Abstract
Tandem repeat expansion is responsible for the Repeat Expansion Diseases, a group of human genetic disorders that includes Fragile X syndrome. FXS results from expansion of a premutation (PM) allele having 55–200 CGG•CCG-repeats in the 5’ UTR of the FMR1 gene. The mechanism of expansion is unknown. We have treated FX PM mice with potassium bromate (KBrO3), a potent DNA oxidizing agent. We then monitored the germline and somatic expansion frequency in the progeny of these animals. We show here that KBrO3 increased both the level of 8-oxoG in the oocytes of treated animals and the germline expansion frequency. Our data thus suggest that oxidative damage may be a factor that could affect expansion risk in humans.
Keywords: Repeat expansion disease; triplet repeat instability; Fragile X syndrome; FMR1; DNA repair; oxidative damage; 7,8-dihydro-8-oxo-guanine (8-oxoG)
Introduction
Repeat expansion is a particular form of DNA instability seen in mammalian cells. This expansion process is responsible for the Repeat Expansion Diseases, a group of ~20 human disorders, that includes the neurodegenerative disorder, Huntington Disease (HD; MIM# 143100), and the leading familial cause of intellectual disability, Fragile X Syndrome (FXS; MIM# 300624) (see (Mirkin, 2006) for review). Unlike the generalized microsatellite instability seen when mismatch repair genes are mutated, instability in the Repeat Expansion Diseases affects just a single tandem array, shows an expansion bias and can involve very large increases in repeat number that occur within a single generation Furthermore, at least in the case of CAG•CTG-repeats that are responsible for HD, functional mismatch repair proteins including MSH2, MSH3 and PMS2 are required for expansion in mouse models (Foiry, et al., 2006; Gomes-Pereira, et al., 2004; Manley, et al., 1999). The repeats responsible for these diseases vary with respect to length and sequence. They also form intrastrand and interstrand secondary structures that are thought to contribute to the propensity of the repeats to expand (reviewed in (Usdin and Grabczyk, 2000)).
Work in yeast and bacteria suggests that problems with replication, repair and recombination can all produce instability (Bhattacharyya and Lahue, 2004; Bhattacharyya, et al., 2002; Bowater, et al., 1997; Bowater, et al., 1996; Callahan, et al., 2003; Collins, et al., 2007; Freudenreich, et al., 1998; Hashem, et al., 2004; Hirst and White, 1998; Ireland, et al., 2000; Iyer and Wells, 1999; Jankowski and Nag, 2002; Jaworski, et al., 1995; Kang, et al., 1996; Miret, et al., 1997; Napierala, et al., 2004; Parniewski, et al., 1999; Parniewski, et al., 2000; Razidlo and Lahue, 2008; Refsland and Livingston, 2005; Schumacher, et al., 1998; Schumacher, et al., 2001; Schweitzer and Livingston, 1998; White, et al., 1999; Yang and Freudenreich, 2007). However, the mechanism responsible for expansion in humans remains unknown. It is not even clear whether different diseases share a common expansion mechanism, or whether the germline expansions occur by the same mechanism as the somatic expansions seen in a subset of these disorders.
We have previously generated a Fragile X premutation (PM) mouse in which the endogenous murine Fmr1 gene was retrofitted with ~130 CGG•CCG-repeats (Entezam, et al., 2007). In humans, expansion of FMR1 (MIM# 309550) alleles with that number of repeats is common, with small expansions predominating on paternal transmission and expansion to >200 repeats occurring at high frequency on maternal transmission and resulting in FX syndrome. We have shown that at least 2 mechanisms of expansion occur in mice (Entezam and Usdin, 2008; Entezam and Usdin, 2009). One of these mechanisms occurs in both males and females but has a paternal bias and may account for the small repeat length changes seen in humans. The second one occurs exclusively on maternal transmission and may correspond to the mechanism responsible for the large repeat length changes seen on maternal transmission of human PM alleles. The DNA damage response (DDR) kinase, Ataxia Telengiectasia Mutated (ATM) normally protects against the expansions with a paternal bias, while a related kinase, AT and Rad 3-related (ATR) protects against those expansions occurring exclusively in females (Entezam and Usdin, 2008; Entezam and Usdin, 2009). A number of compounds such as ethidium bromide, caffeine and aspirin have been shown to affect CTG•CAG-repeat expansion in cells in tissue culture (Gomes-Pereira and Monckton, 2006; Pineiro, et al., 2003; Yang, et al., 2003). However, the mechanisms responsible are uncertain and to date no small molecules of any kind have been tested in animal models.
In this manuscript we describe the effect of exposure of FX premutation mice to the potent DNA oxidizing agent, potassium bromate (KBrO3). Bromate is a byproduct of the ozonation process that is sometimes used for the disinfection of municipal drinking water. It is also a common component of hair permanent wave kits and is still sometimes added to flour, fish paste, beer and cheese. While renal toxicity is a hallmark of KBrO3 exposure, this compound is also found in many other organs of treated rodents including liver, thyroid and testes (Delker, et al., 2006). Bromate treatment also increases the incidence of micronuclei, a sign of DNA damage, in the blood (Awogi, et al., 1992; Hayashi, et al., 1989) and bone marrow (Hayashi, et al., 1988) of treated mice, and increases the incidence not only of kidney tumors, but peritoneal mesotheliomas and thyroid tumors in rats as well (DeAngelo, et al., 1998; Kurokawa, et al., 1986). KBrO3 is unusual amongst oxidizing agents in that the predominant oxidized base it generates in DNA is 7,8-dihydro-8-oxo-guanine (8-oxoG) (Ballmaier and Epe, 2006; Kawanishi and Murata, 2006). Failure to remove this residue leads to the formation of 8-oxoG: A mispairs, which are poorly repaired. OGG1, an enzyme that is responsible for excision of this oxidized base has been shown to be essential for the generation of somatic expansions in a transgenic mouse model of HD, an expansion disease involving a CAG•CTG repeat tract (Kovtun, et al., 2007).
We show here that administration of KBrO3 in drinking water increases the frequency of germline expansions in the FX PM mice. This suggests that KBrO3, perhaps via its ability to cause oxidative damage to DNA, increases the frequency of FX repeat expansions. We also show that mice haploinsufficient for Fen1 (Kucherlapati, et al., 2002) and mice homozygous for a hypomorphic allele of DNA ligase I (LigI) (Harrison, et al., 2002) do not affect repeat instability in the FX premutation mice. This may have implications for the mechanisms responsible for repeat expansion.
Materials and Methods
Mice breeding and maintenance
The generation of the Fragile X premutation, Fen1 knockout and Lig146BR mice have been previously described (Entezam, et al., 2007; Harrison, et al., 2002; Kucherlapati, et al., 2002). Mice were maintained in accordance with the guidelines of the NIDDK Animal Care and Use Committee and with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996). KBrO3 was administered via the drinking water that was given ad libitum at a concentration of 0.5g/l KBrO3. All breeding pairs were derived from litters that had been raised for at least 2 generations on bromated water. The breeding pairs were also raised from birth with this solution as their sole source of drinking water.
Genotyping and repeat length determinations
Genomic DNA is prepared from mouse tail DNA or homogenized mouse tissue as previously described (Entezam, et al., 2007). Fen1 and Lig146BR genotyping was carried out as described elsewhere (Barlow, et al., 1996; Harrison, et al., 2002; Kucherlapati, et al., 2002). The primer pair, frax-c and frax-f (Fu, et al., 1991), was used to detect both wildtype (WT) Fmr1 and FXS premutation alleles. The size of the CGG•CCG-repeat tract was monitored by Polymerase chain reaction (PCR) using frax-m5 (5’-CGGGGGGCGTGCGGTAACGGCCCAA-3’) and 6-carboxyfluorescein (FAM) or 4,7,2′,4′,5′,7′-hexachloro-6-carboxyfluorescein (HEX) labeled frax-m4 (5’-CTTGAGGCCCAGCCGCCGTCGGCC-3’). The binding sites for these primers are located immediately adjacent to the repeat tract and their 3’ ends are unique to the KI allele. The reaction products were then run on a 3130XL Genetic Analyzer and analyzed using GeneMapper® 3.7 (Applied Biosystems, Foster City, CA). Results were confirmed where necessary by Southern blotting. PCR across long repeats typically produces multiple bands and the size of each allele was calculated based on the mobility of the central band in the cluster.
Detection of 8-oxoG
The levels of 8-oxoG in treated and control animals were examined in two ways. Ovaries were immersed in Kahle’s solution (4% formalin, 28% ethanol, and 0.34 M glacial acetic acid) immediately following euthanasia. After at least 24 hr in Kahle’s solution, the tissue was embedded in egg yolk gelatin cooled to solidify the egg-gel, post fixed in 4% paraformaldehyde overnight, and then blocks were sunk in 30% sucrose in 1% buffered paraformaldehyde (Hoffman, et al., 2008). Sections of 30µm were cut on a freezing microtome and placed into cryoprotectant antifreeze (Watson, et al., 1986) until staining was begun. To initiate staining, the sections were rinsed in PBS, placed into 8OH-DG antisera (AB5830, Millipore, Temecula, CA) diluted 1:75,000 in 0.4% triton X 100 for 48 hr at 4°C. Sections were reacted using tyramide signal amplification (with biotinylated tyramide) according to previously published methods (Berghorn, et al., 1994) (Hoffman, et al., 2008). Following the fluorophore reaction with streptavidin Cy3, the sections were mounted onto subbed slides, dried overnight, placed into a 0.0001% solution of Hoechst 33258 (pentahydrate (bis-bisbenzamide)), a DNA stain, for 10 min, dehydrated in ascending series of alcohol, cleared in xylenes, and coverslipped with Histomount (National Diagnostics). Sections were viewed on a Nikon Eclipse microscope. Images were captured using a Retiga cooled CCD camera and iVision software (Biovision Technologies, Exton PA).
The relative levels of 8-oxoG were determined in the ovaries of treated and untreated animals as previously described (Senthil, et al., 2005). The DNA concentration was determined using a Nanodrop spectrophotometer (Nanodrop Products, Wilmington, DE). The quality and amount of this DNA was confirmed by gel electrophoresis in the presence of ethidium bromide. Equal amounts of DNA from the ovaries of bromate treated animals and untreated age-matched controls were tested in triplicate alongside an equivalent mass of a 800 bp PCR fragment from the Fmr1 gene from untreated animals that was used as a negative control. No signal was detected for the PCR product. The amount of antibody binding to the treated and untreated samples was assessed by densitometry using a Chemidoc system (Bio-Rad Laboratories, Hercules CA). The data for each animal was averaged and expressed as the amount of 8-oxoG binding relative to the untreated sample.
Data analysis
Statistical analysis was carried out using a web-based version of the GraphPad QuickCalcs Software (http://www.graphpad.com/quickcalcs). Analysis of the frequency of both deletions and expansions was carried out using Fisher’s exact test. Since the deletion frequency did not change significantly with either treatment or in either of the mutant backgrounds, and since this supports previous data suggesting that expansion and contraction are not reciprocal events but probably occur by completely different mechanisms, the average expansion size was calculated as the sum of the total number of repeats added in all transmissions showing an expansion as a function of the total number of expanded alleles. Because of the presence of a small number of large expansions that could skew the mean expansion size, we calculated the mean initially using the full data set. We then recalculated the mean using a data set that excluded expansions more than 2 standard deviations from the first mean. The significance of the difference between any 2 means was calculated using Student’s T-test.
Results
KBrO3 exposure increases germline expansions of mouse FX premutation alleles
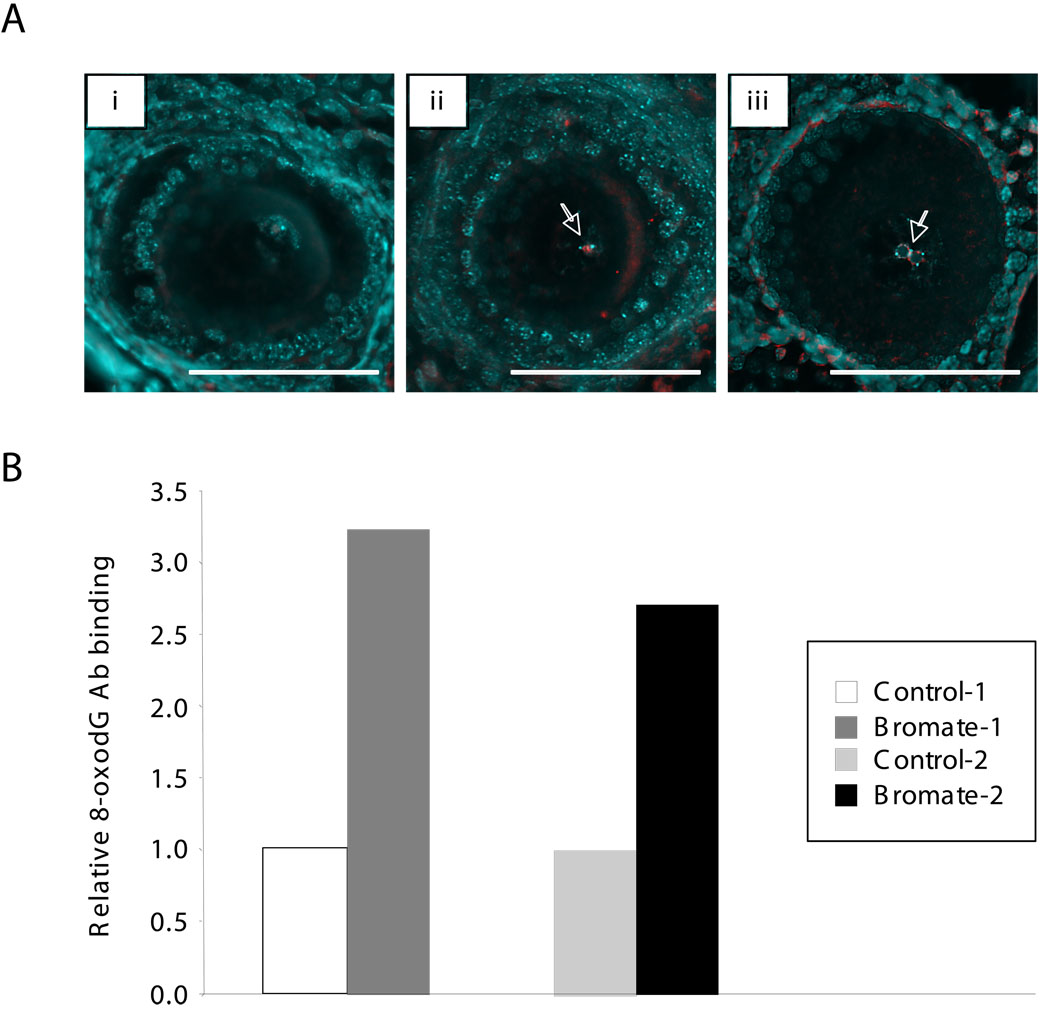
Potassium bromate generates 8-oxoG lesions in DNA and is carcinogenic to rodents when provided in drinking water at a concentration as low as 0.02 g/l (DeAngelo, et al., 1998). While in our experience doses higher than 0.5 g/l impaired survival and fertility, the ad libitum exposure of mice to 0.5 g/l KBrO3 in their drinking water for multiple generations produced no discernable effects on fertility (data not shown). Mice raised for at least 2 generations on water containing 0.5 g/l KBrO3 were used to study the effect of bromate on repeat expansion in their progeny. This bromate dose produced elevated levels of oxidative damage in some cells of the parents as evidenced by the higher level of 8-oxoG in the nuclei of oocytes of bromate-treated animals compared to untreated ones (Fig. 1A) and the elevated amount of 8-oxoG found in total ovary (Fig. 1B).
Fig. 1.
8-oxoG in the ovaries of untreated and bromate-treated mice. Panel A. 8-oxoG in the oocytes of untreated and bromate-treated mice. Sections of the ovaries of untreated mice (i) and bromate-treated animals of the same age (ii and iii) were stained with antibodies to 8-oxoG as described in the Materials and Methods. The DNA was stained with Hoechst 33258. The 8-oxoG staining regions are shown in red with the DNA shown in blue. Bars = 100 microns. The white arrows indicate 8-oxoG-positive signals in the oocyte nuclei. Note that occasional cytoplasmic staining is also seen in bromate-treated ovaries, most likely due to oxidation of mitochondrial DNA and RNA. Note that the oocyte shown in (iii) demonstrates the typical perinucleolar clumping of DNA sometimes seen in oocytes of this stage. The non-uniform distribution of 8-oxoG seen in the nuclei of some cells may reflect the known clustering of 8-oxoG lesions in the human genome (Ohno, et al., 2006). Panel B. Relative 8-oxoG levels in the ovaries of 2 bromate-treated mice and their age matched controls raised on unadulterated water. The 8-oxoG levels in treated animals are expressed relative to the amount of 8-oxoG in the appropriate age-matched control animals. Each bar represents the average of 3 independent determinations.
Consumption of bromate-adulterated water by these animals resulted in litters with fewer error-free transmissions and more expansions than breeding pairs that were not bromate-exposed (Fig. 2). Bromate increased the expansion frequency from 37% to 70% (1.9-fold) and reduced the error-free transmission rate from 35% to 15% (2.3-fold) when the PM allele was maternally transmitted (p=0.0036). Males showed an increase from 62% to 83% (1.34-fold) in the expansion frequency (p=0.049) and a 3-fold decrease in the number of error-free transmissions, from 24% of alleles in the offspring of untreated males, to 8% in the offspring of bromate-exposed males.
Fig. 2.
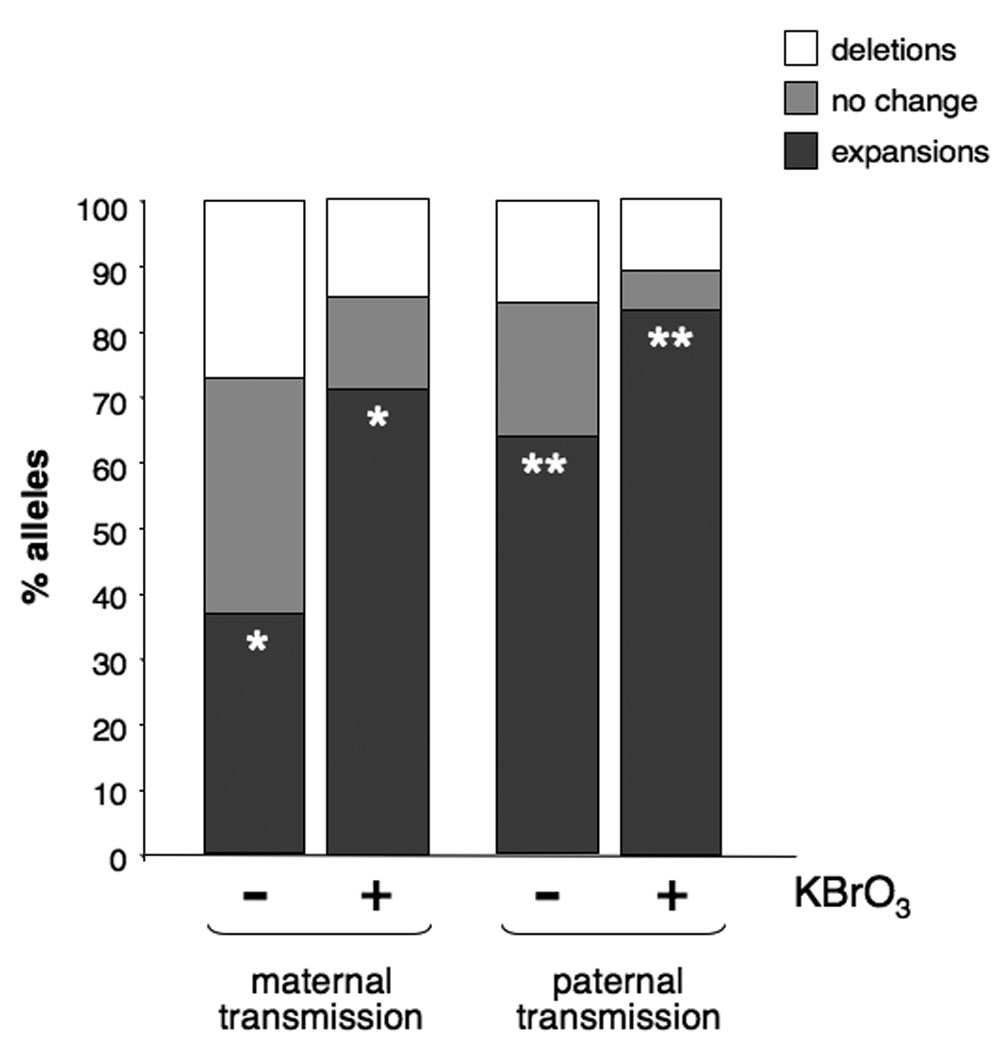
The effect of KBrO3 on the frequency of expansions and deletions seen on maternal and paternal transmission of the PM allele in animals. The single asterisks on the first pair of dark gray bars indicates that the difference between the expansion frequency seen in the offspring of bromate-treated and untreated mothers is significant at a P value of <0.0001. The double asterisks in the 2nd pair of dark gray bars indicates that the difference between the expansion frequency seen in the offspring of bromate-treated and untreated fathers is significant at a P value of 0.0114.
No significant effect of KBrO3 was seen on either maternal or paternal deletions (p=0.131 and p=0.769). This is one more piece of evidence to support the contention that expansions and deletions in the repeat tract are caused by different mechanisms in mice (Entezam, et al., 2007; Entezam and Usdin, 2008; Entezam and Usdin, 2009)..
Untreated males and females show no difference in the distribution of repeat length changes in their progeny (Entezam and Usdin, 2008; Entezam and Usdin, 2009). When the PM allele was paternally transmitted, there was a change in the distribution of repeat lengths (Fig. 3A). In particular, the average size of the expansions changed from 6.58 repeats/expansion on unadulterated water to 8.64 repeats/expansion on KBrO3 water if all the expansions are counted. However, both means were affected by the presence of a small number of large expansions. After correcting for such outliers by removing from consideration any expansion >2 standard deviations from the mean expansion size, the mean expansion size drops to 3.47 and 6.34 respectively. This corresponds to a 1.8-fold increase in mean expansion size in the offspring of bromate-treated males relative to untreated males. In contrast, the average expansion size and distribution of repeat length changes was smaller in offspring with maternally transmitted PM alleles (Fig. 3B) and indistinguishable from what is seen in untreated males (Compare gray bars in Fig. 3B with gray bars in Fig. 3A) and in untreated females (Entezam and Usdin, 2008; Entezam and Usdin, 2009). We also examined the brain, heart, liver, kidney and gonads of animals raised on bromate. Little, if any, effect was seen on somatic expansions in animals 12 months of age (data not shown).
Fig. 3.
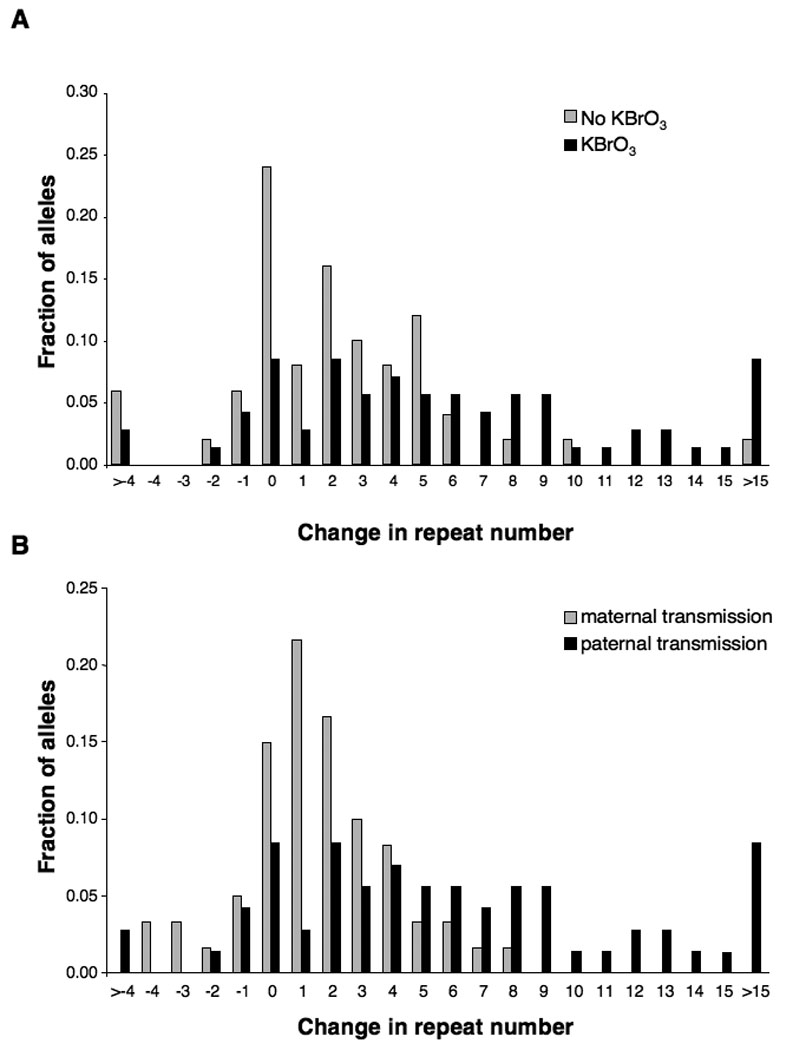
Distribution of repeat length changes on maternal and paternal transmission of the PM allele with and without KBrO3 treatment. The product of the total number of alleles of each repeat size and the number of repeats added or lost to that particular allele class was divided by the total number of expanded alleles examined. A) Transmission of the PM by KBrO3-treated males leads to an increase in the number of larger expansions. The profile of repeat length changes on paternal and maternal transmission in untreated animals is indistinguishable (Entezam, et al., 2007; Entezam and Usdin, 2008). B) Paternal exposure to KBrO3 results in larger repeat length changes than maternal exposure.
We had previously demonstrated the existence of ATR-sensitive expansions that arise both in the diploid and haploid gamete of the FX PM mice (Entezam and Usdin, 2008). Thus these expansions could arise from problems related to DNA damage occurring during gametogenesis. No significant effect of bromate was seen on the expansion or deletion frequency seen in the offspring of ATR+/− heterozygous females (p=0.609 and p=0.201 respectively). However, it is possible that the already high expansion frequency seen in this genetic background, 86%, masks any effect of bromate.
Fen1 and LigI mutations do not affect repeat expansions
Since work in yeast suggests that mutations in the yeast homologs of FEN1 (MIM# 600393) and LIG1 (MIM# 126391) increase the instability of CTG•CAG and CGG•CCG-repeats (Callahan, et al., 2003; Freudenreich, et al., 1998; Ireland, et al., 2000; Refsland and Livingston, 2005; Schweitzer and Livingston, 1998; White, et al., 1999; Yang and Freudenreich, 2007), we decided to test the effects of mutations on these genes on repeat instability. Fen1 null mice die early in embryogenesis. However, since heterozygous Fen1 mutant mice still show rapid tumor progression it suggests that Fen1 is insufficient in these animals (Kucherlapati, et al., 2002). We thus examined the effects of Fen1 heterozygosity on germline and somatic instability in our FX premutation mice. Lig1 null alleles are also embryonic lethal. However, mice homozygous for a Lig1 hypomorphic allele, LigI46BR, are viable but display increased genomic instability and cancer incidence (Harrison, et al., 2002). We thus also examined the germline and somatic instability of the FX repeat in a LigI46BR homozygous background. As can be seen in Table 1, no effect on intergenerational instability was seen in either case. No effect on somatic instability was observed either (data not shown). The lack of an effect of Fen1 heterozygosity on somatic instability we observe in the FX PM mice is consistent with what has been reported in a knockin mouse model of Myotonic dystrophy type I (van den Broek, et al., 2006). In a small study of a transgenic mouse model of HD an increase in the germline expansion frequency was seen, however this increase was not statistically significant (Spiro and McMurray, 2003). However, since some residual enzymatic activity is present in these animals, it may be that despite the other sorts of DNA repair abnormalities seen in these animals, that the levels of these proteins are still high enough to prevent expansion.
Table 1.
Expansion frequency of premutation alleles in Fen1 heterozygote, Lig1 46BR homozygote and WT mice.
| genotype | Expansions | |
|---|---|---|
| paternal transmission* |
maternal transmission* |
|
| WT | 31/51 (67%) | 31/85 (37%) |
| FEN1+/− | 51/76 (71%) | 24/54 (44%) |
| Lig146BR | 25/40 (63%) | 36/107 (34%) |
No significant effect of the genotype of the transmitting parent on expansion frequency is seen using Fisher’s exact test; no effect of genotype was seen on expansion size was seen by Student’s t-test.
Discussion
We have shown that exposure of FX PM mice to KBrO3 results in increased levels of 8-oxoG in cells of the ovary including the oocyte (Fig. 1). This is associated with a significant increase in the expansion frequency and a corresponding decrease in the number of error-free germline transmissions when PM alleles were maternally transmitted (Fig. 2 and 3). An increased expansion frequency was also seen on paternal transmission. However, somatic instability was not affected.
KBrO3 is a powerful oxidizing agent that generates a high proportion of 8-oxoG modified bases in both DNA and RNA. Bromate becomes distributed throughout the bodies of treated animals and is carcinogenic at doses significantly lower than the one used here (DeAngelo, et al., 1998; Delker, et al., 2006). While an effect of bromate on processes other than the generation of 8-oxoG cannot be conclusively ruled out, the presence of increased levels of 8-oxoG in our treated mice raises the possibility that the bromate effect is mediated via its ability to generate this oxidative product in DNA. This possibility is intriguing in light of the demonstration that in a transgenic mouse model of HD, the somatic instability of the CAG•CTG-repeats responsible for disease pathology has been shown to depend on the activity of OGG1 (Kovtun, et al., 2007). Since OGG1 is a DNA glycosylase that is responsible for removal of 8-oxoG as part of the BER process (Nash, et al., 1996), oxidative damage to guanine may be an important trigger for repeat expansion. While, an OGG1 deficiency had no effect on the germline expansion frequency in the HD mouse model (Kovtun, et al., 2007), it is possible that an alternative OGG1-independent pathway is used to reduce the incidence of 8-oxoG lesions during gametogenesis. In principle, our failure to observe an increase in somatic instability could be due to a lack of oxidative damage occurring in these tissues. However, it is noteworthy that even the major target organ for bromate damage, the kidney, showed no evidence of expansion. Thus it may be that somatic cells have efficient error-free repair processes better able to deal with the increased 8-oxoG load than oocytes.
A potential effect of oxidative damage on repeat expansion in FX premutation carriers is important given that oxidative damage is a pervasive source of DNA damage in the environment with ~2×204 oxidative DNA lesions being introduced into each human cell each day. The byproducts of aerobic metabolism may be one source of oxidative damage. This damage may be compounded by external sources of oxidative stress including cigarettes or other tobacco products or pesticides like paraquat (Halliwell, 1987). Genetic variation in the proteins involved in the response to oxidative stress may also be trans-acting modifiers of such expansions.
Although Fen1 heterozygote and Lig1 hypomorphic mice show evidence of a variety of defects including decreased growth rate and hematopoiesis and increased cancer incidence (Harrison, et al., 2002; Kucherlapati, et al., 2002), these deficiencies do not result in changes in either the germline or somatic expansion frequency in FX PM mice. Okazaki fragment processing, which is essential for lagging strand DNA synthesis, and Long Patch BER, a process often used to repair oxidative damage in mammals (Lu, et al., 2001), are strongly dependent on both Fen1 and Lig1. The fact that mutations in these genes had no effect on repeat expansion suggests that these processes are not involved in either promoting or repressing repeat expansions in these mice. However, some residual enzyme activity is present in both mouse mutants. Thus, the possibility that one or both of these processes play a role in repeat expansion but are much less sensitive to reduced levels of these enzymes than other Fen1 or Lig1-dependent processes, cannot be conclusively ruled out.
Whatever the mechanism responsible for the effect of KBrO3 on expansions, our data lend support to the idea that this compound, and others with similar effects, may be environmental modifiers of repeat expansion and that mutations in genes that affect the metabolism of KBrO3 or its products may be modifiers of expansion risk.
Acknowledgments
We would like to thank Dr Debbie Hinton for her reading of our manuscript and the great NIDDK animal care staff that made this work possible. This research was supported by the Intramural Program of the NIDDK (NIH) and the extramural program of the Eunice Kennedy Shriver NICHD (NIH) through cooperative agreement 1U54 HD 36207 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
References
- Awogi T, Murata K, Uejima M, Kuwahara T, Asanami S, Shimono K, Morita T. Induction of micronucleated reticulocytes by potassium bromate and potassium chromate in CD-1 male mice. Mutat Res. 1992;278(2–3):181–185. doi: 10.1016/0165-1218(92)90231-n. [DOI] [PubMed] [Google Scholar]
- Ballmaier D, Epe B. DNA damage by bromate: mechanism and consequences. Toxicology. 2006;221(2–3):166–171. doi: 10.1016/j.tox.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86(1):159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Bonnett JH, Hoffman GE. cFos immunoreactivity is enhanced with biotin amplification. J Histochem Cytochem. 1994;42(12):1635–1642. doi: 10.1177/42.12.7983364. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Lahue RS. Saccharomyces cerevisiae Srs2 DNA helicase selectively blocks expansions of trinucleotide repeats. Mol Cell Biol. 2004;24(17):7324–7330. doi: 10.1128/MCB.24.17.7324-7330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Rolfsmeier ML, Dixon MJ, Wagoner K, Lahue RS. Identification of RTG2 as a modifier gene for CTG*CAG repeat instability in Saccharomyces cerevisiae. Genetics. 2002;162(2):579–589. doi: 10.1093/genetics/162.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowater RP, Jaworski A, Larson JE, Parniewski P, Wells RD. Transcription increases the deletion frequency of long CTG.CAG triplet repeats from plasmids in Escherichia coli. Nucleic Acids Res. 1997;25(14):2861–2868. doi: 10.1093/nar/25.14.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowater RP, Rosche WA, Jaworski A, Sinden RR, Wells RD. Relationship between Escherichia coli growth and deletions of CTG.CAG triplet repeats in plasmids. J Mol Biol. 1996;264(1):82–96. doi: 10.1006/jmbi.1996.0625. [DOI] [PubMed] [Google Scholar]
- Callahan JL, Andrews KJ, Zakian VA, Freudenreich CH. Mutations in yeast replication proteins that increase CAG/CTG expansions also increase repeat fragility. Mol Cell Biol. 2003;23(21):7849–7860. doi: 10.1128/MCB.23.21.7849-7860.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NS, Bhattacharyya S, Lahue RS. Rev1 enhances CAG.CTG repeat stability in Saccharomyces cerevisiae. DNA Repair (Amst) 2007;6(1):38–44. doi: 10.1016/j.dnarep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, George MH, Kilburn SR, Moore TM, Wolf DC. Carcinogenicity of potassium bromate administered in the drinking water to male B6C3F1 mice and F344/N rats. Toxicol Pathol. 1998;26(5):587–594. doi: 10.1177/019262339802600501. [DOI] [PubMed] [Google Scholar]
- Delker D, Hatch G, Allen J, Crissman B, George M, Geter D, Kilburn S, Moore T, Nelson G, Roop B, et al. Molecular biomarkers of oxidative stress associated with bromate carcinogenicity. Toxicology. 2006;221(2–3):158–165. doi: 10.1016/j.tox.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395(1–2):125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezam A, Usdin K. ATR protects the genome against CGG.CCG-repeat expansion in Fragile X premutation mice. Nucleic Acids Res. 2008;36(3):1050–1056. doi: 10.1093/nar/gkm1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entezam A, Usdin K. ATM and ATR protect the genome against two different types of tandem repeat instability in Fragile X premutation mice. Nucleic Acids Res. 2009;37(19):6371–6377. doi: 10.1093/nar/gkp666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foiry L, Dong L, Savouret C, Hubert L, te Riele H, Junien C, Gourdon G. Msh3 is a limiting factor in the formation of intergenerational CTG expansions in DM1 transgenic mice. Hum Genet. 2006;119(5):520–526. doi: 10.1007/s00439-006-0164-7. [DOI] [PubMed] [Google Scholar]
- Freudenreich CH, Kantrow SM, Zakian VA. Expansion and length-dependent fragility of CTG repeats in yeast. Science. 1998;279(5352):853–856. doi: 10.1126/science.279.5352.853. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gomes-Pereira M, Fortune MT, Ingram L, McAbney JP, Monckton DG. Pms2 is a genetic enhancer of trinucleotide CAG.CTG repeat somatic mosaicism: implications for the mechanism of triplet repeat expansion. Hum Mol Genet. 2004;13(16):1815–1825. doi: 10.1093/hmg/ddh186. [DOI] [PubMed] [Google Scholar]
- Gomes-Pereira M, Monckton DG. Chemical modifiers of unstable expanded simple sequence repeats: what goes up, could come down. Mutat Res. 2006;598(1–2):15–34. doi: 10.1016/j.mrfmmm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987;1(5):358–364. [PubMed] [Google Scholar]
- Harrison C, Ketchen AM, Redhead NJ, O'Sullivan MJ, Melton DW. Replication failure, genome instability, and increased cancer susceptibility in mice with a point mutation in the DNA ligase I gene. Cancer Res. 2002;62(14):4065–4074. [PubMed] [Google Scholar]
- Hashem VI, Rosche WA, Sinden RR. Genetic recombination destabilizes (CTG)n.(CAG)n repeats in E. coli. Mutat Res. 2004;554(1–2):95–109. doi: 10.1016/j.mrfmmm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Kishi M, Sofuni T, Ishidate M., Jr Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food Chem Toxicol. 1988;26(6):487–500. doi: 10.1016/0278-6915(88)90001-4. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Sutou S, Shimada H, Sato S, Sasaki YF, Wakata A. Difference between intraperitoneal and oral gavage application in the micronucleus test. The 3rd collaborative study by CSGMT/JEMS.MMS. Collaborative Study Group for the Micronucleus Test/Mammalian Mutagenesis Study Group of the Environmental Mutagen Society of Japan. Mutat Res. 1989;223(4):329–344. doi: 10.1016/0165-1218(89)90081-5. [DOI] [PubMed] [Google Scholar]
- Hirst MC, White PJ. Cloned human FMR1 trinucleotide repeats exhibit a length-and orientation-dependent instability suggestive of in vivo lagging strand secondary structure. Nucleic Acids Res. 1998;26(10):2353–2358. doi: 10.1093/nar/26.10.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Sita LV. The importance of titrating antibodies for immunocytochemical methods. Curr Protoc Neurosci. 2008;Chapter 2(Unit 2):12. doi: 10.1002/0471142301.ns0212s45. [DOI] [PubMed] [Google Scholar]
- Ireland MJ, Reinke SS, Livingston DM. The impact of lagging strand replication mutations on the stability of CAG repeat tracts in yeast. Genetics. 2000;155(4):1657–1665. doi: 10.1093/genetics/155.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer RR, Wells RD. Expansion and deletion of triplet repeat sequences in Escherichia coli occur on the leading strand of DNA replication. J Biol Chem. 1999;274(6):3865–3877. doi: 10.1074/jbc.274.6.3865. [DOI] [PubMed] [Google Scholar]
- Jankowski C, Nag DK. Most meiotic CAG repeat tract-length alterations in yeast are SPO11 dependent. Mol Genet Genomics. 2002;267(1):64–70. doi: 10.1007/s00438-001-0635-4. [DOI] [PubMed] [Google Scholar]
- Jaworski A, Rosche WA, Gellibolian R, Kang S, Shimizu M, Bowater RP, Sinden RR, Wells RD. Mismatch repair in Escherichia coli enhances instability of (CTG)n triplet repeats from human hereditary diseases. Proc Natl Acad Sci U S A. 1995;92(24):11019–11023. doi: 10.1073/pnas.92.24.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Ohshima K, Jaworski A, Wells RD. CTG triplet repeats from the myotonic dystrophy gene are expanded in Escherichia coli distal to the replication origin as a single large event. J Mol Biol. 1996;258(4):543–547. doi: 10.1006/jmbi.1996.0266. [DOI] [PubMed] [Google Scholar]
- Kawanishi S, Murata M. Mechanism of DNA damage induced by bromate differs from general types of oxidative stress. Toxicology. 2006;221(2–3):172–178. doi: 10.1016/j.tox.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kovtun IV, Liu Y, Bjoras M, Klungland A, Wilson SH, McMurray CT. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature. 2007 doi: 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati M, Yang K, Kuraguchi M, Zhao J, Lia M, Heyer J, Kane MF, Fan K, Russell R, Brown AM, et al. Haploinsufficiency of Flap endonuclease (Fen1) leads to rapid tumor progression. Proc Natl Acad Sci U S A. 2002;99(15):9924–9929. doi: 10.1073/pnas.152321699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa Y, Takayama S, Konishi Y, Hiasa Y, Asahina S, Takahashi M, Maekawa A, Hayashi Y. Long-term in vivo carcinogenicity tests of potassium bromate, sodium hypochlorite, and sodium chlorite conducted in Japan. Environ Health Perspect. 1986;69:221–235. doi: 10.1289/ehp.8669221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AL, Li X, Gu Y, Wright PM, Chang DY. Repair of oxidative DNA damage: mechanisms and functions. Cell Biochem Biophys. 2001;35(2):141–170. doi: 10.1385/CBB:35:2:141. [DOI] [PubMed] [Google Scholar]
- Manley K, Shirley TL, Flaherty L, Messer A. Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat Genet. 1999;23(4):471–473. doi: 10.1038/70598. [DOI] [PubMed] [Google Scholar]
- Miret JJ, Pessoa-Brandao L, Lahue RS. Instability of CAG and CTG trinucleotide repeats in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17(6):3382–3387. doi: 10.1128/mcb.17.6.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin SM. DNA structures, repeat expansions and human hereditary disorders. Curr Opin Struct Biol. 2006;16(3):351–358. doi: 10.1016/j.sbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Napierala M, Dere R, Vetcher A, Wells RD. Structure-dependent recombination hot spot activity of GAA.TTC sequences from intron 1 of the Friedreich's ataxia gene. J Biol Chem. 2004;279(8):6444–6454. doi: 10.1074/jbc.M309596200. [DOI] [PubMed] [Google Scholar]
- Nash HM, Bruner SD, Scharer OD, Kawate T, Addona TA, Spooner E, Lane WS, Verdine GL. Cloning of a yeast 8-oxoguanine DNA glycosylase reveals the existence of a base-excision DNA-repair protein superfamily. Curr Biol. 1996;6(8):968–980. doi: 10.1016/s0960-9822(02)00641-3. [DOI] [PubMed] [Google Scholar]
- Ohno M, Miura T, Furuichi M, Tominaga Y, Tsuchimoto D, Sakumi K, Nakabeppu Y. A genome-wide distribution of 8-oxoguanine correlates with the preferred regions for recombination and single nucleotide polymorphism in the human genome. Genome Res. 2006;16(5):567–575. doi: 10.1101/gr.4769606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniewski P, Bacolla A, Jaworski A, Wells RD. Nucleotide excision repair affects the stability of long transcribed (CTG*CAG) tracts in an orientation-dependent manner in Escherichia coli. Nucleic Acids Res. 1999;27(2):616–623. doi: 10.1093/nar/27.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniewski P, Jaworski A, Wells RD, Bowater RP. Length of CTG.CAG repeats determines the influence of mismatch repair on genetic instability. J Mol Biol. 2000;299(4):865–874. doi: 10.1006/jmbi.2000.3796. [DOI] [PubMed] [Google Scholar]
- Pineiro E, Fernandez-Lopez L, Gamez J, Marcos R, Surralles J, Velazquez A. Mutagenic stress modulates the dynamics of CTG repeat instability associated with myotonic dystrophy type 1. Nucleic Acids Res. 2003;31(23):6733–6740. doi: 10.1093/nar/gkg898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razidlo DF, Lahue RS. Mrc1, Tof1 and Csm3 inhibit CAG.CTG repeat instability by at least two mechanisms. DNA Repair (Amst) 2008;7(4):633–640. doi: 10.1016/j.dnarep.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsland EW, Livingston DM. Interactions among DNA ligase I, the flap endonuclease and proliferating cell nuclear antigen in the expansion and contraction of CAG repeat tracts in yeast. Genetics. 2005;171(3):923–934. doi: 10.1534/genetics.105.043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher S, Fuchs RP, Bichara M. Expansion of CTG repeats from human disease genes is dependent upon replication mechanisms in Escherichia coli: the effect of long patch mismatch repair revisited. J Mol Biol. 1998;279(5):1101–1110. doi: 10.1006/jmbi.1998.1827. [DOI] [PubMed] [Google Scholar]
- Schumacher S, Pinet I, Bichara M. Modulation of transcription reveals a new mechanism of triplet repeat instability in Escherichia coli. J Mol Biol. 2001;307(1):39–49. doi: 10.1006/jmbi.2000.4489. [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, Livingston DM. Expansions of CAG repeat tracts are frequent in a yeast mutant defective in Okazaki fragment maturation. Hum Mol Genet. 1998;7(1):69–74. doi: 10.1093/hmg/7.1.69. [DOI] [PubMed] [Google Scholar]
- Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, Roberts R, Marian AJ. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res. 2005;97(3):285–292. doi: 10.1161/01.RES.0000177090.07296.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro C, McMurray CT. Nuclease-deficient FEN-1 blocks Rad51/BRCA1-mediated repair and causes trinucleotide repeat instability. Mol Cell Biol. 2003;23(17):6063–6074. doi: 10.1128/MCB.23.17.6063-6074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin K, Grabczyk E. DNA repeat expansions and human disease. Cell Mol Life Sci. 2000;57(6):914–931. doi: 10.1007/PL00000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek WJ, Nelen MR, van der Heijden GW, Wansink DG, Wieringa B. Fen1 does not control somatic hypermutability of the (CTG)(n)*(CAG)(n) repeat in a knock-in mouse model for DM1. FEBS Lett. 2006;580(22):5208–5214. doi: 10.1016/j.febslet.2006.08.059. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7(1):155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- White PJ, Borts RH, Hirst MC. Stability of the human fragile X (CGG)(n) triplet repeat array in Saccharomyces cerevisiae deficient in aspects of DNA metabolism. Mol Cell Biol. 1999;19(8):5675–5684. doi: 10.1128/mcb.19.8.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Freudenreich CH. Haploinsufficiency of yeast FEN1 causes instability of expanded CAG/CTG tracts in a length-dependent manner. Gene. 2007;393(1–2):110–115. doi: 10.1016/j.gene.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Lau R, Marcadier JL, Chitayat D, Pearson CE. Replication inhibitors modulate instability of an expanded trinucleotide repeat at the myotonic dystrophy type 1 disease locus in human cells. Am J Hum Genet. 2003;73(5):1092–1105. doi: 10.1086/379523. [DOI] [PMC free article] [PubMed] [Google Scholar]