Abstract
Objective
To examine transition readiness, adherence and health outcomes in pediatric liver transplant recipients using a clinically administered screening measure.
Methods
71 pediatric liver transplant recipients (11–20 years) and 58 parents completed a clinic-based transition readiness survey measuring perceived and demonstrated self-management skills, allocation of responsibility for health-related tasks, regimen knowledge, and psychosocial adjustment. Adherence was measured using standard deviations (SDs) of immunosuppressants, proportion of immunosuppressant blood levels out of target range, and clinic attendance. Health outcomes included liver test panels, biopsies, rejection episodes, and hospitalizations.
Results
All domains of transition readiness, with the exception of demonstrated skills, and nonadherence were positively correlated with age. Proportion of immunosuppressant blood levels below target range was positively correlated with self-management skills and increased responsibility for medication tasks. Parent regimen knowledge was associated with clinic attendance. Health outcomes were significantly related to medication nonadherence, but not to transition readiness domains.
Conclusions
Medication adherence is considered to be a key factor in the transition from pediatric to adult-centered transplant care. Nonadherence is associated with an increased risk for medical complications and is potentially modifiable. Interventions to promote self-management skills and adherence should be an essential component of transition planning.
Keywords: Transition, Adolescence, Adherence, Pediatric Liver Transplantation, Self-Management
INTRODUCTION
With increased short-term survival rates among pediatric solid organ transplant recipients, health care providers have shifted focus to long-term outcomes. Long-term patient survival is limited by chronic rejection and graft loss, which is more common in adolescents (1). There is also an increased risk for medical complications following the transfer from pediatric to adult-centered transplant services (2). Thus, there is a critical need to develop strategies to assess the readiness to move from pediatric to adult-centered care (3–6).
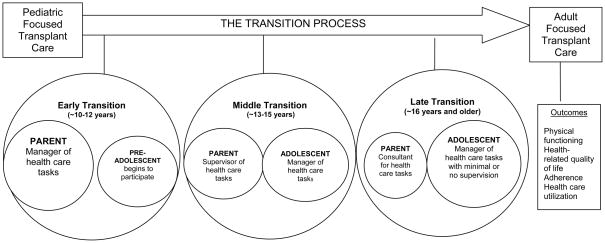
“Transition” is an active process that addresses the medical, psychosocial, and educational/vocational needs of adolescents as they prepare to move from child- to adult-centered health care (7), while “transfer” refers to the change in the location where care is provided (8, 9). The transition process is twofold as it includes the transition of responsibility for health care tasks from the parent to the patient, as well as the preparation to transfer to adult-centered care. Beginning in late childhood/early adolescence, the management of a chronic illness begins to shift from the primary responsibility of the parent to self-management by the adolescent (Figure 1) (10). By shifting responsibility for health related tasks in a developmentally appropriate manner, the adolescent gains the knowledge, skills and experiences necessary to master the independence required to be successful in the adult health care system.
FIGURE 1.
The Transition Process: Transition of Responsibility of Health Management Tasks and Transition from Pediatric to Adult-Centered Care
Self-management skills are integral to the achievement of independence necessary for successful health care transitions (11, 12). Researchers and clinicians agree that adolescents and young adults should not transfer from pediatric to adult health services unless they have the skills necessary for functioning effectively in the adult health care system, including adhering to medication regimens (13, 14). The Pediatric Committee of the American Society of Transplantation has recommended that prior to transferring to adult-centered care, the pediatric transplant recipient should demonstrate the ability to independently manage their health (5). In addition, the pediatric patient should adhere to their immunosuppressant medications to avoid increased risk of graft loss and rejection following the transfer to adult-centered care (3, 4).
The specific aims were to 1) describe adolescent/young adult and parent responses on a clinically developed transition readiness survey (TRS) that assesses perceived and demonstrated skills, as well as the allocation of responsibility for health management tasks; and 2) explore the relationship between the TRS, measures of adherence, and health outcomes. We hypothesized that higher scores on the TRS indicated greater transition-related self-management skills and would be associated with higher rates of medication adherence and better health outcomes.
MATERIALS AND METHOD
Participants
Approval from the medical center’s Institutional Review Board was obtained prior to conducting a retrospective chart review for the present cross-sectional study. A review of the pediatric liver transplant clinic registry identified patients who had completed the transition readiness survey since October 2007 as part of routine follow-up in the Pediatric Liver Transplant Clinic. Patients completed this survey if they were 11 years of age and older and more than 6 months post-transplant. We included patients as young as 11 years given that previous research with pediatric liver transplant recipients found that the transition of responsibility for health related tasks occurs between the ages of 9 and 17 years, with a mean age of 12 years (15). When present, parents completed a parallel version of the measure.
Measures
Transition Readiness
Assessment of transition readiness occurred through the use of a clinically-derived measure designed to guide individualized assessment and intervention as part of transition planning. Survey items were selected by clinical observations, review of the transition, self-management, and medication adherence literature, and discussions with members of the pediatric liver transplant team (13, 16). The team was comprised of a pediatric hepatologist, transplant surgeon, pediatric psychologist, social worker, dietician and transplant nurse coordinator. Administration time for the surveys was approximately 15 minutes. The adolescent/young adult (A/YA) and parent versions are described below.
Transition Readiness Survey – Adolescent/Young Adult Version (TRS:A/YA)
The TRS:A/YA consisted of 38 written items and 4 healthcare provider administrated questions. Responses elicited ranged from self-report using Likert scales to direct demonstration of skills. Items were grouped conceptually into 4 domains: Self-Management, Regimen Knowledge, Demonstrated Skills, and Psychosocial Adjustment (Table 1). The Self-Management domain also included a subscale which assessed Allocation of Responsibility (AoR) in the two weeks preceding the survey. AoR was assessed using 5 items to examine the distribution of medication-taking responsibility between the adolescent/young adult and the parent/caregiver. These items were adapted for use with pediatric transplant recipients from measures used to assess AoR in families of children diagnosed with asthma (17) and type 1 diabetes (18). For each item, respondents indicated the frequency of involvement of the adolescent/young adult or the parent/caregiver.
Table 1.
Domains of the Transition Readiness Survey (TRS)
| Domain | Number of Items | Item(s)Example |
|---|---|---|
| Adolescent Report (TRS:A/YA) | ||
| Self-Management | 12 | I wear a Medialert ID; Who notices when prescriptions needs to be refilled? |
| Allocation of Responsibility | 5 | In the past 2 weeks, how often did you remember to take your medications without an adult reminding you? How often did your parent remind you to take your medication? |
| Perceived Regimen Knowledge | 10 | I can name all of my medications; I know how often I need to come to appointments. |
| Demonstrated Skills | 9 | Name your medications; John took his morning dose of medicine at 7 AM. He takes his 2nd dose 10 hours later. What time does he take his 2nd dose? |
| Psychosocial Adjustment | 11 | I have no control over my health in the future; I am limited in what I can achieve in the future because of my transplant. |
| Parent Report (TRS:P) | ||
| Adolescent Self- Management | 11 | My child wears a Medialert ID; Who notices when prescriptions need to be refilled? |
| Allocation of Responsibility | 5 | In the past 2 weeks, how often did you remind your child to take his/her medication? |
| Adolescent Regimen Knowledge | My child can name all of their medications My child knows how often they need to come to their appointments | |
| Demonstrated Skills | 5 | John took his morning dose of medicine at 7 AM. He takes his 2nd dose 10 hours later. What time does he take his 2nd dose? |
| Adolescent Psychosocial Adjustment | 11 | My child feels they have little control over their health; Because of their transplant, my child cannot pursue certain jobs in the future. |
| Regimen Knowledge | 2 | My child needs to take medications for the rest of their life. |
Scoring of the TRS varied with each item and yielded a total score of 126 with higher scores reflecting increased skill acquisition. For items assessing perceived skills, knowledge or attitudes, patients responded using three- or four-point Likert scales. Higher scores reflected perceived presence of knowledge/skills or agreement with items. Items requiring direct observation of skills included symptom recognition and health literacy. These were scored using percentage correct and assigned a value on a 3-point scale (1=<33% correct, 2=34–65% correct; 3=>66%). To further assess medication knowledge, upon completing the written portion of the survey, patients were asked to verbally list their medications, as well as the dosages, timing of dose administration, and function of the medications. Responses to each question were coded on a 3-point scale (1=none, 2=some, 3= all) indicating extent of patient’s knowledge.
Transition Readiness Survey: Parent Report (TRS:P)
The TRS:P consisted of 36 items yielding a total possible score of 108. Parents were assessed on their perception of their adolescent’s skills using parallel items from the A/YA measure. Similar to the TRS:A/YA, higher scores reflected increased perceived skills needed to transfer from pediatric to adult-centered care. Items were grouped conceptually into five domains (Table 1). Three domains were proxy reports of the adolescent’s skills and the remaining two assessed parents’ knowledge of their adolescent regimen, and demonstration of regimen knowledge.
Adherence
A multi-method assessment of the adolescent’s adherence to immunosuppressant medications and clinic visits in the 12 months preceding administration of the TRS was conducted using a retrospective review of the patient’s medical record. Adherence measures were used as continuous and categorical variables in analyses.
Immunosuppressant levels
The degree of fluctuation in immunosuppressant blood levels has been used to assess the variability of medication administration, with higher fluctuations indicating medication nonadherence (15, 19–24). Data from routine monitoring of tacrolimus and cyclosporine blood levels were obtained from the participant’s medical record and the University of Michigan’s Organ Transplant Information System (OTIS) for the previous year. Immunosuppressant levels obtained during inpatient hospitalization stays were not included in the analyses. Standard deviations (SD) of consecutive blood levels obtained retrospectively were calculated. In addition, the proportion of immunosuppressant blood levels outside of the adolescent’s recommended target therapeutic range (above and below) was also calculated using laboratory values for the preceding 12 months. To avoid the potential introduction of bias, adherence data was collected in a systematic method by a research assistant who was not involved in direct patient care. Based on previous studies of the association between immunosuppressant variability and risk for poor health outcomes, such as late allograft rejection, adherence was defined as a tacrolimus SD <2 and cyclosporine SD<30 (20, 22, 25, 26), and/or <50% of immunosuppressant blood values out of the target range (15, 22). Given the clinical context, our definitions for adherence are conservative as we routinely examine variability in immunosuppressant blood levels to identify patients who may be at risk for medication nonadherence.
Clinic attendance
Adherence to clinic visits was assessed retrospectively by comparing actual clinic attendance to the frequency recommended by the transplant team. The rate of clinic attendance for the year prior to study participation was obtained from the adolescent’s medical record. Adherence was defined as a clinic attendance rate of ≥85%. This definition is consistent with the pediatric adherence literature (27), and previous work in pediatric liver transplantation, wherein the rate of clinic attendance was significantly related to hospital admissions, liver biopsies, and rejection episodes (20).
Health Outcomes
Measures of adolescent/young adult health outcomes including liver panel tests (AST, ALT, TBili), graft function, frequency of hospital admissions, liver biopsies and episodes of rejection for the year prior to study participation were obtained from a review of medical records.
Data Analysis
To examine the factorial structure of the TRS for data interpretation, we conducted a confirmatory factor analysis (CFA) with four factors to test the hypothesis that the TRS measured a unitary construct underlying transition readiness. We hypothesized that the construct was comprised of 4 subdomains: self-management, regimen knowledge, demonstrated skills, and psychosocial adjustment. To accomplish this, a principal components analysis (PCA) with a varimax rotation was performed on the 38 items of the TRS:A/YA and the 36 items of the TRS:P. Given the negatively skewed distribution of the data a nonparametric approach with a varimax rotation was used to isolate factor loadings that discriminate based on the explained variability (28).
Results from the surveys were analyzed using descriptive statistics (e.g. means, standard deviations) to explore patterns of transfer readiness. Pearson’s product moment correlation coefficients were calculated whenever the assumptions underlying parametric statistical testing were met. Given the significant skewness of the TRS, Spearman’s rank correlation coefficients were calculated to examine the associations between scores on the TRS domains, measures of adherence, health outcomes, and demographic factors (age, time since transplantation). All analyses were conducted using the SPSS version 16.0 statistical package.
RESULTS
Participant Characteristics
The total sample consisted of 71 pediatric liver transplant recipients, which represented approximately 98% of the clinic population in the targeted age range. Of these, 58 (82%) adolescents had parallel parent reports. Mean adolescent age was 15.6±2.7 years (range 11–20 years) with an average time since transplant of 9.4±5.4 years (range 1–19 years). Fifty-six percent of the adolescents were female and 37% belonged to a minority group. Most adolescents (85%) used tacrolimus as their primary immunosuppressant with the remainder using cyclosporine. All adolescents were on twice-daily dosages of their immunosuppressant medications.
Transition Readiness Scale Psychometric Properties
TRS:A/YA Version
Each of the original 36 items was examined to determine its contribution to the scale. Items were not omitted from analyses given their apparent conceptual relevance. It is recommended that items assessing construct-relevant information be retained until measures can be evaluated across broader samples (29). Given the skewness of the data, items were entered into a nonparametric PCA with varimax rotation. The joint criteria of Eigen values >1 and Cattell’s elbow criteria on the scree plot (30) indicated that a 4 factor solution accounted for 32.5% of the variance in responses. We examined the number of specific items on each factor that had loadings of >0.4, Factor 1, labeled Demonstrated Skills, included 3/9 proposed items (factor loadings range 0.73–0.85). Factor 2, labeled Self-Management, contains 6/12 proposed items (factor loadings range: 0.60–0.77). Factor 3, labeled Psychosocial Adjustment contains 3/11 proposed items (factor loadings range: 0.48–0.85). Factor 4, labeled Perceived Regimen Knowledge, contains 2/10 proposed items (factor loadings: 0.69, 0.75). All domains of the adolescent version of the TRS had acceptable internal consistency, with Cronbach alphas ranging from 0.68–0.81 (Table 2). The Cronbach alpha for the entire 38-item adolescent version was 0.85.
Table 2.
Descriptive Statistics for Domains of the Transition Readiness Survey
| Domain | Mean | SD | Range | Cronbach’s alpha |
|---|---|---|---|---|
| Adolescent Report (TRS:A/YA) | ||||
| Self Management | 26.23 | 4.98 | 7–35 | 0.68 |
| Allocation of Responsibility | 11.75 | 2.95 | 0–15 | 0.80 |
| Regimen Knowledge | 23.44 | 3.07 | 12–27 | 0.71 |
| Demonstrated Skills | 19.42 | 4.79 | 0–26 | 0.81 |
| Psychosocial Adjustment | 26.82 | 5.12 | 2–33 | 0.82 |
| Total | 95.90 | 14.78 | 28–117 | 0.85 |
| Parent Report (TRS:P) | ||||
| Adolescent Self-Management | 22.66 | 4.21 | 11–32 | 0.63 |
| Allocation of Responsibility | 11.27 | 2.73 | 5–15 | 0.79 |
| Adolescent Regimen Knowledge | 18.26 | 2.32 | 9–21 | 0.60 |
| Demonstrated Skills | 11.90 | 3.69 | 0–15 | 0.75 |
| Adolescent Psychosocial Adjustment | 26.21 | 3.38 | 17–32 | 0.56 |
| Regimen Knowledge | 4.47 | 1.01 | 1–6 | 0.19 |
| Total | 83.48 | 9.13 | 56–97 | 0.75 |
TRS: Parent Version
Similar to the TRS:A/YA, each of the original 36 items was examined to determine its contribution to the scale using the same procedure outlined above. The results indicate that a 4 factor solution accounted for 32.5% of the variance in responses. We examined the number of specific items on each factor that had loadings of >0.4,. Factor 1, labeled Self-Management, contains 5/11 proposed items (factor loading range: 0.45–0.76). Factor 2, labeled Demonstrated Skills, contains 4/5 proposed items (factor loadings range: 0.66-0.9). Factor 3, labeled Psychosocial Adjustment, contains 4/11 proposed items (factor loadings range: 0.41–0.84)/Factor 4, labeled Adolescent Regimen Knowledge, contains 4/7 proposed items (factor loadings range: 0.43–0.54). For the parent version, Cronbach alphas ranged from 0.18–0.75 (Table 2). The Cronbach alpha for the entire 36-item parent version was 0.75.
Transition Readiness Scale Descriptives
TRS:A/YA
The mean total score on the TRS:A/YA was 95.9 (76.1%, SD: 14.8), with a range of 28–117 (22.2%–92.9%). Each domain had a similarly wide range of scores (Table 2). Most adolescents (90%) correctly named their medications; however, 73% could not state dosages, 51% were unable to specify prescribed timing between doses with a minimum acceptable answer being some variation of “in the morning and at night”, and 41% could not identify the basic function of their immunosuppressants, with a minimum acceptable answer being some variation of “they are for my liver.”
TRS:P
The mean total score on the TRS:P was 83.5 (77.3%; SD: 9.1), with a range of 56–97 (51.85%–89.8%). Each domain had a similarly wide range of scores (Table 2).
Transition Readiness and Participant Characteristics
Table 3 presents the correlations between the TRS and participant characteristics. Age at survey was positively correlated with the TRS:A/AYA Total Score and domain scores including Perceived Self-Management, Regimen Knowledge, and Psychosocial Adjustment. Age was also positively correlated with the TRS:A/YA Allocation of Responsibility subscale. Adolescent age was not significantly related to Adolescent Demonstrated Skills.
Table 3.
Correlations between TRS, Participant Age and Adherence Measures
| Age | Rate of Attendance | Cyclosporine SD | IS Out of Range | IS Below Range | |
|---|---|---|---|---|---|
| Age | r =−0.26; p =0.03 | r =0.78; p =0.005 | r = 0.25; p =0.03 | - | |
| Adolescent TRS | |||||
| Total Score | rs =0.51; p =0.000 | - | - | - | - |
| Self-Management | rs =0.44; p =0.000 | - | rs =0.72; p =0.01 | - | rs =0.27; p =0.02 |
| Allocation of Responsibility Subscale | rs =0.44; p =0.000 | - | rs =0.68; p =0.02 | - | rs =0.23; p =0.05 |
| Perceived Regimen Knowledge | rs =0.46; p =0.000 | - | - | - | - |
| Demonstrated Skills | - | - | - | - | - |
| Psychosocial Adjustment | rs =0.51; p =0.000 | - | - | - | - |
| Parent TRS | |||||
| Total Score | rs =0.43; p =0.001 | - | - | - | - |
| Adolescent Self-Management | rs =0.44; p =0.001 | - | - | - | - |
| Allocation of Responsibility Subscale | rs = 0.43; p =0.001 | - | - | - | - |
| Adolescent Regimen Knowledge | rs =0.42; p =0.001 | - | - | - | - |
| Demonstrated Skills Adolescent | - | - | - | - | - |
| Psychosocial Adjustment | rs =0.29; p =0.026 | - | - | - | - |
| Regimen Knowledge | - | rs =0.40; p =0.002 | - | - | - |
Of the 58 dyads, there were positive and significant correlations between adolescent age and TRS:P Total Score, including parent reported domains assessing Adolescent Knowledge, Adolescent Self-Management, and Adolescent Psychosocial Adjustment. Age was also positively correlated with the parent reported AoR subscale. Adolescent age was not related to Parent Demonstrated Skills or Parent Regimen Knowledge.
To further explore the association between age and the AoR subscale, we compared TRS:A/YA and TRS:P scores across adolescents aged 11–13, 14–17, >18 years. Based on adolescent self-reports, the 11–13 year old age group was significantly lower in their responsibility for health-related tasks (Mean=9.5±3.8) compared to the 14–17 year olds (Mean=12.2±2.3, p=0.002) and >18 year olds (Mean=12.7±2.4, p=0.001). There were no differences between the 14–17 year olds and the >18 year old groups. Similarly, based on parent reports, the 11–13 year old age group was significantly lower in their responsibility for health-related tasks (Mean=9.4±3.0) compared to 14–17 year olds (Mean 11.8±2.3, p=0.004) and >18 year olds (Mean=11.3±2.7, p=0.005). There were no differences between the 14–17 year olds and the >18 year old groups.
Adolescent age was significantly related to time since transplantation, though time since transplantation was not significantly related to any domain of the TRS.
Adolescent/Parent Agreement
For the 58 dyads in which adolescent and parent reports on the TRS were available, paired correlations are as follows: Self-Management r=0.47, p=0.000; AoR = r= 0. 47, p= 0.000; Perceived Regimen Knowledge: r=0.58, p=0.000; Adolescent Psychosocial Adjustment r=0.47; p=0.000; Demonstrated Skills: r= −0.26, p=0.84. With the exception of the Demonstrated Skills domain, all correlations are in the moderate agreement range.
Patterns of Adherence
Table 4 displays descriptive statistics for the adherence variables. Thirty-one percent (N=22) of adolescents were nonadherent using standard deviations of tacrolimus (SD>2) or cyclosporine (SD>30) and 26.8% (N=19) were nonadherent based on having >50% of immunosuppressant lab values out of the target range. Thirty-two percent (N=23) of adolescents were nonadherent as measured by clinic attendance <85% over the preceding 12 months. Overall, 43% of adolescents (N=31) were classified as adherent across all measures, (29.6% (N=21) were classified as nonadherent on one variable, 19.7% (N=14) were classified as nonadherent on 2 variables and 7.0% (N=5) were classified as nonadherent on all variables.
Table 4.
Descriptive Statistics for Measures of Adherence
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Tacrolimus standard deviation (n=60) | 2.12 | 2.05 | 0.32–11.45 |
| Cyclosporine standard deviation (n=11) | 23.81 | 10.76 | 8.0–42.0 |
| Percent of immunosuppressant levels out of range | 37.66% | 23.96% | 0.00–100.0% |
| Percent of immunosuppressant levels below range | 15.57% | 17.57% | 0.0–75.0% |
| Percent of immunosuppressant levels above range | 22.10% | 19.42% | 0.0–80.0% |
| Clinic attendance | 84.97% | 24.52% | 0.0–100.0% |
Participant age was significantly related to measures of adherence with older adolescents demonstrating lower rates of clinic attendance (r = −0.26; p=0.03), higher standard deviations of cyclosporine (r = 0.78; p=0.005), and a higher proportion of immunosuppressant blood levels greater than 1 standard deviation out of the target range (r = 0.25; p = 0.03). The association between adolescent age and tacrolimus SD were not significant (r = 0.24; p = 0.06). Follow-up univariate analyses indicated significant differences between adolescents <16 years old and >16 years old with respect to measures of adherence. Compared to the younger adolescents, those who were >16 years (N=36) had lower rates of clinic attendance (79% vs. 90%; p=0.05), higher tacrolimus SDs (2.69 vs. 1.58, p=0.035), and a higher percentage of immunosuppressant blood levels out of target range (42% vs. 32%; p=0.05).
Transition Readiness Domains and Adherence
Table 3 presents correlations between the TRS and measures of adherence. No significant associations were found between the TRS: A/YA and adherence as measured by tacrolimus SDs or rate of clinic attendance. The TRS:A/YA domains measuring Adolescent Perceived Self-Management was positively correlated with the percentage of immunosuppressant levels below the target range, and cyclosporine SD. Within the Self-Management domain, the AoR subscale was positively correlated with cyclosporine SD and the percentage of immunosuppressant levels below the target range. On the TRS:P scale, there were no significant associations between parent reports and adherence as measured by tacrolimus or cyclosporine SDs, or immunosuppressant laboratory values out of target range. However, the rate of clinic attendance was significantly related to the TRS:P domain measuring Parent Regimen Knowledge.
Health Outcomes
Within the 12 months prior to participation, 20% of the adolescents had been hospitalized at least once, 15.5% underwent at least one liver biopsy and 1.4% had a documented biopsy-proven episode of rejection. Across all participants, there were a total of 25 hospital admissions, 155 inpatient days, 12 liver biopsies, and 1 biopsy-proven episode of rejection. In the year prior to participation, average ALT was 50.7 (SD = 50.0), average AST was 48.26 (SD = 54.4) and average TBili was 0.88 (SD = 0.94).
Transition Readiness Domains, Adherence and Health Outcomes
There were no significant associations between TRS:P or TRS:A/YA and measures of health outcomes. Significant associations were found between measures of adherence and health outcomes. Average ALT levels for the year prior to participation were significantly related to tacrolimus SD (r = 0.41; p=0.001) and percentage of immunosuppressant lab values out of target range (r = 0.33; p = 0.005). Average AST levels for the year prior to participation were also significantly associated with tacrolimus SD (r = 0.42; p = 0.001), cyclosporine SD (r = 0.61; p = 0.047) and percentage of immunosuppressant lab values out of target range (r = 0.28; p = 0.017). The number of biopsies, which suggests increased concern for rejection, was significantly related to the percentage of immunosuppressant lab values out of target range (r = 0.34, p = 0.004) and tacrolimus SD (r = 0.33, p=0.01),
DISCUSSION
Results of the current study indicate that nearly 30% of the participants were nonadherent with their immunosuppressant medications, and over 30% were nonadherent to recommended clinic visits. This is consistent with previous reports that medication nonadherence is common among adolescent transplant recipients (15, 31, 32). There was a significant association between age and nonadherence, with older adolescents and young adults demonstrating higher rates of nonadherence compared to the younger children. This is concerning as chronological age is the most common criterion used to determine readiness to transfer from pediatric to adult-centered care, with 16–22 years being the most frequently cited age range to start the transition process (33). As medication adherence and health outcomes may both be affected by changes associated with a transfer to a new medical provider, this is a time of increased risk for adolescent and young adult transplant recipients.
With respect to the assessment of transition readiness, perceived self-management skills, perceived regimen knowledge, and psychosocial adjustment increased with chronological age. Yet, the association between age and performance on the demonstrated skills domain did not reach statistical significance. This suggests that while older adolescents and young adults may perceive that they have sufficient regimen knowledge and self-management skills, their chronological age is not associated with demonstrated self-management skills, including the ability to describe their medical regimen or the ability to recognize critical health symptoms. The ability to recognize symptoms and demonstrate when and how to seek urgent medical care is cited as an important component of the transition process (5).
We hypothesized that higher TRS scores would be associated with higher rates of adherence. On the contrary, the present study found that increased scores on the adolescent/young adult self-management skills scale were associated with higher rates of medication nonadherence. Specifically, higher scores on the self-management scale were associated with higher cyclosporine SDs and increased proportion of immunosuppressant blood values below target range, which is indicative of suboptimal medication intake (34). Within the self-management domain, increased allocation of responsibility for medication-related tasks to the adolescent/young adult was also associated with higher proportion of immunosuppressant blood levels below range.
Although older adolescents/young adults perceived greater self-management, they were at higher risk for medication nonadherence. Moreover, with increasing age, this population is being monitored less by their parents/caregivers. Thus, the transition of medication-related responsibilities from parents to adolescents may not be successful as evidenced by poor medication adherence in the older adolescents/young adults. Likewise, research has demonstrated that increased parental monitoring is associated with improved adherence and health outcomes in other chronic illness groups, including adolescents with diabetes (35), HIV (36) and asthma (17). In addition, a recent report suggests that adolescent liver transplant recipients are inconsistent with their health management tasks (37). Thus, it is critical that attention be given to the role of parental monitoring and supervision of medication related tasks during the transition process as adolescents begin to demonstrate mastery of health management tasks.
Parents’ knowledge of the adolescent’s medical regimen was significantly correlated with the rate of clinic attendance. It is possible that the rate of clinic attendance is a measure of parental adherence to the regimen rather than the adolescent/young adult’s adherence. Further research to investigate the impact of adolescent clinic attendance without their parent on transition readiness and adherence is warranted, particularly as it relates to the allocation of regimen responsibility.
The results of the present study should be viewed in light of study limitations. The transition readiness survey used in the present study was developed for clinical use to guide intervention. This was not a prospective measurement development study, thus the construct and content validity of the TRS were not investigated prior to its administration for clinical purposes. Moreover, given the retrospective nature of this study, the predictive validity of the TRS as it relates to medication adherence and successful transfer of care is not known. Lastly, this retrospective, cross-sectional study was conducted within a single pediatric liver transplant program which limits generalizability. Within this single center study, relatively few participants were receiving cyclosporine as their primary immunosuppressant medication (N=11). Thus, the significant association between higher cyclosporine SDs and higher perceived self-management skills should be interpreted with caution. Further multi-center study of the association between medication adherence as measured by immunosuppressant variability and transition-readiness skills is warranted.
Unfortunately, while many clinical programs strive to enhance the acquisition of self-management skills in adolescents, they do not routinely assess transition readiness, including the ability to independently manage health care needs (38, 39). One barrier to the routine assessment of transition skills may relate to the lack of a validated instrument to assess transition-related self-management skills. There is a critical need for the development and validation of objective assessment tools to empirically evaluate the pediatric patient’s readiness to move from a pediatric to adult-focused transplant health care. We are presently revising the TRS by conducting additional quantitative analyses as well as qualitative research with key informants regarding areas of transition which need further development. These data will inform the development of a revised TRS which will be piloted with a broader sample of adolescent/young adult transplant recipients across transplant centers. Future research will also incorporate other measures of self-management, allocation of health care responsibility, and health outcomes, in order to evaluation the concurrent and predictive validity of the TRS. This will allow for the refinement of questions and domains within the instrument.
Limitations aside, the present study has notable clinical implications. The current literature recommends that medical stability is a critical variable in the transfer from pediatric to adult-centered care (5, 40). Given that medication nonadherence is associated with increased risk for medical complications, particularly among adolescent transplant recipients (41, 42), targeting problems of medication nonadherence should be an essential component of the transition process. While medication adherence may not be sufficient for optimal health care transitions, it is an essential component.
Self-management interventions are effective in promoting medication adherence in children with other chronic health conditions (43, 44), and there is increasing recognition that self-management skills should be the goal of any transition program (11, 12). Interventions to promote transition readiness should also target parental monitoring and the transition of responsibility of health-related tasks from the parent to the adolescent/young adult. The inverse relationship between medication adherence and perceived self-management skills suggests that though adolescents perceive that they have adequate knowledge and management skills, they are not demonstrating the skills necessary for optimal medication management. This may relate to a variety of individual, family, and/or provider factors including motivation, health literacy, and communication with health care provider. In addition, the role of peer support has been suggested to be an important factor for adolescents and young adults transitioning from pediatric to adult-centered care (5, 40). The current study did not investigate the impact of peer and social support on medication adherence or transition-related skills. We are currently conducting prospective studies to examine factors that relate to medication adherence and self-management skills throughout the transition process.
Using this clinical tool, we have developed a transition program in our clinic which involves patient and parent education, behavioral goals and skill building and systematic planning for the transfer to adult-care. Clinic-based adherence interventions incorporate educational, motivational, behavioral and organizational strategies to foster the acquisition of self-management skills. We are currently engaged in ongoing quality improvement efforts to evaluate the effectiveness of our programs to promote medication adherence and transition skills in a systematic and developmentally appropriate manner.
CONCLUSIONS
Medication adherence is a key factor in the transition from pediatric to adult-centered transplant care. The current study demonstrated that chronological age was associated with perceived self-management skills, yet older age was associated with increased risk for nonadherence. Thus, despite increased independence over health-related tasks, age alone should not determine readiness to transfer from pediatric to adult-focused health care given the risk of medical complications. Rather, the timing of transfer from pediatric to adult-focused care should be individualized and based on the acquisition and mastery of self-management skills. Medication nonadherence is associated with an increased risk for medical complications including graft loss and rejection. Therefore, the promotion of medication adherence should be an essential component of transition planning. Interventions to promote transition readiness should also target parental monitoring and the developmentally appropriate transition of responsibility of health-related tasks from the parent to the adolescent/young adult.
Acknowledgments
This publication was made possible by Grant Number UL1RR024986 from NCRR CTSA funding to Dr. Fredericks. We thank Dr. Trivellore Raghunathan for his statistical consultation and thoughtful review of this manuscript.
References
- 1.Sudan DL, Shaw BW, Jr, Langnas AN. Causes of late mortality in pediatric liver transplant recipients. Ann Surg. 1998;227:289–295. doi: 10.1097/00000658-199802000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magee J. Growing up is hard to do: Transitional care from pediatrics to adulthood. 6th Annual ASTS Winter Symposium; Scottsdale, Arizona. 2006. [Google Scholar]
- 3.Annunziato RA, Emre S, Shneider B, Barton C, Dugan CA, Shemesh E. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatr Transplant. 2007;11:608–614. doi: 10.1111/j.1399-3046.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 4.Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14:469–472. doi: 10.1007/s004670050794. [DOI] [PubMed] [Google Scholar]
- 5.Bell LE, Bartosh SM, Davis CL, et al. Adolescent Transition to Adult Care in Solid Organ Transplantation: a consensus conference report. Am J Transplant. 2008;8:2230–2242. doi: 10.1111/j.1600-6143.2008.02415.x. [DOI] [PubMed] [Google Scholar]
- 6.McDonagh JE, Kelly DA. Trans-plan-sition! Transplantation and transition. Pediatr Transplant. 2007;11:578–581. doi: 10.1111/j.1399-3046.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 7.Blum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions. A position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14:570–576. doi: 10.1016/1054-139x(93)90143-d. [DOI] [PubMed] [Google Scholar]
- 8.Sawyer SM, Blair S, Bowes G. Chronic illness in adolescents: transfer or transition to adult services? J Paediatr Child Health. 1997;33:88–90. doi: 10.1111/j.1440-1754.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy A, Sawyer S. Transition from pediatric to adult services: are we getting it right? Curr Opin Pediatr. 2008;20:403–409. doi: 10.1097/MOP.0b013e328305e128. [DOI] [PubMed] [Google Scholar]
- 10.Kieckhefer GM, Trahms CM. Supporting development of children with chronic conditions: from compliance toward shared management. Pediatr Nurs. 2000;26:354–363. [PubMed] [Google Scholar]
- 11.Watson AR. Problems and pitfalls of transition from paediatric to adult renal care. Pediatric Nephrology. 2005;20:113. doi: 10.1007/s00467-004-1763-y. [DOI] [PubMed] [Google Scholar]
- 12.McDonagh JE, Hackett J. Interrelationship of self-management and transitional care needs of adolescents with arthritis: comment on the article by Stinson et al. Arthritis Rheum. 2008;59:1199–1200. doi: 10.1002/art.23921. author reply 1200–1191. [DOI] [PubMed] [Google Scholar]
- 13.McDonagh JE. Growing up and moving on: transition from pediatric to adult care. Pediatr Transplant. 2005;9:364–372. doi: 10.1111/j.1399-3046.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 14.Viner R. Transition from paediatric to adult care. Bridging the gaps or passing the buck? Arch Dis Child. 1999;81:271–275. doi: 10.1136/adc.81.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shemesh E, Shneider BL, Savitzky JK, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113:825–832. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 16.Paone MC, Wigle M, Saewyc E. The ON TRAC model for transitional care of adolescents. Prog Transplant. 2006;16:291–302. doi: 10.1177/152692480601600403. [DOI] [PubMed] [Google Scholar]
- 17.Walders N, Drotar D, Kercsmar C. The allocation of family responsibility for asthma management tasks in African-American adolescents. J Asthma. 2000;37:89–99. doi: 10.3109/02770900009055432. [DOI] [PubMed] [Google Scholar]
- 18.Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol. 1990;15:477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- 19.Shemesh E, Lurie S, Stuber ML, et al. A pilot study of posttraumatic stress and nonadherence in pediatric liver transplant recipients. Pediatrics. 2000;105:E29. doi: 10.1542/peds.105.2.e29. [DOI] [PubMed] [Google Scholar]
- 20.Fredericks EM, Lopez MJ, Magee JC, Shieck V, Opipari-Arrigan L. Psychological Functioning, Nonadherence and Health Outcomes After Pediatric Liver Transplantation. American Journal of Transplantation. 2007;7:1974–1983. doi: 10.1111/j.1600-6143.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 21.Flippin MS, Canter CE, Balzer DT. Increased morbidity and high variability of cyclosporine levels in pediatric heart transplant recipients. The Journal of Heart and Lung Transplantation. 2000;19:343–349. doi: 10.1016/s1053-2498(00)00061-9. [DOI] [PubMed] [Google Scholar]
- 22.Schäfer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic Accuracy of Measurement Methods to Assess Non-Adherence to Immunosuppressive Drugs in Kidney Transplant Recipients. American Journal of Transplantation. 2008;8:616–626. doi: 10.1111/j.1600-6143.2007.02127.x. [DOI] [PubMed] [Google Scholar]
- 23.Venkat VL, Nick TG, Wang Y, Bucuvalas JC. An objective measure to identify pediatric liver transplant recipients at risk for late allograft rejection related to non-adherence. Pediatr Transplant. 2008;12:67–72. doi: 10.1111/j.1399-3046.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 24.Chisholm MA, Lance CE, Williamson GM, Mulloy LL. Development and validation of an immunosuppressant therapy adherence barrier instrument. Nephrol Dial Transplant. 2005;20:181–188. doi: 10.1093/ndt/gfh576. [DOI] [PubMed] [Google Scholar]
- 25.Bucuvalas JC, Ryckman FC, Arya G, et al. A Novel Approach to Managing Variation: Outpatient Therapeutic Monitoring of Calcineurin Inhibitor Blood Levels in Liver Transplant Recipients. The Journal of Pediatrics. 2005;146:744. doi: 10.1016/j.jpeds.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Fredericks EM, Magee JC, Opipari-Arrigan L, Shieck V, Well A, Lopez MJ. Adherence and Health-related Quality of Life in Adolescent Liver Transplant Recipients. Pediatric Transplantation. 2008;12:289–299. doi: 10.1111/j.1399-3046.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 27.Rapoff MA. Adherence to pediatric medical regimens. New York: Kluwer Academic/Plenum Press; 1999. [Google Scholar]
- 28.Rummel RJ. Applied Factor Analysis. Evanston: Northwestern University Press; 1970. [Google Scholar]
- 29.Clark L, Watson D. Constructing validity: basic issues in objective scale development. Psychol Assess. 1995;7:309–319. [Google Scholar]
- 30.DeVellis RF. Scale Development: Theory and Applications. Newbury Park, CA: SAGE Publications; 2003. [Google Scholar]
- 31.Berquist RK, Berquist WE, Esquivel CO, Cox KL, Wayman KI, Litt IF. Non-adherence to post-transplant care: Prevalence, risk factors and outcomes in adolescent liver transplant recipients. Pediatric Transplantation. 2008;12:194–200. doi: 10.1111/j.1399-3046.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 32.Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant. 2005;9:381–390. doi: 10.1111/j.1399-3046.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 33.Betz CL. Transition of adolescents with special health care needs: review and analysis of the literature. Issues Compr Pediatr Nurs. 2004;27:179–241. doi: 10.1080/01460860490497903. [DOI] [PubMed] [Google Scholar]
- 34.Schafer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant. 2008;8:616–626. doi: 10.1111/j.1600-6143.2007.02127.x. [DOI] [PubMed] [Google Scholar]
- 35.Ellis DA, Templin TN, Podolski CL, Frey MA, Naar-King S, Moltz K. The parental monitoring of diabetes care scale: development, reliability and validity of a scale to evaluate parental supervision of adolescent illness management. J Adolesc Health. 2008;42:146–153. doi: 10.1016/j.jadohealth.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Naar-King S, Montepiedra G, Nichols S, et al. Allocation of family responsibility for illness management in pediatric HIV. J Pediatr Psychol. 2009;34:187–194. doi: 10.1093/jpepsy/jsn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annunziato RA, Parkar S, Dugan CA, et al. Brief Report: Deficits in Health Care Management Skills Among Adolescent and Young Adult Liver Transplant Recipients Transitioning to Adult Care Settings. J Pediatr Psychol. 2009 doi: 10.1093/jpepsy/jsp110. [DOI] [PubMed] [Google Scholar]
- 38.McDonagh JE, Kaufman M. Transition from pediatric to adult care after solid organ transplantation. Curr Opin Organ Transplant. 2009;14:526–532. doi: 10.1097/MOT.0b013e32832ffb2a. [DOI] [PubMed] [Google Scholar]
- 39.Scal P, Evans T, Blozis S, Okinow N, Blum R. Trends in transition from pediatric to adult health care services for young adults with chronic conditions. J Adolesc Health. 1999;24:259–264. doi: 10.1016/s1054-139x(98)00127-x. [DOI] [PubMed] [Google Scholar]
- 40.Knauth A, Verstappen A, Reiss J, Webb GD. Transition and transfer from pediatric to adult care of the young adult with complex congenital heart disease. Cardiol Clin. 2006;24:619–629. vi. doi: 10.1016/j.ccl.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Wainwright SP, Gould D. Non-adherence with medications in organ transplant patients: a literature review. J Adv Nurs. 1997;26:968–977. doi: 10.1046/j.1365-2648.1997.00451.x. [DOI] [PubMed] [Google Scholar]
- 42.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77:769–776. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 43.Creer TL. Self-management and the control of chronic pediatric illness. In: Drotar D, editor. Promoting adherence to medical treatment in chronic childhood illness: Concepts, methods and interventions. Hillsdale, NJ: Erlbaum; 2000. pp. 95–129. [Google Scholar]
- 44.Lemanek KL, Kamps J, Chung NB. Empirically supported treatments in pediatric psychology: regimen adherence. J Pediatr Psychol. 2001;26:253–275. doi: 10.1093/jpepsy/26.5.253. [DOI] [PubMed] [Google Scholar]