Abstract
Accumulating evidence suggests that spinal astrocytes play an important role in the genesis of persistent pain, by increasing the activity of spinal cord nociceptive neurons, i.e., central sensitization. But direct evidence of whether activation of astrocytes is sufficient to induce chronic pain symptoms is lacking. We investigated whether and how spinal injection of activated astrocytes could produce mechanical allodynia, a cardinal feature of chronic pain, in naïve mice. Spinal (intrathecal) injection of astrocytes, which were prepared from cerebral cortexes of neonatal mice and briefly stimulated by tumor necrosis factor-alpha (TNF-α), induced a substantial decrease in paw withdrawal thresholds, indicating the development of mechanical allodynia. This allodynia was prevented when the astrocyte cultures were pre-treated with a peptide inhibitor of c-Jun N-terminal kinase (JNK), D-JNKI-1. Of note a short exposure of astrocytes to TNF-α for 15 minutes dramatically increased the expression and release of the chemokine monocyte chemoattractant protein-1 (MCP-1), even 3 hours after TNF-α withdrawal, in a JNK-dependent manner. In parallel, intrathecal administration of TNF-α induced MCP-1 expression in spinal cord astrocytes. In particular, mechanical allodynia induced by TNF-α-activated astrocytes was reversed by a MCP-1 neutralizing antibody. Finally, pretreatment of astrocytes with MCP-1 siRNA attenuated astrocytes-induced mechanical allodynia. Taken together, our results suggest that activated astrocytes are sufficient to produce persistent pain symptom in naïve mice by releasing MCP-1.
Keywords: TNF-α, MCP-1, JNK, astrocytes, central sensitization
Introduction
Chronic pain, such as nerve injury-induced neuropathic pain, is an unmet clinical challenge (Campbell and Meyer, 2006; Costigan et al., 2009; Dworkin et al., 2003). Chronic pain can manifest as spontaneous pain, such as burning pain, and evoked pain, such as hyperalgesia (increased responsiveness to noxious stimuli). Chronic pain is also characterized by allodynia, in particular mechanical allodynia or tactile allodynia, so that normally non-painful low threshold mechanical stimuli can induce painful responses. Mechanical allodynia is a cardinal and intractable symptom of chronic pain (Campbell and Meyer, 2006). It is not only produced in the injured region, but also spread to the adjacent non-injured regions and even contralateral side of body parts (Baron, 2009; Campbell et al., 1988). It is generally believed that hyperactivity in the spinal cord pain circuit, i.e. central sensitization, contributes to the generation of mechanical allodynia and spread of pain (Ji et al., 2003; Woolf and Salter, 2000).
Recent progress in pain research has pointed to an important role of glial cells in the generation of chronic pain. Many lines of evidence indicate that glial cells such as microglia and astrocytes in the spinal cord become reactive in chronic pain conditions and contribute to the development and maintenance of chronic pain by inducing central sensitization (DeLeo and Yezierski, 2001; Garrison et al., 1994; Ji and Strichartz, 2004; McMahon and Malcangio, 2009; Milligan and Watkins, 2009; Ren and Dubner, 2008; Scholz and Woolf, 2007). Accumulating evidence demonstrates that activation of microglia in the spinal cord induces neuropathic pain by producing proinflammatory cytokines and the brain-derived neurotrophic factor (BDNF) (Coull et al., 2005; Inoue and Tsuda, 2009; Suter et al., 2007; Tsuda et al., 2005; Tsuda et al., 2003). The role of astrocytes in chronic pain and the underlying mechanisms have also been investigated (Ji et al., 2006). Astrocytes are organized in gap junction-coupled networks. They not only transmit Ca2+ signaling in the form of oscillations or waves through the networks (Haydon, 2000), but also form a “tripartite” synapse with pre- and post-synaptic membranes and through which modulate synaptic strength (Haydon and Carmignoto, 2006; Jourdain et al., 2007). After injuries, reactive astrocytes express the c-Jun-N-terminal kinase (JNK), a member of the mitogen-activated protein kinase (MAPK), and produce the proinflammatory cytokine interleukin-1beta (IL-1β) and chemokine MCP-1, enhancing and maintaining central sensitization and chronic pain states (Gao et al., 2009; Guo et al., 2007; Kawasaki et al., 2008a; Kawasaki et al., 2008b; Ren and Dubner, 2008; Zhuang et al., 2006).
Spinal injection of ATP-activated microglia has been shown to produce mechanical allodynia via releasing BDNF (Coull et al., 2005; Tsuda et al., 2003). It remains unclear whether and how activated astrocytes are sufficient to induce this persistent pain symptom. Our recent study showed that TNF-α induced a dramatic increase of MCP-1 in astrocytes via the activation of JNK (Gao et al., 2009). In this study we further examined whether TNF-α-activated astrocytes would induce mechanical allodynia by releasing MCP-1.
Materials and Methods
Animals
CD1 mice, obtained from Charles River Laboratories, were used for most experiments. Adult CD1 mice (male, 25–32 g) were used for behavioral studies. Neonatal CD1 mice (P2) were used to prepare primary cultures of astrocytes. TNF receptor (R1/R2) double knockout mice (TNFR1/R2−/−, male, 25–32 g), obtained from Jackson Laboratories, and C57BL/6 wild-type control mice were also used in some experiment. All animal procedures performed in this study were approved by the Animal Care Committee of Harvard Medical School.
Reagents
TNF-α was purchased from R&D. MCP-1 neutralizing antibody and control serum were purchased from Millipore and Invitrogen, respectively. The JNK inhibitor D-JNKI-1 was kindly provided by Dr. Christopher Bonny, University of Lausanne, Switzerland (Borsello et al., 2003). MCP-1 siRNA was purchased from Santa Cruz. Non-targeting siRNA was synthesized by Dharmacon Research Incorporation as a control siRNA. SiRNA was dissolved in RNase-free water at 1 µg/µl as stock solution and mixed with the transfection reagent polyethyleneimine (PEI, Fermentas Inc) and normal saline before use. Specifically, 1 µg siRNA was dissolved in 3.3 µl PEI and 66 µl normal saline (Tan et al., 2009; Tan et al., 2005).
Primary culture of astrocytes
To get high quality and large quantity of astrocytes, we prepared most astrocyte cultures from cerebral cortexes of neonatal mice (P2). We also prepared some astrocyte cultures from spinal cords of neonatal mice. After dissection, we transferred the cerebral hemispheres or spinal cord segments to ice-cold Hank’s buffer and carefully removed the meninges. Tissues were then minced into ~1 mm pieces, triturated, filtered through a 100 µm nylon screen, and collected by centrifugation at ~3000g for 5 min. The cell pellets were broken with a pipette and resuspended in a medium containing 15% fetal bovine serum (FBS) in low glucose Dulbecco's Modified Eagle's Medium (DMEM). After trituration, the cells were filtered through a 10 µm screen and then plated onto 6-well plates at a density of 2.5 × 105 cells/cm2, and cultured for 10–12 days. The medium was replaced twice a week, first with 15% FBS, then with 10% FBS. Once the cells were grown to about 95% confluence, 0.15 mM dibutyryl cAMP (Sigma) was added to induce differentiation. Three days later, the cells were used for experiments.
Intrathecal administration
Before injection, astrocytes were washed with 0.01 M phosphate buffer saline (PBS) for 3 times and centrifuged for 5 min at 3000 g. Astrocytes were then resuspended in PBS. For intrathecal injection, spinal cord puncture was made with a 30G needle between the L5 and L6 level to deliver the cells or reagents (10 µl) to the cerebral spinal fluid (Hylden and Wilcox, 1980).
ELISA
Mouse MCP-1 ELISA kit was purchased from R&D. Culture medium and cells were collected separately after treatment. Astrocytes were homogenized in a lysis buffer containing protease and phosphatase inhibitors (Zhuang et al., 2006). Protein concentrations were determined by BCA Protein Assay (Pierce). For each reaction in a 96-well plate, 100 µg of proteins or 50 µl of culture medium were used, and ELISA was performed according to manufacturer’s protocol. The standard curve was included in each experiment.
Immunohistochemistry
Animals were deeply anesthetized with isoflurane and perfused through the ascending aorta with PBS followed by 4% paraformaldehyde with 1.5% picric acid in 0.16 M PB. After the perfusion, the L4–L5 spinal cord segments were removed and postfixed in the same fixative overnight. Spinal cord sections (30 µm, free-floating) were cut in a cryostat and processed for immunofluorescence as we described previously (Jin et al., 2003; Zhuang et al., 2006). For Iba1 staining, spinal cord sections were first blocked with 2% goat serum for 1 h at room temperature, then incubated overnight at 4°C with rabbit anti-Iba1 primary antibody (1:5000, Wako), followed by incubating with Cy3-conjugated secondary antibody (1:400, Jackson ImmunoResearch) for 1 h at room temperature. For double staining of MCP-1 and GFAP, the spinal cord sections were blocked with 2% goat serum and then incubated with a mixture of primary antibodies against MCP-1 (rabbit, 1:500, Millipore) and GFAP (mouse, 1:5000, Millipore), followed by a mixture of corresponding secondary antibodies conjugated with either Cy3 or FITC (1:400, Jackson ImmunoResearch) (Gao et al., 2009). The stained spinal cord sections were examined with a Nikon fluorescence microscope, and images were captured with a CCD Spot camera.
Behavioral analysis
Animals were habituated to the testing environment daily for at least two days before baseline testing. The room temperature and humidity remained stable for all experiments. For testing mechanical sensitivity, animals were put in boxes on an elevated metal mesh floor and allowed 30 min for habituation before examination. The plantar surface of each hindpaw was stimulated with a series of von Frey hairs with logarithmically incrementing stiffness (0.02–2.56 grams, Stoelting), presented perpendicular to the plantar surface (1–2 seconds for each hair). The 50% paw withdrawal threshold was determined using Dixon’s up-down method (Chaplan et al., 1994).
Quantification and statistics
Five nonadjacent spinal cord sections were randomly selected from a spinal cord segment (L4–L5) and 3 mice were included for each group. The intensity of Iba1 staining in the superficial dorsal horn (laminae I–III) was measured with a computer-assisted imaging analysis system (Image J, NIH). The number of MCP-1 positive cells in the spinal cord dorsal horn (laminae I–III) was also counted under microscope. All the data were expressed as mean ± s.e.m. Differences between groups were compared by one-way ANOVA with repeated measurement and student t-test. The criterion for statistical significance was P<0.05.
Results
Intrathecal administration of TNF-α-stimulated astrocytes induces mechanical allodynia
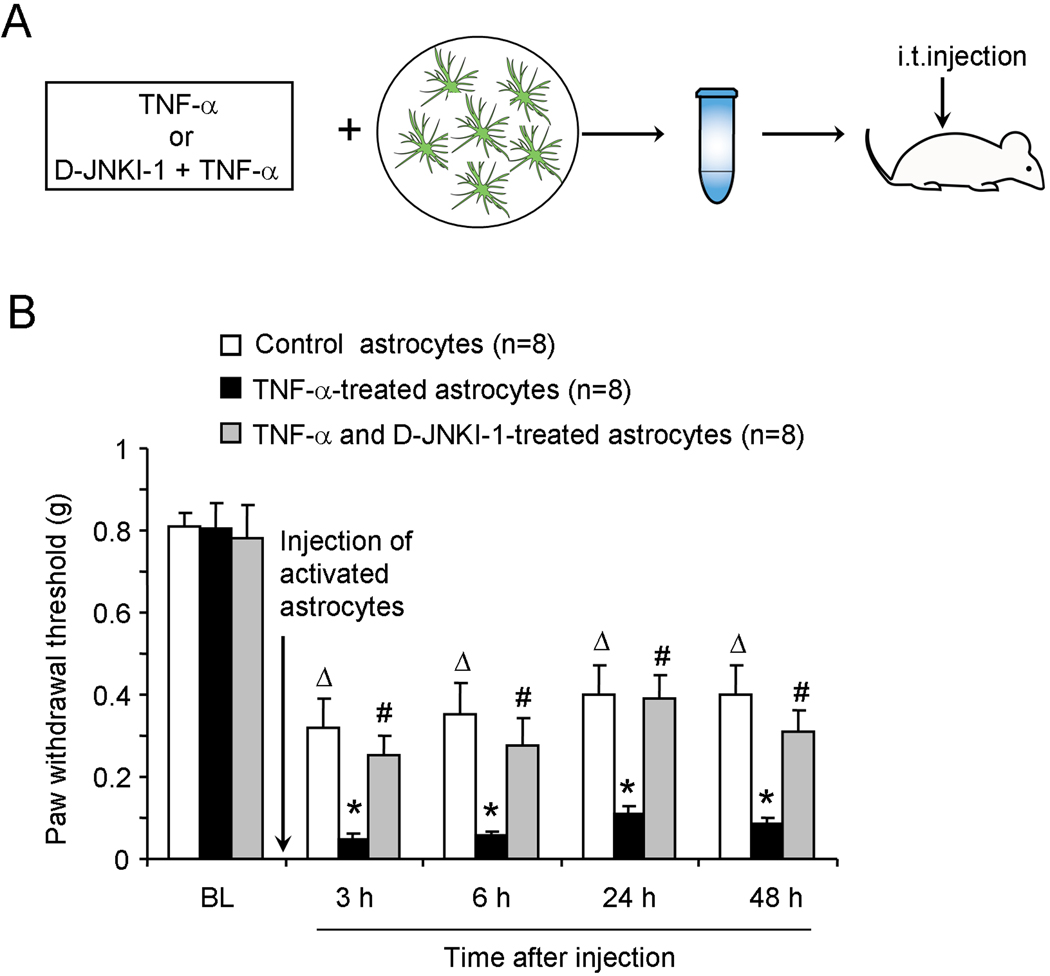
To determine whether activated astrocytes are sufficient to induce pain sensitization, we prepared primary astrocyte cultures from cerebral cortexes of neonatal mice (P2) and differentiated them with 0.15 mM dibutyryl cAMP to mimic mature astrocytes. We activated astrocytes with TNF-α to release MCP-1 (Gao et al., 2009). After a brief incubation with TNF-α (10 ng/ml, 15 min), we washed astrocytes 3 times with PBS to remove TNF-α and collected the astrocytes for intrathecal injection in naïve mice (Fig.1A). We found a dramatic reduction in paw withdrawal threshold (PWT) after inthathecal injection of TNF-α-stimulated astrocytes, indicating the development of mechanical allodynia (P<0.05, one-way ANOVA, Fig.1B). This allodynia developed at 3 h and lasted for more than 48 h (Fig.1B). Notably, intrathecal injection of non-stimulated control astrocytes also significantly reduced PWT (P<0.05, one-way ANOVA). However, the PWT following injection of activated astrocytes was much lower than that following injection of control astrocytes (P<0.05, t-test, Fig. 1B).
Figure 1.
Intrathecal injection of TNF-α-treated astrocytes induces persistent mechanical allodynia in naive mice via the activation of JNK. (A) Experimental protocol showing the preparation of astrocytes for intrathecal injection. The differentiated astrocytes were incubated with TNF-α (10 ng/ml, 15 min), or TNF-α together with the JNK inhibitor D-JNKI-1 (20 µM), then collected and resuspended for intrathecal injection into naïve mice. (B) Paw withdrawal thresholds after intrathecal injection of control astrocytes, TNF-α-treated astrocytes, or astrocytes treated with TNF-α plus D-JNKI-1. Δ P<0.05, compared with baseline; * P<0.05, compared with control group; # P<0.05, compared with TNF-α-treated group. n = 8 mice.
Because JNK is known to be activated by TNF-α and nerve injury in astrocytes and contributes to the development of mechanical allodynia (Gao et al., 2009; Zhuang et al., 2006), we examined whether mechanical allodynia elicited by TNF-α-stimulated astrocytes would require JNK. Pretreatment of cultured astrocytes with the peptide inhibitor of JNK, D-JNKI-1 (20 µM) starting 30 min before TNF-α stimulation, significantly reduced mechanical allodynia evoked by the activated astrocytes (P<0.05, t-test, Fig. 1B). Collectively, these results suggest that TNF-α-activated astrocytes are sufficient to induce mechanical allodynia in naïve mice, in a JNK-dependent manner.
TNF-α induces MCP-1 expression and release in astrocytes via activation of JNK
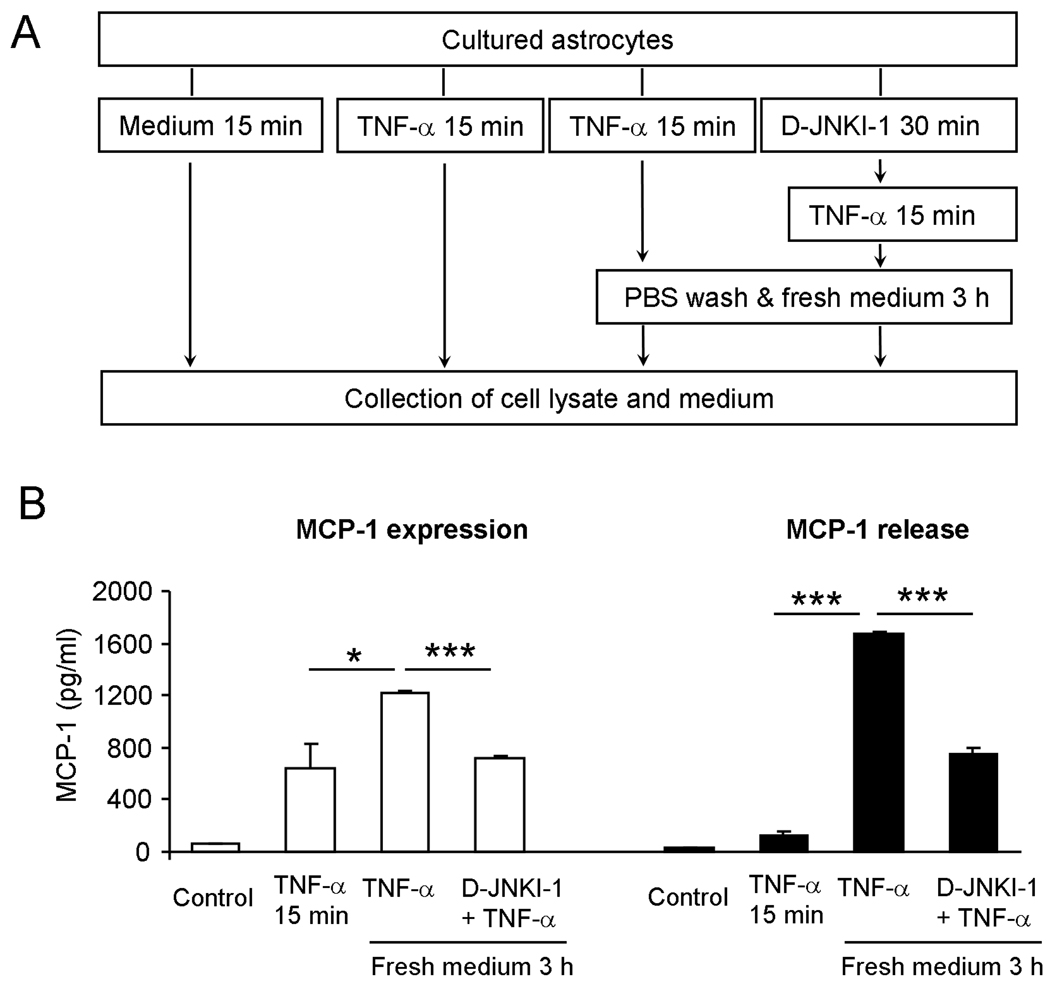
To explore possible mechanisms underlying the astrocytes-induced tactile allodynia, we examined MCP-1 expression and release in cultures using an experimental protocol to mimic in vivo conditions, as shown in Fig.2A. Brief incubation of astrocytes with TNF-α for 15 min increased MCP-1 expression. Interestingly, even after we removed TNF-α by 3 times PBS wash, MCP-1 expression continued to increase 3 h after initial TNF-α stimulation (15 min only). This increase was partially prevented when the astrocytes were pretreated with D-JNKI-1 (20 µM, P<0.05, t-test, Fig.2B). The brief application of TNF-α (15 min) also increased MCP-1 release in culture medium. Strikingly, 3 h after removal of TNF-α and replaced it with fresh medium, MCP-1 release further increased 10 times (P<0.05, t-test), and this increase in release was also reduced by D-JNKI-1 pretreatment (P<0.05, t-test, Fig. 2A, 2B).
Figure 2.
A brief exposure of astrocytes to TNF-α induces robust MCP-1 expression and release via the activation of JNK. (A) Experimental protocol of astrocyte preparation. (B) MCP-1 expression and release in astrocytes after a brief stimulation with TNF-α (10 ng/ml, 15 min). TNF-α evokes MCP-1 expression and release even 3 h after TNF-α withdrawal. D-JNKI-1 pretreatment (20 µM) reduces TNF-α-triggered MCP-1 expression and release. *P<0.05, *** P<0.001, n = 3.
To examine if astrocytes from the spinal cords have similar response to TNF-α as astrocytes from the cortexes, we prepared astrocyte cultures from the spinal cords and incubated the cultures with TNF-α for 15 min. ELISA analysis showed that a brief exposure of TNF-α also induced a substantial increase in MCP-1 expression and release in spinal cord astrocytes (Supporting information Fig.1). Thus, cortical astrocytes and spinal cord astrocytes show similar responses to TNF-α by inducing robust MCP-1 expression and release.
TNF-α induces MCP-1 expression in spinal cord astrocytes
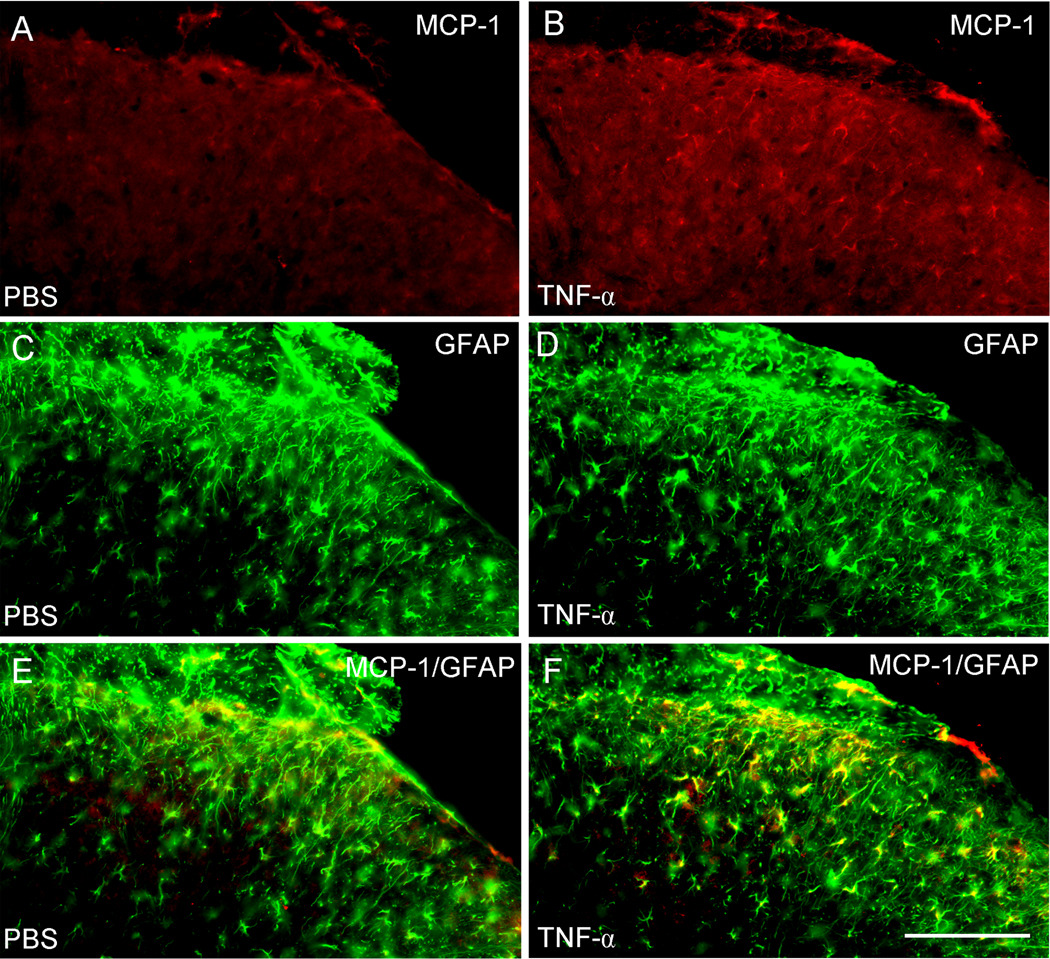
To further determine whether astrocytes in spinal cord in vivo show similar response to TNF-α as cultured astrocytes, we examined whether intrathecal TNF-α can induce MCP-1 expression in the intact spinal cord. Intrathecal TNF-α at a dose (20 ng) that is known to elicit mechanical allodynia (Gao et al., 2009) markedly increased MCP-1 expression in the spinal cord 3 h after the injection (Fig. 3A,B). The number of MCP-1-positive cells in the superficial dorsal horn (laminae I–III) increased from 10.4 ± 0.2 cells per section in the PBS-treated group to 33.5 ± 2.4 cells per section in the TNF-α-treated group (P<0.05, t-test, n=3 mice). Double staining revealed that MCP-1 was co-localized with the astrocyte marker GFAP (Fig.3E, F). These data strongly suggest that TNF-α induces MCP-1 expression not only in cultured astrocytes but also in spinal cord astrocytes in vivo, in support of our previous result that nerve injury induces MCP-1 in spinal cord astrocytes (Gao et al., 2009).
Figure 3.
Intrathecal injection of TNF-α (20 ng) induces MCP-1 expression in spinal astrocytes. (A, B) Immunohistochemistry showing MCP-1 expression in the spinal cord dorsal horn of mice receiving intrathecal injection of PBS (A) and TNF-α (B). (C, D) Immunohistochemistry showing GFAP expression in the spinal cord dorsal horn of mice receiving intrathecal injection of PBS (C) and TNF-α (D). (E, F) Double staining showing the colocalization of MCP-1 and GFAP in the spinal cord dorsal horn of mice receiving intrathecal injection of PBS (E) and TNF-α (F). E is the merge of A and C and F is the merge of B and D. Animals were sacrificed 3 hours after PBS or TNF-α injection. Scale bar, 100 µm.
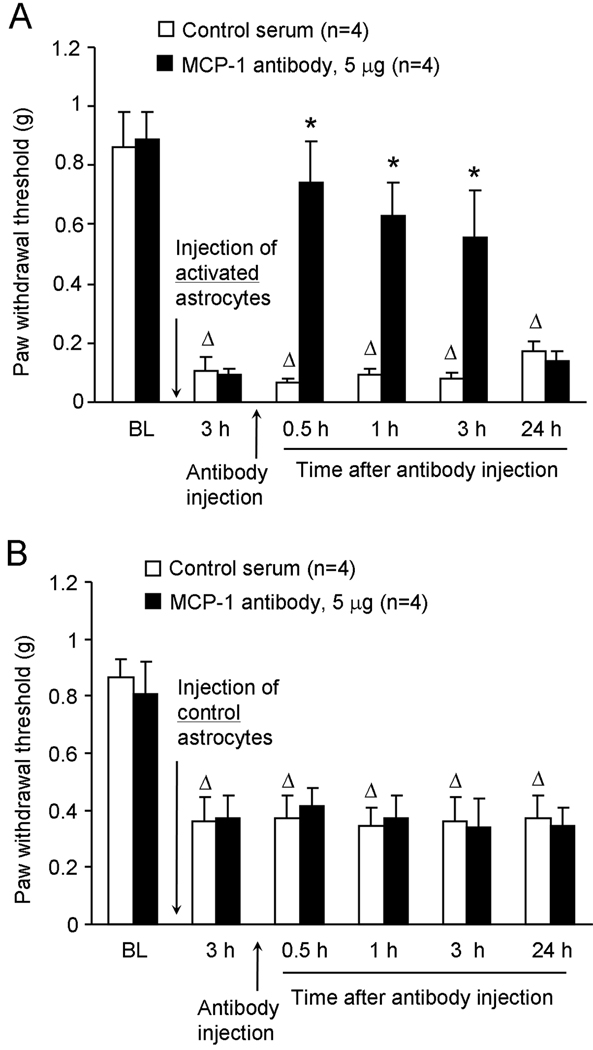
MCP-1 neutralizing antibody reverses mechanical allodynia induced by activated astrocytes but not by control astrocytes
To test the hypothesis that TNF-α-activated astrocytes release MCP-1 to generate tactile allodynia in naïve animals, we intrathecally injected a MCP-1 neutralizing antibody at 3 h after intrathecal injection of TNF-α-treated astrocytes. At a dose (5 µg) that is effective in reducing SNL-induced neuropathic pain (Gao et al., 2009) the neutralizing antibody reversed the mechanical allodynia induced by activated astrocytes (P<0.05, one-way ANOVA, Fig. 4A). This reversal began at 30 min, maintained at 3 h, but diminished at 24 h following the antibody injection (Fig. 4A). In contrast, intrathecal injection of the control serum had no effect on mechanical allodynia (P>0.05, one-way ANOVA, Fig. 4A).
Figure 4.
Effects of intrathecal administration of the MCP-1 neutralizing antibody on mechanical allodynia induced by intrathecal injection of TNF-α-activated astrocytes (A) and non-activated control astrocytes (B). Note that the MCP-1 neutralizing antibody only reverses allodynia induced by the activated astrocytes. * P<0.05, compared with control serum; Δ P<0.05, compared with baseline, n = 4 mice.
Since intrathecal injection of control astrocytes also decreased paw withdrawal threshold in naïve animals (Fig.1B), we further checked if this allodynia might also depend on MCP-1. Intrathecal injection of the MCP-1 neutralizing antibody (5 µg) did not change the PWT at 3 h after injection of control astrocytes (P>0.05, one-way ANOVA, Fig.4B). Thus, mechanical allodynia elicited by control astrocytes does not require MCP-1.
Intrathecal injection of TNF-α is known to elicit mechanical allodynia (Gao et al., 2009). To exclude the possibility that the mechanical allodynia induced by TNF-α-stimulated astrocytes is caused by the residue TNF-α in the culture, we injected the TNF-α-stimulated astrocytes into double knockout mice lacking type I and II TNF receptors (TNFR1/R2 −/−) using C57BL/6 wild-type mice as control. Notably, intrathecal injection of TNF-α-stimulated astrocytes was still able to elicit mechanical allodynia in knockout mice (P>0.05, t-test, supporting information, Fig.2). This finding suggests that mechanical allodynia elicited by activated astrocytes is caused neither by residue TNF-α in the culture medium nor by TNF-α released from astrocytes.
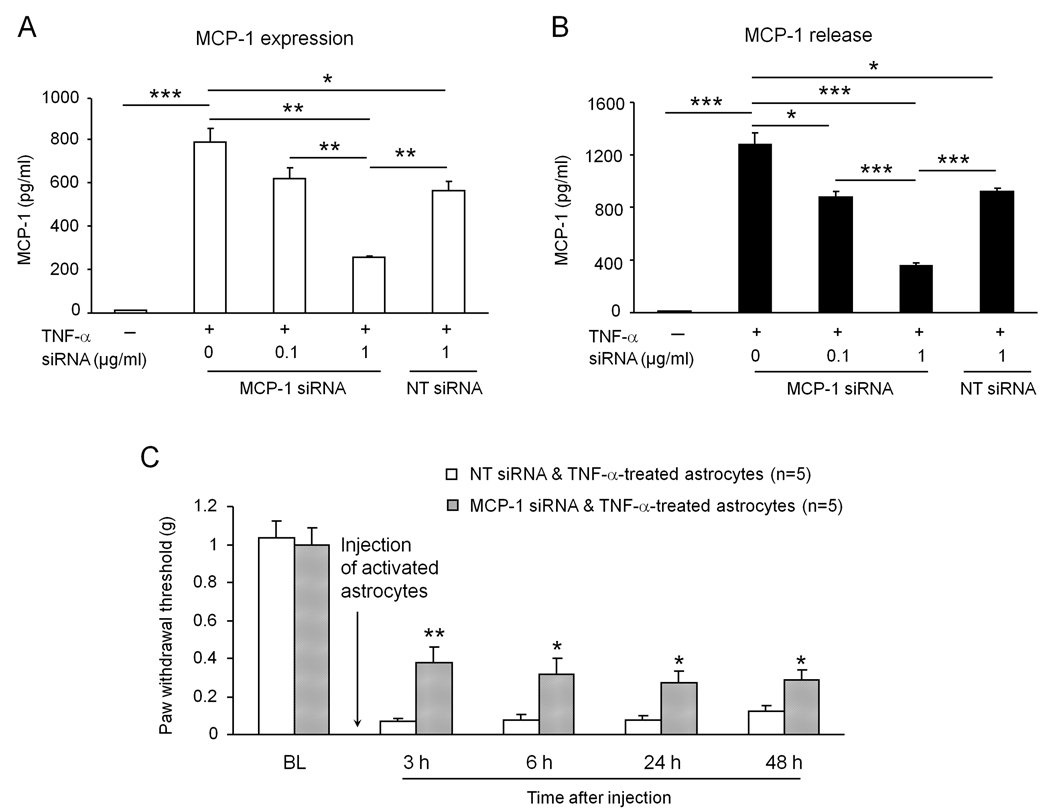
Astrocytes-induced mechanical allodynia is reduced by pretreatment of astrocytes with MCP-1 siRNA
To further confirm the role of astrocytic MCP-1 release in generating mechanical allodynia, we treated astrocyte cultures with a specific small interfering RNA (siRNA) against MCP-1. Astrocytes were incubated with MCP-1 siRNA for 18 h followed by TNF-α stimulation for 15 min, and the medium and cell lysates were collected 3 h later. MCP-1 siRNA treatment inhibited TNF-α-induced MCP-1 expression and release in a dose-dependent manner (Fig. 5A, B). At the concentration of 0.1 and 1 µg/ml, MCP-1 siRNA inhibited MCP-1 expression by 22% and 68% and MCP-1 release by 32% and 73%, respectively, compared to non-treated astrocytes. Notably, the non-targeting siRNA at the high concentration (1µg/ml) also decreased MCP-1 expression and release by 28%. This effect of non-targeting siRNA may be associated with interferon-mediated off-target effects (Sledz et al., 2003). However, MCP-1 siRNA at the high concentration (1 µg/ml) produced more robust inhibition of MCP-1 expression and release (P<0.01, t-test, Fig. 5A, B).
Figure 5.
MCP-1 siRNA treatment in astrocytes reduces TNF-α-induced MCP-1 expression and release and astrocytes-induced mechanical allodynia. (A, B) pretreatment of MCP-1 siRNA dose-dependently reduces TNF-α-induced MCP-1 expression (A) and release (B) in cultured astrocytes. *, P<0.05; **, P<0.01; ***, P<0.001. n = 3 separate cultures from different mice. (C) Mechanical allodynia induced by intrathecal injection of TNF-α-activated astrocytes. Pretreatment of astrocytes with MCP-1 siRNA reduces allodynia induced by activated astrocytes. *, P<0.05; **, P<0.01 vs non-targeting siRNA control. n = 5 mice.
After we confirmed an effective knockdown of MCP-1 expression and release by MCP-1 siRNA in cultured astrocytes, we next examined the effect of this knockdown on pain behavior. Following incubation with MCP-1 siRNA or non-targeting control siRNA (1 µg/ml, 18 h), astrocytes were stimulated with TNF-α for 15 min, washed with PBS and then collected for intrathecal injection in naïve mice. Mechanical allodynia was reduced after the injection of MCP-1 siRNA-treated astrocytes compared with that after the injection of control siRNA-treated astrocytes (P<0.05 or P<0.01, t-test, Fig. 5C).
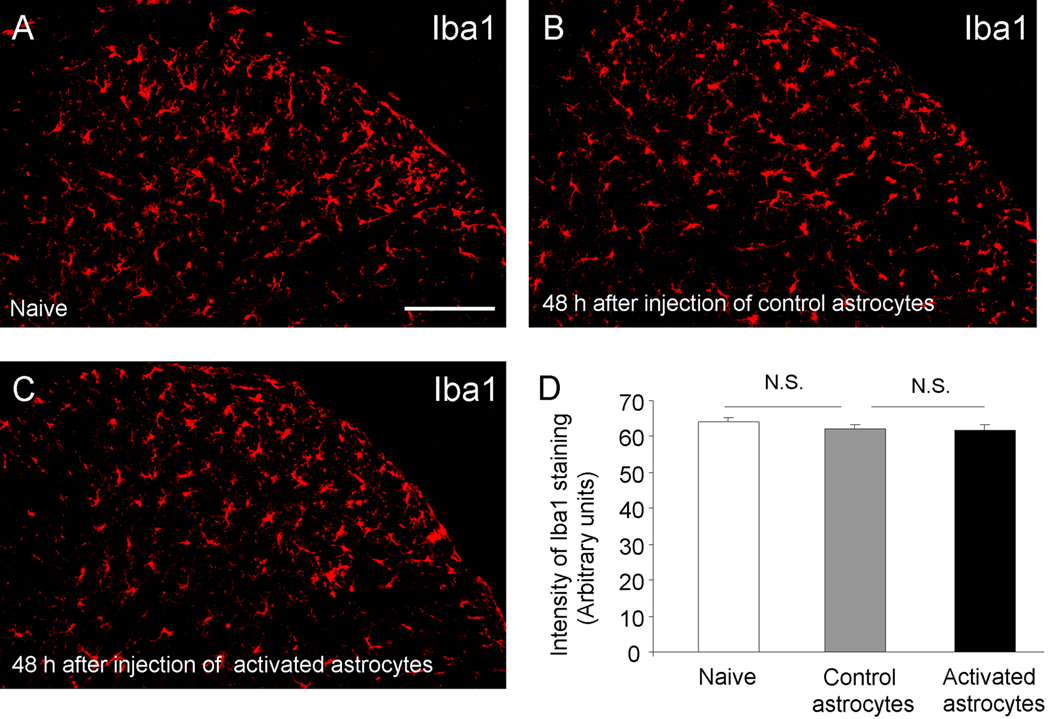
Expression of the microglial marker Iba1 in the spinal cord does not change after intrathecal injection of activated astrocytes
To determine possible mechanisms by which astrocyte-released MCP-1 produces mechanical allodynia, we examine microglial reaction in the spinal cord, because MCP-1 has been shown to promote neuropathic pain by inducing microglial responses in the spinal cord (Thacker et al., 2009; Zhang et al., 2007). We collected spinal cord segments at 48 h after intrathecal injection of TNF-α-stimulated astrocytes or control astrocytes and examined the expression of the microglial marker, Iba1, which is associated with microglial proliferation and migration (Zhang et al., 2007). To our surprise, we did not observe any difference in the intensity of Iba1 immunofluorescence between these two groups (P>0.05, t-test). Neither did we find any differences in the morphology of Iba1-stained microglia (Fig.6A–D). Therefore, there is no evidence of microgliosis in the spinal cord following intrathecal injection of activated astrocytes.
Figure 6.
Iba1 immunostaining in the spinal cord dorsal horn of naïve mice (A) and mice receiving intrathecal injection of control astrocytes (B) or TNF-α treated astrocytes (C). The morphology and density of Iba1-labeled microglia in the spinal cord are comparable among different groups. (D) Intensity of Ibal staining in the spinal cord dorsal horn. N.S., no significance, n=4 mice. Mice were sacrificed 48 hours after the intrathecal injections. Scale bar, 200µm
Discussion
Astrocytes are required for producing chronic pain symptoms
Astrocytes become reactive in chronic pain conditions after nerve injury and tissue injury/inflammation (DeLeo et al., 2004; Garrison et al., 1994; Garrison et al., 1991; Raghavendra et al., 2004; Zhuang et al., 2006). Several lines of evidence suggest that astrocytes are required for the generation of persistent pain. First, intrathecal injection of astroglial toxin fluorocitrate (Milligan et al., 2003) and L-alpha-aminoadipate (Zhuang et al., 2006) inhibits nerve injury- or nerve inflammation-induced mechanical allodynia. Second, astrocytes form astroglial networks by gap junctions (Giaume and McCarthy, 1996), and intrathecal gap junction blocker carbenoxolone suppresses mechanical allodynia in the contralateral paw after nerve inflammation (Spataro et al., 2004). Third, intrathecal administration of the astroglial aconitase inhibitor sodium fluoroacetate inhibits trigeminal neuropathic pain (Okada-Ogawa et al., 2009). Fourth, trigeminal pain and central sensitization induced in nociceptive neurons in trigeminal subnucleus caudalis following tooth pulp inflammation can be attenuated by intrathecal superfusion of methionine sulfoximine, an inhibitor of the astroglial enzyme glutamine synthetase that is involved in the glutamate-glutamine shuttle (Chiang et al., 2007). Finally and importantly, following injuries reactive astrocytes in the spinal cord express multiple signaling molecules such as JNK1 and phosphoJNK (Katsura et al., 2008; Zhuang et al., 2006) , phosphoERK (Zhuang et al., 2005), transforming growth factor-activated kinase-1 (Katsura et al., 2008), matrix metalloprotease-2 (MMP-2) (Kawasaki et al., 2008a), IL-1β (Guo et al., 2007; Wei et al., 2008), IL-18 receptor (Miyoshi et al., 2008), MCP-1 (Gao et al., 2009), and tissue type plasminogen activator (Kozai et al., 2007). These studies further demonstrate that spinal inhibition of these signaling molecules can reduce chronic pain symptoms such as mechanical allodynia.
Astrocytes are sufficient for producing chronic pain symptoms
Accumulating evidence suggests that astrocytes may also be sufficient to produce chronic pain symptoms. Hofstetter et al. reported that implantation of neural stem cells into the injured spinal cord elicited allodynia-like hypersensitivity in the forepaws by generating astrocytes (Hofstetter et al., 2005). Davies et al. demonstrated that transplantation of glial-restricted precursor-derived astrocytes promoted the onset of mechanical allodynia (Davies et al., 2008). Basic fibroblast growth factor (bFGF), which is produced by injured primary sensory neurons (Ji et al., 1995) and reactive astrocytes (Madiai et al., 2005) after nerve injury, induces astrocyte gliosis and proliferation. bFGF also activates JNK in astrocytes and produces persistent mechanical allodynia (Ji et al., 2006). In the present study we provided direct evidence that intrathecal injection of TNF-α-activated astrocytes or even control astrocytes is sufficient to induce persistent mechanical allodynia in naïve animals. It remains to be investigated why injection of non-activated control astrocytes also induce mechanical allodynia. Under stress conditions, astrocytes may release some pain mediators. Intrathecal injection of astrocytes may also cause mild innate immune responses. Nevertheless, mechanical allodynia elicited by TNF-α-activated astrocytes was much more robust. Importantly, only mechanical allodynia produced by TNF-α-activated astrocytes but not by control astrocytes is mediated by MCP-1.
Activated astrocytes produce mechanical allodynia via MCP-1
TNF-α is an essential trigger of an inflammatory cascade that underlies the pathogenesis of pain (Mata et al., 2008; Schafers et al., 2003; Sommer, 2001; Sommer et al., 2001; Xu et al., 2006; Xu et al., 2010). After nerve injuries, activated microglial cells are a major source for TNF-α (Ji and Suter, 2007; Xu et al., 2006; 2007). ATP induces TNF-α release from microglia via P2X7 receptor (Suzuki et al., 2004). TNF-α is a strong activator of astrocytes: even a short exposure of astrocytes to TNF-α for 15 min induced a substantial and persistent increase in the expression and release of MCP-1, which required the activation of the JNK pathway. Our in vivo data also showed that intrathecal TNF-α markedly increased MCP-1 expression in spinal cord astrocytes. Indeed, our previous cytokine array study has identified MCP-1 as one of the most inducible chemokines in TNF-α-stimulated astrocytes (Gao et al., 2009). Of great interest, astrocytes continued to release high levels of MCP-1 even after removal of TNF-α. Intrathecal injection of MCP-1 has been shown to induce chronic pain symptoms such as heat hyperalgesia (Gao et al., 2009) and mechanical allodynia (Thacker et al., 2009). Our data clearly demonstrated that MCP-1 is also critical for astrocytes-induced mechanical allodynia, because this allodynia was attenuated by blocking the MCP-1 signaling either with a neutralizing antibody or with a siRNA (Fig. 4 and Fig. 5).
MCP-1 and microglial responses
After nerve injury MCP-1 is up-regulated in primary sensory neurons (White et al., 2005) and released, in an activity-dependent manner, from the central terminals of these neurons (Thacker et al., 2009). CCR2, a major receptor for MCP-1, may also be induced in spinal microglia after nerve injury (Abbadie et al., 2003; Zhang et al., 2007). Nerve injury-induced microgliosis is prevented by a CCL2 neutralizing antibody (Thacker et al., 2009; Zhang et al., 2007) or in mice lacking CCR2 (Zhang et al., 2007). Nerve injury-induced p38 activation in spinal microglia is also attenuated in CCR2 knockout mice (Abbadie et al., 2003; Zhang and De Koninck, 2006; Zhang et al., 2007). Thus, it has been proposed that MCP-1 promotes neuropathic pain via inducing microglial responses (e.g., migration and gliosis) in the spinal cord (Zhang et al., 2007; Thacker et al., 2008). However, we did not find any difference in the intensity of Iba1 immunofluorescence and the morphology of Iba1-stained microglia after intrathecal injection of TNF-α-stimulated astrocytes, arguing against a microgliosis in this condition. It is likely that the amount of MCP-1 produced by exogenous astrocytes in our experimental setting may not sufficient to increase Iba1 expression and induce morphological changes of microglia, although this amount is sufficient to produce mechanical allodynia via a direct action on neurons. Indeed, perfusion of spinal cord slices with MCP-1 can rapidly (within 5 min) induce phosphorylation of extracellular signal-regulated kinase (ERK) in dorsal horn neurons (Gao et al., 2009). MCP-1 perfusion also increases NMDA currents in dorsal horn neurons (Gao et al., 2009). Notably, a previous study by Zhang et al. (2007) used very high dose of MCP-1 for intrathecal injections (3 injections, 2 µg per injection over 6 d) to induce microgliosis. Therefore, we postulate that astrocyte-produced MCP-1 can induce rapid and direct sensitization of dorsal horn neurons (central sensitization) (Gao and Ji, 2010). MCP-1 may also regulate central sensitization via presynaptic neurotransmitter release (Knerlich-Lukoschus et al., 2008; White et al., 2005) or by regulating GABAergic transmission in dorsal horn neurons (Gosselin et al., 2005). Therefore, MCP-1, released from astrocytes or primary afferents, could enhance central sensitization and chronic pain via a direct action on neurons or an indirect action via microglial cells.
In summary, we have demonstrated that TNF-α-activated astrocytes are sufficient to produce a cardinal symptom of chronic pain, mechanical allodynia via releasing the chemokine MCP-1. We have further identified a signaling pathway for astrocyte-induced central sensitization which involves TNF-α/JNK/MCP-1. Thus, targeting this signaling pathway in astrocytes may offer new therapeutics for the management of the intractable chronic pain conditions.
Supplementary Material
Acknowledgements
This work was supported by NIH R01 grants NS54932, NS67686, and DE17794 to RRJ.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Neuropathic pain: a clinical perspective. Handb Exp Pharmacol. 2009:3–30. doi: 10.1007/978-3-540-79090-7_1. [DOI] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27:9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Carlton SM. GFAP expression in lumbar spinal cord of naive and neuropathic rats treated with MK-801. Exp Neurol. 1994;129:237–243. doi: 10.1006/exnr.1994.1165. [DOI] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565:1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95:1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. Neuroglial networks: neurons and glia talk to each other. Curr Biol. 2000;10:R712–R714. doi: 10.1016/s0960-9822(00)00708-9. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004 doi: 10.1126/stke.2522004re14. reE14. [DOI] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Zhang X, Piehl F, Reilly T, Pettersson RF, Hokfelt T. Prominent expression of bFGF in dorsal root ganglia after axotomy. Eur J Neurosci. 1995;7:2458–2468. doi: 10.1111/j.1460-9568.1995.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Katsura H, Obata K, Miyoshi K, Kondo T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia. 2008;56:723–733. doi: 10.1002/glia.20648. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008a;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008b;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, Juraschek M, Blomer U, Lucius R, Mehdorn HM, Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J Neurotrauma. 2008;25:427–448. doi: 10.1089/neu.2007.0431. [DOI] [PubMed] [Google Scholar]
- Kozai T, Yamanaka H, Dai Y, Obata K, Kobayashi K, Mashimo T, Noguchi K. Tissue type plasminogen activator induced in rat dorsal horn astrocytes contributes to mechanical hypersensitivity following dorsal root injury. Glia. 2007;55:595–603. doi: 10.1002/glia.20483. [DOI] [PubMed] [Google Scholar]
- Madiai F, Goettl VM, Hussain SR, Clairmont AR, Stephens RL, Jr, Hackshaw KV. Anti-fibroblast growth factor-2 antibodies attenuate mechanical allodynia in a rat model of neuropathic pain. J Mol Neurosci. 2005;27:315–324. doi: 10.1385/JMN:27:3:315. [DOI] [PubMed] [Google Scholar]
- Mata M, Hao S, Fink DJ. Gene therapy directed at the neuroimmune component of chronic pain with particular attention to the role of TNF alpha. Neurosci Lett. 2008;437:209–213. doi: 10.1016/j.neulet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci. 2008;28:12775–12787. doi: 10.1523/JNEUROSCI.3512-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci. 2009;29:11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Sommer C. Cytokines in neuropathic pain. Anaesthesist. 2001;50:416–426. doi: 10.1007/s001010100135. [DOI] [PubMed] [Google Scholar]
- Sommer C, Lindenlaub T, Teuteberg P, Schafers M, Hartung T, Toyka KV. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913:86–89. doi: 10.1016/s0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain? Neuron Glia Biol. 2007;3:255–268. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PH, Yang LC, Ji RR. Therapeutic potential of RNA interference in pain medicine. Open Pain J. 2009;2:57–63. doi: 10.2174/1876386300902010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Xu JT, Xin WJ, Wei XH, Wu CY, Ge YX, Liu YL, Zang Y, Zhang T, Li YY, Liu XG. p38 activation in uninjured primary afferent neurons and in spinal microglia contributes to the development of neuropathic pain induced by selective motor fiber injury. Exp Neurol. 2007;204:355–365. doi: 10.1016/j.expneurol.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.