Summary
In order to cause disease, all pathogens must tolerate microenvironmental stresses encountered in vivo during infection. One microenvironmental stress that is known to occur at sites of tissue damage is hypoxia. Yet, the occurrence and impact of hypoxic microenvironments during invasive aspergillosis, caused by the mold Aspergillus fumigatus, are essentially unknown. Here, we briefly review the potential implications of hypoxic microenvironments on the Aspergillus – host interaction. We focus on three areas where hypoxia may play a role in determining the outcome of infection: fungal virulence, host immune responses, and efficacy of current antifungal drug treatments.
Keywords: Invasive Aspergillosis, hypoxia, Aspergillus fumigatus, antifungal drugs, fungal virulence
INTRODUCTION
Interactions between disease causing microorganisms and their respective hosts generate complex in vivo microenvironments that can affect the outcome of infection. Interactions between Aspergillus fumigatus and susceptible hosts are certainly not exceptions. However, our understanding of the stress conditions found in microenvironments generated during Aspergillus infections are largely rudimentary. Moreover, how these microenvironments and associated stress conditions ultimately affect the outcome of the infection from the perspective of both the host and fungus (and how they affect antifungal drug efficacy) is also relatively under appreciated.
Stress conditions that occur within these microenvironments that are most often studied in the context of A. fumigatus pathogenesis include: temperature, oxidative stress, pH changes, and macro and micro-nutrient availability [1-6]. One stress condition that occurs at sites of microbial infections that has generally been overlooked in the context of A. fumigatus pathogenesis is hypoxia. Hypoxic lesions (oxygen levels ≤ 1%) have been well described in tumors, wounds, and sites of necrotic tissue and occur due to decreased tissue perfusion that is often secondary to microvascular damage and thrombosis [7-11]. These changes are typically coupled with the actions of the invasive growth of the fungus and subsequent host responses. Indeed, typical pathology observed with invasive aspergillosis is characterized by infarcted tissue with extensive hyphal growth [12].
The idea that hypoxia could affect the physiology of pathogenic Aspergillus species appears to have been first examined in 1994 by Hall and Denning [13]. In this study, the authors examined the oxygen requirements of various pathogenic Aspergillus species. They observed that Aspergillus species such as A. fumigatus could grow at very low oxygen concentrations with most species capable of growth at 0.1% oxygen. Interestingly, A. fumigatus does not seem capable of growth under strict anaerobic conditions and requires the use of the respiratory chain for conidia germination and initiation of infection [13, 14]. Subsequently in 2005, Tarrand et al. [15] postulated that the low rate of Aspergillus recovery from clinical samples likely reflects the adaptation of the fungus to the host microenvironment, which the authors speculated includes hypoxia.
More circumstantial evidence for the occurrence of hypoxic microenvironments during A. fumigatus infection comes from a recent study that generated a luciferase producing strain of A. fumigatus for use in murine models of invasive aspergillosis [16]. Surprisingly, the results from this study suggested that luminescence from the lungs decreased after reaching a maximum at one day post infection, despite the high number of fungal hyphae present in histopathology examinations. Since oxygen is essential for the light-producing reaction, the authors hypothesized that their results might be due to the severe tissue damage in and through the pulmonary lesions, which are probably severe enough to decrease the oxygen concentration in the lung tissue.
Our laboratory became interested in hypoxia and invasive aspergillosis in 2007 when a 1H-NMR metabolomics profile of broncheoalveolar lavage fluid from Aspergillus infected neutropenic mice revealed the presence of ethanol [Grahl et al. manuscript in preparation]. We hypothesize that the presence of ethanol indicates that the fungus encounters low levels of oxygen at sites of infection and potentially switches its metabolism from aerobic respiration to anaerobic fermentation [17]. Ongoing studies in our laboratory are examining the occurrence and timing of hypoxia in multiple distinct murine models of invasive aspergillosis using hypoxia detecting chemical reagents [Grahl et al. manuscript in preparation].
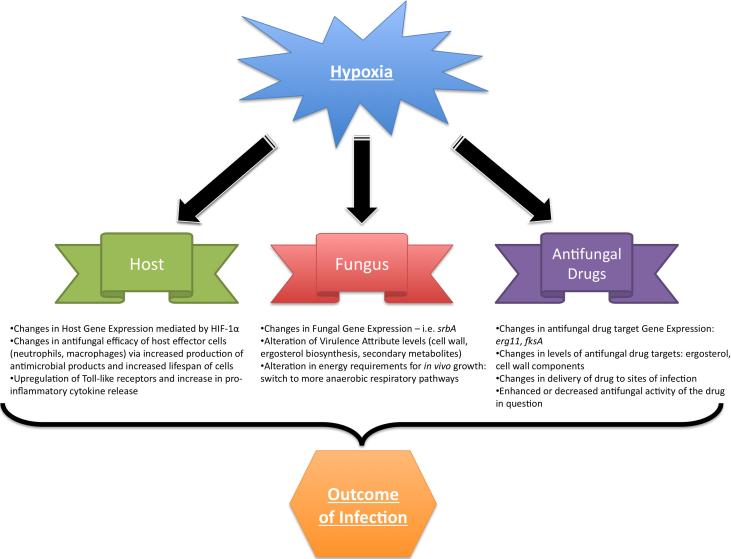
Thus, these described data and evidence from other infectious disease pathosystems strongly suggest that hypoxia is an important component of in vivo microenvironments encountered by both the pathogen and host during invasive aspergillosis. The purpose of this brief review is to discuss the potential implications of these hypoxic microenvironments during invasive aspergillosis (summarized in Figure 1). Our hope is that this small review will spur further discussion and research regarding the clinical significance of hypoxic microenvironments that occur during invasive aspergillosis.
Figure 1.
Summary of key concepts covered in this review. Hypoxia may influence the outcome of the Aspergillus-host interaction on multiple fronts: alterations in fungal virulence, host response to the fungus, and changes in azole drug efficacy at the site of infection. These effects on the host-pathogen interaction are likely mediated at transcriptional, post-transcriptional, and post-translational levels.
EFFECT OF HYPOXIA ON FUNGAL VIRULENCE
The purpose of this section is not to re-visit the “virulence factor” debate with regard to A. fumigatus. It is generally accepted that A. fumigatus virulence is a multigenic trait and is not necessarily attributed to a single gene, gene product, or metabolic pathway. Regardless of whether A. fumigatus contains traditional virulence factors, in order to cause disease, organisms must adapt to the environmental conditions found in the host environment. While attributes that allow in vivo adaptation and growth are not traditionally considered virulence factors, there is a growing appreciation that pathogens like A. fumigatus possess unique attributes that allow them to cause disease in susceptible hosts [1, 2, 6].
To date, evidence that adaptation to hypoxia and subsequent hypoxic growth is a requirement for A. fumigatus virulence comes from studies in our laboratory on the sterol regulatory element binding protein (SREBP) ortholog, SrbA [17-19] (and reviewed in [20]). SrbA null mutants of A. fumigatus are incapable of growth in hypoxic environments, but have normal growth rates on solid media under normoxic conditions. Importantly, the SrbA null mutant is virtually avirulent in two distinct murine models of aspergillosis [19]. The mechanism behind the inability of the SrbA mutant to grow in hypoxic conditions is currently under investigation, but may be related to SrbA transcriptional regulation of key steps in the ergosterol biosynthesis pathway. Discovering the role of SrbA in hypoxic adaptation and growth will be important in definitively determining whether a requirement for hypoxic growth is indeed a critical virulence attribute of A. fumigatus.
To our knowledge, no other A. fumigatus mutants have been observed or reported to be deficient in hypoxic adaptation or growth. As oxygen is such a critical molecule for obligate aerobes like A. fumigatus, it is highly likely that in vivo hypoxic microenvironments affect the overall metabolism of this organism. Indeed, microarray experiments examining gene expression of A. fumigatus under hypoxic conditions reveal a substantial change in the overall gene expression of the organism [Barker et al. unpublished data]. Thus, it is highly probable that mutations in various critical metabolic pathways that respond to hypoxia will influence the ability of the organism to tolerate hypoxic conditions and possibly its ability to cause disease. Thus, adding hypoxia to the list of stress conditions associated with virulence that mutants are tested against in vitro may help to explain the virulence attenuation of certain strains. Discovery of additional genes and proteins required for hypoxic adaptation and growth will allow us to better appreciate and define the role of hypoxia in invasive aspergillosis from the perspective of the fungus.
EFFECT OF HYPOXIA ON HOST RESPONSES TO ASPERGILLUS
As hypoxia affects the metabolism and growth of the invading fungus, limitations on oxygen availability also affect cells of the host. With regard to microbial pathogenesis, there is a growing appreciation of the importance of hypoxia in directing innate immune responses [21-28]. Oxygen levels at sites of infection appear to be playing important roles in determining the outcome of host immune and pathogen cell interactions. The major regulator of host cell hypoxia adaptation is hypoxia inducible factor 1 alpha (HIF-1α). The HIF DNA-binding complex may be composed of one of three α-subunits (HIF-1α, HIF-2α, and HIF-3α) and one constitutively expressed β-subunit (HIF-1β), and is the central regulator of hypoxic gene expression in mammals (reviewed in [29],[30]). Both the degradation and activity of the HIF-1α and HIF-2α subunits are regulated by oxygen-dependent post-translational hydroxyl modifications.
The role of HIF-1α in innate immune responses to human pathogens has been most extensively studied in the context of bacterial pathogenesis. Studies suggest that HIF-1α is required for the survival and bactericidal activities of important phagocytes such as macrophages and neutrophils. For example, macrophages from mice lacking HIF-1α are significantly impaired in their ability to kill bacterial pathogens such as group A and B Streptococcus, Pseudomonas aeruginosa, and Salmonella typhimurium [31, 32]. Mice with HIF-1α deficient myeloid cells are also significantly more susceptible to group A Streptococcus infections [31]. In the eukaryotic pathogen Toxoplasma gondii, it was recently found that the intracellular survival of this parasite relies on host cell expression of HIF-1α [33]. These and other studies on infectious organisms and hypoxia strongly suggest that regulation of host responses to hypoxia is a key factor in determining the outcome of host-pathogen interactions (reviewed in [21]). However, to date, no studies have examined the potential role of hypoxia in influencing the host response to fungal pathogens such as A. fumigatus.
The immune status of the host is a significant variable when potentially addressing the role of hypoxia and immune responses to A. fumigatus. For example, when examining angiogenesis related gene expression in two distinct murine models of invasive aspergillosis, Ben-Ami et al. found that HIF-1α mRNA levels were substantially higher 24 hours after infection in mice treated solely with cortisone acetate as compared to mice treated with cyclophosphamide and cortisone acetate [34]. This result potentially suggests that mice treated solely with cortisone acetate have higher levels of hypoxia in the lung during Aspergillus infection than mice made neutropenic by addition of cyclophosphamide to the immunosuppression regimen. Alternatively, the difference in expression could be due to differences in the numbers of immune effector cells recruited to the sites of infection in the different models. Results from this study also suggested that inhibition of angiogenesis was a direct effect of fungal metabolism mediated by secretion of the potent non-ribosomal peptide gliotoxin. Thus, it seems clear that activities of the mold, in combination with activities of the host, may directly contribute to the development of hypoxia at sites of infection. Future research will likely uncover how hypoxic microenvironments influence the outcome of the fungus-host interaction from the perspective of the host.
EFFECT OF HYPOXIA ON ANTIFUNGAL DRUG TREATMENTS
The generation of hypoxic microenvironments by the invading fungus and subsequent host immune response could potentially alter the efficacy of antifungal agents. It is known that Amphotericin B-mediated lysis of Candida albicans protoplasts is decreased in hypoxia, which suggests that optimal Amphotericin B activity requires oxygen [35]. Conversely, administration of Amphotericin B (AmB) to clinical isolates of Aspergillus species revealed a general increase in susceptibility in hypoxic (1% O2) versus normoxic (21% O2) conditions [36]. Additionally, this study discovered the enhanced inhibitory effect of the echinocandin micafungin under hypoxic conditions, demonstrating a true minimum inhibitory concentration (MIC) instead of the minimum effective concentration (MEC) normally calculated for this class of drugs. Similar results with anidulafungin were observed under hypoxic conditions [37]. Perkhofer et al. [37] observed no difference in susceptibility to the triazole voriconazole (VCZ) in Aspergillus spp. incubated in hypoxic conditions, while Warn et al. [36] observed a small decrease in the MIC when A. fumigatus was treated with itraconazole under hypoxic conditions.
These two studies highlight the importance of in vitro susceptibility to antifungal drugs in the context of hypoxia in invasive aspergillosis. Thus, it seems that due to the complex interactions between host and fungi, the kinetics of antifungal drugs cannot be evaluated solely by the in vitro MIC in normoxic conditions. As demonstrated by Perkhofer et al. [37], even the effect of combination therapy (VCZ + anidulafungin) takes on new light when examining conidial or hyphal treatment in hypoxia. The finding with regard to the MIC versus MEC and the echinocandins is intriguing and begs the question how hypoxia alters the efficacy of these antifungal agents. The answer may be related to the differential regulation of cell wall biosynthesis genes under hypoxic conditions [Barker et al. unpublished data].
Several physiological events could account for the observed in vitro susceptibility differences in hypoxia. First, the ability of the administered drug to access the fungal mass within a hypoxic lesion will vary depending on the fungal rate/stage of growth, the strength of the host response, and the timing of antifungal treatment. It is well known that even in different murine models of invasive aspergillosis the host response is vastly different from model to model. For instance, in the corticosteroid model, host inflammation contributes substantially to the fatality associated with fungal infection [38, 39]. A sizeable host inflammatory response and inhibition of angiogenesis by the invading fungus may decrease the accessibility and diffusion of antifungal drugs within a fungal lesion as has been suggested for hypoxic Mycobacterium tuberculosis granulomas [34, 40].
Second, the differential regulation of drug targets by hypoxic stimuli will vary depending on the progression of the hypoxic lesion and the class of antifungal being administered. Genes encoding drug targets, such as the 14-demethylases (Erg11/cyp51) targeted by the triazoles, are transcriptionally induced in hypoxic conditions [19, 41]. This potential increase in the actual drug target in response to hypoxia could contribute to the observed increase of in vitro susceptibility to certain antifungal drugs that has been detected previously. Thus, identifying additional gene and biochemical pathway targets that are altered by hypoxia at the site of infection could yield new insights into antifungal drug development and efficacy. The example of the echinocandin's increase in activity in hypoxia seems to support this hypothesis. In short, a further understanding of the kinetics and host-pathogen dynamics of antifungal drug administration in the context of hypoxia may improve the delivery and efficacy of current antifungal drugs and foster new approaches to drug development for invasive aspergillosis and other fungal infections.
CONCLUSIONS
Studies on the role and importance of hypoxia during invasive aspergillosis are in their infancy. Clearly, much remains to be learned about how the fungus adapts to hypoxic microenvironments and whether hypoxia adaptation is a critical virulence attribute necessary for in vivo fungal growth and disease development. On the host side, the influence of hypoxia on the immune response to Aspergillus infections has not been studied. Preliminary studies underway in our laboratory will hopefully shed light on this potentially important aspect of the fungus-host interaction. Finally, the recognition that hypoxic microenvironments are a component of the pathophysiology of invasive aspergillosis should improve our ability to determine and improve the efficacies of existing and developmental antifungal agents. It is also intriguing to contemplate what effects minimizing hypoxia via use of hyperbaric oxygen would have on the outcome of invasive aspergillosis both from the perspective of the mold and the host and in the context of an antifungal regimen.
ACKNOWLEDGEMENTS
The authors would like to thank the organizing committee of the 4th Advances Against Aspergillosis meeting for the kind invitation and members of the Cramer laboratory for insightful discussions regarding the content of this manuscript. SJW is supported by a pre-doctoral fellowship from the American Heart Association grant number 10PRE2700014. RAC is supported by NIH/NIAID grant R01AI81838, NIH/NCRR COBRE grant 2P20RR020185, NIH/NCRR INBRE grant 2P20RR16455, and the Montana State University Agricultural Experiment Station.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Askew DS. Aspergillus fumigatus: virulence genes in a street-smart mold. Curr Opin Microbiol. 2008;11:331–337. doi: 10.1016/j.mib.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–389. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- 5.Latge JP, Calderone R. Host-microbe interactions: fungi invasive human fungal opportunistic infections. Curr Opin Microbiol. 2002;5:355–358. doi: 10.1016/s1369-5274(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes JC. Aspergillus fumigatus: growth and virulence. Med Mycol. 2006;44(Suppl 1):S77–81. doi: 10.1080/13693780600779419. [DOI] [PubMed] [Google Scholar]
- 7.Matherne GP, Headrick JP, Coleman SD, Berne RM. Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr Res. 1990;28:348–353. doi: 10.1203/00006450-199010000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Van Belle H, Goossens F, Wynants J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am J Physiol. 1987;252:H886–893. doi: 10.1152/ajpheart.1987.252.5.H886. [DOI] [PubMed] [Google Scholar]
- 9.Dewhirst MW. Concepts of oxygen transport at the microcirculatory level. Semin Radiat Oncol. 1998;8:143–150. doi: 10.1016/s1053-4296(98)80040-4. [DOI] [PubMed] [Google Scholar]
- 10.Arnold F, West D, Kumar S. Wound healing: the effect of macrophage and tumour derived angiogenesis factors on skin graft vascularization. Br J Exp Pathol. 1987;68:569–574. [PMC free article] [PubMed] [Google Scholar]
- 11.Simmen HP, Battaglia H, Giovanoli P, Blaser J. Analysis of pH, pO2 and pCO2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgery. Infection. 1994;22:386–389. doi: 10.1007/BF01715494. [DOI] [PubMed] [Google Scholar]
- 12.Denning DW, Ward PN, Fenelon LE, Benbow EW. Lack of vessel wall elastolysis in human invasive pulmonary aspergillosis. Infect Immun. 1992;60:5153–5156. doi: 10.1128/iai.60.12.5153-5156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall LA, Denning DW. Oxygen requirements of Aspergillus species. J Med Microbiol. 1994;41:311–315. doi: 10.1099/00222615-41-5-311. [DOI] [PubMed] [Google Scholar]
- 14.Taubitz A, Bauer B, Heesemann J, Ebel F. Role of respiration in the germination process of the pathogenic mold Aspergillus fumigatus. Curr Microbiol. 2007;54:354–360. doi: 10.1007/s00284-006-0413-y. [DOI] [PubMed] [Google Scholar]
- 15.Tarrand JJ, Han XY, Kontoyiannis DP, May GS. Aspergillus hyphae in infected tissue: evidence of physiologic adaptation and effect on culture recovery. J Clin Microbiol. 2005;43:382–386. doi: 10.1128/JCM.43.1.382-386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brock M, Jouvion G, Droin-Bergere S, et al. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Appl Environ Microbiol. 2008;74:7023–7035. doi: 10.1128/AEM.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willger SD, Grahl N, Cramer RA., Jr. Aspergillus fumigatus metabolism: clues to mechanisms of in vivo fungal growth and virulence. Med Mycol. 2009;47(Suppl 1):S72–79. doi: 10.1080/13693780802455313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grahl N, Cramer RA., Jr. Regulation of hypoxia adaptation: an overlooked virulence attribute of pathogenic fungi? Med Mycol. 2009:1–16. doi: 10.3109/13693780902947342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willger SD, Puttikamonkul S, Kim KH, et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200. doi: 10.1371/journal.ppat.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bien CM, Espenshade PJ. Sterol regulatory element binding proteins in fungi: hypoxic transcription factors linked to pathogenesis. Eukaryot Cell. 2010;9:352–359. doi: 10.1128/EC.00358-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang HY, Hughes R, Murdoch C, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degrossoli A, Giorgio S. Functional alterations in macrophages after hypoxia selection. Exp Biol Med (Maywood) 2007;232:88–95. [PubMed] [Google Scholar]
- 25.Anand RJ, Gribar SC, Li J, et al. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1{alpha}-dependent manner. J Leukoc Biol. 2007 doi: 10.1189/jlb.0307195. [DOI] [PubMed] [Google Scholar]
- 26.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann AK, Yang J, Biju MP, et al. Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction. Proc Natl Acad Sci U S A. 2005;102:17071–17076. doi: 10.1073/pnas.0506070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cramer T, Johnson RS. A novel role for the hypoxia inducible transcription factor HIF-1alpha: critical regulation of inflammatory cell function. Cell Cycle. 2003;2:192–193. [PubMed] [Google Scholar]
- 29.Schofield CJ, Ratcliffe PJ. Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun. 2005;338:617–626. doi: 10.1016/j.bbrc.2005.08.111. [DOI] [PubMed] [Google Scholar]
- 30.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peyssonnaux C, Datta V, Cramer T, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear W, Chan D, Coppens I, et al. The host cell transcription factor hypoxia- inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–352. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Ami R, Lewis RE, Leventakos K, Kontoyiannis DP. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood. 2009;114:5393–5399. doi: 10.1182/blood-2009-07-231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokol-Anderson ML, Brajtburg J, Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J Infect Dis. 1986;154:76–83. doi: 10.1093/infdis/154.1.76. [DOI] [PubMed] [Google Scholar]
- 36.Warn PA, Sharp A, Guinea J, Denning DW. Effect of hypoxic conditions on in vitro susceptibility testing ofamphotericin B, itraconazole and micafungin against Aspergillus and Candida. J Antimicrob Chemother. 2004;53:743–749. doi: 10.1093/jac/dkh153. [DOI] [PubMed] [Google Scholar]
- 37.Perkhofer S, Jost D, Dierich MP, Lass-Florl C. Susceptibility testing of anidulafungin and voriconazole alone and in combination against conidia and hyphae of Aspergillus spp. under hypoxic conditions. Antimicrob Agents Chemother. 2008;52:1873–1875. doi: 10.1128/AAC.01572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens-Romero SD, Mednick AJ, Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect Immun. 2005;73:114–125. doi: 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun. 2005;73:494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klinkenberg LG, Sutherland LA, Bishai WR, Karakousis PC. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J Infect Dis. 2008;198:275–283. doi: 10.1086/589515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todd BL, Stewart EV, Burg JS, Hughes AL, Espenshade PJ. Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Mol Cell Biol. 2006;26:2817–2831. doi: 10.1128/MCB.26.7.2817-2831.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]