Abstract
The ocular environment has been shown to induce tolerance to locally administered antigens. We therefore investigated whether there was a systemic immune response against adenoviral vectors injected into the vitreous of retinoblastoma patients enrolled in a phase 1 clinical trial of adenoviral-mediated thymidine kinase gene transfer. Sections of enucleated eyes were immunostained with antibodies against inflammatory cells. A trend toward increasing numbers of plasma cells, T cells, macrophages, and antigen-presenting cells was observed in the injected subjects' eyes, but systemically, there was no significant increase in the number of adenovirus-specific cytotoxic T lymphocytes (CTLs) or in adenovirus neutralizing antibodies. Therefore, in contrast to studies showing significant immunogenicity of Ad-RSVtk following injection into extraocular tumors, injection into the eye produces only a mild local inflammatory response without evidence of systemic cellular or humoral immune responses to adenovirus.
Introduction
The use of adenoviral vectors to obtain prolonged transgene expression in human subjects has fallen out of favor due to induction of a host immune response primarily directed to antigenic viral proteins.1 First-generation adenoviral vectors induce both innate and acquired immune responses that are responsible for the clearance of the vector. The interaction of the viral particle with the epithelium induces release of cytokines such as tumor necrosis factor-α and interleukin-6, and upregulation of adhesion molecules required for lymphocyte homing followed by infiltration of inflammatory cells, mostly neutrophils, into the transduced tissue. This phase of immune activation has been shown to be independent of viral or transgene expression. When the viral proteins of adenoviral vectors are presented in the context of the MHC1 molecule, a cellular immune response is induced. This immune response is dominated by cytotoxic T lymphocytes (CTLs) that are responsible for the destruction of transduced cells2 and by neutralizing antibodies that reduce the effectiveness of vector readministration.
The systemic response to adenovirus may contrast with the consequences of local administration to the eye. Delivery of antigens into the ocular environment usually induces host tolerance, an effect that has been termed anterior chamber–associated immune deviation.3,4,5,6 Consistent with this concept, the systemic immune response against adenoviral vectors was absent following delivery to the ocular environment of immunocompetent mice,7 allowing prolonged and sustained expression in photoreceptors of adenoviral vector–delivered transgenes by the chimeric first-generation vector Ad5/F35 after a single injection.8
We have therefore measured the systemic immune response against adenoviral vectors injected into the vitreous of human retinoblastoma patients who received a first-generation adenoviral vector encoding the thymidine kinase gene (tk). We compared the immunological consequences to previously published observations from a phase 1 clinical trial in which the same vector was injected into human prostate tumors.9 We find that, unlike extraocular administration, administration of Ad-RSVtk to the eye does not induce systemic immune responses, even in individuals in whom the malignancy and its treatment had disrupted the normal tissue architecture, including the disruption of the blood–retinal barrier.10,11
Results
Characterization of ocular inflammatory response in Ad-RSVtk-treated retinoblastoma subjects
Nine retinoblastoma patients were enrolled in an institutional review board (IRB)–, Institutional Biosafety Safety Committee (IBC)–, and U S Food and Drug Administration (FDA)–approved phase 1 clinical trial to study the effects of adenoviral vector delivery of the herpes simplex thymidine kinase gene into the retinoblastomas of children who had previously failed conventional therapy. Patients received an intraocular injection of the vector followed by intravenous ganciclovir for 7 days. A mononuclear inflammatory response was observed in fluid obtained by paracentesis from the anterior chamber as early as 2 weeks following the injection of adenoviral vector (data not shown). Patients received topical steroids immediately following the procedure and the anterior chamber inflammatory response resolved within 1 month following the last injection of the adenoviral vector with no adverse effects on vision.
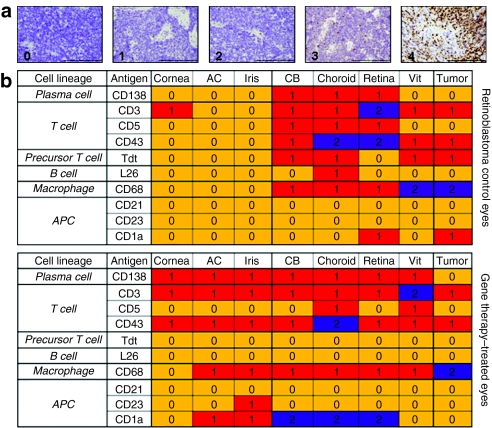
Although the targeted vitreous seeds were completely eradicated in all children who received intraocular Ad-RSVtk followed by systemic ganciclovir, all but one child eventually came to enucleation because of progressive disease in untreated areas of the eye.12 The remaining child was enucleated because of suspected recurrent seeds, although this was subsequently shown by histopathology to be inflammatory cells masquerading as vitreous tumor seeds. Tissue blocks of the enucleated eyes from seven of the children enrolled on the phase 1 trial were available for study. The tissues were sectioned and examined for inflammatory cells using the panel of antibodies listed in Table 1. The standard treatment for patients with unilateral retinoblastoma includes enucleation of the affected eye without prior treatment. Eyes from these patients were used as controls. The sections obtained from the fixed, paraffin-embedded eyes were graded for immune response using a 0–4 scale (Figure 1a). Differences in grading and localization of inflammatory cells within the eye structures were noted between patients who were injected with viral vector and patients who underwent primary enucleation (Figure 1b). An increase in grade 1 response was observed in the cornea, anterior chamber, and iris for CD138+, CD3+, CD43+, CD68+, CD23+, and CD1a+ cells when compared to eyes from patients who underwent enucleation alone. Furthermore, an increase in grade 2 response of CD1a+ cells was observed in the ciliary body, choroid, and retina of patients who received Ad-RSVtk when compared to eyes from patients whose only treatment was enucleation. When the Fisher's exact test and outcomes of negative (grade 0) versus positive (grade ≥1) response were used to compare the overall median grade between patients who had received adenoviral vector to those who were enucleated alone, there was only a statistically significant increase (P = 0.029) in CD138+ cells. Taken together, these results suggest that, although there are no statistically significant changes (possibly due to the small number of patients enrolled) in the total number of T cells (CD3+, CD5+, CD43+, and Tdt+), B cells (L26+), macrophages (CD68+), or antigen-presenting cells (CD23+, and CD1a+) detected, there is a change in the localization of these cells within the structures of the eyes.
Table 1. Antibodies utilized in immunohistochemistry staining of retinoblastoma samples.
Figure 1.
Characterization of the inflammatory response in the eyes of retinoblastoma patients injected with the Ad-RSVtk vector. (a) Pathological definitions of immunostaining grades for retinoblastoma eyes. Enucleated eyes from unilateral retinoblastoma patients and Ad-RSVtk-treated retinoblastoma subjects were stained with antibodies against different cell surface antigens. Based on staining, trained pathologists assigned grades of 0–4. Bar = 200 µm. (b) Median grade observed for different markers in retinoblastoma patients who received (n = 7) or did not receive (n = 6) the Ad-RSVtk vector followed by ganciclovir. Grading was determined for each characteristic area of the eye. AC, anterior chamber; APC, antigen-presenting cell; CB, ciliary body; Vit, vitreous.
Delivery of an Ad-RSVtk first-generation vector to the eye of retinoblastoma patients does not induce a systemic adenovirus-specific cytotoxic T-cell (CTL) immune response
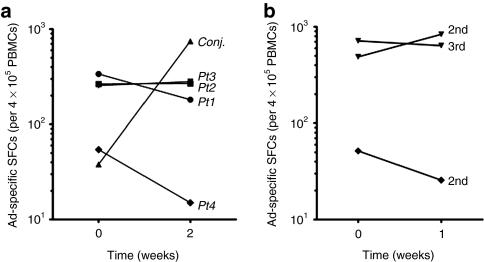
To determine whether the modest local inflammatory response observed in retinoblastoma subjects who received Ad-RSVtk vector is associated with a CTL immune response against adenovirus vector antigens, peripheral blood mononuclear cells (PBMCs) were isolated before and after injection from the patients who received 2.5 × 1010 to 1 × 1011 viral particles. The frequency of Ad-reactive cells in the patients' peripheral blood was compared with a subject suffering from adenovirus conjunctivitis who had not received local or systemic steroids (used as the positive control). We used an enzyme-linked immunosorbent spot (ELISpot) assay and stimulated patient T cells with an immunogenic adenovirus hexon PepMix containing a pool of peptides spanning the entire sequence of hexon from serotype 5 (ref. 13). This assay has been used by several groups to detect ex vivo-activated T cells from human patients.14,15,16,17 Baseline activation was measured using unstimulated PBMCs alone. No secretion of IFN-γ was induced in these cells. Patients who received the adenovirus vector had no increase of adenovirus-specific CTLs at 2 weeks after vector injection (Figure 2a), unlike the patient with adenoviral conjunctivitis who developed a significant increase in the frequency of adenovirus-specific CTLs 2 weeks after diagnosis. The wide variation observed in the baseline CTL response has been reported previously from healthy seropositive donors, and does not appear to impede further rises on exposure to the virus.13,14 Of note, patients 3 and 4 received three or two injections, respectively, of the adenoviral vector several weeks apart, but even after this repeat exposure, the patients had no significant increase in adenovirus-specific CTLs (Figure 2b). Furthermore, ELISpot assays were performed in all four patients for at least 2 months with no significant increase in Ad-specific spot-forming cells in any of the patients (data not shown).
Figure 2.
Delivery of Ad-RSVtk vector to the eye does not induce an adenovirus cytotoxic T lymphocyte (CTL)–specific immune response. (a) Presence of adenovirus-specific CTLs in the peripheral blood of four patients who received eye injections of Ad-RSVtk vector, and a patient diagnosed with adenovirus conjunctivitis was measured by enzyme-linked immunosorbent spot assay. PBMCs collected before the injection or at the time of diagnosis, and cells collected 2 weeks after the first acquired sample were challenged with an immunogenic hexon peptide common to all known serotypes of human adenovirus or PBMCs alone as a control (data not shown). None of the patients who received Ad-RSVtk vector injection had a significant increase in adenovirus-specific CTLs at 2 weeks after injection, whereas the patient with adenoviral conjunctivitis had a significant increase in adenovirus-specific CTLs at 2 weeks after diagnosis. This adenovirus conjunctivitis patient had not received steroid treatment. (b) Comparison of the adenovirus-specific CTL response of patients 3 (inverted closed triangles) and 4 (closed diamonds) who received multiple injections of adenovirus vector. No significant increase in adenovirus-specific CTL response was observed after 1 week of either of the subsequent vector injections. Plotted data were standardized to spot-forming cells (SFCs) per 4 × 105 PBMCs. PBMC, peripheral blood mononuclear cell.
Humoral immune response in Ad-RSVtk-treated retinoblastoma subjects
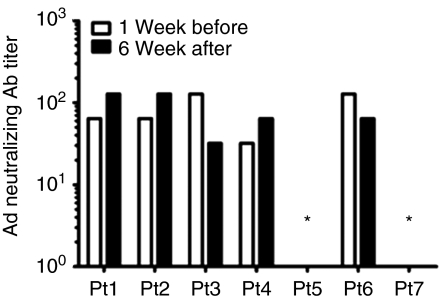
We measured neutralizing antibodies to adenovirus in serum samples prior to injection and 6 weeks following vector injection using a clinical laboratory complement fixation assay. Two of the retinoblastoma subjects had no detectable neutralizing IgG antibodies against adenovirus before or after injection of Ad-RSVtk. None of the subjects developed IgM (data not shown) or an increase in IgG antibodies against adenovirus (Figure 3).
Figure 3.
Delivery of Ad-RSVtk vector to the eye does not induce an adenovirus humoral immune response. Detection of adenovirus neutralizing antibodies was performed 1 week before the first injection of Ad-RSVtk and 6 weeks after the last injection of Ad-RSVtk vector. Titer is shown as the inverse of the highest dilution of serum showing a positive fixation of complement. All IgM values were <1:10 (data not shown). *Patients 5 and 7 had a titer <4 both before and after the injection and are considered to be negative for neutralizing antibodies.
Discussion
After intraocular administration of adenoviral vectors, retinoblastoma patients in a phase 1 clinical trial of adenoviral-mediated gene transfer showed a mild local inflammatory response characterized by changes in the localization without any substantive increase in absolute numbers of T cells, B cells, macrophages, and antigen-presenting cells. In contrast to reports after extraocular administration of the same vector,9 our patients did not develop systemic cellular or humoral immunity directed to the vector. These results support our previously published data that showed that the intraocular injection of adenoviral vectors in normal mice does not induce a systemic immune response.7 Interestingly, our current data suggest that, unlike in mice, the immune tolerance observed in our human retinoblastoma patients may be independent of the integrity of the blood–retinal barrier.
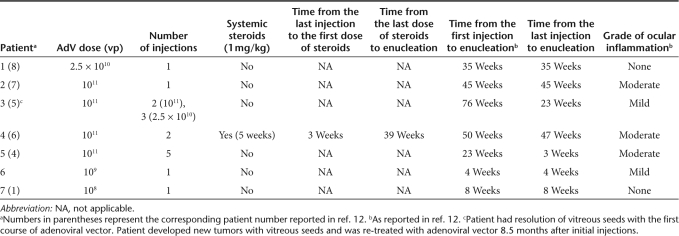
Adenoviral-mediated suicide gene transfer has been explored as a novel therapy for several malignancies.18 We previously reported a phase 1 clinical trial for children with retinoblastoma that explored the safety of using an intraocular injection of adenoviral vector to deliver a herpes simplex thymidine kinase (HS-tk) gene followed by systemic treatment with ganciclovir.12 Patients had completed any chemotherapy or radiation therapy course at least 1 month before their inclusion and were facing imminent enucleation. In this dose-escalation phase 1 clinical trial, patients received intravitreal injections of 1 × 108 to 1 × 1011 adenoviral vector particles in 100 µl of balanced salt solution using a microscope or indirect ophthalmoscope to visualize as a 30-gauge needle was inserted through the peripheral cornea, iris, and zonules avoiding the lens. These patients received postoperative ciprofloxacin (0.3%), scopolamine (0.25%), and prednisolone (1%) to prevent infections and manage the inflammation that might be caused by the procedure. Patients who developed a grade 1 or greater ocular inflammatory response were treated with oral prednisolone at 1 mg/kg/day. Steroids are frequently administered after intraocular injections (including just before and after ocular gene therapy) to reduce local inflammation caused by the procedure. Indeed, such an approach was used to manage postsurgical inflammation in all patients enrolled on an ocular gene replacement protocol using an adeno-associated viral vector to deliver the RPE65 transgene to patients with Leber's amaurosis.19 It is unlikely that such low total doses of steroids per se would cause systemic immunosuppression. When we analyzed the treatment time line of our patients, only patient 4 received systemic steroids at a dose of 1 mg/kg/day 3 weeks after the last injection of the Ad-RSVtk vector (Table 2). Patients 1, 2, and 3 did not receive systemic steroids, and the time from their first injection of the viral vector to enucleation was 35, 45, and 76 weeks, respectively, without reports of grade 1 or greater ocular inflammation. The results of our trial demonstrated no dose-limiting toxicity, and retinoblastoma vitreous seeds were completely eradicated in the targeted region following treatment in all of the eight patients who presented with this complication.
Table 2. Treatment time line for retinoblastoma patients injected with Ad-RSVtk followed by ganciclovir.
The injection of viral vectors into the mouse eye has previously shown to induce immune tolerance.1,7,20 Hoffman et al.20 reported that after subretinal delivery of an adenoviral vector, transgene expression was similar in immunodeficient and immunocompetent mice. Furthermore, subretinal and intravitreal delivery of adenoviral vectors appeared to actively inhibit systemic humoral and cell-mediated immune responses toward the adenoviral vectors and the expressed transgene, in part, by inducing the secretion of immunosuppressive cytokines such as IL-10 or TGF-β, and to allow sustained expression of the delivered transgene.7,8,21 However, this inhibition of immune response required the presence of an intact Bruch's membrane, the membrane that acts as the blood–retinal barrier. Suber et al.7 reported that when an adenoviral vector is injected intravitreally or subretinally into mice with inherited photoreceptor degeneration (rd-/rd- mice), a delayed-type hypersensitivity is induced that is not seen in control normal mice. In rd-/rd- mice, the integrity of the Bruch's membrane is compromised and the vector antigens can cross into the blood. Conventional treatment for unilateral retinoblastoma is usually enucleation, whereas both local and systemic treatment strategies are used in an attempt to save at least one eye when the disease is bilateral.22,23 All of the patients enrolled in our phase 1 trial had chorioretinal scarring and disruption of Bruch's membrane. This was caused by either the invasive tumor itself or by local therapies such as radiation, cryo- or laser treatments that compromised the integrity of the blood–retinal barrier.10,11 The lack of demonstrable systemic cellular or humoral immune responses to the adenoviral vector described in this article was therefore unexpected. Because the blood–retinal barrier was disrupted, the absence of a systemic immune response suggests that the local ocular environment is sufficient to prevent a systemic immune response to the adenoviral vector.
The results reported here contrast with those reported by van der Linden et al.9 This group used the same Ad-RSVtk vector at similar doses (2 × 1010 or 2 × 1011 vp) as a neoadjuvant therapy for prostate cancer patients. After administration of either dose of the vector, they reported an increase in the secretion of IFN-γ by the patients' CTLs after exposure to the adenoviral vector and an increase in adenovirus neutralizing antibodies. Increases in adenovirus neutralizing antibodies have also been reported by Xu et al.24 after administration of a similar vector containing the HS-tk gene. The fact that the retinoblastoma patients treated with the Ad-RSVtk vector at 1 × 1011 viral particles did not show any of the changes in adenovirus neutralizing antibodies or adenovirus-specific CTLs suggests that elements of the intraocular environment decreased the immunogenicity of the adenoviral vector. The response to intraocular exposure to foreign antigens is a unique immunologic tolerance that has been termed anterior chamber–associated immune deviation.3,4,5,6 This phenomenon occurs not only because of the local anatomic blood–retinal barrier of the eye and a lack of intraocular lymphatic drainage but also because of an immunosuppressive biochemical environment that induces local apoptosis of immunomodulatory cells and a systemic tolerance to the foreign antigen.25 Although this phenomenon is called anterior chamber–associated immune deviation, further evidence has suggested that it is not a property of the anterior chamber exclusively but of the internal structures of the eye such as the vitreous body and the retina as well.26,27 Intraocular injection of antigen in mice induces the expansion of CD4+CD25+FoxP3+ T regulatory cells,28 which further supports anterior chamber–associated immune deviation as an active process of immune tolerance. Nonetheless, we do not know in our own patient group whether a similar active process is occurring, or whether we are witnessing unresponsiveness for other reasons.
Although adenoviral vectors have many advantages over other vector delivery systems including a relatively large gene packaging capability, high transgene expression, efficient delivery into a large variety of both dividing and nondividing cells, and a relative ease of high titer production, the use of adenoviral vectors for systemic gene replacement therapy has fallen out of favor because of local and systemic immune responses that shorten the duration of transgene expression and increase morbidity.29,30,31,32,33 Of note, subretinal delivery of transgenes into immunocompetent mice using first-generation adenoviral vectors such as these demonstrated sustained, undiluted expression of the transgene in retinal photoreceptors for at least 8 months without any evidence of an inflammatory response.8 The favorable preliminary results of the phase 1 clinical trial for children with retinoblastoma,12 including the lack of a significant local or systemic immunologic response described in this article, should provide a new impetus to study the potential use of adenoviral vectors for not only short-duration cancer therapy but also for long-term gene replacement therapy in the ocular environment especially in photoreceptors and retinal pigmented epithelium that have previously been shown to be effective targets of these vectors. Furthermore, understanding the mechanisms that suppress the immune response after ocular injection of antigens such as adenoviral vectors may lead to innovations that could improve the outcomes for systemic gene therapy.34
Materials and Methods
Clinical protocol. An IRB, IBC, and FDA approved phase 1 clinical trial was initiated to examine the safety of injecting into the eye an adenoviral vector to deliver a herpes thymidine kinase gene (Ad-RSVtk) followed by systemic administration of ganciclovir to treat retinoblastoma.12 Blood samples were drawn immediately prior to the intraocular injections, and 1 and 2 weeks after each injection to determine T-cell responses measured by IFN-γ ELISpot and 6 weeks after the last injection to examine adenovirus-specific antibody titers. All patients eventually required enucleation, and eyes from Ad-RSVtk-treated patients were compared to those from nontreated retinoblastoma patients using histology and immunohistochemistry.
Immunohistochemistry on paraffin-embedded tissue. Histology sections mounted on slides were deparaffinized and rinsed with deionized water. Dako Target Retrieval Solution (Carpinteria, CA), diluted 1:10 according to the manufacturer's directions, was heated to 95 °C in a steamer. Slides were placed in the solution for 25 minutes, removed, allowed to cool for 20 minutes and rinsed in deionized water. Endogenous peroxidase was blocked by placing slides in 3% H2O2 in deionized water for 8 minutes. Slides were rinsed in deionized water and stained using the Dako Autostainer Universal Staining System. Primary antibodies (Table 1) were diluted in Dako Antibody Diluent, and slides were incubated for 20 minutes. Antibodies were visualized using the appropriate rabbit or mouse Dako EnVision HRP DAB+ Kit. The Envision System consists of an HRP-conjugated polymer with the appropriate rabbit or mouse secondary antibody. Slides were incubated with the labeled polymer for 25 minutes and with DAB+ for 5 minutes. Dako Wash Buffer, a Tris buffered saline with 0.05% Tween 20, was used for all rinses on the Autostainer except for a rinse with deionized water after DAB substrate incubation. After slides were removed from the Autostainer, they were rinsed in deionized water, counterstained with hematoxylin 2 (Thermo Fisher Richard-Allan Scientific, Kalamazoo, MI), rinsed in tap water, dehydrated, and coverslipped.
Isolation of PBMCs. Blood was drawn from subjects and diluted with phosphate-buffered saline (PBS 1X; Sigma, St Louis, MO). Sample was then layered over 20 ml of Lymphoprep (Axis-Shield, Oslo, Norway) and centrifuged at 4,000g for 45 minutes. The PBMC layer was removed and washed with PBS followed by centrifugation at 450g for 10 minutes. Cell pellets were resuspended in PBS, and viability was determined using Trypan blue exclusion. After a final centrifugation at 450g for 5 minutes, PBMCs were resuspended in freezing media (RPMI-1640, 10% DMSO) at 5 × 106 PBMCs/ml. Cells were stored at −80 °C until assayed.
Adenovirus-specific ELISpot assay. PBMCs were thawed and placed in complete culture media (RPMI-1640; Sigma; 1% Pen-Strep 10% human serum) in a 24-well plate (2 × 106 cell/well) overnight at 37 °C with 5% CO2. The MultiScreen plate (Millipore, Billerica, MA) was washed once with 75% ethanol and twice with PBS 1×. The wells were then coated with anti-IFN-γ (100 µl/well; Diapharma, West Chester, OH) and stored at 4 °C overnight. Excess antibody was decanted, and the plate was washed twice with PBS 1× at 5-minute intervals. Following the second wash, 150 µl/well of complete culture media were added and the plate was incubated for 1 hour at 37 °C. PBMCs were counted and resuspended at a final density of either 4 × 106 or 2 × 106 PBMCs/ml in complete culture media. Media was removed from the wells and replaced with a total of 2 × 105 or 4 × 105 PBMCs per well. Cells were challenged with a hexon PepMix that contained a pool of peptides spanning the entire sequence of hexon from serotype 5 (JPT Technologies, Berlin, Germany) or a control peptide from the Epstein-Barr virus (EBV) protein LMP-1 for 24 hours. Finally, cells were removed, and IFN-γ spots were developed using a biotinylated anti-IFN-γ that was detected with an avidin–peroxidase complex. Developed spots were counted by a third party, ZellNet Consulting (Fort Lee, NJ). Plotted data were standardized to spot-forming cells per 4 × 105 PBMCs by doubling the numbers of spots obtained in assays using 2 × 105 PBMCs. All spot-forming cells were well within the quantifiable range, as reported by ZellNet.
Detection of adenovirus neutralizing antibodies. Neutralizing antibody titers determined by complement fixation were performed by clinical laboratories designated by Texas Children's Hospital. The values reported here are lower than the saturating value of the assay (≥256); if increases had occurred, they would have been detected.
Statistical analysis. Median grade observed between the cornea and the vitreous was calculated for each marker. Considering median values between 0 as negative and ≥1 as positive, a Fisher's exact test was performed comparing Ad-RSVtk-treated versus -nontreated retinoblastoma eyes based on the numbers of eyes allocated to each category. This test was chosen due to the small sample size of the trial. P values ≤0.05 were considered significant.
Acknowledgments
This research was funded, in part, by NIH grants GM085793 (C.J.I.), CA103698 (R.L.H.), CA97762 (R.L.H.), Clayton Foundation for Research, Retina Research Foundation, Golfers Against Cancer, and the Howard Hughes Medical Institutes Med Into Grad Initiative.
REFERENCES
- Christ M, Lusky M, Stoeckel F, Dreyer D, Dieterlé A, Michou AI, et al. Gene therapy with recombinant adenovirus vectors: evaluation of the host immune response. Immunol Lett. 1997;57:19–25. doi: 10.1016/s0165-2478(97)00049-7. [DOI] [PubMed] [Google Scholar]
- Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Franklin RM., and, Prendergast RA. Primary rejection of skin allografts in the anterior chamber of the rabbit eye. J Immunol. 1970;104:463–469. [PubMed] [Google Scholar]
- Kaplan HJ., and, Streilein JW. Analysis of immunologic privilege within the anterior chamber of the eye. Transplant Proc. 1977;9:1193–1195. [PubMed] [Google Scholar]
- Raju S, Grogan JB., and, Hardy JD. A new test of rejection for implants in the anterior chamber of the eye: application for the study of immunological mechanisms operative in a privileged site. Am Surg. 1969;35:856–863. [PubMed] [Google Scholar]
- Taylor AW. Ocular immune privilege. Eye (Lond) 2009;23:1885–1889. doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suber ML, Hurwitz MY, Chévez-Barrios P., and, Hurwitz RL. Immune consequences of intraocular administration of modified adenoviral vectors. Hum Gene Ther. 2001;12:833–838. doi: 10.1089/104303401750148801. [DOI] [PubMed] [Google Scholar]
- Mallam JN, Hurwitz MY, Mahoney T, Chévez-Barrios P., and, Hurwitz RL. Efficient gene transfer into retinal cells using adenoviral vectors: dependence on receptor expression. Invest Ophthalmol Vis Sci. 2004;45:1680–1687. doi: 10.1167/iovs.03-0730. [DOI] [PubMed] [Google Scholar]
- van der Linden RR, Haagmans BL, Mongiat-Artus P, van Doornum GJ, Kraaij R, Kadmon D, et al. Virus specific immune responses after human neoadjuvant adenovirus-mediated suicide gene therapy for prostate cancer. Eur Urol. 2005;48:153–161. doi: 10.1016/j.eururo.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Abramson DH, Frank CM, Chantada GL, de Totah AB, de Pifano IT, Ramírez GT, et al. Intraocular carboplatin concentrations following intravenous administration for human intraocular retinoblastoma. Ophthalmic Genet. 1999;20:31–36. doi: 10.1076/opge.20.1.31.2302. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG. The blood-retinal barriers. Doc Ophthalmol. 1976;41:287–327. doi: 10.1007/BF00146764. [DOI] [PubMed] [Google Scholar]
- Chévez-Barrios P, Chintagumpala M, Mieler W, Paysse E, Boniuk M, Kozinetz C, et al. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol. 2005;23:7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- Leen AM, Sili U, Vanin EF, Jewell AM, Xie W, Vignali D, et al. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Ohrmalm L, Lindblom A, Omar H, Norbeck O, Gustafson I, Lewensohn-Fuchs I, et al. Bone Marrow Transplant. epub ahead of print; 2010. Evaluation of a surveillance strategy for early detection of adenovirus by PCR of peripheral blood in hematopoietic SCT recipients: incidence and outcome. [DOI] [PubMed] [Google Scholar]
- Myers GD, Bollard CM, Wu MF, Weiss H, Rooney CM, Heslop HE, et al. Reconstitution of adenovirus-specific cell-mediated immunity in pediatric patients after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:677–686. doi: 10.1038/sj.bmt.1705645. [DOI] [PubMed] [Google Scholar]
- Olive M, Eisenlohr L, Flomenberg N, Hsu S., and, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- Smythe WR, Hwang HC, Amin KM, Eck SL, Davidson BL, Wilson JM, et al. Use of recombinant adenovirus to transfer the herpes simplex virus thymidine kinase (HSVtk) gene to thoracic neoplasms: an effective in vitro drug sensitization system. Cancer Res. 1994;54:2055–2059. [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LM, Maguire AM., and, Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol Vis Sci. 1997;38:2224–2233. [PubMed] [Google Scholar]
- Anand V, Duffy B, Yang Z, Dejneka NS, Maguire AM., and, Bennett J. A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Mol Ther. 2002;5:125–132. doi: 10.1006/mthe.2002.0525. [DOI] [PubMed] [Google Scholar]
- Hurwitz RL, Shields CL, Shields JA, Chevez-Barrios P, Hurwitz MY., and, Chintagumpala M. Pizzo PA, Poplack DG. Retinoblastoma. Principles and Practice of Pediatric Oncology. Lippincott-Raven: Philadelphia, PA; 2002. pp. 825–846. [Google Scholar]
- Hurwitz RL, Chévez-Barrios P, Boniuk M, Chintagumpala M., and, Hurwitz MY. Retinoblastoma: from bench to bedside. Expert Rev Mol Med. 2003;5:1–14. doi: 10.1017/S1462399403005520. [DOI] [PubMed] [Google Scholar]
- Xu F, Li S, Li XL, Guo Y, Zou BY, Xu R, et al. Phase I and biodistribution study of recombinant adenovirus vector-mediated herpes simplex virus thymidine kinase gene and ganciclovir administration in patients with head and neck cancer and other malignant tumors. Cancer Gene Ther. 2009;16:723–730. doi: 10.1038/cgt.2009.19. [DOI] [PubMed] [Google Scholar]
- Camelo S, Kezic J., and, McMenamin PG. Anterior chamber-associated immune deviation: a review of the anatomical evidence for the afferent arm of this unusual experimental model of ocular immune responses. Clin Experiment Ophthalmol. 2005;33:426–432. doi: 10.1111/j.1442-9071.2005.01044.x. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Wilbanks GA., and, Cousins SW. Immunoregulatory mechanisms of the eye. J Neuroimmunol. 1992;39:185–200. doi: 10.1016/0165-5728(92)90253-h. [DOI] [PubMed] [Google Scholar]
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- Saban DR, Cornelius J, Masli S, Schwartzkopff J, Doyle M, Chauhan SK, et al. The role of ACAID and CD4+CD25+FOXP3+ regulatory T cells on CTL function against MHC alloantigens. Mol Vis. 2008;14:2435–2442. [PMC free article] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Bowen GP, Borgland SL, Lam M, Libermann TA, Wong NC., and, Muruve DA. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-kappa B. Hum Gene Ther. 2002;13:367–379. doi: 10.1089/10430340252792503. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Gahery-Segard H, Mehtali M, Le Boulaire C, Ribault S, Boulanger P, et al. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J Virol. 2000;74:7678–7682. doi: 10.1128/jvi.74.16.7678-7682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michou AI, Santoro L, Christ M, Julliard V, Pavirani A., and, Mehtali M. Adenovirus-mediated gene transfer: influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE., and, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J Neurosci. 1996;16:3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner MK. The eyes have it. Mol Ther. 2010;18:451–452. doi: 10.1038/mt.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]