Abstract
Cytokinesis is regulated to ensure the precise partitioning of cytoplasm and duplicated chromosomes to daughter cells. The NACK-PQR pathway, which includes NACK1 kinesin-like protein (KLP) and a mitogen-activated protein kinase (MAPK) cascade, plays a key role in cytokinesis in tobacco cells. Although HINKEL/AtNACK1 (HIK) KLP, ANP MAP kinase kinase kinases (MAPKKKs) and MKK6/ ANQ MAP kinase kinase (MAPKK) have been identified independently as regulators of cytokinesis in Arabidopsis thaliana, the involvement of HIK, ANPs and MKK6/ANQ in a regulatory cascade remains to be demonstrated. Here we provide details of the protein kinase pathway that controls cytokinesis in A. thaliana. Analysis of the subcellular distribution of six MAPKKs of A. thaliana that had been fused to green fluorescent protein revealed that only MKK6/ANQ protein was concentrated at the equatorial plane of the phragmoplast, at the site of localization of HIK. Expression of MKK6/ANQ in yeast cells replaced the growth-control function of the MAPKK encoded by yeast PBS2, provided that both ANP1 MAPKKK and HIK [or TETRASPORE/AtNACK2 (TES)] KLP were coexpressed, suggesting that ANP1 activates MKK6/ANQ in the presence of HIK (or TES). Coexpression of HIK and ANP3 (another member of the ANP MAPKKK family) weakly activated MKK6/ANQ but that of TES and ANP3 did not. MKK6/ANQ phosphorylated MPK4 MAPK in vitro to activate the latter kinase. Thus cytokinesis in A. thaliana is controlled by a pathway that consists of ANP MAPKKKs that can be activated by HIK and MKK6/ANQ MAPKK, with MPK4 MAPK being a probable target of MKK6/ANQ.
Keywords: Arabidopsis, Cytokinesis, Kinesin-like protein, MAP kinase cascade, Phragmoplast, Tobacco
Introduction
Cytokinesis is essential for the appropriate distribution of cytoplasmic components and chromosomes to daughter cells. Cytokinesis of plant cells requires a plant-specific structure known as the phragmoplast, which consists mainly of complex arrays of microtubules (MTs) and microfilaments and expands centrifugally to the parental cell walls. Expansion of the phragmoplast is mediated by depolymerization of MTs within the phragmoplast and polymerization of tubulins at its periphery (Asada et al. 1991, Hush et al. 1994, Nishihama and Machida 2001). Fusion of Golgi-derived vesicles occurs within and in the equatorial region of the phragmoplast, resulting in the eventual formation of new membranes and the cross walls known as cell plates (Samuels et al. 1995, Mayer and Jürgens 2004, Reichardt et al. 2007).
Various components of the mitogen-activated protein kinase (MAPK) cascade are involved in biotic and abiotic stress-signaling pathways, responses to hormones, and developmental processes in plants (Nakagami et al. 2005). In particular, the NACK-PQR pathway, which plays a key role in cytokinesis of tobacco cells (Nishihama et al. 2001, Ishikawa et al. 2002, Nishihama et al. 2002, Soyano et al. 2003), includes a MAPK cascade that consists of NPK1 MAP kinase kinase kinase (MAPKKK; Banno et al. 1993), NQK1/NtMEK1 MAP kinase kinase (MAPKK) and NRK1/NTF6 MAPK (Soyano et al. 2003). In this pathway, NRK1/NTF6 MAPK phosphorylates MT-associated protein MAP65-1, which decreases the ability of MAP65-1 to bundle MTs and stimulates expansion of the phragmoplast (Sasabe et al. 2006). In addition, two M-phase-specific kinesin-like proteins (KLPs), designated NPK1-activating KLP1 (NACK1) and NACK2, interact with NPK1 MAPKKK to activate NPK1 and control the localization, at the phragmoplast equator, of NPK1, which is required for cytokinesis of tobacco cells (Nishihama et al. 2002). The stalk region of NACK1 (NACK1-ST) binds to the carboxyl-terminal region of NPK1 (Ishikawa et al. 2002).
In Arabidopsis thaliana, the syntaxin-related protein KNOLLE (KN), which accumulates only in dividing cells, mediates the fusion of vesicles during formation of the cell plate, as does KEULE (KEU), a member of the Sec1/Munc18 family, with which KN interacts (Lauber et al. 1997, Assaad et al. 2001). Both kn and keu, single mutant cells accumulate unfused membrane vesicles at the plane of cell division (Lauber et al. 1997, Waizenegger et al. 2000). In addition, each of the A. thaliana homologs of components of the NACK-PQR pathway in tobacco has been identified as a regulator of cytokinesis in Arabidopsis, with the exception of NRK1/NTF6 MAPK. Genes for two KLPs, AtNACK1 and AtNACK2, which are identical to HINKEL (HIK) and STUD (STD)/TETRASPORE (TES), respectively, are required for somatic and meiotic cytokinesis, respectively (Nishihama et al. 2002, Strompen et al. 2002, Yang et al. 2003). Mutations in HIK/AtNACK1 interfere with cytokinesis and affect organization of the phragmoplast. A. thaliana has three genes for MAPKKKs in the ANP family: ANP1, ANP2, and ANP3 (Nishihama et al. 1997), and hypocotyls and embryos of anp2anp3 double-mutant plants exhibit abnormal cytokinesis (Krysan et al. 2002). A. thaliana also has 10 genes for MAPKKs (Ichimura et al. 2002), and a mutation in the MKK6/ANQ gene causes dwarfism, with unusually large cells that contain multiple nuclei and cell wall stubs in various organs, indicative of defects in cytokinesis (Soyano et al. 2003). Thus MAPKKKs in the ANP family and MKK6/ANQ MAPKK appear to be positive regulators of cytokinesis. However, the relationship between HIK (or TES), ANPs and MKK6/ANQ remains to be examined.
In the present study we investigated functional relationships among HIK and TES, ANPs, and MKK6/ANQ using yeast cells. We also attempted to locate MAPKs downstream of MKK6/ANQ by phosphorylation of group B MAPKs by MKK6/ANQ in vitro. Our results demonstrate that activation of MKK6/ANQ requires the presence of HIK (or TES) and at least one ANP and that active MKK6/ANQ efficiently phosphorylates only MPK4 in vitro, suggesting that HIK (or TES), ANPs, MKK6/ANQ and MPK4 constitute a single pathway that controls cytokinesis in A. thaliana.
Results
Plants with mutations in the MKK6/ANQ gene exhibit severe defects in cytokinesis
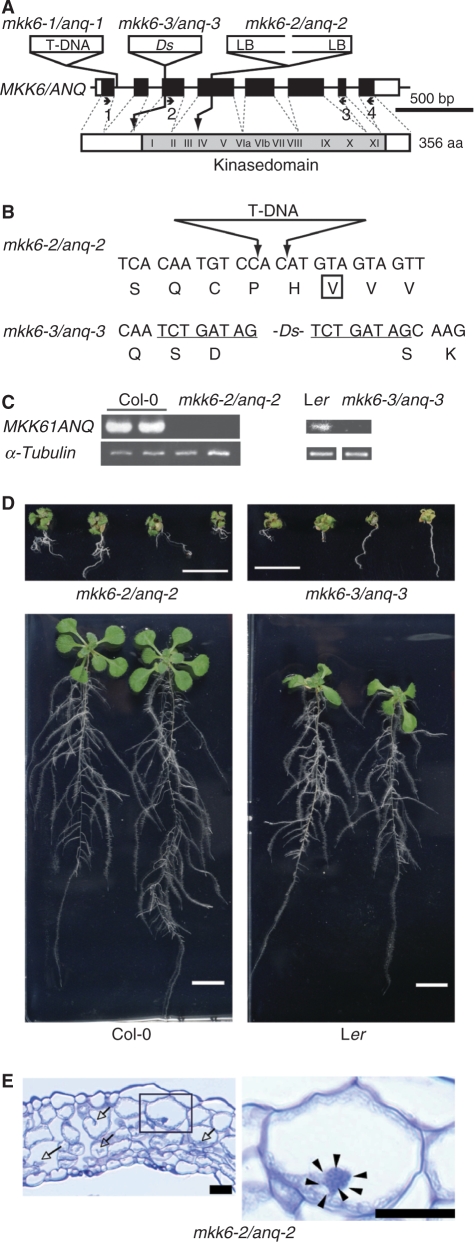
In a previous study by Soyano et al. (2003), the mkk6-1/anq-1 mutation did not appear to be a null allele. Therefore, in the present study we used two recently isolated new alleles of mkk6/anq [mkk6-2/anq-2 (SALK_117230) and mkk6-3/anq-3 (CSHL_GT5338)]. The mkk6-2/anq-2 allele on the Col-0 ecotype background corresponded to the MKK6/ANQ gene with a T-DNA in the fourth exon, which disrupted kinase subdomain IV (Fig. 1A, B). Analysis by reverse transcription polymerase chain reaction (RT-PCR) with a pair of primers that spanned the insertion failed to detect the relevant transcript in mkk6-2/anq-2 plants (Fig. 1C). Thus this allele most likely corresponds to a null mutation and therefore we used it in this study as a representative mkk6/anq mutant allele. Homozygous mkk6-2/anq-2 plants exhibited severe dwarfism (Fig. 1D) with typical defects in cytokinesis (Fig. 1E), and most homozygous mutants stopped growing before bolting occurred. Growth was restored by a transgene that included MKK6/ANQ cDNA (see Fig. 4A).
Fig. 1.
The dwarf phenotype of mkk6/anq mutant plants. (A) Schematic illustration of the structure of the MKK6/ANQ gene. White boxes correspond to exons and black boxes to coding regions. Sites of insertion of T-DNA and the transposon (Ds) are indicated above the gene (not drawn to scale). LB indicates the left border of the T-DNA. The insertion in mkk6-2/anq-2 consisted of two left borders of T-DNA. The structure of the MKK6/ANQ protein is depicted below the gene. The gray region and Roman numerals indicate the kinase domain and subdomains that are conserved in members of the family of protein kinases, respectively. Numbers refer to primers. (B) Sequences of nucleotides around the insertions. Predicted amino acids in the wild-type protein are depicted below triplets. A box indicates a conserved nonpolar amino acid in kinase subdomain IV. Underlining indicates nucleotides that are duplicated in mkk6-3/anq-3. (C) Analysis by RT-PCR of mkk6/anq mutants. Primer pairs 2 plus 3 and 1 plus 4, as depicted in (A), were used for the analysis of mkk6-2/anq-2 and mkk6-3/anq-3 mutants, respectively. RNA was extracted from entire aboveground parts of plants that had been grown on plates for 24 days. Two independent samples were used for the analysis of Col-0 and mkk6-2/anq-2. The gene for α-tubulin was used as the control. (D) The mkk6-2/anq-2 and mkk6-3/anq-3 mutations were on the Col-0 and Ler backgrounds, respectively. Mutant and wild-type plants were grown for 14 days on plates. Scale bars = 1 cm. (E) A transverse section of the fourth leaf of an mkk6-2/anq-2 mutant plant. A magnified view of the boxed region is shown in the right panel. Arrows indicate incomplete cell walls. Arrowheads indicate nuclei in a multinucleate cell. Scale bars = 20 μm.
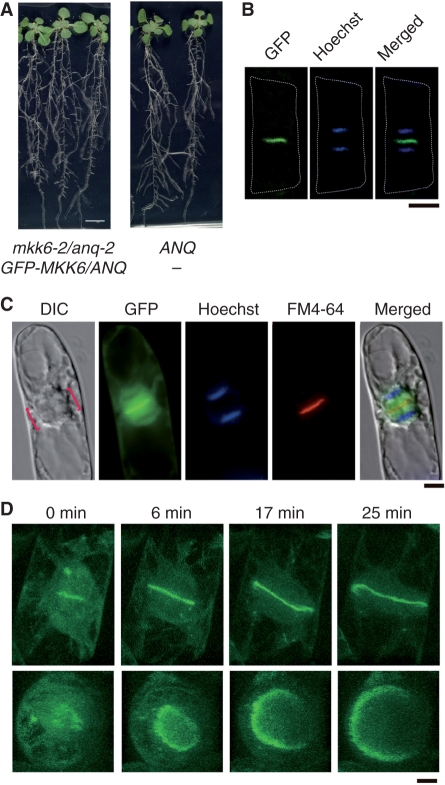
Fig. 4.
Subcellular localization of GFP-MKK6/ANQ. (A) Complementation of the mkk6-2/anq-2 mutation with GFP-MKK6/ANQ. Heterozygous mkk6-2/anq-2 mutant plants were transformed with a plasmid that harbored the GFP-MKK6/ANQ gene under the control of the 35S promoter of cauliflower mosaic virus. T2 transgenic plants were grown without antibiotic-resistant selection and their genotypes were determined. Photographs show T2 plants after growth on plates for 17 days. Siblings that were homozygous for mkk6-2/anq-2 and carried the transgene (left) and that were homozygous for MKK6/ANQ without the transgene (right) are shown at the same magnification. (B) Localization of GFP-MKK6/ANQ. Root epidermal cells of the mkk6-2/anq-2 mutant plants that expressed GFP-MKK6/ANQ and are shown in panel (A) were stained with Hoechst 33342. Fluorescence of GFP and Hoechst 33342-stained chromosomes in a single cell at telophase are shown. (C) Localization of GFP-MKK6/ANQ in BY-2 cells. BY-2 cells expressing GFP-MKK6/ANQ under the control of the 35S promoter were stained with Hoechst 33342 and FM4-64. Brackets in the Nomarski image (DIC) indicate the phragmoplast. The merged image is shown on the right. (D) Confocal microscopic analysis of GFP-MKK6/ANQ in BY-2 cells. Photographs show a single cell from late anaphase to telophase and were taken at the indicated times after the appearance of fluorescence due to GFP at the equatorial region of the phragmoplast. “Horizontal” views (top panels) and “vertical” views (bottom panels) are shown. Bars represent 1 cm in (A) and 10 μm in (B)–(D).
To determine whether mkk6/anq gametes are viable, we performed reciprocal crosses between an mkk6-2/anq-2 heterozygote and a wild-type parent. When we used mutant pollen from a heterozygous mkk6-2/anq-2 parent for crosses with a wild-type female parent, mutant alleles were transmitted to approximately 50% of the offspring, as expected, indicating that male gametophytes with an mkk6-2/anq-2 single mutation were viable. However, when we pollinated heterozygous parents with pollen from wild-type plants, only 43 of 192 offspring contained the mkk6-2/anq-2 allele, which must have been derived from female gametes. These results suggested that approximately 71% of female gametes with the mkk6-2/anq-2 mutation were not viable in heterozygous plants.
We also used the mkk6-3/anq-3 allele on the Landsberg erecta ecotype background. In this mutant allele, the Ds transposon was integrated into the third exon of the MKK6/ANQ gene, resulting in a duplication of eight nucleotides upstream of the kinase domain (Fig. 1A, B). We did not detect a transcript that spanned the integration site in mkk6-3/anq-3 plants, Thus this allele also probably corresponds to a null mkk6/anq mutation (Fig. 1C). Homozygous mkk6-3/anq-3 plants exhibited severe defects in growth and cytokinesis (data not shown).
Elevated levels of MKK6/ANQ mRNA in shoot apices and flowers that contain dividing cells
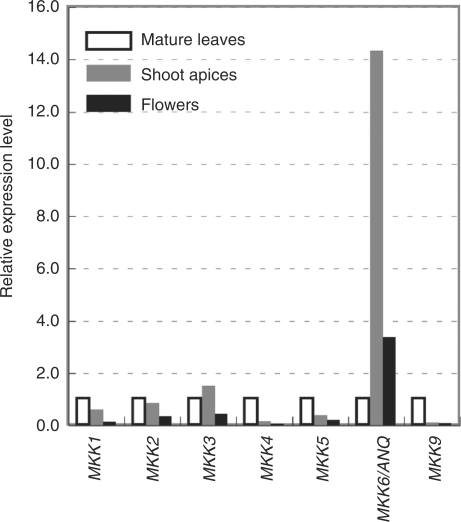
The Arabidopsis genome includes10 genes for MAPKKs (Ichimura et al. 2002). We examined the levels of transcripts of each of these genes in mature leaves, shoot apices (including flower buds) and flowers (including developing young siliques). We failed to detect transcripts of MKK8 and MKK10 by RT-PCR. Levels of the MKK7 transcript were also relatively low in mature leaves (see Supplementary Fig. S1).
Next we analyzed the expression of MKK1, MKK2, MKK3, MKK4, MKK5, MKK9 and MKK6/ANQ by quantitative real-time RT-PCR (Fig. 2). The transcript of MKK6/ANQ accumulated at much higher levels in shoot apices and flowers than in mature leaves, as did transcripts of Cyclin B1;1 (see Supplementary Fig. S1), indicating that these tissues are rich in proliferating cells.
Fig. 2.
Expression of genes for MAPKKs in A. thaliana. Quantitative RT-PCR was performed to determine the levels of transcripts of genes for MAPKKs in mature leaves, shoot apices (including flower buds) and flowers (including young developing siliques). RNA was isolated from plants that had been grown in soil for 49 days. After the reverse transcription reaction, the resultant cDNAs were amplified with primers specific for each respective gene. Quantitative RT-PCR data were normalized by reference to the gene for β-tubulin and are shown relative to the values obtained from mature leaves.
ANP1 and ANP3 activate MKK6/ANQ in the presence of HIK
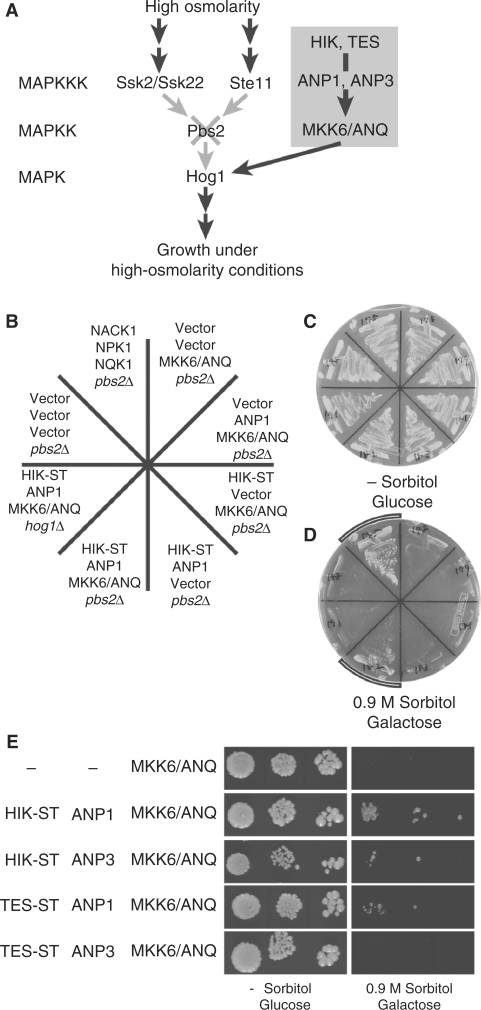
As noted above, two kinesin-like proteins, HIK/AtNACK1 and TES/AtNACK2, and the MAPKKKs ANP1, ANP2 and ANP3, which are homologs of tobacco NPK1, are required for cytokinesis in A. thaliana (Krysan et al. 2002, Nishihama et al. 2002, Strompen et al. 2002, Yang et al. 2003, Tanaka et al. 2004). In the tobacco BY-2 system, NACK1 binds to NPK1 (as does NACK2) to activate the NPK1 (Nishihama et al. 2002), which activates NQK1 MAPKK (Soyano et al. 2003). In the present study we examined the relationships among these factors and MKK6/ANQ in A. thaliana. To investigate these relationships, we used one of the modules in the MAPK cascades of Saccharomyces cerevisiae. The chosen module includes the Pbs2p MAPKK and the Hog1p MAPK, which function in the osmosensing MAPK cascade (Fig. 3A). Yeast cells with the pbs2Δ mutation cannot grow under high-osmolarity conditions (Brewster et al. 1993). If MKK6/ANQ were to activate Hog1, pbs2Δ cells expressing MKK6/ANQ would then be expected to grow under these restrictive conditions. To test this hypothesis, we fused MKK6/ANQ cDNA to the galactose-inducible promoter of the GAL1 gene from yeast and introduced the fusion construct into pbs2Δ mutant cells. We also made chimeric genes for the expression of HIK cDNA and ANP1 cDNA using the constitutively active promoters of the GAP gene from yeast (see Materials and Methods). As shown in Fig. 3B, D, expression of MKK6/ANQ cDNA did not confer osmotolerance on pbs2Δ mutant cells, even in the presence of galactose, when the HIK and ANP1 fusion genes were absent from pbs2Δ cells. However, when MKK6/ANQ cDNA was coexpressed with both the HIK and the ANP1 fusions, pbs2Δ cells were able to grow at elevated osmolarity (Fig. 3B, D, Table 1). Thus MKK6/ANQ required HIK and ANP1 for suppression of the osmosensitive phenotype of the pbs2Δ mutant.
Fig. 3.
Suppression of the osmosensitive growth defects of yeast pbs2Δ and hog1Δ mutants. (A) Schematic illustration of the basis for the experiment with the yeast pbs2Δ mutant in which we examined the activation of MKK6/ANQ, exploiting the osmoregulatory pathway in S. cerevisiae. Osmotic signals activate the MAPKKKs Ssk2/Ssk22 and Ste11, which in turn activate the Hog1p MAPK via the Pbs2p MAPKK (Brewster et al. 1993, Maeda et al. 1994, Maeda et al. 1995, Posas and Saito 1997). We used yeast pbs2Δ cells, which lack Pbs2p and cannot grow under high-osmolarity conditions. If a plant MAPKK, such as MKK6/ANQ, was able to activate Hog1 in pbs2Δ cells, the cells were able to grow under high-osmolarity conditions (Soyano et al. 2003). (B) The pbs2Δ and the hog1Δ mutants were transformed with various combinations of plasmids that harbored cDNAs for HIK, ANP1, and MKK6/ANQ, or with the corresponding empty vectors. Expression of MKK6/ANQ was driven by a galactose-inducible promoter. (C, D) Transformants were grown on plates that were supplemented either with glucose but no sorbitol (C) or with galactose plus 0.9 M sorbitol (D) at 30°C for 10 days. The two arcs in (D) indicate yeast cells that grew under high-osmolarity conditions. (E) Serial 10-fold dilutions of cultures of transformants, as indicated, were spotted on plates supplemented with either glucose or galactose plus 0.9 M sorbitol. Cells were cultured for 7 days at 30°C.
Table 1.
Summary of the suppression of growth defects related to osmosensitivity of yeast cells
| KLP | MAPKKK | MAPKK | Host strain | Growth | |
|---|---|---|---|---|---|
| 1 | Vector | Vector | Vector | pbs2Δ | − |
| 2 | Vector | Vector | MKK6/ANQ | pbs2Δ | − |
| 3 | HIK | ANP1 | MKK6/ANQ | pbs2Δ | + |
| 4 | HIK-ST | ANP1 | MKK6/ANQ | pbs2Δ | + |
| 5 | Vector | ANP1 | MKK6/ANQ | pbs2Δ | − |
| 6 | HIK-ST | Vector | MKK6/ANQ | pbs2Δ | − |
| 7 | HIK-ST | ANP1 | Vector | pbs2Δ | − |
| 8 | HIK-ST | ANP1 | MKK6/ANQ | hog1Δ | − |
| 9 | HIK | ANP3 | MKK6/ANQ | pbs2Δ | + |
| 10 | HIK-ST | ANP3 | MKK6/ANQ | pbs2Δ | + |
| 11 | Vector | ANP3 | MKK6/ANQ | pbs2Δ | − |
| 12 | HIK-ST | ANP3 | Vector | pbs2Δ | − |
| 13 | TES-ST | ANP1 | MKK6/ANQ | pbs2Δ | + |
| 14 | TES-ST | ANP1 | Vector | pbs2Δ | − |
| 15 | TES-ST | ANP3 | MKK6/ANQ | pbs2Δ | − |
| 16 | TES-ST | ANP3 | Vector | pbs2Δ | − |
Symbols + and − indicate growth and the absence of growth under high-osmolarity conditions, respectively. “Vector” indicates the corresponding empty vector.
NACK1 and HIK include putative motor domains in the amino-terminal halves and stalk domains in the carboxy- terminal halves and the stalk domain (ST) of NACK1 (NACK1-ST) is, by itself, sufficient for the activation of NPK1 (Nishihama et al. 2002). We examined the effect of the stalk domain of HIK (HIK-ST) in a similar set of experiments. Our results indicated that HIK-ST was able to replace intact HIK in this assay.
We examined whether the HOG1 gene, which encodes a MAPK that acts downstream of Pbs2p, might be required for the suppression of the osmosensitive phenotype of pbs2Δ cells by expression of the combination of HIK, ANP1 and MKK6/ANQ cDNAs in the hog1Δ mutant yeast cells. Fig. 3B, D show that even when all three constructs were expressed together, they did not suppress the osmosensitive phenotype of hog1Δ cells, indicating that HIK, ANP1, and MKK6/ANQ stimulate yeast growth through the function of Hog1p MAP kinase.
Although the predicted amino acid sequences of tobacco NPK1, ANP1 and ANP2 are very similar, ANP3 is rather different from other members of the ANP family (Nishihama et al. 1997). In addition, the amino acid sequence of the carboxy-terminal region of tobacco NPK1 is required for the interaction of NPK1 with NACK1 (Ishikawa et al. 2002). The carboxy-terminal region of ANP3 exhibits a low similarity in terms of amino acid sequences (about 50% identical) to those of other members, although corresponding regions of other members show higher similarities (about 75% identical; see Discussion). We examined the effects of expression of HIK and ANP3 separately and together on the activation of MKK6/ANQ in yeast cells. Coexpression of (HIK) HIK-ST, ANP3 and MKK6/ANQ only weakly supported the growth of pbs2Δ cells under high- osmolarity conditions (Fig. 3E). The weak suppression depended on the presence of (HIK) HIK-ST and MKK6/ANQ (see lines 9–12 in Table 1).
We also examined the effect of TETRASPORE (TES)/AtNACK2 KLP, which is an Arabidopsis ortholog of tobacco NACK2 (see Introduction), on the activation of MKK6/ANQ. Since we were unable to obtain transformed yeast cells when we introduced full-length TES cDNA into the expression vector, we tested the effect of TES-ST. Yeast pbs2Δ cells that expressed MKK6/ANQ with the combination of TES-ST plus ANP1 were able to grow under high-osmolarity conditions, with growth depending on the presence of both TES-ST and ANP1 (line 13 in Table 1). Experiments with serial dilutions of transformants showed that the osmotolerance of pbs2Δ cells that expressed TES-ST cDNA was weaker than that of pbs2Δ cells that expressed HIK-ST cDNA (Fig. 3E). Coexpression of TES-ST, ANP3, and MKK6/ANQ cDNAs did not confer osmotolerance on pbs2Δ cells under the conditions of our assays (Fig. 3E; line 15 in Table 1).
On the basis of the previous results, we postulated that MKK6/ANQ might be activated by ANP1 and that activity might be increased by the expression of HIK in pbs2Δ yeast cells. Our data also suggest that HIK and TES might be weak activators of ANP3 and ANP1, respectively. TES did not activate ANP3.
Localization of GFP-MKK6/ANQ at the equatorial plane of the phragmoplast
We generated a cDNA that encoded green fluorescent protein (GFP) fused to MKK6/ANQ (GFP-MKK6/ANQ) under the control of the cauliflower mosaic virus 35S promoter. We introduced the fusion gene into mkk6-2/anq-2 mutant plants and we confirmed that the mutant phenotype was rescued by this construct (Fig. 4A). Moreover, we were able to detect GFP-MKK6/ANQ at the putative plane of division of root cells. Hoechst staining revealed that GFP-MKK6/ANQ was localized between the two sets of daughter chromosomes, at sites that appeared to correspond to the plane of division of the root cells (Fig. 4B).
We examined the localization of GFP-MKK6/ANQ in greater detail using cultured tobacco BY-2 cells (Fig. 4C). At telophase, GFP-MKK6/ANQ was localized at the equatorial plane of the phragmoplast and colocalized with FM-46-stained cell plates. Confocal microscopy revealed that fluorescence due to GFP-MKK6/ANQ had a ring-like profile (Fig. 4D). Time-lapse analysis demonstrated, moreover, that the ring-like profile expanded centrifugally over time (Fig. 4D).
We examined the localization of five other MAPKKs (MKK1, MKK2, MKK3, MKK4 and MKK5) from Arabidopsis in BY-2 cells using GFP-fused derivatives. None of them was localized similarly to the MKK6/ANQ fusion protein (Supplementary Fig. S5). Thus MKK6/ANQ appeared to be the only MAPKK of Arabidopsis that is localized in the equatorial region of the phragmoplast.
Molecular relationships among MKK6/ANQ MAPKK and group B MAPKs
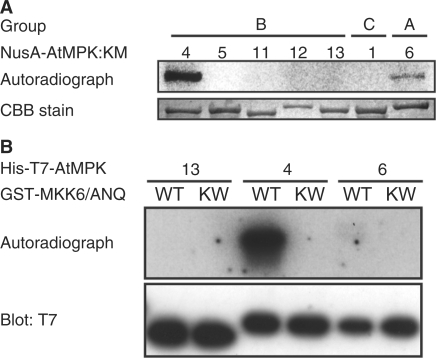
There are 20 genes for MAPKs in the Arabidopsis genome (Ichimura et al. 2002). We tested five MAPKs in group B (MPK4, MPK5, MPK11, MPK12 and MPK13) for phosphorylation and activation by MKK6/ANQ in vitro. We also examined one member of group C (MPK1) and one member of group A (MPK6) for activation in vitro. We generated GST-fused MKK6/ANQ [GST-MKK6/ANQ] in Escherichia coli cells because the GST-fused NQK1 efficiently phosphorylates NRK1 MAPK, for reasons unknown (Soyano et al. 2003). We examined whether GST-MKK6/ANQ was able to phosphorylate the inactive form of each MAPK mentioned above. In vitro, GST-MKK6/ANQ phosphorylated MPK4 but failed to phosphorylate other MAPKs in group B (Fig. 5A). MPK6 (group A) was also phosphorylated, but at a very low level (Fig. 5A). In-gel kinase assays revealed that wild-type MPK4 that had been phosphorylated by GST-MKK6/ANQ phosphorylated MBP efficiently (Fig. 5B). In contrast, we failed to detect the activity of MPK13 and MPK6 in vitro (Fig. 5B). GST-MKK6/ANQ did not phosphorylate MPK1 (group C).
Fig. 5.
Biochemical identification of a MAPK that acts downstream of MKK6/ANQ. (A) Phosphorylation of MAPKs in groups A, B and C by MKK6/ANQ in vitro. Recombinant GST-fused MKK6/ANQ (active MAPKK) and NusA-tagged kinase-inactive MAPKs were generated in E. coli. The phosphorylation reaction was performed as described in Materials and Methods. Phosphorylation of MAPKs was detected by autoradiography after SDS-PAGE. Numbers above the autoradiograph correspond to those of MPKs. NusA-MPKs were detected by staining with Coomassie Brilliant Blue (CBB; lower panel). (B) Activation of MPK4 by MKK6/ANQ in vitro. His-T7 tagged MPK13 (13), MPK4 (4), and MPK6 (6) were incubated with unlabeled ATP plus either GST- MKK6/ANQ (WT) or GST- MKK6/ANQ:KW (kinase inactive MAPKK; KW). Reaction products were subjected to the kinase assay in polyacrylamide gels that contained MBP (myelin basic protein). After renaturation of proteins, gels were incubated with [γ-32P]ATP. The upper panel shows the autoradiograph and the lower panel shows the results of immunoblotting with T7-specific antibodies.
We searched for MAPKs in group B that were able to interact with MKK6/ANQ using the yeast two-hybrid system. We fused five cDNAs for group B MAPKs individually to the sequence of the LexA DNA-binding domain and coexpressed each of them with MKK6/ANQ cDNA that had been fused to the DNA sequence that encodes the VP16 activation domain [VP16- MKK6/ANQ]. Yeast cells expressing LexA-MPK12 and LexA-MPK13 were able to proliferate under restrictive conditions, with concomitant coexpression of VP16-MKK6/ANQ, in the presence of 0 and 10 mM 3-amino-1,2,4-triazol (3AT), respectively (see lines 5 and 7 in Table 2). Since yeast cells expressing LexA-MPK4 or LexA-MPK11 were able to grow on plates prepared with 40 mM 3AT, irrespective of the presence or absence of VP16-MKK6/ANQ (data not shown), we also examined interactions between each VP16-fused MAPK and LexA-MKK6/ANQ. We observed the efficient growth of yeast cells that expressed VP16-MPK4 and VP16-MPK12 under restrictive conditions with 10 and 5 mM 3AT, respectively, only when LexA-MKK6/ANQ was also expressed (see lines 10 and 14 in Table 2). These results demonstrated that MKK6/ANQ interacts with MPK4, MPK12 and MPK13 in yeast cells.
Table 2.
Yeast two-hybrid analysis of MKK6/ANQ and MAPKs
| LexA | VP16 | 3-amino-1,2,4-triazole (mM) |
||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 40 | |||
| MAPKs | MKK6/ANQ | |||||
| 1 | LexA | VP16-MKK6/ANQ | − | − | − | − |
| 2 | LexA-MPK5 | VP16 | − | ND | ND | ND |
| 3 | LexA-MPK5 | VP16-MKK6/ANQ | − | ND | ND | ND |
| 4 | LexA-MPK12 | VP16 | − | − | ND | ND |
| 5 | LexA-MPK12 | VP16-MKK6/ANQ | + | − | − | ND |
| 6 | LexA-MPK13 | VP16 | + | ± | − | ND |
| 7 | LexA-MPK13 | VP16-MKK6/ANQ | + | + | + | ND |
| MKK6/ANQ | MAPKs | |||||
| 8 | LexA-MKK6/ANQ | VP16 | + | − | − | ND |
| 9 | LexA | VP16-MPK4 | ND | ND | ± | ± |
| 10 | LexA-MKK6/ANQ | VP16-MPK4 | ND | ND | + | + |
| 11 | LexA | VP16-MPK11 | ND | − | − | ND |
| 12 | LexA-MKK6/ANQ | VP16-MPK11 | ND | − | − | ND |
| 13 | LexA | VP16-MPK12 | ND | − | − | − |
| 14 | LexA-MKK6/ANQ | VP16-MPK12 | ND | + | ± | − |
The interaction between MKK6/ANQ and group B MAPKs was examined by monitoring the growth of yeast cells under restrictive conditions (histidine-free medium) at the indicated concentrations of 3AT. Symbols +, ±, and − indicate copious, limited, and absent growth, respectively. ND indicates that the corresponding combination was not tested.
We also examined the interaction between MKK6/ANQ and selected MAPKs using a pull-down assay. While MPK4 coprecipitated with MKK6/ANQ, MPK13 did not (Supplementary Fig. S2). This result demonstrated the specific interaction between MPK4 and MKK6/ANQ in vitro.
Discussion
Cytokinesis in A. thaliana is controlled by a pathway that includes HIK and a MAP kinase cascade that consists of ANPs and MKK6/ANQ
The newly isolated alleles of mkk6/anq used in the present study were associated with clear defects in growth and cytokinesis (Fig. 1), indicating that MKK6/ANQ MAPKK is required for cytokinesis in Arabidopsis. Our experiments with the yeast pbs2Δ mutant revealed that the activation of MKK6/ANQ required a combination of ANP1 MAPKKK plus HIK KLP, ANP1 plus TES KLP, or ANP3 plus HIK (Fig. 3), indicating that MKK6/ANQ is activated by each respective combination of MAPKKK and KLP. Since, even in the presence of ANP1 and HIK, activated MKK6/ANQ did not rescue the growth defect of the yeast cells with a mutant hog1 gene under high-osmolarity conditions (Fig. 3), MKK6/ANQ appears to act as a MAPKK upstream of Hog1 MAPK. The results of assays of the activation by HIK are consistent with the observations that both GFP-MKK6/ANQ (Fig. 4) and HIK (Strompen et al. 2002) are localized at the equator of the phragmoplast.
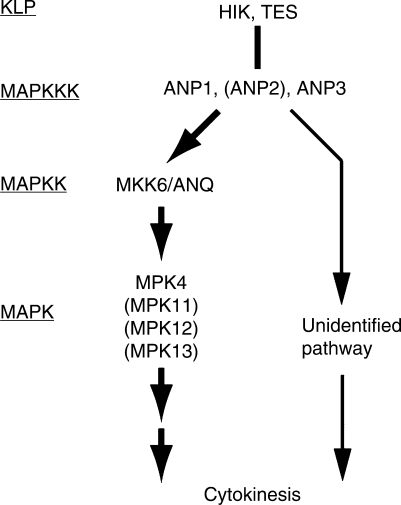
The phosphorylation experiments in vitro showed clearly that MKK6/ANQ phosphorylates and activates MPK4 MAPK (Fig. 5). In addition, our preliminary observations indicate that the mpk4-2 mutant has defects in cytokinesis (our unpublished observation). These results predict the involvement of a cytokinesis-controlling MAPK cascade in A. thaliana that includes ANP1, with activation by HIK, MKK6/ANQ and MPK4 (Fig. 6). Since mkk6/anq mutant cells remain viable, additional combinations of MAPKKs and KLPs might be involved in the control and maintenance of cytokinesis. Some unknown pathway(s) other than the NACK-PQR pathway also might be involved in cytokinesis in A. thaliana plants (see below).
Fig. 6.
Possible pathways of for the control of cytokinesis in A. thaliana. MKK6/ANQ plays a major role in cytokinesis of A. thaliana cells. However, the presence of a factor with redundant function is probable. A MAPKK other than MKK1, MKK2 and MKK3 might function redundantly with MKK6/ANQ in cytokinesis (see Discussion). Alternatively, ANP1, ANP2 and ANP3 might have functions distinct from the activation of MAPKK that are also involved in the control of cytokinesis.
ANP1 is activated by HIK and TES, while ANP3 is only weakly activated by HIK
Nishihama et al. (1997) reported that in yeast cells, ANP1 is activated by NACK1 KLP (a tobacco ortholog of HIK). Our present results related to the activation of ANP1 and ANP3 by HIK shed light on the mechanism of their activation. Tobacco NPK1 MAPKKK is activated via its interaction with NACK1 and the sequence of 60 amino acids near the carboxy-terminal end of NPK1 is essential for its binding to NACK1 (Ishikawa et al. 2002). This region includes a coiled-coil structure, which is required for the interaction with NACK1. Amino acid sequences of the C-terminal regions of ANP1 and NPK1are highly conserved (75% identical) (Nishihama et al. 1997), and ANP1 also includes a similar coiled-coil structure, so it seems plausible that ANP1 might respond to similar regulation by HIK (Nishihama et al. 1997). The C-terminal region of ANP3 is, however, rather different from those of NPK1 and ANP1: 10 amino acid residues are absent from the C-terminal end of ANP3, and the similarity between these regions of ANP3 and NPK1 (50% identical) is lower than that between the corresponding regions of ANP1 and NPK1 (see above) (Nishihama et al. 1997). Although the C-terminal region of ANP3 was also predicted to form a coiled-coil structure, the probability of the formation in ANP3 was low compared to that in ANP1 (data not shown). The results of our yeast complementation studies indicate that the effects of ANP3 and HIK on the activation of MKK6/ANQ are very weak when compared to those of ANP1 and HIK (Fig. 3E). The weak activation of MKK6/ANQ by the coexpression of ANP3 plus HIK might have been due to the difference in the amino acid sequence of the C-terminal region of ANP3. Coexpression of ANP3 and TES did not activate MKK6/ANQ. In A. thaliana, the activity of ANP3 might be controlled by unidentified factors that must be different from the regulator for ANP1.
Possible involvement of factors other than MKK6/ANQ in cytokinesis
The observation that the anq mutation was not lethal indicates the presence of factors other than MKK6/ANQ that might function redundantly in cytokinesis. For example, in gametogenesis, as when multiple redundant genes for products that act upstream of MKK6/ANQ are disrupted, mutant plants exhibit severe lethality during gametogenesis. The hik tes double mutation (Tanaka et al. 2004, Oh et al. 2008) and the anp1 anp2 anp3 triple mutation result in lethality during female and male gametogenesis (Krysan et al. 2002). Since all known mkk6/anq mutant alleles are associated with severe defects in growth and cytokinesis (Fig. 1; Soyano et al. 2003), and since most corresponding homozygous mutants stop growing prior to bolting, it seems that MKK6/ANQ must play a major role in the cytokinesis of A. thaliana cells. However, since a few mutant plants set flowers and even produced seeds, some factor(s) with a function equivalent to that of MKK6/ANQ probably exists.
The most likely candidates for such factors, whose functions overlap that of MKK6/ANQ in cytokinesis, are the closely related members of the MAPKK family in A. thaliana, namely MKK1, MKK2 and MKK3. However, the phenotypes of double mutants that harbored the mkk6/anq mutation in addition to the mkk1, mkk2, or mkk3 mutation were indistinguishable from those of single mkk6/anq mutants (see Supplementary Fig. S3). Triple-mutant plants, with mutations in genes for all the MAPKKs in group A (MKK1, MKK2 and MKK6) were also indistinguishable from mkk6/anq single mutants (see Supplementary Fig. S4). Although other MAPKKs that have not yet been tested might be involved in cytokinesis, an unidentified pathway that is controlled by the HIK-ANP pathway might also exist (Fig. 6). In addition to the activation of downstream MAPKKs, other roles for MEKK1, a MAPKKK in Arabidopsis, have been reported (Miao et al. 2007). Certain ANPs might also participate directly in the control of cytokinesis.
MPK4 is a biochemical target of MKK6/ANQ
Although the kinase reactions in vitro revealed that MKK6/ANQ phosphorylated only MPK4 among tested MPKs, assays using the yeast two-hybrid system demonstrated that MKK6/ANQ interacts with MPK4, MPK12 and MPK13 in yeast cells (Table 2). Melikant et al. (2004) reported that MPK13 is activated by MKK6/ANQ in yeast cells, and their observation is consistent with ours. In contrast, while Lee et al. (2008) reported that MPK11 interacts strongly with MKK6 in the yeast two- hybrid system, we failed to detect such an interaction. Recently Lee et al. (2009) reported that MPK12 is phosphorylated in vitro by constitutively active mutant forms of several MAPKKs, including MKK6/ANQ, but again, the results of our kinase assays in vitro are inconsistent with theirs (Fig. 5). Although the activity of MPK4 has been shown to respond to various external stimuli, such as infection by pathogens (Petersen et al. 2000, Brader et al. 2007, Qiu et al. 2008); a certain type of cell death (Ichimura et al. 2006); treatment with the bacterial elicitor flagellin (Mészáros et al. 2006, Suarez-Rodriguez et al. 2007); and cold, salt and osmotic stresses (Teige et al. 2004, Droillard et al. 2004), the results of the present study indicate that MPK4 is one of the biochemical targets of MKK6/ANQ in A. thaliana. It is also likely that other MAPKs in group B are substrates of this MAPKK in vivo. Clarification of the entire MAPK cascade that controls cytokinesis in A. thaliana will require further extensive investigations.
Materials and Methods
Plant materials and transformation of cells and plants
The mkk6-2/anq2 mutant of A. thaliana with the Col-0 background was provided by the SALK Institute (SALK_117230; La Jolla, CA, USA). The mkk6-3/anq-3 mutant with the Landsberg erecta background (CSHL_GT5338) was provided by Cold Spring Harbor Laboratory. The original strains were outcrossed three times with wild-type plants of the respective ecotype. Suspension-cultured tobacco BY-2 cell lines were maintained according to a previously reported method (Banno et al. 1993). Transformed BY-2 cells were generated by Agrobacterium-mediated transformation (Nishihama et al. 2001). Transformation of A. thaliana was performed by the floral dip method (Clough and Bent 1998). For analysis of the gross morphology of plants, sterilized seeds were sown on plates prepared with 0.2% gellan gum-solidified Murashige and Skoog medium. Plates with seeds were stored for 2 days at 4°C for vernalization and then transferred to a growth chamber (22°C; light for 16 h and dark for 8 h daily).
Isolation of RNA and RT-PCR
All techniques for isolation of RNA and RT-PCR were described previously (Ishikawa et al. 2008). Quantitative real-time RT-PCR (qRT-PCR) was performed as described by Terakura et al. (2007). Primers are listed in Supplementary Table S1.
Functional complementation of yeast cells
Functional complementation experiments were performed as described by Soyano et al. (2003). S. cerevisiae TM344 (MATa, ura3, leu2, trp1, his3, lys2, pbs2Δ::HIS3; a gift from K. Matsumoto, Nagoya University, Nagoya, Japan) was transformed with combinations of pSK12 that carried HIK or TES, YEpGAP that carried ANP1 or ANP3, and pNV7-MKK6/ANQ. Plasmids derived from pSK12 and YEpGAP included constitutively active promoters, and pNV7 had a galactose-inducible promoter. For analysis of cell growth under high-osmolarity conditions, yeast cells were streaked on a synthetic medium that contained 0.9 M sorbitol. For serial-dilution experiments, cultures of transformed cells were diluted to an OD600 of 1.0. A set of 10-fold serial dilutions of each of these cultures was prepared and 5 μl of each dilution were spotted onto plates. The hog1Δ mutant was provided by Dr. K. Matsumoto.
Fluorescence microscopy
All techniques for microscopic analysis were described previously (Nishihama et al. 2001). GFP-MKK6/ANQ was expressed using the pGWB6 vector (Nakagawa et al. 2007). To stain DNA and cell plates in BY-2 cells, we added Hoechst 33342 (Dojin, Kumamoto, Japan), and FM4-64 (Invitrogen, Carlsbad, CA, USA) to cell cultures at final concentrations of 0.5 μg/ml and 1 μM, respectively. To stain DNA, we soaked Arabidopsis plants in Murashige and Skoog liquid medium supplemented with 2 μg/ml Hoechst 33342. Fluorescence was observed with a confocal microscope (LSM510; Carl Zeiss, Oberkochen, Germany).
Site-directed mutagenesis
To produce genes for kinase-defective forms of MAPKs, we introduced mutations in the sequence of each gene that encoded the adenosine triphosphate (ATP)-binding site of each MAPK with a QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene; http://www.protocol-online.org/biology-forums/posts/33869.html). All reactions were performed according to the manufacturer’s instructions.
Production and purification of recombinant proteins
We produced His-T7-tagged and NusA-tagged recombinant proteins, as well as the MKK6/ANQ protein fused to GST, in E. coli BL21(DE3) using the vectors pET28 (Novagen; http://www.csun.edu/∼hcbio027/biotechnology/lec4a/petsys.html), pET50 (Novagen), and pGEX (GE Healthcare; http://www .gelifesciences.com/aptrix/upp01077.nsf/Content/Products? OpenDocument&ModuleId=38859&zone=Proteomics), respectively. His-T7-tagged and NusA-tagged recombinant proteins were purified with Ni-NTA agarose (Qiagen; http://www1 .qiagen.com/literature/handbooks/literature.aspx?id= 1000137&lid=JA). GST-fused MKK6/ANQ was purified with glutathione-Sepharose 4B (GE Healthcare).
Kinase assays in vitro and in gels
Recombinant MAPKs (200 ng each) and recombinant GST-MKK6/ANQ (15 ng) were incubated in 20 μl of kinase buffer (Nishihama et al. 2001) that contained 50 μM ATP and 10 μCi of [γ-32P]ATP (GE Healthcare) at 25°C for 30 min. Reactions were terminated by the addition of 20 μl of 2× sample buffer for SDS-PAGE, and half of each resultant reaction mixture was fractionated by SDS-PAGE. Radioactivity was visualized with an imaging analyzer (BAS-1800; Fuji Film, Tokyo). Kinase assays in gels were performed as described previously (Soyano et al. 2003).
Yeast two-hybrid analysis
Yeast two-hybrid analysis was performed as described by Soyano et al. (2003). S. cerevisiae L40 [MATα his3-200 trp1-901 leu2-3,112 ade2 LYS::(lexAop)4-HIS3 URA3::(lexAop)8-lacZ] was cotransformed with a combination of pBTM116-MKK6/ANQ and pVP16 that carried the gene for a MAPK and with a combination of pBTM116 that carried the gene for a MAPK and pVP16-MKK6/ANQ. To analyze cell growth, we streaked yeast cells on a synthetic medium that contained 3-amino-1,2,4-triazole (3AT) without histidine.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported in part by a grant from the Program for the Promotion of Basic Research Activities for Innovative Biosciences (BRAIN), by a Grant-in-Aid for Scientific Research on Priority Areas (no. 19060003), and by a Grant-in-Aid for the 21st Century COE Program (Systems Bioscience) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Supplementary Material
Acknowledgments
Y.T. was supported by a grant from the Research Program for the Promotion of BRAIN.
Glossary
Abbreviations
- GFP
green fluorescent protein
- KLP
kinesin-like protein
- MAPK
mitogen-activated protein kinase
- MAPKK
mitogen-activated protein kinase kinase
- MAPKKK
mitogen-activated protein kinase kinase kinase.
References
- Asada T, Sonobe S, Shibaoka H. Microtubule translocation in the cytokinesis apparatus of cultured tobacco cells. Nature. 1991;350:238–241. [Google Scholar]
- Assaad FF, Huet Y, Mayer U, Jürgens G. The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE. J. Cell Biol. 2001;152:531–543. doi: 10.1083/jcb.152.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno H, Hirano K, Nakamura T, Irie K, Nomoto S, Matsumoto K, et al. NPK1, a tobacco gene that encodes a protein with a domain homologous to yeast BCK1, STE11 and Byr2 protein kinases. Mol. Cell Biol. 1993;13:4745–4752. doi: 10.1128/mcb.13.8.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brader G, Djamei A, Teige M, Palva ET, Hirt H. The MAP kinase kinase MKK2 affects disease resistance in Arabidopsis. Mol. Plant Microbe Interact. 2007;20:589–596. doi: 10.1094/MPMI-20-5-0589. [DOI] [PubMed] [Google Scholar]
- Brewster JL, De Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Droillard MJ, Boudsocq M, Barbier-Brygoo H, Laurière C. Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Lett. 2004;574:42–48. doi: 10.1016/j.febslet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Hush JM, Wadsworth P, Callaham DA, Hepler PK. Quantification of microtubule dynamics in living plant cells using fluorescence redistribution after photobleaching. J. Cell Sci. 1994;107:775–784. doi: 10.1242/jcs.107.4.775. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, et al. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K. MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J. Biol. Chem. 2006;281:36969–36976. doi: 10.1074/jbc.M605319200. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Soyano T, Nishihama R, Machida Y. The NPK1 mitogen-activated protein kinase kinase kinase contains a functional nuclear localization signal at the binding site for the NACK1 kinesin-like protein. Plant J. 2002;32:789–798. doi: 10.1046/j.1365-313x.2002.01469.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Machida C, Yoshioka Y, Ueda T, Nakano A, Machida Y. EMBRYO YELLOW gene, encoding a subunit of the conserved oligomeric Golgi complex, is required for appropriate cell expansion and meristem organization in Arabidopsis thaliana. Genes Cells. 2008;13:521–535. doi: 10.1111/j.1365-2443.2008.01186.x. [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Jester PJ, Gottwald JR, Sussman MR. An Arabidopsis mitogen-activated protein kinase kinase kinase gene family encodes essential positive regulators of cytokinesis. Plant Cell. 2002;14:1–12. doi: 10.1105/tpc.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 1997;39:1485–1493. doi: 10.1083/jcb.139.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Huh KW, Bhargava A, Ellis BE. Comprehensive analysis of protein-protein interactions between Arabidopsis MAPKs and MAPK kinases helps define potential MAPK signaling modules. Plant Signal. Behav. 2008;3:1037–1041. doi: 10.4161/psb.3.12.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Wang S, Sritubtim S, Chen JG, Ellis BE. Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant J. 2009;57:975–985. doi: 10.1111/j.1365-313X.2008.03741.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast Pbs2 MAPKK by MAPKKKs or binding of an SH3-containing osmosenser. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Mayer U, Jürgens G. Cytokinesis: lines of division taking shape. Curr. Opin. Plant Biol. 2004;7:599–604. doi: 10.1016/j.pbi.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Melikant B, Giuliani C, Halbmayer-Watzina S, Limmongkon A, Heberle-Bors E, Wilson C. The Arabidopsis thaliana MEK AtMKK6 activates the MAP kinase AtMPK13. FEBS Lett. 2004;576:5–8. doi: 10.1016/j.febslet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Mészáros T, Helfer A, Hatzimasoura E, Magyar Z, Serazetdinova L, Rios G, et al. The Arabidopsis MAP kinase kinase MKK1 participates in defence responses to the bacterial elicitor flagellin. Plant J. 2006;48:485–498. doi: 10.1111/j.1365-313X.2006.02888.x. [DOI] [PubMed] [Google Scholar]
- Miao Y, Laun TM, Smykowski A, Zentgraf U. Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol. Biol. 2007;65:63–76. doi: 10.1007/s11103-007-9198-z. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Banno H, Kawahara E, Irie K, Machida Y. Possible involvement of differential splicing in regulation of the activity of Arabidopsis ANP1 that is related to mitogen-activated protein kinase kinase kinases (MAPKKKs) Plant J. 1997;12:39–48. doi: 10.1046/j.1365-313x.1997.12010039.x. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Ishikawa M, Araki S, Soyano T, Asada T, Machida Y. The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev. 2001;15:352–363. doi: 10.1101/gad.863701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihama R, Machida Y. Expansion of the phragmoplast during plant cytokinesis: a MAPK pathway may MAP it out. Curr. Opin. Plant Biol. 2001;4:507–512. doi: 10.1016/s1369-5266(00)00208-9. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Soyano T, Ishikawa M, Araki S, Tanaka H, Asada T, et al. Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell. 2002;109:87–99. doi: 10.1016/s0092-8674(02)00691-8. [DOI] [PubMed] [Google Scholar]
- Oh SA, Bourdon V, Das Pal M, Dickinson H, Twell D. Arabidopsis kinesins HINKEL and TETRASPORE act redundantly to control cell plate expansion during cytokinesis in the male gametophyte. Mol. Plant. 2008;1:794–799. doi: 10.1093/mp/ssn042. [DOI] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, et al. Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/s0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, et al. Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol. 2008;148:212–222. doi: 10.1104/pp.108.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt I, Stierhof YD, Mayer U, Richter S, Schwarz H, Schumacher K, et al. Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr. Biol. 2007;1:7204–7205. doi: 10.1016/j.cub.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH, Jr, Staehelin LA. Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J. Cell Biol. 1995;130:1345–1357. doi: 10.1083/jcb.130.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, et al. Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006;20:1004–1014. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Nishihama R, Morikiyo K, Ishikawa M, Machida Y. NQK1/NtMEK1 is a MAPKK that acts in the NPK1 MAPKKK-mediated MAPK cascade and is required for plant cytokinesis. Genes Dev. 2003;17:1055–1067. doi: 10.1101/gad.1071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strompen G, El Kasmi F, Richter S, Lukowitz W, Assaad FF, Jürgens G, et al. The Arabidopsis HINKEL gene encodes a kinesin-related protein involved in cytokinesis and is expressed in a cell cycle-dependent manner. Curr. Biol. 2002;12:153–158. doi: 10.1016/s0960-9822(01)00655-8. [DOI] [PubMed] [Google Scholar]
- Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, et al. MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol. 2007;143:661–669. doi: 10.1104/pp.106.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Ishikawa M, Kitamura S, Takahashi Y, Soyano T, Machida C, et al. The AtNACK1/HINKEL and STUD/TETRASPORE/AtNACK2 genes, which encode functionally redundant kinesins, are essential for cytokinesis in Arabidopsis. Genes Cells. 2004;9:1199–1211. doi: 10.1111/j.1365-2443.2004.00798.x. [DOI] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Terakura S, Ueno Y, Tagami H, Kitakura S, Machida C, Wabiko H, et al. An oncoprotein from the plant pathogen Agrobacterium has histone chaperone-like activity. Plant Cell. 2007;19:2855–2865. doi: 10.1105/tpc.106.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizenegger I, Lukowitz W, Assaad F, Schwarz H, Jürgens G, Mayer U. The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr. Biol. 2000;10:1371–1374. doi: 10.1016/s0960-9822(00)00775-2. [DOI] [PubMed] [Google Scholar]
- Yang CY, Spielman M, Coles JP, Li Y, Ghelani S, Bourdon V, et al. TETRASPORE encodes a kinesin required for male meiotic cytokinesis in Arabidopsis. Plant J. 2003;34:229–240. doi: 10.1046/j.1365-313x.2003.01713.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.