Abstract
Objective
Oxidation of plasma cysteine/cystine (Cys/CySS) redox potential (EhCySS) has been associated with risk factors for cardiovascular disease in humans. Cys and CySS are derived from dietary sulfur amino acids (SAA), but the specific effects of SAA depletion and repletion on Cys/CySS redox indices are unknown. The present study examined the effect of dietary SAA intake level on free Cys, free CySS and EhCySS in human plasma under fasting conditions.
Research Methods and Procedures
Healthy individuals aged 18–36 y (n=13) were equilibrated to foods providing the RDA for SAA and then fed chemically defined diets without SAA (0 mg·kg−1·d−1; n=13) followed by SAA at levels approximating the mean (56 mg·kg−1·d−1; n=8) or 99th percentile (117 mg·kg−1·d−1; n=5) intake levels of Americans. Fasting plasma samples were collected daily during 4-d study periods and analyzed for free Cys, free CySS and the EhCySS.
Results
The SAA-free diet significantly (p<0.05) decreased plasma free Cys concentrations and oxidized EhCySS values after 4 days of SAA depletion. With SAA repletion at 56 mg·kg−1·d− 1, plasma free Cys increased significantly and values for EhCySS became more reducing. Administration of a diet providing a higher dose of SAA (117 mg·kg−1·d−1) resulted in a significantly higher level of free Cys and a more reducing EhCySS.
Conclusions
These results show that free Cys and Cys/CySS redox potential (EhCySS) in fasting plasma are affected by dietary SAA intake level in humans. Significant changes occur slowly over 4 days with insufficient SAA intake, but rapidly (after 1 day) with repletion.
Keywords: Oxidative stress, amino acid balance, cardiovascular disease, methionine
Introduction
Proteins contain Cys residues which readily undergo reversible oxidation-reduction reactions. In vitro studies show that controlled variation in extracellular thiol/disulfide redox state alters cell signaling of monocyte adhesion to endothelial cells [1], platelet activation [2], cell proliferation [3, 4] and apoptosis [5] apparently mediated through integrins, metalloproteinases, growth factor receptors and ion channels. In vivo studies in humans show that oxidized thiol/disulfide redox potential is associated with increased carotid intima media thickness [6], decreased flow mediated dilation [7], reversible myocardial perfusion defects [8] and persistent atrial fibrillation [9]. Thus, the dietary factors affecting extracellular thiol/disulfide redox potential in human plasma could be important in cardiovascular disease.
The predominant low molecular weight thiol/disulfide system present in plasma is cysteine/cystine (Cys/CySS). CySS concentration in human plasma is considerably higher than Cys concentration under most conditions [10–12], and accumulating evidence indicates that the balance between Cys and CySS could be an important health determinant. Because the stoichiometry is 2 Cys per CySS, the balance is often expressed in terms of the redox potential (EhCySS). The reducing force of the plasma Cys/CySS couple is quantitatively expressed by its redox potential (EhCySS), related to Cys and CySS concentrations by the Nernst equation [13]. EhCySS in healthy individuals aged 25–35 y was found to be −80 ± 9 mV [10], and cross-sectional studies show that this value is more positive (oxidized) in association with aging [14], cigarette smoking [15], chronic alcohol abuse [16] and anticancer therapy [17]. Increased plasma CySS concentration and/or oxidized plasma EhCySS have been associated with human disease risk, e.g., persistent atrial fibrillation [9], peripheral vascular disease [6] and age-related macular degeneration [18].
In a study of dietary intake of SAA in rats, we found that SAA-deficient food (providing no Cys and 17% of control diet SAA intake as Met) resulted in oxidation of plasma EhCySS, with corresponding changes in gut GSH/GSSG redox potentials and cell growth indices [19]. A study of diurnal variation of GSH and Cys in human plasma showed that plasma EhCySS varied in an apparent meal-related pattern [20]. EhCySS was maximally oxidized at early hours of the morning (0430–0630 AM), became transiently reduced 2–3 h after each meal, and was maximally reduced at 2030, which was 3 h after the largest meal [20]. Together, these data suggest that in humans, EhCySS could vary as a consequence of dietary intake of SAA.
The present study was designed to determine the effects of dietary SAA depletion and repletion on the fasting plasma cysteine/cystine redox potential in humans. The study used a semisynthetic, chemically defined diet design based upon the studies of Young and coworkers [21, 22], which allows specific changes in L-amino acid content. To minimize contributions of other environmental factors which could affect oxidative stress and thereby alter EhCySS, fasting plasma levels were obtained at a consistent time in the controlled setting of a metabolic research unit.
Materials and methods
Materials
Sodium heparin, bathophenanthroline disulfonate sodium salt (BPDS), sodium iodoacetate, dansyl chloride, L-serine, Cys, CySS, and sodium acetate trihydrate were from Sigma Chemical Corp. (St. Louis, MO, USA). γ-Glutamylglutamate (γ-Glu-Glu) was from MP Biomedicals Corp. (Irvine, CA, USA). Boric acid, sodium tetraborate, potassium tetraborate, perchloric acid, and acetic acid were reagent grade and purchased locally. Methanol, acetone, and chloroform were HPLC grade.
Subjects
This study was reviewed and approved by the Emory Investigational Review Board. A total of 13 volunteers, self-described as healthy, were recruited beginning January 1, 2005 by posting fliers in public locations in the Atlanta/Emory University community. Following informed consent, all subjects were screened in the outpatient unit of the Emory University Hospital GCRC, where a medical history and physical examination, body height and weight, fasting standard blood chemistry and hematology tests and a urinalysis were performed (a serum pregnancy test was also performed in females). Indirect calorimetry was used to determine resting energy expenditure (REE). Eligibility was established by the absence of evidence of acute or chronic illness, no current smoking history, and a body mass index (BMI) < 30.
Within one month following screening, the subjects were scheduled to begin the study. Subjects taking antioxidants, nutrient supplements (with the exception of once-daily multivitamin-mineral supplements) or acetaminophen were asked to discontinue these two weeks prior to the onset of the studies. A 3-day equilibration period was used to normalize the 13 subjects with regard to diet (Figure 1). During equilibration, the standardization diet was based on the U.S. Recommended Dietary Allowances [23] providing maintenance energy and protein intake, approximating the RDA for SAA intake (12.2 mg/kg body weight Met per day plus 6.6 mg/kg body weight Cys per day). During the subsequent study periods, the subjects were given chemically defined oral diets providing maintenance energy intake and 1.0 g/kg body weight/day protein equivalents as an L-amino acid mixture (Figure 1, see below). All study meals were prepared in the GCRC metabolic kitchen, given at standardized mealtimes and consumed over 20 min, and intake was monitored by the GCRC Bionutrition Unit staff. The study period consisted of two consecutive 4-day study periods. During the first phase, all subjects received a chemically defined diet devoid of Met and Cys (SAA depletion phase). This period was followed by an SAA repletion phase which was isocaloric based upon gross energy content and isonitrogenous with the chemically defined diets providing either 56 mg/kg body weight SAA per day (n=8) or 117 mg/kg body weight per day (n=5) respectively (Figure 1). The chemically defined diets contained a distribution of Met:Cys of 2:1 by gravimetric weight. The intake of 56 and 117 mg/kg body weight SAA per day levels approximate the mean and 99th percentile daily intake of SAA in the American diet, respectively, as determined in the National Health and Nutrition Examination Survey (NHANES III) [23]. Overnight fasted plasma was obtained at 0830 AM for study endpoints.
Fig 1. Study scheme for dietary sulfur amino acid (SAA) effects on plasma cysteine, cystine and cysteine/cystine redox potential.
The 13 healthy adult study subjects were each admitted into the Emory General Clinical Research Center (GCRC) for a 13-day inpatient study period. The first 3 days were an equilibration phase, during which conventional food items approximating the RDA for SAA (18.7 mg·kg−1) were given. The subjects were given chemically defined oral diets for a subsequent 10-day study period providing maintenance energy intake and 1.0 g/kg body weight/day protein equivalents. During the study period without SAA, all 13 subjects were fed a chemically defined diet devoid of Met and Cys (SAA insufficiency phase). This was followed by a SAA repletion phase during which the chemically defined diet providing either 56 mg SAA per kg body weight per day (n=8), similar to average SAA intake based on U.S. consumption data, or 117 mg·kg−1·d−1 SAA (n=5), similar to 99 percentile intake of Americans, was given. Protein equivalents were provided as an L-amino acid mixture containing 9 essential amino acids, without or with methionine (Met) and 9 non-essential amino acids, without or with cysteine (Cys). The SAA repletion diets were made isocaloric and isonitrogenous to the SAA insufficient diet by proportional adjustment of the 9 non-essential amino acids in the L-amino acid mixture. Arrows designate time points for overnight fasting plasma sample collection for free Cys, free CySS and EhCySS, which were obtained at 0830 AM.
Diet and nutrient intake
The protein equivalents of diets were supplied in the same forms of specific L-amino acid mixtures (Ajinomoto USA, Teaneck, NJ), providing 1.0 g/kg body weight per day as outlined in detail [21, 22]. The standard mixture was patterned after hen’s egg protein and provides all 9 indispensable (essential) amino acids, including Met, in amounts sufficient for the mean requirements of healthy young adults [21, 22], but which are higher than the requirements proposed by the World Health Organization [21, 24]. The standard amino acid mixture also contained 9 dispensable (non-essential) amino acids, including Cys and glutamate, and was glutamine- and taurine-free. To compensate for the difference in Met + Cys between the 0, 56 and 117 mg/kg body weight per day SAA diets, the amount of all non-essential amino acids were proportionally changed to maintain a constant dietary nitrogen content while at the same time maintaining them as isoenergetic. Similarly, for all diets, the proportion by gravimetric weight of Met:Cys was constant (2:1). To improve palatability, a powdered flavoring agent was added to the liquid amino acid mixture (provided as a sherbet-based drink) (19,20). The Cys was added immediately prior to consumption to minimize Cys oxidation to CySS prior to consumption. The dietary energy (1.3 times measured REE) was mainly derived from lipid and carbohydrate sources provided in the form of protein-free wheat starch and butter/safflower oil cookies and a sherbet-based drink, as outlined [21, 22]. All subjects were completely compliant with all research meals as verified by the GCRC bionutrition staff.
Adequate oral hydration and vitamin, mineral and electrolyte requirements were provided to all subjects to meet or exceed recommended allowances [21]. Water was provided ad libitum with monitoring to ensure urine output of at least 700 mL during each 24-h period. All subjects received on a daily basis one multivitamin-multimineral capsule with iron (One-A-Day; Miles Inc., Elkhart, IN); three potassium tablets (K-LYTE;20 meq each, generic); four calcium tablets (TUMS; SKB Corp., Pittsburgh, PA); two sodium chloride tablets (1 gram tablets; Eli Lilly and Co., Indianapolis, IN); two choline capsules (250-mg; Lee Nutrition Inc., Cambridge, MA); and one magnesium oxide tablet (400 mg). All supplements were administered on a regular schedule by the GCRC research nurses. Stool softener (oral docusate) was provided as needed to a few subjects. Body weights were determined daily and vital signs were obtained every 8 h. Low-level activity was allowed and restricted to walking on the GCRC.
Sampling and redox analyses of EhCyS and EhGSSG
Blood was collected at 0830 AM each day of the depletion and repletion period before the morning meal being provided using a standardized and validated procedure for the further analyses of free Cys, CySS, GSH, and GSSG. The blood (1.35 mL) was collected via a heparinized 5 mL syringe with a butterfly needle and immediately transferred to a microcentrifuge tube containing 0.15 mL of preservation solution designed to alkylate Cys and prevent its oxidation. The preservation solution included 0.504 mol/L L-serine, 9.3 mmol/L batho-phenanthroline disulfonic acid disodium salt(BPDS), 0.165 mol/L γ-glutamylglutamate, 0.401 mol/L boric acid, 0.144 mol/L iodoacetic acid, and 2.5 mg (per mL) heparin. The BPDS, a chelator, was added to minimize autoxidation. The serine and borate were included to inhibit γ-glutamyltranspeptidase and to buffer pH to a value where iodoacetate rapidly carboxymethylated thiols. These samples were immediately centrifuged in a microcentrifuge at 13,000 g for 30 s, and 200 μL of plasma was aliquoted to a tube containing 10% ice-cold perchloric acid and 0.2 mol/L boric acid solution. The entire sampling procedure took less than 2 min for each sample, avoiding artifacts from delays in processing [12]. Samples were stored at −80° C and analyzed within 1 month. Stability tests showed that samples are stable under these conditions for at least 2 months [25].
A 300-μl aliquot of each supernatant was transferred to a fresh microcentrifuge tube. The pH was adjusted to 9.0 ± 0.2 with a KOH/tetraborate solution. After 20 min, 300 μl of the dansyl chloride solution was added, and the samples were mixed and placed in the dark at room temperature for 16 to 26 h. Chloroform (500 μl) was then added to each tube to extract the unreacted dansyl chloride, and samples were assayed by HPLC [25].
EhCySS was calculated using the Nernst equation, Eh = Eo + RT/nF ln[CySS]/([Cys]2), where Eo is the standard potential (−250 mV for CySS/Cys at pH 7.4), R is the gas constant, T is the absolute temperature, n is 2 for the number of electrons transferred, and F is Faraday’s constant [10]. Protein cysteinylation was found to correlate with plasma CySS and was not measured in the current study [10]. In previous studies of subjects with both normal homocysteine and homocysteinemia, total free and protein-bound homocysteine have been shown to correlate [26]. In the current study, study subjects were not screened for homocysteinemia. EhGSSG was calculated as above using respective GSH and GSSG concentrations and an Eo of −264 mV for pH 7.4.
Statistics
Minitab software (version 15; Minitab, Inc, State College PA) was used for all analyses. The protocol was designed so that each individual was studied without SAA (depletion) and with either 56 or 117 mg·kg−1·d−1 (repletion). With this design, all 13 subjects were treated identically for the first study period but differed in dose for the second study period. The fasting morning blood sample immediately following the 3-day equilibration phase represented the baseline sample for the SAA depletion period (day 0). The subsequent four fasting morning samples represented the SAA depletion phase (depletion days 1–4; Figure 1). The fasting morning blood sample following the SAA depletion represented the baseline timepoint for the SAA repletion phase (day 0 of repletion phase). The subsequent four fasting morning samples represented the SAA repletion phase (repletion days 1–4; Figure 1). One-way ANOVA was used to evaluate effects of time on plasma Cys, CySS and EhCySS in all 13 subjects during SAA depletion. One way ANOVA was also used to assess the effects of SAA repletion of redox indices within the lower SAA repletion dose group (n = 8 for the 56 mg·kg−1·d−1). To assess possible SAA repletion dose-responses, we performed two-way repeated measures ANOVA to compare the repletion data between the 8 subjects receiving SAA repletion at 56 mg·kg−1·d−1 versus the 5 subjects receiving SAA repletion at 117 mg·kg−1·d−1. Tukey simultaneous tests were used for post-hoc comparisons. Data are expressed as mean ± SEM. Results were considered significant at P ≤ 0.05.
Results
Study subject characteristics
Thirteen subjects aged 18 to 36 y were studied, eight in Group 1 with 56 mg/kg body weight SAA (18.7 mg/kg Cys) and five in Group 2 with 117 mg/kg body weight SAA (39 mg/kg Cys) daily intake (see Table 1). The 13 subjects included 8 males and 5 females, with 6 Whites, 5 African-Americans, 1 Asian and 1 other. Groups 1 and 2 did were not different in mean age (± SEM) at 23.6 ± 2.7 y and 24.9 ± 2.2 y, respectively. BMI ranged from 20 to 26 with mean (± SEM) values of 22.5 ± 1.8 and 22.5 ± 1.5, for Groups 1 and 2, respectively, and were not statistically different. Subjects reported no acute or chronic illness and none were taking regular prescription medications.
TABLE 1.
Characteristics of the study participants
| Group 1: Subjects receiving sulfur amino acids at 56 mg·kg−1·d−1 | |||
|---|---|---|---|
| Age | Sex | Race | BMI |
| 18 | F | White | 22.5 |
| 20 | M | White | 22.3 |
| 21 | M | Black | 20.7 |
| 23 | F | Black | 24.8 |
| 23 | M | White | 23.2 |
| 25 | M | Asian | 21.0 |
| 33 | F | Black | 26.0 |
| 36 | M | Black | 20.0 |
| Group 2: Subjects receiving sulfur amino acids at 117 mg·kg−1·d−1 | |||
|---|---|---|---|
| Age | Sex | Race | BMI |
| 18 | F | White | 26.3 |
| 19 | M | Other | 23.6 |
| 21 | F | Black | 20.3 |
| 29 | M | White | 24.7 |
| 31 | M | White | 25.6 |
Effect of SAA depletion on plasma free Cys, free CyS and EhCyS
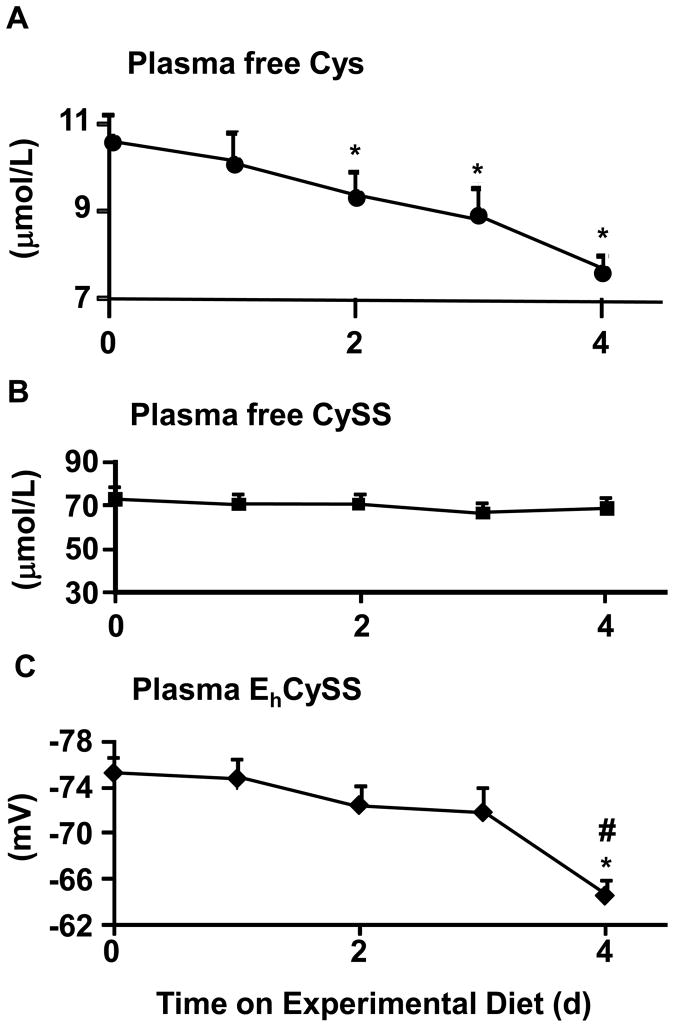
At baseline (following equilibration with the 18.7 mg·kg−1·d−1 SAA diet), the mean fasting Cys value was 10.6 ± 0.6 μmol/L for all 13 subjects (Fig 2A). The free Cys concentrations by one-way ANOVA decreased with time during SAA depletion phase (Fig 2A; p <0.05) with the minimum value occurring after 4 days (7.6 ± 0.4 μmol/L). The free Cys values were significantly different from baseline on days 2 to 4 by post-hoc testing (Fig 2A). The data show a gradual plasma free Cys decrease occurs with an approximate rate of 0.6 μmol/L per day.
Fig 2. The free cysteine (Cys), free cystine (CySS) and Cys/CySS redox potential (EhCySS) in human plasma under fasting conditions following intake of SAA-free diet.
The study protocol is as outlined in Figure 1. Data shown are derived from fasting plasma samples (n=8) obtained at morning time points after completion of the equilibration period and for the next 4 days during consumption of diets devoid in methionine, Cys and CySS. A. The free Cys in fasting plasma. A significant effect of time was observed by one-way ANOVA (P<0.05). *Significant difference from baseline values (P<0.05). B. The free CySS in fasting plasma. No change over time was observed. C. Fasting plasma EhCySS. A significant effect of time was observed by one-way ANOVA (P<0.05). *Significant difference from baseline values and #from day 1 values by Tukey’s post-hoc test (P<0.05). Data are expressed as mean ± SEM.
The free CySS concentration in fasting plasma at baseline was (74 ± 5 μmol/L). Plasma free CySS concentrations did not change significantly over time in subjects fed the SAA depleted diet (Fig 2B).
Fasting plasma EhCySS was −75 ± 2 mV (n = 13) at baseline. This value is similar to the previously reported mean values of −72 to −77 mV for fasting samples taken between 06:30 AM and 08:30 AM in a diurnal variation study [20]. EhCySS became significantly more positive (less reducing) during the SAA-depletion period, with values at day 4 (−67 ± 2) significantly more oxidized than baseline and depletion day 1 values by post-hoc testing (p < 0.05) (Fig 2C).
Effect of SAA repletion on plasma free Cys, free CyS and EhCyS
The effects of dietary SAA repletion on plasma Cys/CySS redox was determined in 8 of the 13 subjects studied above. These individuals were fed isoenergetic, isonitrogenous, chemically defined diets providing SAA intake approximating the mean American intake (56 mg·kg−1·d−1) from NHANES III) [27]. Figure 3 shows the free Cys values in fasting plasma for these 8 subjects during their SAA repletion phase. SAA at this dose significantly increased plasma free Cys concentrations over time (Figure 3A; P<0.005), with values rising significantly above the post-depletion baseline values by day 1 of SAA repletion and then plateauing by day 2 of repletion. Plasma free CySS levels did not change during SAA repletion (Figure 3B). However, due to the increase in plasma free Cys, the EhCySS became more reducing during repletion over time from the post-depletion baseline value of −70 ± 3 mV (P<0.05) (Figure 3C). The most reduced values occurred by day 2 of SAA repletion (−81 ± 2 mV; P < 0.05 versus baseline).
Fig 3. Free Cys, free CySS and EhCySS in human plasma obtained under fasting conditions following SAA repletion.
The study protocol is as outlined in Figure 1. Data shown are derived from fasting plasma samples (N = 8) obtained at morning time points following completion of SAA depletion period and for the next 4 days during repletion with diets providing 56 mg·kg−1·d−1 SAA. A. The free Cys in fasting plasma. A significant effect of time was observed by one-way ANOVA (P<0.05). *Significant difference from baseline values (P<0.05). B. The free CySS in fasting plasma. No change over time was observed. C. Fasting plasma EhCySS. A significant effect of time was observed by one-way ANOVA (P<0.05). *Significant difference from baseline values by Tukey’s post-hoc test (P<0.05). Data are expressed as mean ± SEM.
SAA repletion dose-responses
To explore potential dose-response effects of SAA repletion on the free Cys, free CySS and EhCySS in fasting plasma, we compared the repletion data between the 8 subjects receiving SAA repletion at 56 mg·kg−1·d−1 and the 5 subjects receiving SAA repletion at 117 mg·kg−1·d. Two-way repeated measures ANOVA were performed to determine the dose response of SAA on free Cys, free CySS and EhCySS. This repeated measures ANOVA showed the increase in plasma free Cys concentrations over time with the higher SAA repletion dose was marginally significant compared to the lower dose (p=0.055) (Figure 4A). In contrast, plasma free CySS levels were not statistically different with the lower versus the higher SAA repletion doses (Figure 4B). Redox potential (EhCySS) for the Cys-CySS couple was significantly affected by SAA repletion dose; the values for EhCySS were more reducing with the 117 mg·kg−1·d SAA repletion dose over time compared to the 56 mg·kg−1·d− dose (Figure 4C).
Fig 4. Dose response to SAA repletion on free Cys, free CySS and EhCySS in human fasting plasma following SAA repletion.
The study protocol is as outlined in Figure 1. Data shown are derived from fasting plasma samples obtained at morning time points following completion of SAA depletion period and for the next 4 days during repletion with diets providing either 56 mg·kg−1·d−1 SAA (solid lines: n=8) or 117 mg·kg−1·d−1 SAA (dotted line: n=5). A. The free Cys in fasting plasma. A trend for an effect of SAA dose was observed by two-way repeated measures ANOVA (P=0.055). B. The free CySS in fasting plasma. No significant effect of SAA dose was observed by two-way repeated measures ANOVA. C. Fasting plasma EhCySS. A significant effect of SAA dose was observed by two-way repeated measures ANOVA (P<0.05). Data are expressed as mean ± SEM.
Effects on free GSH redox couple in fasting plasma
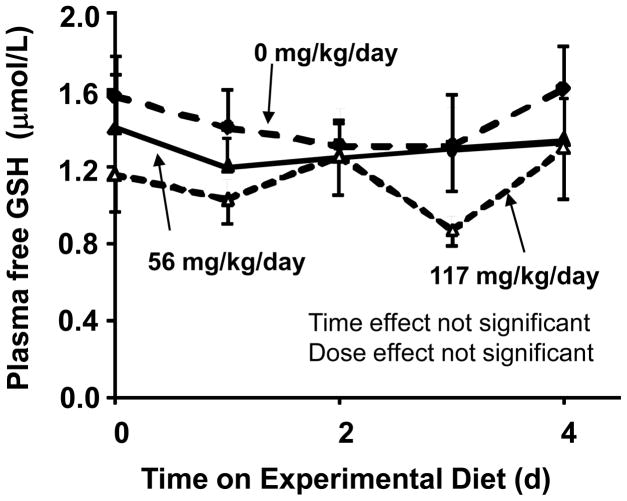
For all conditions described, analyses were also performed to determine free GSH, free GSSG and the redox potential for the GSH/GSSG couple (EhGSSG). These data showed the GSH and GSSG concentrations were comparable to those obtained previously for baseline of fasting morning conditions (GSH: 1.6 ± 0.2 μmol/L, GSSG:0.08 ± 0.03 μmol/L expressed as mean ± SEM) [25]. The plasma free GSH did not change during depletion and repletion period (Figure 5). No significant effect of SAA intakes on GSSG or EhGSSG in fasting plasma was detected (data not shown).
Fig 5. Dose response of SAA repletion on free GSH in human fasting plasma following SAA repletion.
The study protocol is as outlined in Figure 1. Data shown are derived from fasting plasma samples obtained at morning time points from baseline (completion of SAA depletion period, closed circle, dotted line) and for the next 4 days during repletion with diets providing either 56 mg·kg−1·d−1 SAA (closed triangle, solid lines: n=8) or 117 mg·kg−1·d−1 SAA (open triangle, dotted line: n=5). No significant effect of SAA dose was observed by two-way repeated measures ANOVA. Data are expressed as mean ± SEM.
Discussion
Accumulating evidence indicates that plasma GSH/GSSG and Cys/CySS pools could be important in disease risk in humans and be especially relevant to cardiovascular disease as possible biomarkers of risk. For instance, both EhGSSG and EhCySS were found to be oxidized in association with age [14], cigarette smoking [15] and increased BMI [7]. EhGSSG was also found to be oxidized in association with type 2 diabetes [28] and alcohol abuse [16], and EhCySS was oxidized in association with increased proinflammatory cytokines, IL-1β and TNF-α [29], and reduced in individuals consuming a Mediterranean diet [30]. The concentration of CySS, which is a co-variable with EhCySS, is also increased in association with TNF-α [29], endothelial dysfunction [7], and increased carotid intima media thickness [6]. In addition, a substantial literature shows that dietary inducers can affect tissue levels of GSH and related metabolites [31]. Thus, there is a need to understand dietary factors which affect plasma EhGSSG, EhCySS or CySS concentration in humans.
The present study addressed whether recent history of dietary sulfur amino acid intake affected fasting plasma EhGSSG, EhCySS or CySS concentration. The data show no significant effects on EhGSSG or CySS concentration. However, the results show that low SAA intake decreased fasting blood levels of free Cys and resulted in an oxidation of EhCySS over a period of 4 days. In contrast, 1 day with SAA at the mean American intake level was sufficient to restore the free Cys and EhCySS in fasting plasma. These data indicate that if fasting plasma EhCySS is used for assessment of cardiovascular disease risk, the previous 24-h intake of SAA could mask oxidation due to a long-term inadequate SAA intake. On the other hand, surveillance data show that long-term inadequate SAA intake is not common [27], and 1 day of inadequate SAA intake is not sufficient to result in a falsely oxidized EhCySS value. Thus, although variations in recent history of SAA intake can affect EhCySS, this effect is insufficient to seriously compromise use of EhCySS as a biomarker. The lack of effects of SAA intake on fasting plasma EhGSSG and CySS concentration indicate that these parameters are minimally affected by the recent history of SAA intake.
In vitro studies indicate that oxidation of plasma EhCySS could be mechanistically important in disease development. While plasma EhGSSG is thought to indicate tissue oxidative stress, EhCySS appears to reflect extracellular oxidative processes [14]. A more oxidized EhCySS in culture media was found to increase sensitivity of retinal pigment epithelial cells [5] and endothelial cells (Y-M Go and DP Jones, unpublished observation) to apoptosis, enhance binding of monocytes [1, 32] and neutrophils [33] to endothelial cells and increase production of proinflammatory cytokines in monocytes [29]. At least some of these effects are mediated through cell surface thiols [1], indicating that oxidation of plasma thiol/disulfide couples could directly affect function of extracellular or cell surface proteins, including receptors, integrins and metalloproteinases [34].
The magnitudes of the diet-induced changes after 4 d further suggest that fasting plasma EhCySS could be mechanistically important in cell signaling through cell surface protein dithiol/disulfide couples; an 8 mV oxidation in EhCySS (as observed after 4 days of SAA depletion) is equivalent to an approximately 2-fold change in protein dithiol/disulfide ratio while an 18 mV change (difference between 4 d with high SAA versus no SAA) is equivalent to a 4- fold change [35]. The magnitude of the EhCySS oxidation observed with SAA depletion is similar to differences associated with aging [14, 18], smoking [15], or alcohol consumption [16]. Also, the mean difference in EhCySS for individuals with high and low risk of persistent atrial fibrillation was 10 mV [9]. The similarities in magnitude of EhCySS in these disorders and with SAA depletion in the present study suggest that diet-induced changes at extreme levels of SAA intake may have pathophysiologic effects.
Deficient intake of SAA could have effects other indirect effects. For instance, taurine, a product of Cys oxidation, was found to protect against hyper-homocysteinemia-induced toxicity apparently through an antioxidant mechanism [36]. Insufficient intake could also have effects through altered transport of Cys and CySS in vivo because the transporters for these amino acids are common to other amino acids. Changes in SAA would thereby affect the balance of amino acids required for cellular protein synthesis. Furthermore, the GSH turnover is decreased by SAA intake [21, 22], indicating that transport of precursors could be limiting. In vitro studies show that culture of HT29 cells in Cys- and CySS-free media results in oxidation of the cellular EhGSSG and increased GSH synthesis upon re-addition of the amino acids to the culture media [11]. The xc− system functions in uptake of CySS and is transcriptionally induced through the Nrf-2 transcription factor by oxidative conditions [11]. Thus, it appears likely that altered transport occurs in response to SAA insufficiency.
The present study of healthy, young individuals does not allow conclusions concerning older individuals who are more at risk of disease. The present study included young, healthy individuals, but clinical data for EhCySS and disease largely include middle age and older individuals. Some of these latter studies provide stronger associations of disease risk with the free CySS concentration than with EhCySS [6, 37, 38]. Thus, experiments are warranted to determine whether similar or greater effects on the free CySS and EhCySS occur in older individuals and in individuals with disease risk.
In conclusion, the data show that for young adults with previously sufficient SAA intake, the free Cys, free CySS and EhCySS in fasting plasma are affected by SAA intake over a 4-day period. The results show that SAA repletion rapidly improves Cys/CySS redox status (within 24 h) after a diet devoid of methionine, cysteine or cystine. The magnitude of differences between intake of insufficient and sufficient diets were similar to previously reported differences associated with aging, oxidative stress and disease, suggesting that SAA intake at extreme levels could contribute to disease processes associated with these redox parameters.
Acknowledgments
The authors thank the GCRC nursing staff and Jennifer Terry, R.D., Margaret Pedersen, R.D, Vera Hull and Diane Harris of the GCRC Bionutrition Unit for preparation of the study diets. This research was supported by NIH grants ES012929 and ES011195 (DPJ), DK55850 and K24 RR023356 (TRZ) and the Emory General Clinical Research Center grant M01 RR00039/UL1 RR025008.
Footnotes
The authors had no conflicts of interest.
Role of each author in work: DJ and TZ conceived and designed the study; NG, CA and TZ provided oversight for the study; NG, YP and YL conducted the study and collected the data; YP and TY provided statistical analysis; DJ, YP, CA and TZ drafted and revised the paper; DJ, YP and TZ had primary responsibility for approval of final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111(22):2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 2.Essex DW, Li M. Redox control of platelet aggregation. Biochemistry. 2003;42(1):129–136. doi: 10.1021/bi0205045. [DOI] [PubMed] [Google Scholar]
- 3.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33(11):1499–1506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 4.Nkabyo YS, Go YM, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G70–78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 5.Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jr, Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46(3):1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- 6.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Harrison DG, Quyyumi AA. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol. 2006;47(5):1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 7.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Alexander RW, Harrison DG, Quyyumi AA. Endothelial Function and Aminothiol Biomarkers of Oxidative Stress in Healthy Adults. Hypertension. 2008;52:80–85. doi: 10.1161/HYPERTENSIONAHA.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramson JLJD, Bremner JD, Quyyumi AA, Goldberg J, Vaccarino V. Oxidative stress, as indicated by the redox states of plasma thiol/disulfide couples, is associated with myocardial perfusion defects in men without clinical CAD. JACC. 2005;45 (3):401A. [Google Scholar]
- 9.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC., Jr Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53(9):1652–1657. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28(4):625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 11.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 12.Torres CL, Miller JW, Rogers QR. Determination of free and total cyst(e)ine in plasma of dogs and cats. Vet Clin Pathol. 2004;33(4):228–233. doi: 10.1111/j.1939-165x.2004.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. Faseb J. 2004;18(11):1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 14.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33(9):1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 15.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35(12):1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med. 2007;176(3):270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas CR, Puckett AB, Jones DP, Griffith DP, Szeszycki EE, Bergman GF, Furr CE, Tyre C, Carlson JL, Galloway JR, et al. Plasma antioxidant status after high-dose chemotherapy: a randomized trial of parenteral nutrition in bone marrow transplantation patients. Am J Clin Nutr. 2000;72(1):181–189. doi: 10.1093/ajcn/72.1.181. [DOI] [PubMed] [Google Scholar]
- 18.Moriarty-Craige SE, Adkison J, Lynn M, Gensler G, Bressler S, Jones DP, Sternberg P., Jr Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am J Ophthalmol. 2005;140(6):1020–1026. doi: 10.1016/j.ajo.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Nkabyo YS, Gu LH, Jones DP, Ziegler TR. Thiol/Disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino Acid intake. J Nutr. 2006;136(5):1242–1248. doi: 10.1093/jn/136.5.1242. [DOI] [PubMed] [Google Scholar]
- 20.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86(4):1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 21.Raguso CA, Regan MM, Young VR. Cysteine kinetics and oxidation at different intakes of methionine and cystine in young adults. Am J Clin Nutr. 2000;71(2):491–499. doi: 10.1093/ajcn/71.2.491. [DOI] [PubMed] [Google Scholar]
- 22.Lyons J, Rauh-Pfeiffer A, Yu YM, Lu XM, Zurakowski D, Tompkins RG, Ajami AM, Young VR, Castillo L. Blood glutathione synthesis rates in healthy adults receiving a sulfur amino acid-free diet. Proc Natl Acad Sci U S A. 2000;97(10):5071–5076. doi: 10.1073/pnas.090083297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients) Washington, DC: National Academies Press; 2005. [DOI] [PubMed] [Google Scholar]
- 24.Di Buono M, Wykes LJ, Ball RO, Pencharz PB. Dietary cysteine reduces the methionine requirement in men. Am J Clin Nutr. 2001;74(6):761–766. doi: 10.1093/ajcn/74.6.761. [DOI] [PubMed] [Google Scholar]
- 25.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47(10):1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueland PM, Mansoor MA, Guttormsen AB, Muller F, Aukrust P, Refsum H, Svardal AM. Reduced, oxidized and protein-bound forms of homocysteine and other aminothiols in plasma comprise the redox thiol status--a possible element of the extracellular antioxidant defense system. J Nutr. 1996;126(4 Suppl):1281S–1284S. doi: 10.1093/jn/126.suppl_4.1281S. [DOI] [PubMed] [Google Scholar]
- 27.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients) doi: 10.1016/s0002-8223(02)90346-9. [ http://www.nap.edu/catalog/10490.html] [DOI] [PubMed]
- 28.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24(5):699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 29.Iyer SS, Accardi CJ, Ziegler TR, Blanco RA, Ritzenthaler JD, Rojas M, Roman J, Jones DP. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS One. 2009;4(3):e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2008;88(5):1364–1370. doi: 10.3945/ajcn.2008.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez Alvarez JR, Belles VV, Lopez-Jaen AB, Marin AV, Codoner-Franch P. Effects of alcohol-free beer on lipid profile and parameters of oxidative stress and inflammation in elderly women. Nutrition. 2009;25(2):182–187. doi: 10.1016/j.nut.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Go YM, Craige SE, Orr M, Gernert KM, Jones DP. Gene and Protein Responses of Human Monocytes to Extracellular Cysteine Redox Potential. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyer SS, Jones DP, Brigham KL, Rojas M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. American Journal of Respiratory Cell & Molecular Biology. 2009;40(1):90–98. doi: 10.1165/rcmb.2007-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go Y-M, Park H, Koval M, Orr M, Reed M, Liang Y, Smith D, Pohl J, Jones DP. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radical Biology & Medicine. 2009 doi: 10.1016/j.freeradbiomed.2009.10.050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 36.Yalcinkaya S, Unlucerci Y, Giris M, Olgac V, Dogru-Abbasoglu S, Uysal M. Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet: protective effect of taurine. Nutrition. 2009;25(4):436–444. doi: 10.1016/j.nut.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Iyer SS, Jones DP, Brigham KL, Rojas M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am J Respir Cell Mol Biol. 2009;40(1):90–98. doi: 10.1165/rcmb.2007-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriarty-Craige SE, Ha KN, Sternberg P, Jr, Lynn M, Bressler S, Gensler G, Jones DP. Effects of long-term zinc supplementation on plasma thiol metabolites and redox status in patients with age-related macular degeneration. Am J Ophthalmol. 2007;143(2):206–211. doi: 10.1016/j.ajo.2006.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]