hyphal tip in the wA and wB mutants, indicating that they fail to undergo dynein-mediated retrograde transport in these mutants. (D) Similar to wild-type GFP-HC, GFP-wB with the wB mutation in the HC highlights microtubule plus-ends, and this localization disappears in the alcA-ARP1 mutant, where the expression of the ARP1 subunit of dynactin is shut off. Note that besides the decoration at the plus-ends, faint decoration by GFP-wB along microtubules can also be observed. (E) An image of GFP-wA showing GFP signals along a microtubule and a kymograph showing that the GFP signals are static, suggesting that the wA mutant dynein is unable to move along the microtubule. (F) GFP-wA decorates along microtubules in the alcA-ARP1 and ΔkinA mutants. Scale bar: 5 μm.
Dynein isolated from the ΔkinA mutant has normal ATPase activity
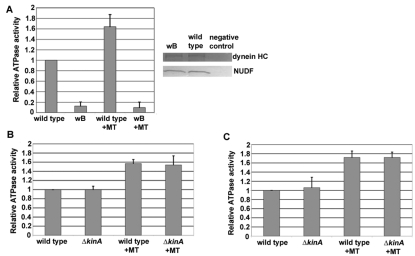
Because the accumulation of dynein at the microtubule plus-ends is diminished in the ΔkinA mutant (Zhang et al., 2003), we determined whether dynein from ΔkinA mutant cells has a normal ATPase activity. Dynein was isolated from A. nidulans extracts using S-tag affinity purification as previously described (Zhuang et al., 2007; Zhang et al., 2008). We first used mild extraction conditions (see Materials and Methods) under which regulatory proteins such as dynactin and NUDF/LIS1 co-purify with dynein (Zhuang et al., 2007; Zhang et al., 2008), thus minimizing the possibility that crucial regulators are lost during protein isolation. We previously measured the basal ATPase activity of dynein isolated under similar conditions and found that the NUDAR3086C mutation in AAA4 causes a 50% reduction, strongly suggesting that the ATPase activity we measured is due to dynein (Zhuang et al., 2007). Thus, we measured ATPase activity of the wB mutant dynein heavy chain, which showed a dramatic reduction in both the basal and microtubule-stimulated ATPase activities relative to the wild type (Fig. 2A; P<0.001), further demonstrating that the ATPase activity we measured is dynein-specific. By contrast, both basal and microtubule-stimulated ATPase activities of dynein isolated from the ΔkinA mutant resembled the wild type (Fig. 2B). To ensure that we were taking into account not only dynein isolated from the soluble fraction but also dynein bound to membrane cargoes, we additionally used conditions aimed at facilitating extraction of membrane-bound proteins (see Materials and Methods). Under these conditions, dynein from the wild-type and ΔkinA strains still showed similar ATPase activities (Fig. 2C). Together, these results support the notion that the microtubule plus-end-localization of dynein is not a prerequisite for activation of its ATPase.
Fig. 2.
Dynein from ΔkinA cells has ATPase activity similar to that of wild type (WT). (A) ATPase values of dynein from wild type and the wB mutant. Values were all relative to the wild-type value without microtubules (MT; basal level), which was set at 1 (same for B and C). The mean and standard deviation (s.d.) values were calculated from 6 enzyme assays with dynein purified from two independent experiments. A western blot analysis on dynein HC and NUDF from these two strains is shown on the right. The negative control strain was GR5, which does not contain the affinity tag S-IC. (B) ATPase values of dynein isolated from the wild type and the ΔkinA mutant with a low-salt and detergent buffer. The mean and s.d. values were calculated from 11 enzyme assays with dynein purified from 3 independent experiments. (C) ATPase values of dynein isolated from the wild type and the ΔkinA mutant with a higher-salt and detergent buffer. The mean and s.d. values were calculated from 6 enzyme assays with dynein purified from 2 independent experiments.
Wild-type dynein does not colocalize with hyphal-tip-accumulated early endosomes in the ΔkinA mutant but the AAA1 Walker B mutation allows dynein to colocalize with these accumulated early endosomes
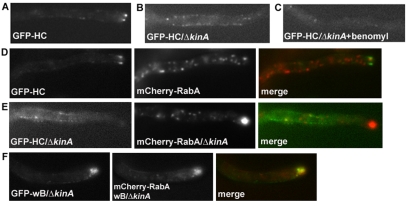
We previously reported that GFP-NUDA (dynein heavy chain), driven by the alcA promoter, accumulates at the microtubule plus-ends (Han et al., 2001), and that this accumulation is diminished in the ΔkinA mutant (Zhang et al., 2003). We recently constructed a strain in which the GFP-nudA allele is introduced into the nudA locus by gene replacement and expression of GFP-NUDA is under the control of the nudA promoter (Zhuang et al., 2007). We introduced the GFP-NUDA fusion (GFP-HC) into the ΔkinA background by crossing. Consistent with previous results, the bright comets representing plus-end accumulation in wild type (Fig. 3A) were no longer observed in the ΔkinA mutant (Fig. 3B). The new strain combined with improvements in our imaging system allowed us to determine that, in the ΔkinA mutant, GFP-HC signals were located along microtubule-like structures throughout the hyphae (Fig. 3B), and that these signals disappeared after microtubules were depolymerized by benomyl (Fig. 3C). These results indicate that although the plus-end accumulation of dynein requires kinesin 1, dynein molecules are able to interact with microtubules in its absence.
Fig. 3.
Dynein does not colocalize with hyphal-tip-accumulated early endosomes in the ΔkinA mutant, but dynein–early-endosome colocalization is observed in the wB/ΔkinA mutant. (A) GFP-dynein HCs in wild-type cells form comet-like structures reflecting their microtubule plus-end localization. (B) In the ΔkinA mutant, GFP-dynein HCs fail to accumulate at the plus-end but instead are seen as dots along microtubule-like structures. (C) As expected for signals along microtubules, the signals of GFP-HC in the ΔkinA mutant disappear after a 10-minute treatment with 2.4 μg/ml of a microtubule-depolymerizing drug, benomyl. (D) In a wild-type cell, the mCherry-RABA-labeled early endosomes distribute along the hyphae more or less evenly and the plus-end comets of GFP-HCs can be observed. (E) In a ΔkinA cell, the mCherry-RABA-labeled early endosomes accumulate as a cloud at the hyphal tip, reflecting a failure in their retrograde movement, whereas GFP-HC localizes along microtubules and does not show any colocalization with the hyphal-tip-accumulated early endosomes. (F) GFP-wB colocalizes with hyphal-tip-accumulated early endosomes in the ΔkinA mutant. Scale bar: 5 μm.
As described previously by Abenza et al. (Abenza et al., 2009), RabA-associated early endosomes accumulate at the hyphal tip in the ΔkinA mutant (Fig. 3D,E). Observation of GFP-HC and mCherry-labeled RabA did not reveal any colocalization of GFP-HC and mCherry-RabA signals at the hyphal tip (Fig. 3E). However, introducing the AAA1 wB mutation into the GFP-HC produced an unexpected colocalization between dynein and the hyphal-tip-accumulated early endosomes in the ΔkinA background. In the ΔkinA cells, GFP-wB did not highlight the plus-ends, although faint dots along microtubule-like structures were still observed (Fig. 3F). Unexpectedly, GFP-wB in the ΔkinA cells formed a prominent cloud-like accumulation at the hyphal tip (Fig. 3F). To determine whether this accumulation represents colocalization with accumulated early endosomes, we introduced the mCherry-RabA fusion into the GFP-wB/ΔkinA background by crossing. As shown in Fig. 3F, mCherry-RabA signals also accumulate at the hyphal tip and the signals of mCherry-RabA and of GFP-wB largely overlap in the same cell. Thus, presence of the AAA1 wB mutation allows dynein to localize with hyphal-tip-accumulated early endosomes in the ΔkinA mutant. The observation of the dynein–early-endosome colocalization in the wB/ΔkinA cells, but not in the ΔkinA cells, is not due to an increased buildup of early endosomes at the hyphal tip in the wB/ΔkinA cells relative to the ΔkinA cells. In fact, the average intensity sum of the mCherry-RabA cloud at the hyphal tips of wB/ΔkinA cells is lower, and is only about 37% of that in ΔkinA cells (n=12 for each group, P<0.005). This is consistent with the idea that because endocytosis is coupled with hyphal tip growth (Araujo-Bazán et al., 2008; Taheri-Talesh et al., 2008; Upadhyay and Shaw, 2008; Harris, 2010), it might be less efficient in the slowly growing wB/ΔkinA cells, which form small colonies resembling those of previously described double-mutant strains defective in both KINA and NUDA (Requena et al., 2001; Zhang et al., 2003). We speculate that GFP-wB molecules might reach the hyphal-tip-accumulated early endosomes by diffusion within the slow-growing hyphae of the wB/ΔkinA double mutant. We also speculate that because ATP hydrolysis is implicated in dynein rebinding to microtubules after ATP-mediated release from the microtubules (Hackney, 1996; Tsygankov et al., 2009; Hook, 2010), wB dynein might be unable to bind to microtubules as strongly as wild-type dynein, which further facilitates diffusion. By contrast, as wild-type dynein molecules bind along microtubules as active minus-end-directed motors before they arrive at the plus-ends, they are unable to diffuse efficiently to the hyphal tip and, thus, in wild-type cells, KINA-mediated plus-end accumulation of the dynein is crucial for dynein–early-endosome interaction.
Dynein colocalizes with abnormally accumulated early endosomes in the absence of NUDF
A. nidulans NUDF is a homolog of LIS1 involved in neuronal migration (Xiang et al., 1995; Reiner et al., 1993). LIS1 is clearly involved in dynein function in nuclear migration (Tsai and Gleeson, 2005; Vallee and Tsai, 2006; Kardon and Vale, 2009; McKenney et al., 2010), and is also involved in vesicle trafficking (Liang et al., 2004; Lenz et al., 2006). In ΔnudF cells, the plus-end accumulation of dynein is clearly visible (Zhang et al., 2003) but early endosomes labeled with GFP-RabA accumulate at the hyphal tip (Fig. 4A), indicating that the retrograde movement of early endosomes is impaired. These results are remarkably similar to the results obtained in the filamentous hyphae of U. maydis (Lenz et al., 2006). Interestingly, a cloud of GFP-HC was also observed at the hyphal tips of the ΔnudF mutant (Fig. 4A). This phenomenon was mentioned previously in Zhang et al. (Zhang et al., 2003) but we did not provide a good explanation for it. In the current work, we found that in the ΔnudF mutant expressing both GFP-HC and mCherry-RabA, the fluorescent signals largely overlap, indicating colocalization of dynein and early endosomes (Fig. 4B). Thus, we believe that the cloud of GFP-HC at the hyphal tip represents dynein molecules that are attached to early endosomes but are unable to move towards the minus-ends. Together, these results suggest that although KINA mediates plus-end accumulation of dynein to facilitate dynein-early-endosome interaction, NUDF affects dynein function through a different mechanism.
Fig. 4.
An analysis of NUDF function in A. nidulans. (A) GFP-RABA abnormally accumulates at the hyphal tip in the ΔnudF mutant. The image on the right shows GFP-HC forming comet-like structures as well as a cloud at the hyphal tip. Scale bar: 5 μm. (B) Colocalization of the GFP-HC cloud and abnormally accumulated mCherry-RABA signals at the hyphal tip in a ΔnudF cell. (C-E) Dynein isolated from the alcA-nudF mutant under repressive conditions has a normal ATPase activity but a weaker affinity to microtubules. In these experiments, dynein was isolated with a low-salt and detergent buffer to ensure that NUDF is bound to dynein in wild-type extracts. (C) Western blots showing that NUDF is depleted in the alcA-nudF mutant grown on YUU (with glucose) overnight. (D) Dynein from alcA-nudF cells has ATPase activity similar to that of wild type (WT). Values were all relative to the wild-type value without microtubules (MT; basal level), which was set at 1. The mean and s.d. values were calculated from 11 enzyme assays with dynein purified from 3 independent experiments. (E) Results of microtubule pelleting assays shown by a silver-stained gel (top) and by a western blot probed with an anti-HC antibody (bottom). Samples with no microtubules added (−MT) were used as negative controls. (F) The ratio of dynein HC in pellet (P) to dynein HC in supernatant (S) is significantly lower in alcA-nudF than in wild type (P<0.001). Values were relative to the wild-type value, which was set at 1. The mean and s.d. values were calculated from 3 independent dynein isolation experiments.
LIS1 is unlikely to significantly affect the ATPase activity of free dynein given that dynein isolated from the null mutant of Pac1p (LIS1 homolog in budding yeast) cells moves with a normal speed along microtubules (Reck-Peterson et al., 2006). Studies using purified dynein and LIS1 from brain showed that adding LIS1 to dynein does not affect dynein ATPase activity but enhances dynein-microtubule interaction in vitro (Yamada et al., 2008; McKenney et al., 2010). However, an earlier study indicated that purified LIS1 causes moderate but obvious increases of dynein ATPase activity (Mesngon et al., 2006). In order to test in A. nidulans whether dynein ATPase and/or dynein-microtubule interaction are defective when NUDF/LIS1 is deficient, we performed ATPase assays and microtubule pelleting assays using dynein isolated from the conditional nudF-null mutant, alcA-nudF. Unlike ΔnudF, this conditional mutation allows normal conidiation (i.e. asexual spore production) under non-repressing conditions (glycerol as carbon source), facilitating large-scale protein isolation. After the alcA-nudF cells were grown overnight in the glucose-containing rich medium YUU, NUDF was largely undetectable in the fraction containing dynein (Fig. 4C). In spite of this fact, we were unable to detect any significant defect in the ATPase activity of dynein isolated from these NUDF-deficient alcA-nudF mutant cells (Fig. 4D). This result is inconsistent with the report by Mesngon et al. (Mesngon et al., 2006) but consistent with results from two recent studies by Yamada et al. (Yamada et al., 2008) and McKenney et al. (McKenney et al., 2010). Moreover, in agreement with these two studies, dynein isolated from the alcA-nudF cells has decreased affinity for microtubules (Fig. 4E,F). We therefore suggest that NUDF enhances dynein-microtubule interaction, which facilitates the early-endosome-bound dynein to undergo minus-end-directed movement. In vitro, LIS1 significantly improves the processivity of dynein under a heavy load (McKenney et al., 2010). Our results are consistent with this idea and suggest that, in fungal hyphae, with viscosity and cytoplasmic streaming towards the hyphal tip (Bloom, 2008; Lang et al., 2010), early endosomes are heavy enough loads to make NUDF necessary for dynein to carry them along microtubules.
Discussion
In this work, our data suggest that although KINA-mediated microtubule plus-end localization of Aspergillus dynein is important for dynein–early-endosome interaction, it is not required for ATPase activation. As early endosome cargoes largely accumulate at the hyphal tip, whereas dynein localizes along microtubules in the ΔkinA mutant, these results suggest that cargo binding is not necessary for activation of dynein ATPase. Previous reports indicate that kinesin 1 and myosin V motors are in a folded, inactive conformation in the absence of cargo binding but unfold and become active upon cargo binding (Coy et al., 1999; Thirumurugan et al., 2006; Wang et al., 2004). Thus, our results suggest that the regulatory mode of dynein might differ from that of kinesin 1 and myosin V. The idea that plus-end accumulation is not a prerequisite for ATPase activation is also consistent with data showing that single dynein molecules isolated from the budding yeast pac1Δ (LIS1) mutant defective in dynein plus-end accumulation exhibit normal velocity along microtubules (Reck-Peterson et al., 2006). However, because Pac1p can be considered as a factor that retains dynein at the plus-end (Moore et al., 2009), dynein from the pac1Δ mutant might have had a chance to interact with the plus-end.
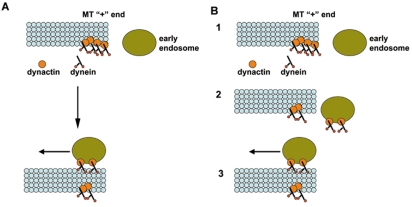
The result that dynein is an active ATPase before arriving at the plus-end has implications on how we interpret the accumulation of dynein at the plus-ends. As the steady-state accumulation is best explained by a higher arrival than departure rate, it has been proposed that dynein must remain inactive at the plus-end until being switched on by other factors or by cargoes (Lee et al., 2003; Sheeman et al., 2003; Zhang et al., 2003; Lenz et al., 2006; Steinberg, 2007). If the ATPase activity is not the target of regulation, then how is the switch of dynein activity achieved? We suggest that, at the microtubule plus-ends, dynein might be tethered to other proteins that preclude its movement toward the minus-end (Fig. 5A). In A. nidulans and U. maydis, dynactin might play this tethering role as dynactin is required for the plus-end accumulation of dynein (Zhang et al., 2003; Zhang et al., 2008; Lenz et al., 2006). One possible model (Fig. 5A) is that interaction with cargo, for example, an early endosome, releases the tether, thereby allowing dynein to move toward the minus-end (Fig. 5A). An alternative model is that endosome binding might cause the dynein/dynactin complex to be transiently off-loaded from the plus-end to the endosome, followed by reattachment of endosome-bound dynein to a microtubule track as a motor (Fig. 5B). This model is analogous to that explaining budding yeast spindle orientation, which also involves off-loading of plus-end dynein to the cortical sites, which is then followed by dynein moving toward the minus-end of an astral microtubule to reel in the spindle (Lee et al., 2003; Sheeman et al., 2003). Importantly, there is a major difference between filamentous fungi and budding yeast in the requirement for LIS1 in dynein-cargo interaction. In budding yeast, because the plus-end accumulation of dynein requires the LIS1 homolog Pac1p (Lee et al., 2003; Sheeman et al., 2003), the cortical localization of dynein also becomes Pac1p-dependent (Markus et al., 2009). By contrast, LIS1 homologs (such as NUDF in A. nidulans) in filamentous fungi are not required for dynein accumulation at the plus-ends (Zhang et al., 2003; Lenz et al., 2006). As early endosomes abnormally accumulate at the hyphal tip in the absence of NUDF, the colocalization of dynein and early endosomes becomes very conspicuous in ΔnudF cells (Fig. 4A,B).
Fig. 5.
Model of dynein regulation at the microtubule plus-end. (A) A diagram showing that dynein at the plus-end is inhibited by a dynactin tether and early endosome binding releases the tether, thereby allowing the endosome-bound dynein to move toward the minus-end. (B) An alternative model showing an additional step (step 2) in which interaction of the endosome with dynactin off-loads dynein-dynactin from the plus-end, which is followed by dynein reattaching to a microtubule as a motor to move the endosome towards the minus-end.
It should be pointed out that although dynein accumulation at the microtubule plus-end is clearly an important component of dynein regulatory strategy in fungi, there must exist other independent regulatory mechanisms. In fungi and higher eukaryotic cells, dynein and kinesin might bind to the same vesicle or to each other directly, and vesicles along a microtubule often undergo bidirectional movement, which could be achieved by tug-of-war between kinesin and dynein or by other regulatory strategies (Ma and Chisholm, 2002; Ligon et al., 2004; Ha et al., 2008; Muller et al., 2008; Shubeita et al., 2008; Soppina et al., 2009; Ally et al., 2009; Hendricks et al., 2010). Nevertheless, the dynamic microtubule plus-end represents a cargo-loading site not only in fungi but also in higher eukaryotic cells (Vaughan et al., 2002; Lenz et al., 2006; Lomakin et al., 2009). Moreover, plus-end localization of dynein and its regulators such as dynactin and LIS1 has been found not only in fungi but also in mammalian cells (Valetti et al., 1999; Vaughan et al., 1999; Vaughan et al., 2002; Han et al., 2001; Ma and Chisholm, 2002; Coquelle et al., 2002; Lee et al., 2003; Sheeman et al., 2003; Lenz et al., 2006; Vogel et al., 2009). Thus, a detailed understanding of dynein regulation at the microtubule plus-end is relevant to many organisms.
Our current study supports the notion that KINA-mediated accumulation of dynein to the microtubule plus-end, rather than KINA per se, facilitates dynein interaction with early endosomes. The failure of wild-type dynein to interact with early endosomes in the ΔkinA mutant might be largely due to the fact that dynein moves along microtubules as active motors, thereby unable to meet endosomes near the plus-ends. In U. maydis, early endosomes undergo kinesin-3-mediated transport toward the plus-end, and a large percentage of early endosomes (~75%) undergo dynein-mediated retrograde transport only after they arrive at the plus-ends near the hyphal tip (Lenz et al., 2006). In the absence of dynein function, kinesin 3 would presumably carry early endosomes all the way to the plus-ends, causing a severe early endosome accumulation at the hyphal tip. In A. nidulans, endocytosis occurs primarily at a collar behind the hyphal tip (Araujo-Bazán et al., 2008; Taheri-Talesh et al., 2008; Upadhyay and Shaw, 2008). However, some endocytic vesicles might be generated along hyphae and, as proposed by Abenza et al., (Abenza et al., 2009), these vesicles might first need to be transported to the microtubule plus-end where they can start their dynein-mediated journey, and the UNCA kinesin 3 might play an important role in this process (Zekert and Fischer, 2009). It is probable that although dynein is active before arriving at the plus-end, the chance for individual dynein molecules located along microtubules to meet early endosomes is relatively small, whereas the plus-end accumulation of dynein significantly increases the chance of productive interaction between an early endosome and a dynein motor. At a low frequency, however, dynein motors present along microtubules could still bind to an endosome and power its retrograde movement. This might explain why a low percentage of early endosomes (~25%) do start their minus-end-directed journey before arriving at the plus-ends (Lenz et al., 2006).
Materials and Methods
Aspergillus strains and growth media
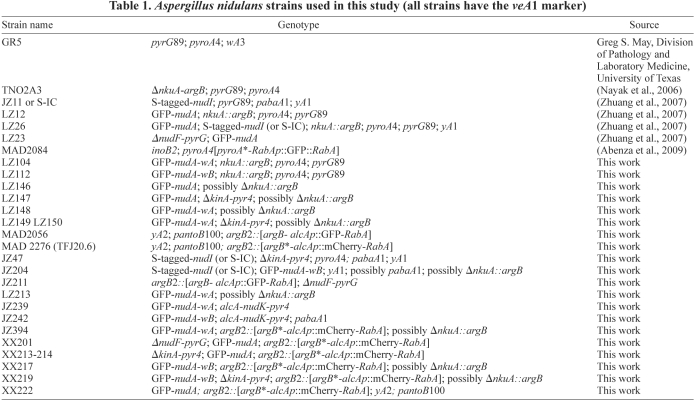
Aspergillus nidulans strains used in this study are listed in Table 1. For biochemical experiments involving dynein isolation for ATPase assays, YG (yeast extract plus glucose) + UU (or YUU) liquid medium was used. The YUU medium was also used for growing the alcA-nudF strain for microtubule pelleting assays as a repressive medium for the alcA promoter. For live-cell imaging experiments, minimal medium with either glucose or glycerol plus supplements was used as previously described (Zhang et al., 2003).
Table 1.
Aspergillus nidulans strains used in this study (all strains have the veA1 marker)
Construction of AAA1 mutants
To make the AAA1-Walker A (wA) mutant, we changed the amino acid lysine (K) in the first P-loop to alanine (A), similar to that described in Reck-Peterson and Vale (Reck-Peterson and Vale, 2004). The fragment containing the mutation was constructed by a fusion PCR strategy using high-fidelity polymerases such as Accuprime Pfx and Accuprime Taq Hi Fi polymerases (Szewczyk et al., 2006). We first amplified genomic DNA with two sets of primers: nudA55 (5′-ATCACTGAGGCTATTGCGCAG-3′) and wA3 (5′-GCCTTGACGGACTCTGTCGCTCCGGTACCTGCAGGACCGTAA-3′); and wA5 (5′-GACAGAGTCCGTCAAGGC-3′) and nudA38 (5′-CATCGGTGGGAGGGTTACAA-3′). Fusion PCR was then performed with primers nudA55 and nudA38 to obtain the final PCR product of about 4 kb. This fragment was transformed into the GFP-nudA strain (LZ12) with the Δku70 background (Nayak et al., 2006; Zhuang et al., 2007). The strain that contains the correct replacement (GFP-NUDAK1940A) exhibited a typical nud colony phenotype and was confirmed by sequencing of the genomic DNA. A similar strategy was used to make the GFP-AAA1-Walker B (wB) mutant in which the glutamic acid (E) in the wB region is replaced by a glutamine (Q; GFP-NUDAE1987Q). The wB primers wB3 (5′-GTTCCTCAAGACGGTTGAACTGATCGAAACAACCCCATGCAC-3′) and wB5 (5′-GTTCAACCGTCTTGAGGAAC-3′) were used in the fusion PCR. The alcA-nudK (or alcA-ARP1) allele was introduced into the GFP-NUDAK1940A (GFP-wA) and GFP-NUDAE1987Q (GFP-wB) backgrounds by genetic crossing. The presence of the alcA-ARP1 allele was verified by western analyses on p150 of dynactin, as the p150 level is drastically lowed in the alcA-ARP1 mutant grown on glucose (Zhang et al., 2008). The ΔkinA allele was introduced into the GFP-wA and GFP-wB backgrounds by genetic crossing. The double mutants were selected based on the tiny colony phenotype (the colony of the double mutant was significantly smaller than that of the wA or wB single mutant), similar to that produced by the previously described double mutants of ΔkinA and nudA loss-of-function mutants (Requena et al., 2001; Zhang et al., 2003).
Image analyses
Samples were prepared as previously described (Zhang et al., 2003; Zhang et al., 2008). Images were captured using an Olympus IX70 inverted fluorescence microscope (with a 100× objective) linked to a PCO/Cooke Corporation Sensicam QE cooled charge-coupled device camera. A filter wheel system with GFP/mCherry-ET Sputtered series with high transmission (Biovision Technologies) was used. The IPLab software (BioVision Technologies) was used for image acquisition and analysis.
Aspergillus dynein purification, ATPase assay and microtubule pelleting assay
Dynein purification was done using two different buffers. Besides the low-salt Tris buffer described in Zhuang et al. (Zhuang et al., 2007) [25 mM Tris (pH 8.0), 0.4% Triton X-100, 10 μM ATP, 1 mM DTT and a protease inhibitor cocktail (Sigma, St Louis, MO)], we also used a Tris-based buffer with 50 mM NaCl and 1% NP-40 to facilitate extraction of dynein from membranous vesicles. Dynein isolation was done using an S-tag-based method as previously described (Zhuang et al., 2007). ATPase assays and protein quantifications were performed as described previously (Zhuang et al., 2007), except that 0.3 mg/ml microtubules were used in the microtubule-stimulated assays and an Opsys MR plate reader from Dynex Technologies was used to read the optical density (O.D.) values at 630 nm. We made sure that the ATPase activity we measured was specific to dynein (Zhuang et al., 2007) by using a strain in which the IC is not S-tagged as a negative control for the dynein purification procedure. Eluate from the negative control strain was subjected to the same ATPase assay in every experiment. For measuring microtubule-stimulated ATPase activity, the same amount of microtubules were added to the eluate of the negative control and O.D. values from these samples were considered as background values and subtracted from the experimental values obtained with the S-IC strains. Because fungal dynein is much less abundant than brain dynein, it is hard to measure the absolute amount of purified dynein. Although we have used BSA as a standard in silver-stained gels as BSA could stain differently than dynein heavy chain, the protein levels of dynein calculated based on band intensity in silver-stained gels are not accurate (Zhuang et al., 2007). Therefore, we only used values relative to wild-type values in this study and relative dynein amounts were measured by quantitative analyses of heavy chain bands on western blots. Polymerized microtubules, the kits for the ATPase and microtubule pelleting assays were all purchased from Cytoskeleton, Inc. Microtubule pelleting assays were performed according to the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Berl Oakley for the Δku70 strain and Reinhard Fischer for the ΔkinA strain. We are grateful to Erika Holzbaur, Jenny Ross, Adam Hendricks and Mike Welte for helpful discussions and suggestions, especially Erika Holzbaur for critical readings of the manuscript that significantly improved the work and its presentation. We thank Xuanli Yao and Henry Zhou for much help in the laboratory and Xuanli Yao for helpful comments on the paper. This work was supported by an NIH grant (GM069527 to X.X.), a USUHS intramural grant (RO71GO to X.X.), DGICYT BIO2009-07281 (to M.A.P.) and Comunidad de Madrid S2006-SAL-0246 (to M.A.P.). J.F.A. is holder of a CSIC I3P predoctoral contract. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/20/3596/DC1
References
- Abe N., Cavalli V. (2008). Nerve injury signaling. Curr. Opin. Neurobiol. 18, 276-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza J. F., Pantazopoulou A., Rodriguez J. M., Galindo A., Peñalva M. A. (2009). Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic 10, 57-75 [DOI] [PubMed] [Google Scholar]
- Abenza J. F., Galindo A., Pantazopoulou A., Gil C., de Los Rios V., Penalva M. A. (2010). Aspergillus RabBRab5 integrates acquisition of degradative identity with the long distance movement of early endosomes. Mol. Biol. Cell. 21, 2756-2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally S., Larson A. G., Barlan K., Rice S. E., Gelfand V. I. (2009). Opposite-polarity motors activate one another to trigger cargo transport in live cells. J. Cell Biol. 187, 1071-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo-Bazan L., Peñalva M. A., Espeso E. A. (2008). Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol. Microbiol. 67, 891-899 [DOI] [PubMed] [Google Scholar]
- Babst M., Wendland B., Estepa E. J., Emr S. D. (1998). The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17, 2982-2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. S. (2008). Beyond the code: the mechanical properties of DNA as they relate to mitosis. Chromosoma 117, 103-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho P., Gupta M. L., Jr, Hoyt M. A., Pellman D. (2004). Cell cycle control of kinesin-mediated transport of Bik1 (CLIP-170) regulates microtubule stability and dynein activation. Dev. Cell 6, 815-829 [DOI] [PubMed] [Google Scholar]
- Caudron F., Andrieux A., Job D., Boscheron C. (2008). A new role for kinesin-directed transport of Bik1p (CLIP-170) in Saccharomyces cerevisiae. J. Cell Sci. 121, 1506-1513 [DOI] [PubMed] [Google Scholar]
- Coquelle F. M., Caspi M., Cordelieres F. P., Dompierre J. P., Dujardin D. L., Koifman C., Martin P., Hoogenraad C. C., Akhmanova A., Galjart N., et al. (2002). LIS1, CLIP-170's key to the dynein/dynactin pathway. Mol. Cell. Biol. 22, 3089-3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy D. L., Hancock W. O., Wagenbach M., Howard J. (1999). Kinesin's tail domain is an inhibitory regulator of the motor domain. Nat. Cell Biol. 1, 288-292 [DOI] [PubMed] [Google Scholar]
- Gee M. A., Heuser J. E., Vallee R. B. (1997). An extended microtubule-binding structure within the dynein motor domain. Nature 390, 636-639 [DOI] [PubMed] [Google Scholar]
- Gibbons I. R., Lee-Eiford A., Mocz G., Phillipson C. A., Tang W. J., Gibbons B. H. (1987). Photosensitized cleavage of dynein heavy chains. Cleavage at the “V1 site” by irradiation at 365 nm in the presence of ATP and vanadate. J. Biol. Chem. 262, 2780-2786 [PubMed] [Google Scholar]
- Greber U. F., Way M. (2006). A superhighway to virus infection. Cell 124, 741-754 [DOI] [PubMed] [Google Scholar]
- Ha J., Lo K. W., Myers K. R., Carr T. M., Humsi M. K., Rasoul B. A., Segal R. A., Pfister K. K. (2008). A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J. Cell Biol. 181, 1027-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney D. D. (1996). The kinetic cycles of myosin, kinesin, and dynein. Annu. Rev. Physiol. 58, 731-750 [DOI] [PubMed] [Google Scholar]
- Han G., Liu B., Zhang J., Zuo W., Morris N. R., Xiang X. (2001). The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics. Curr. Biol. 11, 719-724 [DOI] [PubMed] [Google Scholar]
- Harris S. D. (2010). Hyphal growth and polarity. In Cellular and Molecular Biology of Filamentous Fungi (ed. Borkovich K., Ebbole D.), pp. 238-259 Washington, DC: ASM Press; [Google Scholar]
- Hendricks A. G., Perlson E., Ross J. L., Schroeder H. W., 3rd, Tokito M., Holzbaur E. L. (2010). Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr. Biol. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook P. (2010). The mechanical components of the dynein motor. ScientificWorldJournal 10, 857-864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L. M., Leipe D. D., Koonin E. V., Aravind L. (2004). Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 146, 11-31 [DOI] [PubMed] [Google Scholar]
- Kardon J. R., Vale R. D. (2009). Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 10, 854-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S., Holzbaur E. L. (1999). Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 11, 45-53 [DOI] [PubMed] [Google Scholar]
- Kon T., Nishiura M., Ohkura R., Toyoshima Y. Y., Sutoh K. (2004). Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry 43, 11266-11274 [DOI] [PubMed] [Google Scholar]
- Lang C., Grava S., van den Hoorn T., Trimble R., Philippsen P., Jaspersen S. L. (2010). Mobility, microtubule nucleation and structure of microtubule-organizing centers in multinucleated hyphae of Ashbya gossypii. Mol. Biol. Cell 21, 18-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. L., Oberle J. R., Cooper J. A. (2003). The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J. Cell Biol. 160, 355-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J. H., Schuchardt I., Straube A., Steinberg G. (2006). A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J. 25, 2275-2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Yu W., Li Y., Yang Z., Yan X., Huang Q., Zhu X. (2004). Nudel functions in membrane traffic mainly through association with Lis1 and cytoplasmic dynein. J. Cell Biol. 164, 557-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon L. A., Tokito M., Finklestein J. M., Grossman F. E., Holzbaur E. L. (2004). A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J. Biol. Chem. 279, 19201-19208 [DOI] [PubMed] [Google Scholar]
- Liu B., Xiang X., Lee Y. R. (2003). The requirement of the LC8 dynein light chain for nuclear migration and septum positioning is temperature dependent in Aspergillus nidulans. Mol. Microbiol. 47, 291-301 [DOI] [PubMed] [Google Scholar]
- Lomakin A. J., Semenova I., Zaliapin I., Kraikivski P., Nadezhdina E., Slepchenko B. M., Akhmanova A., Rodionov V. (2009). CLIP-170-dependent capture of membrane organelles by microtubules initiates minus-end directed transport. Dev. Cell 17, 323-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Chisholm R. L. (2002). Cytoplasmic dynein-associated structures move bidirectionally in vivo. J. Cell Sci. 115, 1453-1460 [DOI] [PubMed] [Google Scholar]
- Markus S. M., Punch J. J., Lee W. L. (2009). Motor- and tail-dependent targeting of dynein to microtubule plus-ends and the cell cortex. Curr. Biol. 19, 196-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney R. J., Vershinin M., Kunwar A., Vallee R. B., Gross S. P. (2010). LIS1 and NudE induce a persistent dynein force-producing state. Cell 141, 304-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesngon M. T., Tarricone C., Hebbar S., Guillotte A. M., Schmitt E. W., Lanier L., Musacchio A., King S. J., Smith D. S. (2006). Regulation of cytoplasmic dynein ATPase by Lis1. J. Neurosci. 26, 2132-2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. K., Stuchell-Brereton M. D., Cooper J. A. (2009). Function of dynein in budding yeast: mitotic spindle positioning in a polarized cell. Cell Motil. Cytoskeleton 66, 546-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. J., Klumpp S., Lipowsky R. (2008). Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc. Natl. Acad. Sci. USA 105, 4609-4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak T., Szewczyk E., Oakley C. E., Osmani A., Ukil L., Murray S. L., Hynes M. J., Osmani S. A., Oakley B. R. (2006). A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172, 1557-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald A. F., Aravind L., Spouge J. L., Koonin E. V. (1999). AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27-43 [PubMed] [Google Scholar]
- Pause A., Sonenberg N. (1992). Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 11, 2643-2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson S. L., Vale R. D. (2004). Molecular dissection of the roles of nucleotide binding and hydrolysis in dynein's AAA domains in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 101, 1491-1495 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reck-Peterson S. L., Yildiz A., Carter A. P., Gennerich A., Zhang N., Vale R. D. (2006). Single-molecule analysis of dynein processivity and stepping behavior. Cell 126, 335-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W. B., Caskey C. T., Ledbetter D. H. (1993). Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature 364, 717-721 [DOI] [PubMed] [Google Scholar]
- Requena N., Alberti-Segui C., Winzenburg E., Horn C., Schliwa M., Philippsen P., Liese R., Fischer R. (2001). Genetic evidence for a microtubule-destabilizing effect of conventional kinesin and analysis of its consequences for the control of nuclear distribution in Aspergillus nidulans. Mol. Microbiol. 42, 121-132 [DOI] [PubMed] [Google Scholar]
- Roberts A. J., Numata N., Walker M. L., Kato Y. S., Malkova B., Kon T., Ohkura R., Arisaka F., Knight P. J., Sutoh K., et al. (2009). AAA+ Ring and linker swing mechanism in the dynein motor. Cell 136, 485-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samso M., Koonce M. P. (2004). 25 Angstrom resolution structure of a cytoplasmic dynein motor reveals a seven-member planar ring. J. Mol. Biol. 340, 1059-1072 [DOI] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. (1990). The P-loop-a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 15, 430-434 [DOI] [PubMed] [Google Scholar]
- Schroer T. A. (2004). Dynactin. Annu. Rev. Cell Dev. Biol. 20, 759-779 [DOI] [PubMed] [Google Scholar]
- Sheeman B., Carvalho P., Sagot I., Geiser J., Kho D., Hoyt M. A., Pellman D. (2003). Determinants of S. cerevisiae dynein localization and activation: implications for the mechanism of spindle positioning. Curr. Biol. 13, 364-372 [DOI] [PubMed] [Google Scholar]
- Shubeita G. T., Tran S. L., Xu J., Vershinin M., Cermelli S., Cotton S. L., Welte M. A., Gross S. P. (2008). Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell 135, 1098-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider J., Houry W. A. (2008). AAA+ proteins: diversity in function, similarity in structure. Biochem. Soc. Trans. 36, 72-77 [DOI] [PubMed] [Google Scholar]
- Soldati T., Schliwa M. (2006). Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 7, 897-908 [DOI] [PubMed] [Google Scholar]
- Soppina V., Rai A. K., Ramaiya A. J., Barak P., Mallik R. (2009). Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc. Natl. Acad. Sci. USA 106, 19381-19386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G. (2007). On the move: endosomes in fungal growth and pathogenicity. Nat. Rev. Microbiol. 5, 309-316 [DOI] [PubMed] [Google Scholar]
- Szewczyk E., Nayak T., Oakley C. E., Edgerton H., Xiong Y., Taheri-Talesh N., Osmani S. A., Oakley B. R. (2006). Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1, 3111-3120 [DOI] [PubMed] [Google Scholar]
- Taheri-Talesh N., Horio T., Araujo-Bazan L., Dou X., Espeso E. A., Peñalva M. A., Osmani S. A., Oakley B. R. (2008). The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell 19, 1439-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumurugan K., Sakamoto T., Hammer J. A., 3rd, Sellers J. R., Knight P. J. (2006). The cargo-binding domain regulates structure and activity of myosin 5. Nature 442, 212-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L. H., Gleeson J. G. (2005). Nucleokinesis in neuronal migration. Neuron 46, 383-388 [DOI] [PubMed] [Google Scholar]
- Tsygankov D., Serohijos A. W., Dokholyan N. V., Elston T. C. (2009). Kinetic models for the coordinated stepping of cytoplasmic dynein. J. Chem. Phys. 130, 025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S., Shaw B. D. (2008). The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans. Mol. Microbiol. 68, 690-705 [DOI] [PubMed] [Google Scholar]
- Valetti C., Wetzel D. M., Schrader M., Hasbani M. J., Gill S. R., Kreis T. E., Schroer T. A. (1999). Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell 10, 4107-4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Tsai J. W. (2006). The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 20, 1384-1393 [DOI] [PubMed] [Google Scholar]
- Vaughan K. T., Tynan S. H., Faulkner N. E., Echeverri C. J., Vallee R. B. (1999). Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at microtubule distal ends. J. Cell Sci. 112, 1437-1447 [DOI] [PubMed] [Google Scholar]
- Vaughan P. S., Miura P., Henderson M., Byrne B., Vaughan K. T. (2002). A role for regulated binding of p150(Glued) to microtubule plus-ends in organelle transport. J. Cell Biol. 158, 305-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. K., Pavin N., Maghelli N., Julicher F., Tolic-Norrelykke I. M. (2009). Self-organization of dynein motors generates meiotic nuclear oscillations. PLoS Biol. 7, e1000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Thirumurugan K., Stafford W. F., Hammer J. A., 3rd, Knight P. J., Sellers J. R. (2004). Regulated conformation of myosin V. J. Biol. Chem. 279, 2333-2336 [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Rossnagel K., Buhrow S. A., Brunner M., Jaenicke R., Rothman J. E. (1994). N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol. 126, 945-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Osmani A. H., Osmani S. A., Xin M., Morris N. R. (1995). NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol. Biol. Cell 6, 297-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Toba S., Yoshida Y., Haratani K., Mori D., Yano Y., Mimori-Kiyosue Y., Nakamura T., Itoh K., Fushiki S., et al. (2008). LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J. 27, 2471-2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Toba S., Takitoh T., Yoshida Y., Mori D., Nakamura T., Iwane A. H., Yanagida T., Imai H., Yu-Lee L. Y., et al. (2009). mNUDC is required for plus-end-directed transport of cytoplasmic dynein and dynactins by kinesin-1. EMBO J. 29, 517-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekert N., Fischer R. (2009). The Aspergillus nidulans kinesin-3 UncA motor moves vesicles along a subpopulation of microtubules. Mol. Biol. Cell 20, 673-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Han G., Xiang X. (2002). Cytoplasmic dynein intermediate chain and heavy chain are dependent upon each other for microtubule end localization in Aspergillus nidulans. Mol. Microbiol. 44, 381-392 [DOI] [PubMed] [Google Scholar]
- Zhang J., Li S., Fischer R., Xiang X. (2003). Accumulation of cytoplasmic dynein and dynactin at microtubule plus-ends in Aspergillus nidulans is kinesin dependent. Mol. Biol. Cell 14, 1479-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang L., Zhuang L., Huo L., Musa S., Li S., Xiang X. (2008). Arp11 affects dynein-dynactin interaction and is essential for dynein function in Aspergillus nidulans. Traffic 9, 1073-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Li S., Musa S., Zhou H., Xiang X. (2009). Dynein light intermediate chain in Aspergillus nidulans is essential for the interaction between heavy and intermediate chains. J. Biol. Chem. 284, 34760-34768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., Zhang J., Xiang X. (2007). Point mutations in the stem region and the fourth AAA domain of cytoplasmic dynein heavy chain partially suppress the phenotype of NUDF/LIS1 loss in Aspergillus nidulans. Genetics 175, 1185-1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.