Abstract
Mitochondrial outer membrane (MOM) proteins in parasitic protozoa like Trypanosoma brucei are poorly characterized. In fungi and higher eukaryotes, Tob55 is responsible for the assembly of β-barrel proteins in the MOM. Here we show that T. brucei Tob55 (TbTob55) has considerable similarity in its primary and secondary structure to Tob55 from other species. TbTob55 is localized in T. brucei MOM and is essential for procyclic cell survival. Induction of Tob55 RNAi decreased the level of the voltage-dependent anion channel (VDAC) within 48 h. Although the primary effect is on VDAC, induction of TbTob55 RNAi for a longer time period also decreased the levels of other nucleus encoded mitochondrial proteins. In addition, the mitochondrial membrane potential was reduced at this later time point possibly due to a reduction in the level of the proteins involved in oxidative phosphorylation. However, mitochondrial structure was not altered due to depletion of Tob55. In vitro protein import of VDAC into mitochondria with a 50-60% reduction of TbTob55 was reduced about 40% in comparison to uninduced control. In addition, the import of presequence-containing proteins such as, cytochrome oxidase subunit 4 (COIV) and trypanosome alternative oxidase (TAO) was affected by about 20 % under this condition. Depletion of VDAC levels by RNAi did not affect the import of either COIV or TAO. Furthermore, TbTob55 over expression increased the steady state level of VDAC as well as the level of the assembled protein complex of VDAC, suggesting that similar to other eukaryotes TbTob55 is involved in assembly of MOM β-barrel proteins and plays an indirect role in the biogenesis of mitochondrial preproteins destined for the mitochondrial inner membrane.
Keywords: Tob55, Trypanosoma brucei, RNAi, VDAC biogenesis, mitochondrial protein import
1. Introduction
Trypanosoma brucei, a haemoflagelated parasitic protozoan, causes a fatal disease in humans and domestic animals known as African trypanosomiasis [1]. T. brucei belongs to a group of earliest eukaryotes, which diverge very early during evolution [2]. The parasite possesses a single tubular mitochondrion with many unique characteristics [3]. In spite of various complexities, the parasite’s mitochondrial genome encodes a handful of mitochondrial proteins. Thus, similar to other eukaryotes, a vast majority of mitochondrial proteins are nuclear encoded and are imported after their synthesis in cytosol [3, 4]. However, the mitochondrial protein import machinery in T. brucei has been poorly characterized. Recently a homolog of Tim17, a component of the translocase of mitochondrial inner membrane (TIM) in other eukaryotes, has been identified and characterized in T. brucei [5, 6]. Searches in the T. brucei genome database found homologs for a few small Tims of the intermembrane space (IMS) [5]. However, none of the subunits of the translocase of mitochondrial outer membrane (TOM) have been identified in T. brucei. Thus, how proteins cross the mitochondrial outer membrane (MOM) in T. brucei remains enigmatic.
In other eukaryotes, MOM possesses several β-barrel proteins [7, 8]. These includes Tom40 (9, 10), VDAC (voltage dependent anion channel, also called porin) [11, 12], Tob55 (topogenesis of β-barrel protein; also called Sam50) [13, 14], and Mdm10 and Mmm2 (mitochondrial morphology proteins) [8, 15]. Among these, Tom40 and Tob55 are crucial for biogenesis of nuclear encoded mitochondrial proteins [13, 14, 16]. Tom40 is the major component of the TOM complex and responsible for import of virtually all types of mitochondrial proteins [16].
Tob55 is needed for biogenesis of mitochondrial β-barrel proteins such as VDAC and Tom40 [13, 14]. The TOB complex in fungi possesses two more proteins Sam35/Tob38 and Sam37/Mas37. The Tob55 and Tob38 are essential OM proteins in fungi. Tob55 is an integral β-barrel protein with the helical N-terminal containing a polypeptide-transport-associated (POTRA) domain [17]. Tob55 belongs to the family of bacterial Omp85 that is responsible for the assembly of β-barrel proteins on bacterial OM [18]. The protein translocator of chloroplast OM, Toc75, also belongs to this group [19]. Tob55 is structurally and functionally conserved among all eukaryotes investigated so far.
Here, we identified and characterized the Tob55 homolog in T. brucei. TbTob55 is a MOM β-barrel protein and is crucial for survival of the procyclic form. Similar to other eukaryotes, TbTob55 is responsible for import and assembly of VDAC. In addition, TbTob55 is involved indirectly in mitochondrial presequence-containing proteins or preproteins biogenesis. These results indicate that a major protein translocator in the MOM exists in T. brucei.
2. Materials and methods
2.1. Strains, media, and growth
The procyclic form of Trypanosoma brucei 427 (29-13) cell line, resistant to hygromycin and neomycin (G418), expressing the tetracycline repressor gene (TetR) and T7RNA polymerase (T7RNAP), were grown in SDM-79 medium (JRH Biosciences) containing 10% heat inactivated fetal bovine serum and appropriate antibiotics (hygromycin; 50 μg/ml; G418; 15 μg/ml) [20]. For measurement of cell growth, the procyclic cells were inoculated at a cell density of 2-3 × 106/ml in fresh medium containing appropriate antibiotics in the presence and absence of doxycycline. Cells were re-inoculated in fresh medium at each time the density reached 1-1.5 × 107/ml. Cells were harvested at different time points of growth (0-264 h) and the number of cells was counted in a Neubauer hemocytometer. To assess growth rates cumulative cell number was plotted versus time of incubation in culture.
2.2. Sequence comparison and secondary structure analysis
Amino acid sequence of TbTob55 (Tb927.3.4380) from the GeneDB database was compared for homology using BLAST analysis. Sequence comparison among Tob55s from T. brucei, Saccharomyces cerevisiae, Neurospora crassa and Homo sapiens was performed using ClustalW alignment program [21] in MacVector 10.0. The Hidden Markov Models (HMM) were built using HMMER 2.3.2 (http://hmmer.janelia.org). The prediction of secondary and tertiary structures of TbTob55 was performed using PRED TMββ [22] and TMBPro prediction tools [23], respectively. The phylogenetic analysis was carried out by maximum likelihood alignments employing PhyML 3.0 [24]. WAG substitution matrix with eight rate categories with the proportion of invariable sites was estimated from the data. Tree-Puzzle 5.2 [25] was used to calculate distances between sequences and puzzleboot (shell script by A. Roger and M. Holder, http:/www.tree-puzzle.de) was used to calculate bootstrap values using the same conditions. Trees were inferred using weighbor 1.2 [26]. MrBayes 3.1.2 [27] was used for performing Bayesian analysis and the Markov Chain Monte Carlo analysis was carried out for 100,000 cycles (sampling every 1000 cycles) after which the significance values were less than 0.05. The tree was drawn using Dendroscope 2.3 [28]
2.3. Generation of the inducible TbTob55RNAi and TbTob55 over-expressing cell lines
To prepare the construct for TbTob55 double stranded RNA expression, the 554 bp fragment of the coding region of TbTob55 was PCR amplified from the T. brucei genomic DNA using high fidelity Pfu polymerase (Stratagene). Sense and antisense primers containing restriction sites at 5′ ends were as follows; TbTob55 For: 5′-ATGGATCCACATTCATCCCCGTGTTATCAGTC-3′ and TbTob55 Rev: 5′-GAAAGCTTAGGCAGATTCGTTCCTTCCCTC-3′. The amplified product was cloned into the BamHI/HindIII sites of a tetracycline inducible dual promoter plasmid vector p2T7Ti-177 [29]. This construct generated TbTob55 double stranded RNA from two opposing tetracycline regulated T7 promoter/primer and expressed the phleomycin resistant gene constitutively for selection purposes. TbVDAC RNAi cell line was developed using the same vector as described [30]. The TbTob55 over-expression construct was generated using the pLew-Myc vector [31]. The entire open reading frame of TbTob55 was PCR amplified from T. brucei genomic DNA using primers TbTob55-Myc For: 5′-GATCAAGCTTATGACCTTTTCTAGCGAGAGTAGTG -3′ and TbTob55-Myc Rev: 5′-CTAGTCTAGACATGGAAAAAGCGGATGACCATG -3′. The PCR product was cloned between HindIII and Xba1 site. From this construct, the C-terminal Myc-tagged Tob55 was expressed upon induction with doxycycline. The construct for TbTob55 RNAi and TbTob55-Myc was verified by sequencing. The purified plasmid DNA was linearized by Not I. The linearized plasmid was used for transfection into procyclic cells (Tb427 29-13 expressing T7 polymerase and tetracycline repressor proteins) according to standard protocols [20].
2.4. RNA and protein analysis
RNA was isolated from the procyclic trypanosomes grown for 4 days with or without doxycycline, using Trizol reagent (Invitrogen) and Northern blot analysis was performed as described [32]. Total cellular proteins and proteins from isolated mitochondria were analyzed on SDS-PAGE (10 or 15%) as described (33), and transferred to nitrocellulose membranes at 4 °C (100 V with 25 mM Tris-HCl and 192 mM glycine, 20% v/v methanol pH 8.3) [34]. Blots were treated with polyclonal antiserum against T. brucei Cyt c1 [35], COIV [36], Cyt c [37], VDAC [30], Hsp70 [38], Tim17 [6], TbPP5 [39], and the Saccharomyces cerevisiae Tob55 [40] (all at 1:1000 dilution in blocking buffer). TAO [41] was detected with the corresponding monoclonal hybridoma supernatants (1:50 dilution in 10 mM Tris-HCl, 150 mM NaCl, pH 8.0 (TBST)). Monoclonal antibody against T. brucei β-tubulin [42] was used in 1:20,000 dilution in TBST. Blots were treated with appropriate secondary antibody and developed using enhanced chemiluminiscence (ECL) detection system (Amersham).
2.5. Isolation and post-isolation treatments of mitochondria
Mitochondria were isolated after lysis of cells via nitrogen cavitation in isotonic buffer as described [43, 44]. The isolated mitochondria were stored at a protein concentration of 10 mg/ml in SME (250 mM sucrose, 20 mM MOPS/KOH, 2 mM EDTA, pH 7.4) buffer containing 50% glycerol at −70°C. Before using, mitochondria were washed twice with 9 volumes of SME to remove glycerol. For alkali extraction, isolated mitochondria (100 μg) were treated with 100 μl of 100 mM Na2CO3 at pH 11.5 for 30 min on ice [43]. The supernatant and pellet fractions were collected after centrifugation (13,000 rpm for 10 min at 4 °C) and analyzed by SDS-PAGE and immunobloting. Protease digestion of isolated mitochondria was performed by treating mitochondria (50 μg) with various concentrations of proteinase K (0-150 μg/ml) in 100 μl reaction volume of SME for 30 min on ice. After the treatment, proteinase K was inhibited by PMSF (4 mM final concentration). Mitochondria were re-isolated and the proteins were analyzed by immunoblot analysis.
2.6. In vitro protein import assay
The open reading frames (ORFs) for the COIV, TAO, and VDAC were amplified from T. brucei genomic DNA using the following forward and reverse primer pairs, COIVFor: 5′AGAAGCTTATGTTTGCTCGCCGCT3′; and COIVRev: 5′AAGAATTCCTAAATCTTGTTTGA3′, TAOFor: 5′AGAAGCTTATGTTTCGTAAC3′; TAORev: 5′AAGAATTCTTACTCGTGTTTG3′; and VDACFor: 5′GATCAAGCTTATGTTTGCACGTGCAAAATCC3′, and VDACRev: 5′GATCGGATCCCTACGAATGGGTAATAAGG3′, respectively. The PCR product was subcloned in pGEM 4Z vector between HindIII and EcoRI sites for COIV. TAO and VDAC was subcloned at HindIII and BamHI sites. Radiolabeled precursors COIV, TAO and VDAC were synthesized in vitro using a coupled transcription/translation rabbit reticulocyte system (TNTR, coupled reticulocyte lysate systems, Promega) according to the manufacturer’s protocol using 35S methionine for COIV and TAO. 35S cysteine was used to label VDAC (DuPont NEN). Radiolabeled precursor proteins were used for in vitro import into isolated mitochondria from T. brucei as described [6, 44]. Mitochondria (100 μg) were washed with 9 volumes of SME buffer and resuspended in 90 μl of import buffer (250 mM sucrose, 80 mM KCl, 5 mM MgCl2, 5 mM dithiothreitol, 1.0 mg/ml fatty acid free bovine serum albumin and 10 mM MOPS/KOH at pH 7.2, 2 mM ATP, 10 mM creatine phosphate, 0.1 mg/ml creatine kinase, 8 mM potassium ascorbate, 0.2 mM N,N,N’,N’-tetramethylphenylenediamine, and 5 mM NADH). The mitochondrial suspension was mixed with 5-10 μl of rabbit reticulocyte translation mixture containing the radiolabeled precursor protein and incubated at room temperature for up to 10 min. Import of VDAC was performed at 15 °C to obtain a linear increase of imported protein with time. Proteins were separated by SDS-PAGE and transferred onto nitrocellulose membrane as described [34]. After transfer, the blot was dried at 37 °C for 30 minutes and exposed to an X-ray film (Biomax Film, Kodak) for detection of radioactive proteins.
2.7. Blue-Native PAGE
Mitochondrial proteins from the parental, TbTob55 KD, and TbTob55 over expressed cells were solubilized in 72 μl of the ice cold buffer containing 20 mM Tris, pH 7.0, 0.1 mM EDTA, 50 mM NaCl, 10% glycerol, 1 mM PMSF, 1 μg/ml leupeptin and 1% digitonin. The solubilized supernatants were clarified by centrifuging at 100,000 X g for 30 min at 4 °C. The supernatants were supplemented with 7.5 μl of sample buffer (750 mM amino caproic acid, 5% Coomassie blue) and were electrophoresed on a linear 6-16% polyacrylamide gradient gel [30, 43]. Protein complexes were detected by immunoblot analysis. Molecular size marker proteins apoferritin (400 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), and bovine serum albumin (66 kDa) were run on the same gel and visualized by Coomassie staining.
2.8. Mitotracker staining
Cells were harvested and suspended in fresh culture medium at a density of 2 × 106 /ml. Mitotracker Red CMXRos (Molecular probe) was dissolved in dimethyl sulfoxide at a concentration of 1 mM and added to a final concentration of 0.5 μM. The mixture was incubated at the normal growth temperature for 10 min. Cells were washed and incubated in fresh culture medium for additional 30 min. Cells were then washed twice with PBS and cell suspension was spread on a poly-lysine coated slides. Slides were washed with PBS to remove the unbound cells. Cells attached on the slides were fixed with 3.7% paraformaldehyde for 10 min. After a brief wash the slides were mounted in Fluoromount G and images were acquired with a Nikon TE200-U C1 laser scanning confocal microscope outfitted with a 60X 1.4 N.A. Plan Apo oil immersion objective lense. MitoTracker Red was excited with a 578 nm argon laser, and the emitted light was collected at 599 nm. All images were collected with exactly the same detector gain, laser strength, and number of scans in order for the data to be examined for differences in fluorescence intensity.
2.9. Electron microscopy
Cells were fixed in 2% (vol/vol) glutaraldehyde, and 2% (wt/vol) paraformaldehyde in 0.1 M sodium cacodylate buffer (SCB), pH 7.2. Cells were then washed with SCB, postfixed with 1% (wt/vol) osmium tetroxide in SCB, stained with 0.5% aqueous magnesium uranyl acetate, dehydrated, and embedded in Spurr’s resin [45]. Blocks were sectioned at 50-70 nm thickness and stained with 5% (wt/vol) uranyl acetate in 1% acetic acid, and 0.4% lead citrate in 0.1 N NaOH. Section grids were inserted into FEI CM12 twin lens 420 transmission electron microscope (FEI) to capture images.
3. Results
3.1. The Tob55 homolog is structurally conserved in T. brucei
A homolog of Tob55/Sam50 (Tb927.3.4380) was identified in T. brucei genome database Gene DB (www.genedb.org). Tb927.3.4380 contains an open reading frame of 1440 bp that encodes a protein of 53.5 kDa. A clustal W alignment of the predicted protein sequence with Tob55/Sam50 from different species showed 17-22% identity and 30-38% similarity (Fig. 1a and supplementary Table 1). Similar to Tob55 from other species TbTob55 shows more conservation in the C-terminal region than the N-terminal region. Pairwise alignment with human and fungal Tob55 proteins showed that TbTob55 possesses more similarity to the human Sam50 than the fungal counterpart (supplementary Table 1). To further confirm that the predicted protein sequence for Tb927.3.8340 is orthologous to other known Tob55 proteins, HMM analysis was carried out using twenty two characterized proteins belonging to this group. Subjecting this model to the T. brucei database recognized the same sequence. Orthologs of Tb927.3.4380 are syntenic in Trypanosoma cruzi and different Leishmania species e.g., L. major, L. infantum, and L. braziliensis. A phylogenetic analysis was also carried out using Tob55, Tom40 and Toc75 proteins from different species by maximum likelihood alignments (Fig. 1b). The Tob55, Tom40, and Toc75 are known to be involved in protein biogenesis in mitochondria and chloroplast, respectively. Since Tom40 has not been discovered in any of the kinetoplastids we wanted to determine the similarity of the putative TbTob55 with other Tob55 versus Tom40 homologs. Results revealed that TbTob55 is grouped with Tob55 from other species rather than Toc75s and Tom40s. All the known Tob55 and Toc75 proteins belong to the bacterial surface antigen family of proteins. Omp85 is one of the ancient members in this family [7]. TbTob55 is found closer to OMP85 in comparison to Tob55 from fungi and human (Fig. 1b).
Fig. 1.
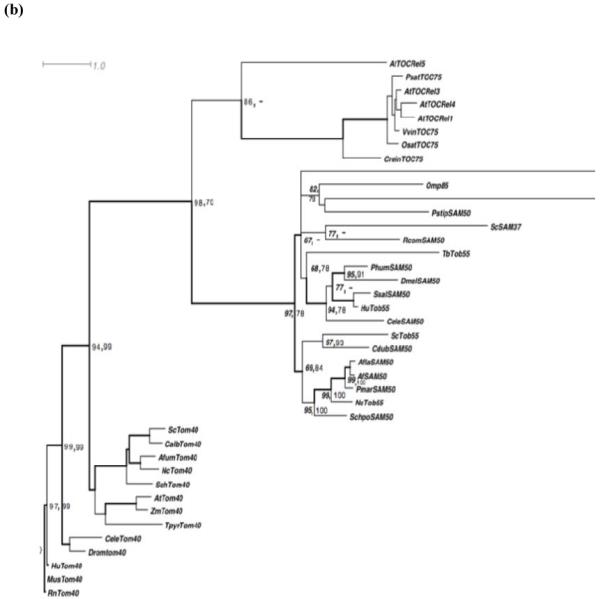
Analysis of the primary and secondary structures of Trypanosoma brucei Tob55. (a) ClustalW alignment of the predicted protein sequence of TbTob55 with that from other species. The accession number of the sequences are Trypanosoma brucei (Tb)Tob55; Tb927.3.4380, Saccharomyces cerevisiae (Sc)Tob55; DAA05204, Neurospora crassa (Nc)Tob55; AAS76651; and Homospienes (Hu)Tob55; NP056195. The conserved β-signal is boxed. (b) Phylogenetic analysis of Tob55, Toc75, and Tom40 from different species. Numbers at nodes are the maximum likelihood (left) and neighbor joining (right) values at bootstrap support of more than 50%. The thickened nodes represent Bayesian posterior probabilities greater than 0.95. The species and the accession number of proteins considered for analysis are as follows; AtTOCRel1, 3, 4 and 5: Arabidopsis thaliana, NP_174821, Q9STE8, Q5IZC8, Q9C5J8, respectively; PsatTOC75: Pisum sativum, Q43715; VvinTOC75: Vitis vinifera, CAN81047; OsatTOC75: Oryza sativa, Q84Q83; CreinTOC75: Chlamydomonas reinhardtii, EDP05963; ProtoTOC75: Prototheca wickerhamii, ABC24940; OMP85: Neisseria meningitides, NP_273240; BnatTOC75: Bigelowiella natans, ABA27321; PstipSAM50: Pichia stipitis, XP_001386142; ScSAM37: Saccharomyces cerevisiae, P50110; RcomSAM50: Ricinus communis, XP_002528327; TbTob55: Trypanosoma brucei, AAZ10471; PhumSAM50: Pediculus humanus corporis, XP_002432616; DmelSAM50: Drosophila melanogaster, Q9V784; SsalSAM50: Salmo salar, ACI66168; HuTob55: Homo sapiens, NP_056195; CeleSAM50: Caenorhabditis elegans, P46576; ScTob55: Saccharomyces cerevisiae, NP_014372; CdubSAM50: Candida dubliensiis, XP_002419475; AflaSAM50: Aspergillus flavus, XP_002385583; AfSAM50: Aspergillus fumigatus, EAL84610; PmarSam50: Penicillium marneffei, XP_002148120; NcTob55: Neurospora crassa, AAS76651; SchpoSAM50: Schizosaccharomyces pombe, NP_594600.1; ScTom40: Saccharomyces cerevisiae, P23644; CalbTom40: Candida albicans, XP_720849; AfumTom40: Aspergillus fumigatus, XP_747566; NcTom40: Neurospora crassa, P24391; SchTom40: Schizosaccharomyces pombe, O13656; AtTom40: Arabidopsis thaliana, Q9LHE5; ZmTom40: Zea mays, NP_001149485; TpyrTom40: Trimastix pyriformis, ABW76113; CeleTom40: Caenorhabditis elegans, Q18090; Dromtom40: Drosophila melanogaster, Q9U4L6; HuTom40: Homo sapiens, O96008; MusTom40: Mus musculus, Q9QYA2; and RnTom40: Rattus norvegicus, Q75Q40.
The secondary structure analysis using PRED-TMββ prediction tools revealed that TbTob55 possesses a β-barrel structure containing multiple β-strands. It also possesses the characteristic N-terminal α-helices, similar to Tob55 from fungi and human. Analysis of the predicted 3D-structure using TMB-Pro software program showed the structure of Tob55 from T. brucei, Saccharomyces cerevisiae and human to be similar (Supplementary Fig.1). Mitochondrial β-barrel proteins possess a sorting signal at the extreme C-terminal known as the β-signal, which consists of a conserved glycine and several hydrophobic residues [46]. During sorting of the β-barrel proteins in OM, the β-signal is recognized by Tob38, an essential component of the TOB complex. TbTob55 also possesses a similar signal in the last C-terminal β-strands (Fig.1a), suggesting that a similar sorting mechanism exists in T. brucei.
3.2. TbTob55 is localized in the mitochondrial outer membrane
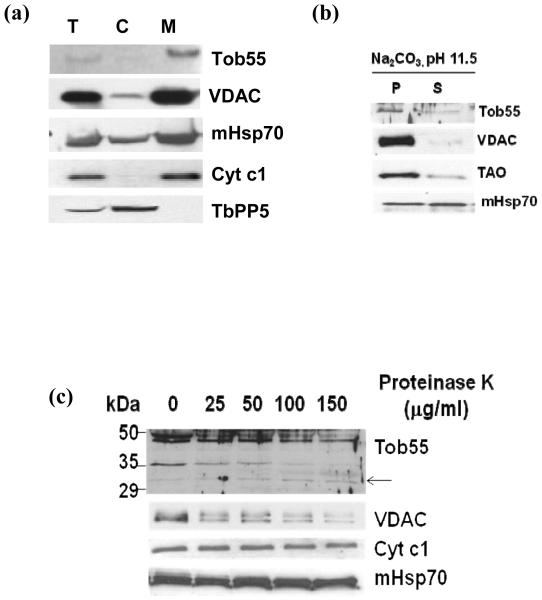
In order to verify the sub cellular location of TbTob55, cell fractionation followed by immunoblot analysis was performed. T. brucei procyclic cells were lysed in isotonic buffer and cytosolic and mitochondrial fractions were separated by differential centrifugation as described [43]. Analysis of the equal amount of proteins from total lysate, cytosolic and mitochondrial fractions by immunoblot using antibodies for S. cerevisiae Tob55 revealed that TbTob55 is enriched in the mitochondrial fraction similar to other mitochondrial proteins e.g., VDAC, heat shock protein 70 (mHsp70) and cytochrome c1 (Cyt c1) (Fig. 2a). Whereas, protein phosphatase 5 (TbPP5), a cytosolic protein is exclusively present in the cytosolic fraction as expected. This showed that TbTob55 is localized in mitochondria. The extraction of the mitochondrial protein with sodium carbonate at pH 11.5 followed by immunoblot analysis demonstrated that TbTob55 is primarily present in the alkali-resistant membrane pellet, similar to VDAC and trypanosome alternative oxidase (TAO). However, Hsp70, a matrix protein is found in the soluble fraction (Fig. 2b), suggesting that TbTob55 is membrane integrated. Proteinase K treatment of isolated T. brucei mitochondria followed by immunoblot analysis of mitochondrial proteins showed that the TbTob55 level is gradually decreased with an increasing concentration of Proteinase K (Fig. 2c). Antibodies against ScTob55 recognized a pair of bands with apparent molecular sizes of 48 and 49 kDa. Both are reduced during proteinase K treatment. These could be the isomeric forms of TbTob55. This antibody also recognized a 35 kDa protein band, which may be a cross-reacting protein or the degradation product of TbTob55 generated during the isolation process. A 30 kDa fragment of TbTob55 was detected when mitochondria were treated with the higher concentration of PK (50-150 μg/ml). The PK treatment of S. cerevisiae mitochondria also generates two fragments of Tob55, 25 kDa and 30 kDa (47). We did not notice the 25 kDa protein band after a similar treatment of T. brucei mitochondria however this could be due to weak detection of this band by ScTob55 antibody. Therefore, above results suggesting that the membrane topology of TbTob55 is similar to that in yeast. During PK treatment, the level of TbVDAC was reduced 50% and 70-80% at Proteinase K concentrations of 100 and 150 μg/ml, respectively. On the other hand, the levels of Tim17, Cyt c1, and mitochondrial Hsp70 remain unchanged during proteinase K digestion, clearly indicating that TbTob55 is localized in the MOM.
Fig. 2.
Sub-cellular localization of TbTob55. (a) Immunoblot analysis of sub cellular fractions using ScTob55-, TbVDAC-, mitochondrial Hsp70 (mHsp70)-, Cyt c1-, and TbPP5-antibodies as probes. Different fractions are denoted as total lysate: T, cytosol: C, and mitochondria: M. Ten micrograms of protein from each fraction were loaded per lane. (b) Sodium carbonate extraction followed by immunoblot analysis of mitochondrial proteins from T. brucei procyclic form. After treatment with Na2CO3 at pH 11.5, mitochondria were re-isolated by centrifugation. The supernatant: S and the pellet: P was analyzed using antibodies specific for ScTob55, TbVDAC, and mHsp70. (c) Proteinase K digestion of isolated mitochondria. Concentration of proteinase K is shown at the top. After digestion, mitochondria were re-isolated by centrifugation and proteins were analyzed by SDS-PAGE and immunoblot using ScTob55-, T. brucei VDAC-, Cyt c1-, and mHsp70-antibodies as indicated. The 30 kDa fragment of TbTob55 generated during proteinase K digestion was indicated by arrow.
3.3. TbTob55 is essential for cell survival
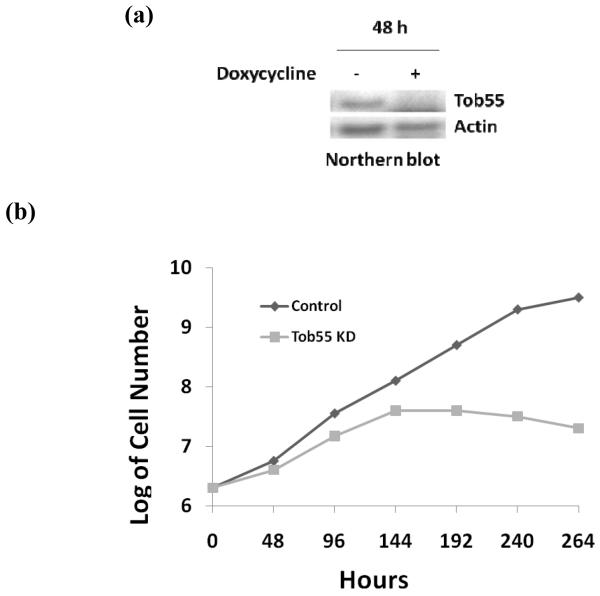
To understand the function of TbTob55, RNA interference studies were performed. Induction of the TbTob55 double stranded RNA in the procyclic form reduced its transcript level more than 95 % within 48 h (Fig. 3a). Depletion of TbTob55 affected cell growth dramatically. Induced cells grew at a reduced rate in comparison to uninduced control up to 96-144 h post induction. The growth of the induced cells ceased at day 144 h and after that it rapidly declined, whereas the uninduced control cells grew exponentially as expected (Fig. 3b). These results revealed that TbTob55 is essential for cell survival in the T. brucei procyclic form.
Fig. 3.
TbTob55 RNAi. The procyclic cell line that was transfected with the TbTob55 RNAi construct and selected by phleomycin was allowed to grow in presence (+) and absence (−) of doxycycline (1 μg/ml). (a) Cells were harvested at 48 h after induction with doxycycline for RNA analysis by Northern blot. Actin was used as the control. (b) The procyclic cell number was counted at different time points during induction and the log of cumulative cell number was plotted versus time. The results are representative of three independent experiments.
3.4. TbTob55 is crucial for mitochondrial protein biogenesis
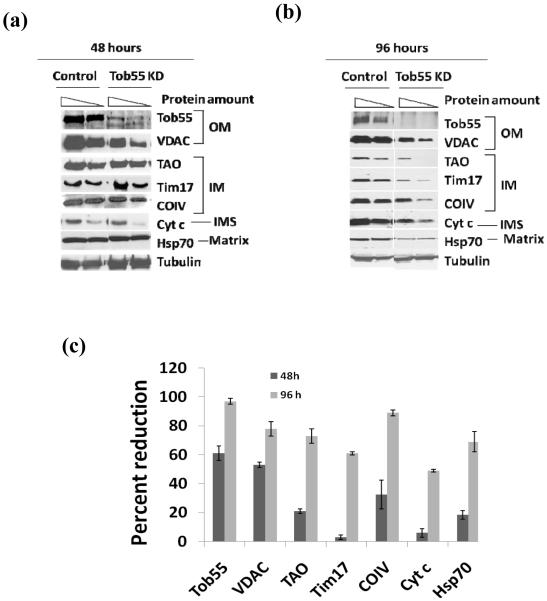
To see the effect of TbTob55 depletion on the level of mitochondrial proteins a semi-quantitative immunoblot analysis was performed using mitochondria isolated from TbTob55 RNAi cells grown for 48 and 96 h in the presence and absence of doxycycline. Our results revealed that TbTob55 depletion decreased the level of VDAC more than 50% within 48 h (Fig. 4a and c). In contrast to VDAC, the levels of several other nucleus encoded mitochondrial proteins such as TAO, Tim17, Cyt c, and mHsp70 were reduced only 5-20% within 48 h, except for the level of COIV, which was decreased more than 30% at this time point (Fig. 4a and c). However, a continued induction of TbTob55 RNAi for 96 h showed 60-80% reduction of most of these protein levels including VDAC (Fig. 4b and c). These results indicated that Tob55 depletion primarily affects the level of VDAC, an MOM β-barrel protein, however it also reduced gradually many other nucleus encoded proteins destined for the inner membrane, inter membrane space, and matrix in mitochondria. During this 96 h time period, the TbTob55 RNAi induced cells were in the growth phase. Therefore it is unlikely that the reduction in the levels of the above proteins was due to cell morbidity. In addition, we analyzed total cellular proteins by immunoblot after growing TbTob55 RNAi cells at various concentrations of doxycycline (0 to 1 μg/ml) for 96 h, we did not find a significant reduction in the levels of proteins that are located either in mitochondria or in the cytosol (Supplementary Fig. 2), except for VDAC, which was reduced about 30-40% at a doxycycline concentration of 1 μg/ml. Together, these results suggest that TbTob55 depletion did not have an overall effect on cellular protein biosynthesis.
Fig. 4.
The effect of TbTob55 RNAi on the levels of nucleus encoded proteins in mitochondria. The TbTob55 RNAi induced with 1.0 μg/ml of doxycycline and the uninduced control cells were grown for 48 h (a) and 96 h (b) and mitochondria were isolated as described in the materials and methods. Mitochondrial proteins were analyzed by immunoblot analysis using various antibody probes as follows; the outer membrane (OM) proteins: Tob55 and VDAC; the inner membrane (IM) proteins: TAO, COIV, and Tim17; intermembrane space (IMS) protein: Cyt c; and the matrix localized mHsp70. Mitochondria-associated protein β-tubulin was used as the control. (c) Estimation of the percent reduction of the levels of various mitochondrial proteins in TbTob55 KD mitochondria in comparison to that in the uninduced control. The intensity of the respective protein bands was quantitated using imaging densitometry as described in Section 2 and normalized with the corresponding β-tubulin protein bands. Percent reduction of the level of proteins in TbTob55 KD mitochondria in comparison to that in the control mitochondria were calculated for each protein. The histogram shows the results of three independent experiments.
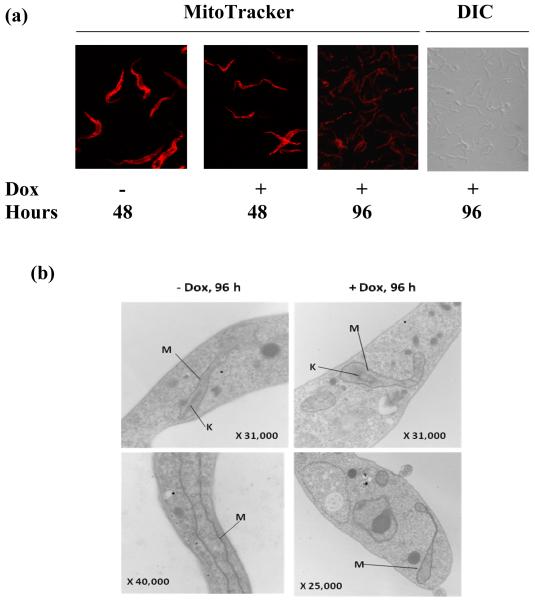
To visualize if there is any effect of Tob55 RNAi on mitochondrial morphology, we stained cells with MitoTracker, a specific dye, which accumulates in mitochondria depending on the presence of a mitochondrial membrane potential. During 48h of induction of TbTob55 RNAi, dye accumulation in mitochondria was reduced. However, the reticular structure of mitochondria remained unaltered. Induction of RNAi for 96h showed a significant reduction of MitoTracker staining. As seen in Fig.5a, the staining pattern appeared dotted rather than a continuous thread-like structure. These results indicate that TbTob55 depletion causes a reduction in the mitochondrial membrane potential possibly due to the reduction of several respiratory proteins as seen in our previous experiment. As shown in the phase-contrast image, the overall cell morphology was not altered at 96h of induction of TbTob55 RNAi (Fig. 5a). To examine further mitochondrial structure, we performed electron microscopy. We found double membrane-containing tubular mitochondrial structure with cristae in both induced and uninduced cells. We did not notice any differences in the kDNA structures inside mitochondria in induced versus uninduced cells (Fig. 5b), suggesting that TbTob55 RNAi induction for 96 h didn’t have any effect on mitochondrial structure. Therefore, a simultaneous reduction in the levels of several nuclear encoded proteins in mitochondria at 96h of induction of TbTob55 RNAi is possibly due to the reduction of import of these proteins into mitochondria.
Fig. 5.
The effect of TbTob55 RNAi on mitochondrial structure and membrane potential. (a) MitoTracker staining of the T. brucei procyclic TbTob55 RNAi cells uninduced (− Dox) and induced (+ Dox) for 48 and 96 h. Cells were stained with MitoTracker Red, fixed with paraformaldehyde and visualized by Confocal microscopy. A phase contrast image (DIC) of the cells induced for 96 h were presented to show that no significant changes observed in cell morphology. (b) Electron microscopic images of the TbTob55 RNAi cells uninduced (−Dox) and induced (+Dox) for 96 h. Mitochondria (M) and the kDNA (K) were indicated by solid lines. Magnification scales were indicated at the bottom right corner of the images.
3.5. Effect of TbTob55 depletion on mitochondrial protein import
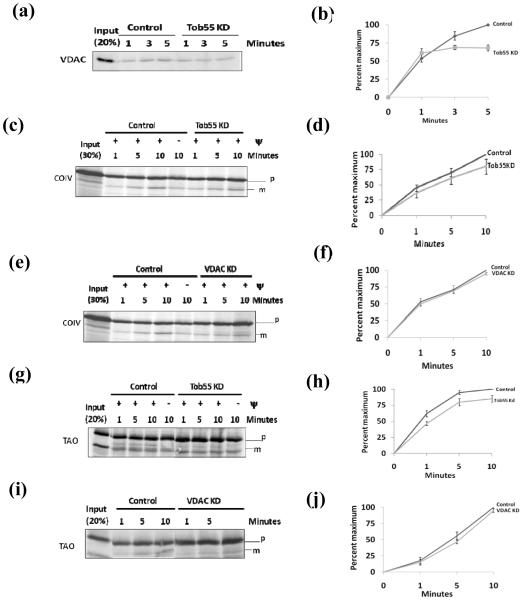
In vitro import assays were performed to understand the role of TbTob55 on mitochondrial protein biogenesis. VDAC, the MOM β-barrel protein and the presequence-containing proteins, such as COIV and TAO, were used as the substrate for in vitro import assays. Mitochondria were isolated from cells uninduced and induced for 48 h for TbTob55 RNAi. The induction period of 48 h was selected because at this time point other mitochondrial proteins and mitochondrial membrane potential are minimally affected. Import and membrane integration of VDAC was assessed by post-import alkali-extraction. We found that VDAC was imported in a time-dependent manner in control mitochondria, however, almost no increase of import was observed with time in TbTob55-depleted mitochondria (Fig. 6a). Quantitation of our results revealed that import of VDAC was reduced about 40% within 5 min into mitochondria isolated from cells induced for Tob55 RNAi in comparison to mitochondria from the uninduced control (Fig. 6b).
Fig. 6.
The effect of TbTob55 RNAi on mitochondrial protein import. (a) In vitro import of radiolabelled VDAC in mitochondria isolated from TbTob55 RNAi induced (TbTob55 KD) and uninduced (Control) cells. After import mitochondria were extracted with 0.1 M Na2CO3 and analyzed by SDS-PAGE and autoradiography. Input lanes show the percentage of radioactive protein loaded in each reaction. (b) The intensity of the imported proteins were quantitated and presented as the percent of maximum import in control. In vitro import of radiolabelled COIV (c-f) and TAO (g-j) into mitochondria isolated from Tob55 KD and control cells (c and g) and mitochondria from VDAC KD and respective uninduced control cells (e and i), respectively. Aliquots were collected at different time points (1-10 minutes). Mitochondria were re-isolated by centrifugation at 4 °C, washed with ice cold buffer, and analyzed by autoradiography. For disruption of mitochondrial membrane potential (Ψ), mitochondria were preincubated with valinomycin (5 μM) and CCCP (50 μM) for 10 minutes at 25 °C before the addition of radiolabelled proteins. The precursor (p) and the matured (m) protein bands were visualized by SDS-PAGE and autoradiography. The input lanes represent the percentage of radiolabelled precursor proteins used for each reaction. The intensity of the matured COIV (c and e) and TAO (g and i) protein bands was quantitated from three independent experiments for each sets by densitometry. The intensity of the matured proteins in the respective control mitochondria at 10 minutes was used as maximum (100%) and the calculated percent of maximum import into the Tob55 KD (d and h), and VDAC KD (f and j) mitochondria was plotted versus time.
The T. brucei COIV possess a cleavable presequence at the N-terminus consisting of 44 amino acids [49]. We found that COIV is imported into mitochondria and produced a mature protein of expected size (Fig. 6 c-f). The intensity of the precursor protein bands indicates its binding capacity to the mitochondria and the intensity of the processed matured protein indicates the efficiency of the import. The lower molecular size proteins detected in the input lanes were possibly generated from internal start sites during in vitro translation. In the control mitochondria, formation of mature COIV was time-dependent and inhibited upon disruption of mitochondrial membrane potential as expected. Similar to the uninduced control, in Tob55 knockdown (KD) mitochondria the precursor COIV bound and processed to the mature form, an effect which increased with time (Fig. 6c). Quantitation of the imported COIV as a percent of maximum import in the control mitochondria showed that the reduction was about 15-20% (Fig. 6d). Longer incubation did not show any further reduction of the COIV import into TbTob55 KD mitochondria in comparison to controls. To assess if TbVDAC plays any role in the import of COIV, in vitro import analysis was also performed in parallel using mitochondria isolated from cells, where VDAC protein level was depleted about 50% by RNAi (30). VDAC depletion showed no effect on the level of TbTob55 (not shown). In vitro import analysis revealed that the precursor protein binding and processing were not inhibited due to depletion of VDAC (Fig. 6e and f).
In vitro import of TAO into Tob55 KD, VDAC KD, and the respective control mitochondria was performed to assess the effect of depletion of Tob55 and VDAC on import of another presequence-containing protein. TAO possesses a predicted N-terminal presequence of 24 amino acid residues. TAO precursor protein was imported and processed to its matured form in a time-dependent manner (Fig. 6g-j). Quantitation of the imported protein bands revealed that depletion of Tob55 reduced TAO import about 20% (Fig. 6g and h), whereas VDAC knockdown did not show any effect on TAO import (Fig. 6i and j).The overall import rate of TAO in VDAC KD and the respective control mitochondria was relatively less in comparison to rates in Tob55 KD and the respective control mitochondria. This is possibly due to differences in the quality of mitochondria or other factors. Together, these results indicated that Tob55 is required for mitochondrial protein import.
3.6. TbTob55 is crucial for VDAC assembly
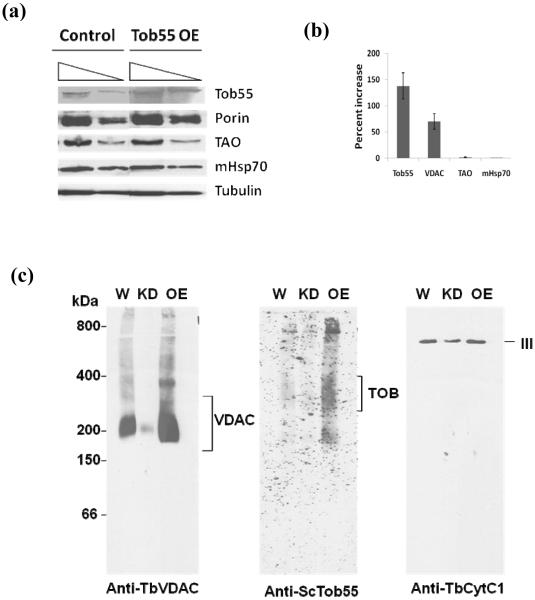
The VDAC is the only OM β-barrel protein identified so far in trypanosomes [30, 48]. The T. brucei VDAC is crucial for mitochondrial metabolite transport and thus essential for its energy production. Since in other eukaryotes Tob55 is required for the assembly of the OM β-barrel proteins we tested further the role of TbTob55 on biogenesis of VDAC complex. We found that TbTob55 over expression increased the level of VDAC about 2 fold in comparison to that in control mitochondria (Fig. 7a and b). However, the levels of TAO and mHsp70 were unaltered due to an increased level of TbTob55. This further suggests that TbTob55 expression is directly correlated with VDAC biogenesis. Next we analyzed the effect of TbTob55 KD and over expression on VDAC protein complex by BN-PAGE. T. brucei VDAC was present in an apparent 212 kDa complex as detected by BN-PAGE of wild type mitochondria. TbTob55 KD depleted this complex almost 90% (Fig. 7c). However, over expression of TbTob55 increased the level of this complex about 2 fold. In addition, higher molecular size complexes of VDAC were also found in the mitochondria sample where TbTob55 was over expressed. Probing the blot with ScTob55 antibody revealed that TbTob55 is present in a protein complex showing a broad band around 250-300 kDa. In wild type mitochondria, this protein complex was recognized weakly by ScTob55 antibody. The intensity of this band diminished in TbTob55 KD mitochondria and increased about 1.5 fold when TbTob55 is over expressed (Fig. 7c), verifying that this protein complex possesses TbTob55. Besides this major band there are few minor bands of around 180 kDa and 800 kDa, which were seen particularly in the OE lane; these may be the disintegration and aggregation products of this complex, produced during preparation of the sample. Furthermore, the result also showed that ectopically expressed TbTob55 is assembled in the endogenous TOB complex. The Cyt c1 antibody detected the Complex III or the bc1 reductase complex at about 670 kDa, as expected [50]. In TbTob55 KD mitochondria, the intensity of this complex slightly decreased (Fig. 7c) since a relatively lower level of Cyt c1 is present in these mitochondria (not shown). TbTob55 over expression did not show any significant effect on the level of complex III. All these results clearly indicate that similar to other eukaryotes, TbTob55 is involved in the membrane integration and assembly of VDAC in the MOM.
Fig. 7.
TbTob55 is required for VDAC assembly. An ectopic copy of TbTob55 was expressed from an inducible expression vector in the procyclic form. Mitochondria were isolated from the uninduced (Control) and induced (Tob55 OE) cells. (a) Mitochondrial proteins were analyzed by immunoblot using antibodies for T. brucei VDAC, TAO, mHsp70, tubulin, and S. cerevisiae Tob55. (b) The intensity of the respective protein bands was quantitated using imaging densitometry as described in Section 2 and normalized with the corresponding β-tubulin protein bands. Percent increase of the level of proteins in TbTob55 OE mitochondria in comparison to that in the control mitochondria were calculated for each protein. The histogram shows the results of three independent experiments. (c) Mitochondria (100 μg) isolated from the wild type control (W), TbTob55 knock-down (KD), and TbTob55 over-expressed (OE) cells were solubilized with digitonin (1.0%). The solubilized supernatants were clarified by centrifugation at 100,000 X g. The samples were electrophoresed on a Blue-Native PAGE and immunodecorated with antibodies specific for ScTob55, TbVDAC, and TbCyt c1. Molecular size marker proteins apoferritin dimer (800 kDa), apoferritin monomer (400 kDa), β-amylase (200 kDa), alcohol dehydogenase (150 kDa), and bovine serum albumin (66 kDa) were run on the same gel and visualized by coomassie staining. The VDAC, TOB, and the respiratory complex III were indicated by the side of the gel.
4. Discussion
We elucidated the role of TbTob55 on mitochondrial protein biogenesis in T. brucei. Similar to other eukaryotes, TbTob55 is involved in mitochondrial OM β-barrel protein biogenesis and is essential for cell growth. TbTob55 depletion causes reduction in the levels of several nuclear encoded mitochondrial proteins and it is indirectly involved in the import of presequence containing precursor proteins.
The homolog of Tob55 in T. brucei showed a significant similarity in its predicted primary, secondary, and tertiary structure with that from other species. The phylogenetic analysis also showed that TbTob55 belongs to the group of Tob55 from various organisms and not within the group of Tom40 proteins. Tom40 and Tob55 are both β-barrel mitochondrial OM proteins. However, Tom40 and Tob55 possess two distinct functions in mitochondrial protein import in fungi and humans [51, 52]. Various searches in trypanosome genome databases failed to identify either the homolog of Tom40 or any other components of the TOM complex, suggesting either these proteins are absent or highly divergent in trypanosomes.
Here we found that the TbTob55 is localized in the MOM. The antibody developed against yeast Tob55 recognized the T. brucei Tob55 protein, which further supported its identity. Protease digestion of isolated mitochondria followed by immunoblot revealed that TbTob55 possesses a similar membrane topology with Tob55 in yeast mitochondria. TbTob55 was found to be essential for cell survival. Depletion of TbTob55 protein ceased cell growth within 144 h in culture and the cell number decreased upon further incubation of the RNAi cells in the presence of doxycycline.
TbTob55 depletion and over expression concomitantly reduced and increased the level of VDAC in mitochondria, respectively, suggesting that similar to other eukaryotes TbTob55 is involved in β-barrel protein biogenesis. Although, the primary effect is on VDAC, induction of TbTob55 RNAi for 96h reduced the levels of several other nuclear encoded proteins in mitochondria. However, TbTob55 depletion did not show any effect on cytosolic proteins like TbPP5 and tubulin which clearly indicates that TbTob55 is linked to the biogenesis of proteins in mitochondria. In other eukaryotes, Tob55 depletion reduced the level of Tom40, the major protein translocator of mitochondrial OM. Although the ortholog of Tom40 was not found in trypanosome genome databases we couldn’t exclude the possibility that a divergent homolog of Tom40 exists in T. brucei. Therefore, it is possible that TbTob55 depletion reduced the level of the unidentified TbTom40 and thus reduced the import of mitochondrial proteins. The other possibility is that TbTob55 acts as the protein translocator in T. brucei MOM.
In vitro import analysis of VDAC into mitochondria showed that import of VDAC is significantly reduced due to depletion of TbTob55, which further indicates the involvement of TbTob55 in VDAC biogenesis. We also found that the import of the two presequence containing proteins destined for the inner membrane is partially inhibited due to TbTob55 RNAi. Knockdown of VDAC did not show any inhibition of the import of these proteins into mitochondria revealing that the effect of TbTob55 RNAi is specific. For in vitro import assays, we isolated mitochondria from cells harvested at 48h post-induction to reduce the secondary effects that may be caused by reduction of other mitochondrial proteins as well as reduction of mitochondrial membrane potential as a consequence of TbTob55 RNAi. Therefore, inhibition of import of COIV and TAO is most likely not due to these secondary effects. However, even at this earlier time point, VDAC level was decreased about 50% while TbTob55 was reduced about 60-65%. Therefore it is likely that another unidentified MOM β-barrel protein could be reduced, which may be acting as the major protein translocator. Furthermore, the range of inhibition that we found for import of COIV and TAO is not sufficiently high to conclude that TbTob55 is acting as the protein translocator in the MOM of T. brucei. Our previously published results with Tim17 knockdown mitochondria showed about 70-80% inhibition of import for these proteins in comparison to the respective control mitochondria [6]. Therefore, it is highly likely that TbTob55 is indirectly involved for the import of preproteins such as COIV and TAO.
Furthermore, TbTob55 depletion not only reduced the steady state level of VDAC but also reduced the level of the matured complex of VDAC. In addition, upregulation of TbTob55 level by expression of an ectopic copy of TbTob55 increased the level and sizes of the VDAC oligomeric complexes, which clearly indicates that TbTob55 plays a role in MOM β-barrel protein assembly. On a BN-PAGE, TbTob55 is found in a protein complex with an apparent molecular size of 250-300 kDa, which is similar to the size of TOB complex found in fungi [13]. This protein complex possibly contains other proteins besides Tob55.
Together, these results indicate TbTob55 is crucial for mitochondrial protein biogenesis thus essential for cell survival. Similar to other eukaryotes, TbTob55 is involved in the assembly of the MOM β-barrel proteins. Since VDAC is not involved in mitochondrial protein translocation and TbTob55 RNAi showed an auxiliary effect for this process, it suggest that another β-barrel protein, most likely a divergent homolog of Tom40 is present in T. brucei, which is possibly acting as the major protein importer of the MOM.
Supplementary Material
Acknowledgements
We thank George Cross and Elizabeth Wirtz for pLew100 vector, Pro427 (29-13) and SM427 cell lines, Doron Rapaport for ScTob55 antibody, Paul Englund for Crithidia Hsp70 antibody, Steve Hajduk for anti-Cyt c1, Julius Lukes for anti-COIV, Andre Schneider for anti-Cyt c, Laurie Read for pLew-Myc vector, Keith Gull for tubulin antibody and p2T7-177 RNAi vector. The morphology core facility in Meharry Medical College is supported by grants U54NS041071. Electron Microscopy work and data analysis were performed in part through use of the VUMC Cell Imaging Shared Resource. The work was supported by NIH Grants 3SO6GM08037-30S1 and 1SC1GM081146.
Abbreviations
- MOM
Mitochondrial outer membrane
- VDAC
voltage dependent anion channel
- TbTob55
T. brucei Tob55
- TAO
Trypanosome alternative oxidase
- COIV
cytochrome oxidase subunit 4
- RNAi
RNA interference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Discloser Statement: The authors have no conflicts of interest to disclose
References
- [1].World Health Organization Human African trypanosomiasis (sleeping sickness); epidemiological update. Wkly Epidemiol Rec. 2006;81:71–80. [PubMed] [Google Scholar]
- [2].Simpson AG, Inagaki Y, Roger AJ. Comprehensive multigene phylogenies of excavate protests reveal the evolutionary positions of primitive eukaryotes. Mol Biol Evol. 2005;23:615–25. doi: 10.1093/molbev/msj068. [DOI] [PubMed] [Google Scholar]
- [3].Lukes J, Hashimi H, Zikova A. Unexplained complexity of the mitochondrial genome and transcriptome in kinetoplastid flagellates. Curr Genet. 2005;48:277–99. doi: 10.1007/s00294-005-0027-0. [DOI] [PubMed] [Google Scholar]
- [4].Schneider A, Bursać D, Lithgow T. The direct route: a simplified pathway for protein import into the mitochondrion of trypanosomes. Trends Cell Biol. 2008;18:12–18. doi: 10.1016/j.tcb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- [5].Gentle IE, Perry AJ, Alcock FH, Likic VA, Dolezal P, Ng ET, Purcell AW, McConnville M, Naderer T, Chanez AL, Charier F, Aschinger C, Schneider A, Tokatlidis K, Lithgow T. Conserved motifs reveal details of ancestry and structure in the small TIM chaperones of the mitochondrial inter membrane space. Mol Biol Evol. 2007;24:1149–60. doi: 10.1093/molbev/msm031. [DOI] [PubMed] [Google Scholar]
- [6].Singha UK, Peprah E, Williams S, Walker R, Saha L, Chaudhuri M. Characterization of the mitochondrial inner membrane translocator Tim17 from Trypanosoma brucei. Mol Biochem Parasitol. 2008;159:30–43. doi: 10.1016/j.molbiopara.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gentle IE, Burri L, Lithgow T. Molecular architecture and function of the Omp85 family of proteins. Mol Microbiol. 2005;58:1216–25. doi: 10.1111/j.1365-2958.2005.04906.x. [DOI] [PubMed] [Google Scholar]
- [8].Paschen SA, Neupert W, Rapaport D. Biogenesis of beta-barrel membrane proteins of mitochondria. Trends Biochem Sci. 2005;30:575–82. doi: 10.1016/j.tibs.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [9].Neupert W, Herrmann JM. Translocation of Proteins into Mitochondria. Ann Rev Biochem. 2007;76:723–49. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- [10].Rapaport D. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J Cell Biol. 2005;171:419–23. doi: 10.1083/jcb.200507147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Colombini M, Blachly-Dyson E, Forte M. VDAC, a channel in the outer membrane. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- [12].Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator-thinking outside the box. Biochim Biophys Acta. 2006;1762:181–90. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- [13].Becker T, Vogtle FN, Stojanovski D, Meisinger C. Sorting and assembly of mitochondrial outer membrane proteins. Biochim Biophys Acta. 2008;1777:557–63. doi: 10.1016/j.bbabio.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [14].Koziak V, Wiedemann N, Milenkovic D, Meyer HE, Guiard B, Meisinger C, Pfanner N. An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem. 2003;278:48520–23. doi: 10.1074/jbc.C300442200. [DOI] [PubMed] [Google Scholar]
- [15].Meisinger C, Rissler M, Chacinska A, Szklarz LK, Milenkovic D, Koziak V, Schonfisch B, Lohaus C, Meyer HE, Yaffe MP, Guiard B, Wiedemann N, Pfanner N. The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev Cell. 2004;7:61–71. doi: 10.1016/j.devcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- [16].Rapaport D. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J Cell Biol. 2005;171:419–23. doi: 10.1083/jcb.200507147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nachez-pulido L, Devos D, Genevrois P, Vicente M, Valencia A. POTRA: a conserved domain in the FtsQ family and a class of β-barrel outer membrane proteins. Trends Biochem Sci. 2003;28:523–26. doi: 10.1016/j.tibs.2003.08.003. [DOI] [PubMed] [Google Scholar]
- [18].Schleiff E, Soll J. Membrane protein insertion: mixing eukaryotic and prokaryotic concepts. EMBO Rep. 2005;6:1023–27. doi: 10.1038/sj.embor.7400563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oreb M, Tews I, Scleiff E. Policing Tic ‘n’ Toc, the doorway to chloroplast. Trend Cell Biol. 2007;18:19–27. doi: 10.1016/j.tcb.2007.10.002. 2007. [DOI] [PubMed] [Google Scholar]
- [20].Biebinger S, Wirtz LE, Lorenz P, Clayton C. Vectors for inducible expression of toxic gene products in bloodstream and procyclic Trypanosoma brucei. Mol Biochem Parasitol. 1997;85:99–112. doi: 10.1016/s0166-6851(96)02815-0. [DOI] [PubMed] [Google Scholar]
- [21].Higgins DG, Thompson, Gibson TJ. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. 1996. [DOI] [PubMed] [Google Scholar]
- [22].Bagos PG, Liakopoulos TD, Spyropoulos IC, Hamodrakas AJ. PRED-TMββ: a web-sever for predicting the topology of β-barrel outer membrane proteins. Nuc. Acid. Res. 2005;32:W400–4. doi: 10.1093/nar/gkh417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Randall A, Cheng J, Sweredoski M, Baldi P. TMBPro: secondary structure, beta contact and tertiary structure prediction of transmembrane beta-barrel proteins. Bioinformatics. 2008;24:513–20. doi: 10.1093/bioinformatics/btm548. [DOI] [PubMed] [Google Scholar]
- [24].Guindon S, Gascuel S. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- [25].Strimmer K, von Haeseler A. Quartet puzzling: A quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–9. [Google Scholar]
- [26].Bruno WJ, Socci ND, Halpern AL. Weighted neighbor joining: a likelihood-based approach to distance-based phylogeny reconstruction. Mol Biol Evol. 2000;17:189–97. doi: 10.1093/oxfordjournals.molbev.a026231. [DOI] [PubMed] [Google Scholar]
- [27].Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–5. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- [28].Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics. 2007;8:460. doi: 10.1186/1471-2105-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wickstead B, Ersfeld K, Gull K. Targeting of a tetracycline-inducible expression system to the transcriptionally silent minichromosomes of Trypanosoma brucei. Mol Biochem Parasitol. 2002;125:211–6. doi: 10.1016/s0166-6851(02)00238-4. [DOI] [PubMed] [Google Scholar]
- [30].Singha UK, Sharma S, Chaudhuri M. Downregulation of mitochondrial porin inhibits cell growth and alters respiratory phenotype in Trypanosoma brucei. Euk Cell. 2009;8:1418–28. doi: 10.1128/EC.00132-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hayman ML, Read LK. Trypanosoma brucei RBP16 is a mitochondrial Y-box family protein with guide RNA binding activity. J Biol Chem. 1999;274:12067–74. doi: 10.1074/jbc.274.17.12067. [DOI] [PubMed] [Google Scholar]
- [32].Sambrook J, Fritsch E, Maniatis T. Molecular cloning, A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor NY: 1994. [Google Scholar]
- [33].Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [34].Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some application. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Priest JW, Hajduk SL. Developmental regulation of Trypanosoma brucei cytochrome c reductase during bloodstream to procyclic differentiation. Mol Biochem Parasitol. 1994;65:291–304. doi: 10.1016/0166-6851(94)90080-9. [DOI] [PubMed] [Google Scholar]
- [36].Maslov DA, Zinkova A, Kyselova I, Lukes J. A putative novel nuclear-encoded subunit of the cytochrome c oxidase complex in trypanosomatids. Mol Biochem Parasitol. 2002;125:113–25. doi: 10.1016/s0166-6851(02)00235-9. 2002. [DOI] [PubMed] [Google Scholar]
- [37].Esseiva AC, Chanez AL, Bochud-Allemann N, Martinou JC, Hemphil A, Schneider A. Temporal dissection of Bax-induced events leading to fission of the single mitochondrion in Trypanosoma brucei. EMBO Rep. 2004;5:268–73. doi: 10.1038/sj.embor.7400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Effron PN, Torri AF, Engman DM, Donelson JE, Englund PT. A mitochondrial heat shock protein from Crithidia fasciculata. Mol Biochem Parasitol. 1993;59:191–200. doi: 10.1016/0166-6851(93)90217-l. [DOI] [PubMed] [Google Scholar]
- [39].Chaudhuri M. Cloning and characterization of a novel serine/threonine protein phosphatase type 5 from Trypanosoma brucei. Gene. 2001;266:1–13. doi: 10.1016/s0378-1119(01)00367-5. [DOI] [PubMed] [Google Scholar]
- [40].Habib SJ, Waizenegger T, Lech M, Neupert W, Rapaport D. Assembly of the TOB complex of mitochondria. J Biol Chem. 2005;280:6434–40. doi: 10.1074/jbc.M411510200. [DOI] [PubMed] [Google Scholar]
- [41].Chaudhuri M, Ajayi WU, Hill GC. Biochemical and Molecular properties of the Trypanosoma brucei alternative oxidase. Mol Biochem Parasitol. 1998;95:53–68. doi: 10.1016/s0166-6851(98)00091-7. [DOI] [PubMed] [Google Scholar]
- [42].Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- [43].Chaudhuri M, Nargang FE. Import and assembly of Neurospora crassa Tom40 into mitochondria of Trypanosoma brucei in vivo. Curr Genet. 2003;44:85–94. doi: 10.1007/s00294-003-0427-y. [DOI] [PubMed] [Google Scholar]
- [44].Williams S, Saha L, Singha UK, Chaudhuri M. Trypanosoma brucei: Differential requirement of membrane potential for import of proteins into mitochondria in two developmental stages. Exp Parasitol. 2008;118:420–33. doi: 10.1016/j.exppara.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hyde GJ, Davis DS, Cole L, Ashford AE. Retention of fluorescent probes during aldehyde free anhydrous freeze-substitution. J Microscience. 2003;210:125–130. doi: 10.1046/j.1365-2818.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- [46].Kutik S, Stojanovski D, Becker L, Becker T, Meinecke M, Kruger V, Prinz C, Meisinger C, Guiard B, Wagner R, Pfanner N, Wiedemann N. Dissecting membrane insertion of mitochondrial β-barrel proteins. Cell. 2008;132:1011–24. doi: 10.1016/j.cell.2008.01.028. [DOI] [PubMed] [Google Scholar]
- [47].Habib SJ, Waizenegger T, Niewienda A, Paschen SA, Neupert W, Rapaport D. The N-terminal domain of Tob55 has a receptor-like function in the biogenesis of mitochondrial β-barrel proteins. J Cell Biol. 2007;176:77–88. doi: 10.1083/jcb.200602050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pusnik M, Charriere F, Maser P, Waller RF, Dagley MJ, Lithgow T, Schneider A. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol Biol Evol. 2009;26:671–80. doi: 10.1093/molbev/msn288. [DOI] [PubMed] [Google Scholar]
- [49].Mayho M, Fenn K, Cruddy P, Crosthwaite S, Matthews K. Post-transcriptional control of nuclear-encoded cytochrome oxidase subunits in Trypanosoma brucei: evidence for genome-wide conservation of life-cycle stage-specific regulatory elements. Nuc Acids Res. 2006;34:5312–24. doi: 10.1093/nar/gkl598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zikova A, Horakova E, Jirku M, Dunajcikova P, Lukes J. The effect of down-regulation of mitochondrial RNA-binding proteins MRP1 and MRP2 on respiratory complex in procyclic Trypanosoma brucei. Mol Biochem Parasitol. 2006;149:65–73. doi: 10.1016/j.molbiopara.2006.04.007. [DOI] [PubMed] [Google Scholar]
- [51].Ahting U, Thieffry M, Engelhardt H, Hegerl R, Neupert W, Nussberger S. Tom40, the pore-forming component of the protein conducting TOM channel in the outer membrane of mitochondria. J Cell Biol. 2001;153:1151–60. doi: 10.1083/jcb.153.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Becker L, Bannwarth M, Meisinger C, Hill K, Model K, Krimmer T, Casadio R, Truscott KN, Schulz GE, Pfanner N, Wagner R. Preprotein translocase of the outer mitochondrial membrane: reconstituted Tom40 forms a characteristic TOM pore. J Mol Biol. 2005;353:1011–20. doi: 10.1016/j.jmb.2005.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.