Abstract
Choroidal neovascularization (CNV) is a common cause of severe and irreversible visual loss; however, the treatment of CNV has been hindered by its complex and poorly understood pathogenesis. It has been postulated that bone marrow (BM)–derived cells (BMCs) contribute to CNV, but little is known about the role of mesenchymal stem cells (MSCs) in CNV and their therapeutic potential for CNV treatment. We found that BM-derived MSCs transplanted by intravenous injection into laser-induced CNV mouse models were specifically recruited into CNV lesions, where they differentiated into multiple cell types and participated in the development of neovascularization, without stagnation in other organs. By taking advantage of this recruitment potential, engineered MSCs were used to produce the antiangiogenic pigment epithelial-derived factor (PEDF) at the CNV sites, thereby inhibiting the growth of CNVs and stimulating regressive features. Further studies indicated that the effect may be mediated, at least partly, by retinal pigment epithelial (RPE) cells, which function as important regulators for CNV development. These results suggest that MSCs contribute to CNV and could serve as delivery vehicles of antiangiogenic agents for the treatment of a range of CNV-associated diseases.
Introduction
Pathological angiogenesis in the eye often leads to serious consequences, including intractable high intraocular pressure, visual impairment, and even irreversible blindness. One major manifestation of ocular angiogenesis is choroidal neovascularization (CNV). CNV is characterized by the formation of new blood vessels that arise from the choriocapillaris through Bruch's membranes into the subretinal space, causing exudation of fluid and hemorrhaging. Furthermore, CNV is often accompanied by the atrophy and senescence of retinal pigment epithelial (RPE) cells and microfractures in Bruch's membranes. Consequently, the overlying neurosensory retina may detach, and the ensuing damage to the retinal photoreceptors could lead to irreversible visual loss.1 CNV is now known to be a common process in nearly 40 ophthalmic diseases affecting people of all ages, especially the elderly.2 The most common condition associated with CNV is age-related macular degeneration, which has emerged as the leading cause of blindness among people aged ≥50 (ref. 3).
In light of the serious social and economic costs of CNV-related diseases, several CNV treatment options, such as ionizing radiation, laser photocoagulation, surgical removal, and photodynamic therapy, have been developed.4 Among them, pharmacotherapy with antiangiogenic agents that target the angiogenic vascular endothelial growth factor (VEGF) pathway has shown relatively high efficacy. Most other therapies, however, are largely ineffective. Even in the case of pharmacotherapy, regression of neovascularization is rarely permanent, and the regrowth of new vessels, often within a few months, requires multiple treatments. Moreover, frequent, invasive, intravitreal injections of antiangiogenic agents may be associated with serious side effects, such as endophthalmitis.3,5 Therefore, it is a pressing issue to develop innovative therapeutic strategies that are less invasive and safer, with enhanced specificity and efficacy.
Mesenchymal stem cells (MSCs) have been shown to differentiate into endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) and incorporate into the new blood vessel wall and form vascular tubes.6 On the other hand, MSCs play distinct roles in different angiogenic models. In contrast to angiogenic activities in various organs other than the eyes,6,7,8 MSCs display antiangiogenic effects in the cornea.9 Recently, MSCs, which were recruited into tumors and function as potential precursors for tumor stroma, have been used as delivery vehicles for anticancer agents via the systemic circulation.10,11,12,13,14,15 Yet, little is known about the contribution of MSCs to CNV, although accumulating evidence has indicated that bone marrow (BM)–derived cells (BMCs), a heterogeneous cell population comprised multiple types of stem/progenitor cells, participate in CNV formation.16,17,18,19 Accordingly, the purpose of this study was to investigate whether MSCs contribute to CNV formation and to explore the potential application of MSCs in CNV treatment.
Results
Isolation and characterization of MSCs
Using the well-established method described above, we enriched plastic-adherent mouse BMCs expressing surface markers characteristic of multipotent MSCs. Following their third passage, cell cultures were devoid of hematopoietic cells and highly enriched for MSCs, as judged by the lack of the hematopoietic markers CD34 and CD45, and the expression of CD44, CD29, and CD105. The multipotent nature of the MSCs was further confirmed by their capacity to differentiate into the adipogenic and osteogenic lineages in vitro. Thus, we concluded that the enriched stem cells were bona fide MSCs, which were subsequently used in the following experiments.
Specific recruitment of MSCs to CNV and their differentiation in CNV
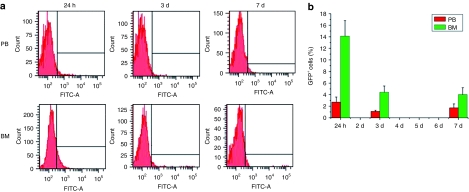
We first examined whether MSCs can be specifically recruited to sites of CNV. We evaluated whether MSCs may remain in other tissues outside the eyes. After laser photocoagulation and transplantation of MSCs with a green fluorescent protein (GFP) reporter, the number of MSCs in the peripheral blood (PB) slightly increased on day 1 after treatment and decreased afterwards. Some MSCs underwent a transient retention in the BM. The number of GFP-labeled cells in the BM peaked on day 1. Afterwards, however, MSCs decreased rapidly (Figure 1). GFP+ cells were not found in lung, liver, spleen, or heart tissues. Recruitment of MSCs to sites of CNV was confirmed by choroidal flat mount. On day 1, a large number of green cells were found to be dispersed around the laser photocoagulation sites (Figure 2, 1 day). On day 3, cell rings composed of green cells were found to surround the laser spots, indicating that the MSCs were moving directionally closer toward the CNV lesions (Figure 2, 3 days). On day 7, GFP-labeled MSCs were found at the sites of CNV and appeared to participate in vascular structure formation (Figure 2, 7 days).
Figure 1.
Quantitative analysis of GFP-expressing mesenchymal stem cells (MSCs) in PB and BM by flow cytometry after laser photocoagulation and GFP-expressing MSC transplantation. (a) Representative data from three experiments are shown. (b) The quantity of GFP-expressing MSCs in BM increased obviously in the first 24 hours of the experiment and decreased rapidly thereafter. The number of MSCs in PB slightly increased on day 1 (error bars, SEM, n = 6). BM, bone marrow; GFP, green fluorescent protein; PB, peripheral blood.
Figure 2.
Representative choroidal flat mount preparations after laser photocoagulation and GFP-expressing mesenchymal stem cell (MSC) transplantation. Blood vessels were stained by rhodamine-conjugated agglutinin (red) in choroidal flat mounts. Panels show the recruitment of GFP-expressing MSCs (green) to the choroidal neovascularization (CNV) lesion (red) on days 1, 3, and 7, indicating a directional movement of the MSCs into the CNV lesions. On day 7, GFP-expressing MSCs in CNV participated in vascular structure formation (yellow). Bar = 50 µm. GFP, green fluorescent protein.
Next, we conducted confocal microscopy to examine the location and differentiation of MSCs in the eye. Most GFP-labeled MSCs appeared to be integrated into CNV between the choroid and the photoreceptor cell layer at the laser spots. We detected MSCs that had differentiated into ECs, VSMCs, macrophages, and RPE cells, which are the major cell types in CNV.20 Expression of all five cell markers (CD31, α-smooth muscle actin (αSMA), F4/80, vimentin, and keratin) were detected in the MSCs examined (Figure 3).
Figure 3.
Immunofluorescence staining of eye sections showing differentiated GFP-expressing mesenchymal stem cells (MSCs) in choroidal neovascularization (CNV) 1 week after CNV induction. The differentiation of MSCs in CNV was analyzed by cell maker staining. Representative confocal images show that MSCs (green) express (a) the mature vascular endothelial cell marker CD31 (red), (b) the vascular smooth muscle cell marker αSMA (red), (c) the macrophage marker F4/80 (red), (d) the fibroblast marker vimentin (red), and (e) the epithelial cell marker keratin (red). Blue: DAPI-stained nuclei. Bar = 20 µm. αSMA, α-smooth muscle actin; GFP, green fluorescent protein.
Transduction efficiency and PEDF expression in MSCs
The transduction efficiency of adenoviral vectors in mouse MSCs was analyzed. Reporter GFP expression in MSCs was detected 24 hours after transduction using an inverted fluorescent microscope (Figure 4a,b). Flow cytometry analyses further showed that 73.6 ± 5.3% of the total cell population was GFP+ (Figure 4c). Adenoviral-expressed pigment epithelial-derived factor (PEDF) persisted for at least 8 days in MSCs in vitro, with the maximum production observed during the first 24 hours after transduction (Figure 4d). In contrast, human PEDF was absent in the supernatant from MSCs transfected with control vectors (AdNull) and untransduced MSCs throughout the entire experimental period.
Figure 4.
Analysis of adenoviral transduction efficiency 24 hours after transduction and enzyme-linked immunosorbent assays of human pigment epithelial-derived factor (PEDF) released by adenoviral vectors expressing PEDF (AdPEDF)–transduced mesenchymal stem cells (MSCs). Representative inverted microscopy images show reporter green fluorescent protein (GFP) expression in MSCs (a, light microscope image; b, fluorescent image). (c) A representative flow cytometry analysis shows that 78.1% cells within the total cell population expressed GFP. (d) Human PEDF production by AdPEDF-transduced MSCs was detected in vitro during an 8-day experimental period. Maximum production was observed during the first 24 hours after infection (error bars, SEM, n = 9). Bar = 20 µm.
In vivo PEDF expression was examined by immunofluorescent staining and enzyme-linked immunosorbent assays (ELISAs). Human PEDF+ staining was found in MSCs and in the nearby extracellular matrix in serial eye sections from mice transplanted with adenoviral vectors expressing PEDF (AdPEDF)-transduced MSCs (Figure 5a). ELISAs (Figure 5b) further showed that, in contrast to the pattern observed in vitro, the average production of human PEDF in the eyes from the group transduced with AdPEDF was constant throughout the experimental period and more than fourfold higher than the quantity required to elicit antiangiogenic effects and CNV inhibition.21 In contrast, human PEDF was absent in the blood samples from all experimental mice and eye samples from the AdNull, nontransduced, and control groups.
Figure 5.
Immunofluorescent staining and enzyme-linked immunosorbent assays (ELISAs) of human PEDF expression in the eyes of mice treated with adenoviral vectors expressing PEDF (AdPEDF)–transduced MSCs. (a) In eye sections of the AdPEDF-expressing mice, positive human PEDF staining (red) was found in AdPEDF-transduced MSCs (green) and the extracellular matrix nearby. Blue: DAPI-stained nuclei. (b) ELISAs show that the average production of human PEDF in eyes of the AdPEDF-expressing mice was relatively steady for 1 week (error bars, SEM, n = 6). Bar = 20 µm. GFP, green fluorescent protein; PEDF, pigment epithelial-derived factor.
Reduction in the severity of CNV in mice treated with AdPEDF-transduced MSCs
Based on the confirmation that MSCs were recruited to sites of CNV, we then examined whether MSCs could be utilized to inhibit CNV growth. CNV lesion severity was measured by quantitative analyses of both histopathology and flat mount. First, the effect of MSCs themselves was examined. The nontransduced group showed a slight increase in CNV severity compared to the control group, although there was no statistically significant difference. Therefore, we subsequently explored the effect of the antiangiogenic factor PEDF on CNV. There was a significant reduction in CNV thickness, diameter (Figure 6a), and surface area (Figure 6b) in the group treated with AdPEDF-transduced MSCs. There was no statistically significant difference between the control group, the AdNull group, and nontransduced group, and we confirmed that it was PEDF secreted by MSCs that affected CNV severity.
Figure 6.
Hematoxylin and eosin (H&E) staining, choroidal flat mount, and quantitative analysis of choroidal neovascularization (CNV) severity 1 week after laser photocoagulation. (a) Representative H&E images show that CNVs from the AdPEDF group were encapsulated by pigmented cells (arrows), whereas CNVs in other groups were not. CNVs are indicated by dotted lines. The average thickness and diameter of CNVs from the AdPEDF group significantly decreased compared to other groups. No statistical difference was found among the other groups (error bars, SEM, n = 8). Bar = 20 µm. (b) The images of choroidal flat mounts show smaller surface areas of CNV (red) in the AdPEDF group. CNV area was significantly reduced in the AdPEDF group. No statistically significant difference was found among the other groups (error bars, SEM, n = 8). Bar = 50 µm. AdPEDF, adenoviral vectors expressing pigment epithelial-derived factor.
Using histopathological analyses, we noticed that 7 days after the injection of AdPEDF-transduced MSCs, many CNVs were encapsulated by pigmented cells, suggesting a regression in neovascularization;22 however, on the other hand, this phenomenon was rarely observed in the other groups (Figure 6a). As a result, we investigated the effect of AdPEDF-transduced MSCs on RPE cells in vitro.
Enhanced proliferation and migration of RPE cells by PEDF produced by MSCs
We examined whether AdPEDF-transduced MSCs affected proliferation and migration of RPE cells in vitro. When cocultured with AdPEDF-transduced MSCs, RPE cells displayed a markedly increased migration rate (Figure 7a), suggesting that the AdPEDF-transduced MSCs were chemotactic for RPE cells. Furthermore, RPE cells that were cocultured with AdPEDF-transduced MSCs were found to proliferate more rapidly relative to cells under other coculture conditions (Figure 7b). Meanwhile, the migration and proliferation of RPE cells were compared to RPE cells that were cocultured with untransduced MSCs and AdNull-transduced MSCs, but there was no statistically significant difference. Therefore, we confirmed that the migration and proliferation of RPE cells were stimulated by PEDF.
Figure 7.
Proliferation and migration assays of RPE cells cocultured with MSCs. (a) Migration of RPE cells was stimulated by the secretion of AdPEDF-transduced MSCs after coculture for 8 hours. (b) Three days after coculture, proliferation of RPE cells cocultured with AdPEDF-transduced MSCs was markedly enhanced. There was no statistically significant difference between the control group, the AdNull group, and the nontransduction group in both assays (error bars, SEM, n = 10). AdPEDF, adenoviral vectors expressing pigment epithelial-derived factor.
Discussion
In this study, we demonstrated that BM-derived MSCs were specially recruited into CNV lesions to participate in CNV development. As an antiangiogenic therapy, engineered MSCs were exogenously administered by intravenous injection, and they locally produced a therapeutic dose of the antiangiogenic factor PEDF to inhibit CNV growth in vivo. Furthermore, the MSC-derived PEDF also enhanced RPE cells, which regulate CNV development, proliferation, and migration.
Both vasculogenesis and angiogenesis play roles in neovascularization in the eye and elsewhere. Previous studies have provided accumulating evidence for a role of BMCs in CNV. In these studies, following lethal irradiation, labeled BMCs were transplanted into animal models, and laser photocoagulation was conducted after BM reconstruction. Laser injury alone was sufficient to induce recruitment of BMCs,20 which may have contributed up to 50% of the total vasculature.17 Our study specifically examined the role of MSCs, a major cell type of the BM. Compared to previous studies, we performed intravenous injection of labeled MSCs, but we avoided BM re-establishment to allow direct and special detection of MSCs.
We found that MSCs contributed to CNV formation. CNV is a complex tissue composed of both vascular components (ECs, VSMCs, and pericytes) and extravascular cells (inflammatory cells, myofibroblasts, glial cells, and RPE cells).3,23 ECs, VSMCs, macrophages, and RPE cells are the major cell types of CNV.20 In light of the multilineage potential of MSCs, and the demonstrated differentiation of MSCs to vascular cells6 and connective tissue cells,12 it is conceivable that MSCs can differentiate into both vascular and extravascular cells in CNV. In this study, we examined cell markers for five cell types, including ECs, VSMCs, macrophages, fibroblasts, and RPE cells, and found that MSCs differentiated into these cell types in CNV. Considering that GFP+ MSCs were transplanted without irradiation, BMCs from the recipient, which were undetectable, must have also participated in CNV formation. Accordingly, some cells of each cell type in CNV were probably derived from the autologous MSCs. Therefore, we do not presume that the differentiated cells detected in this study represented the overall contribution of MSCs to CNV.
In contrast to the antiangiogenic effect of MSCs in corneal wound healing following chemical injury,9 MSCs alone did not inhibit CNV in this model. In alcohol-injured corneas, the role of MSCs was attributed to their anti-inflammatory effects.9 Although CNV involves some degree of inflammation, the inflammatory component varies in intensity from minimal inflammation to pronounced inflammation depending on the underlying disease and dynamic stage of CNV development.2 Therefore, although MSCs might exert antiangiogenic effects under certain conditions in CNV or with an increased transplantation of MSCs, we turned to explore a more efficient way of treating CNV, which is to use MSCs as delivery vehicles of antiangiogenic factors.
The application of MSC vectors in CNV treatment is their recruitment to the lesion. A putative CNV tropism is based primarily on the innate physiological ability of MSCs to move to the sites of inflammation and tissue repair,11 and CNV appears to be a component of several key processes that can be referred to as wound healing or tissue repair.24 Two main chemokines, VEGF and stromal-derived factor-1, that navigate BMC trafficking and homing in CNV, contain receptors on MSCs.6,25,26,27,28 In the current study, we revealed the specific and rapid recruitment of MSCs to the sites of laser injury without movement into other organs (except for very short retention in the BM). Moreover, expression of PEDF in MSCs was absent in the blood, which further suggests the potential to avoid possible side effects. Previous reporters showed other advantages of MSCs as therapeutic candidates. MSCs are amenable to genetic manipulation in vitro,10 and have high metabolic activities and efficiently secrete therapeutic proteins. MSCs exhibit low intrinsic mutation rates.29 Finally, MSCs show little or low immunogenicity due to the lack of expression of co-stimulatory molecules.11 Therefore, MSCs probably act as cellular vectors and transgenic protein factories to safely and efficiently deliver therapeutic agents in CNV. This administration strategy may be especially suitable for ocular diseases because the existence of the blood–ocular barrier confines agents in the circulation. Moreover, delivery of proteins, which are not suitable for intravitreous injection due to poor permeability through the retina to CNV lesions, would be improved by using MSCs.
It is believed that CNV may be attributed to an imbalance in the expression of angiogenic and antiangiogenic factors. Among the endogenous inhibitors of neovascularization, PEDF is a strong inhibitor of angiogenesis.1,30 Compared with other antiangiogenic agents, PEDF may be ideally suited for CNV treatment. PEDF targets aberrant neovascularization and has no known deleterious effects on mature blood vessels.1,5 Furthermore, PEDF is the only known therapeutic agent for CNV inhibition, which can cause regression of already established ocular neovascularization.22 It has been reported that 6 ng of PEDF per eye was sufficient to inhibit CNV.21 In the present study, sufficient PEDF was delivered by transduced MSCs recruited to CNV. Consequently, the growth of CNV was inhibited.
PEDF is known as an antiangiogenic factor because it can induce apoptosis and inhibit migration of ECs,1,31 downregulate VEGF expression, and inhibit VEGF–VEGF receptor 2 binding.32 In fact, the effects of PEDF are complex, and its target cells are various.33,34,35 PEDF can also induce proliferation1 and migration,33 among other effects,1,34,35 which suggests that distinct signal transduction pathways are affected. It is possible that the biological activities of PEDF rest with the differential binding and dissociations of multiple receptors, which are affected by different microenvironments.1,33 A lipase-linked cell membrane receptor for PEDF has been found on RPE cells,36 although the exact signal that would be transduced is still unknown. Under normal conditions, RPE is a major source of PEDF in the eye.1,34,35,37 Under pathological conditions, however, with the atrophy of RPE, reduced PEDF production may permit the formation of CNV.1,30,38 Our study showed improved proliferation and migration of RPE cells when cocultured with AdPEDF-transduced MSCs. Therefore, we presume that a positive feedback loop may exist, in which PEDF improves RPE proliferation, and PEDF expression by RPE cells increases accordingly, causing enhanced antiangiogenic effects. CNV inhibition by AdPEDF-transduced MSCs may be partly mediated by RPE cells; however, the detailed mechanism by which this occurs needs further investigation.
The laser-induced Bruch's membrane photocoagulation model, in which the course of CNV is characterized by a tissue repair response, is the most widely accepted and most frequently utilized experimental CNV model by virtue of its recapitulation of many important features of human age-related macular degeneration.3 However, there are multiple causative factors for CNV in patients, such as pathological myopia, trauma, and infectious diseases, which mandate further studies using distinct experimental systems. In addition, although it remains unclear whether BMCs that can develop into MSCs under in vitro conditions are identical to circulating mesenchymal precursors in PB, it is assumed that cultured BM adherent cells possess MSCs potentials.10,39 The potential concerns over the use of MSCs as delivery vehicles mainly stem from studies that have shown that MSCs can be precursors of tumor stroma.10,11 Yet, the possibility that MSCs are able to enhance or initiate tumor growth is unlikely because of the intrinsic homeostasis of MSCs.29 MSCs may possess tumorigenic properties only after extensive passage, whereas lower-passage MSCs do not form tumors in vivo.29 In this study, third-passage MSCs were used. As a result, we could exclude the possibility that MSCs were single-handedly tumorigenic.
In summary, we have demonstrated that MSCs play a role in CNV and serve as a powerful cellular delivery system for antiangiogenic therapeutic agents in CNV. Our findings may facilitate further understanding of the mechanisms that underlie CNV and represent new therapeutic strategy for CNV treatment.
Materials and Methods
Isolation, culture, and characterization of MSCs. Isolation and enrichment of mouse MSCs was carried out using standard protocols.40 Briefly, BM cells from tibias and femurs of 4-week-old female C57BL/6 mice were cultured on plastic dishes in Dulbecco's modified Eagle's medium supplemented with 15% fetal calf serum (Gibco, Grand Island, NY) and incubated at 37 °C with 5% CO2 in an incubator. After 3 days, nonadherent cells were removed by washing with phosphate-buffered saline, and the remaining monolayer cells were cultured in fresh medium until they reached confluence. After 6–7 days, cells were trypsinized (0.25% trypsin with 0.1% EDTA) and subcultured. The third-passage cells were used for experiments.
To validate enrichment of MSCs, flow cytometry was carried out to characterize the surface antigens, and in vitro differentiation of cultured MSCs was performed as previously reported.41 Briefly, flow cytometry analysis was performed on a BD FACSAria Flow Cytometer (BD Biosciences, San Diego, CA). When the cells became confluent, MSCs were trypsinized and stained using fluorescein isothiocyanate–labeled antibodies against CD44, CD45, CD34, or phycoerythrin-labeled CD105, and CD29 (all from eBioscience, San Diego, CA). Negative control immunofluorescence experiments were performed in parallel with unrelated antibodies. Adipogenic differentiation was induced with dexamethasone, isobutylmethylxanthine, hydrocortisone, indomethacin, and insulin in low-glucose Dulbecco's modified Eagle's medium and 10% fetal calf serum. After 16 days, cells were fixed with 10% formalin and stained with Oil Red O for the detection of lipid vacuoles. Osteogenic differentiation was induced with dexamethasone, ascorbic acid, and β-glycerol phosphate in low-glucose Dulbecco's modified Eagle's medium and 10% fetal calf serum. Cultures were harvested at day 18. Calcium deposition was evaluated qualitatively by von Kossa staining of formalin-fixed cultures. All chemicals used for the induction of differentiation were obtained from Sigma-Aldrich (St Louis, MO). Cultures grown in MSC medium for the entire period served as negative controls.
CNV induction in mice and MSC transplantation. Laser induction of CNV was performed as previously reported.42 Briefly, recipient mice were anesthetized, and their pupils were dilated. Laser photocoagulation (532 nm wavelength, 75 µm spot size, 0.1 second duration, and 90 mW intensity) was delivered with a slit lamp and a cornea contact lens. Six burns were performed in positions 1–1.5 disc diameters from the optic nerve. Only laser spots where rupture of Bruch's membrane was confirmed with a vaporization bubble without hemorrhage were considered effective and included in the study.
To investigate the role of MSCs in experimental CNV, MSCs derived from GFP+ transgenic C57BL/6 homozygous mice were cultured and transplanted into wild-type C57BL/6 mice. The third-passage GFP+ MSCs were trypsinized, and the concentration of cells was assessed by microscopy. The cell suspensions were adjusted to 1.0 × 107 cells/ml with saline, and the GFP+ MSCs (4.0 × 106 cells/0.4 ml/mouse) were injected into adult C57BL/6 mice tail veins 0.5–1 hour after laser photocoagulation.
Detection of MSC recruitment. To investigate the recruitment of MSCs, PB, BM, some highly vascularized organs, and CNV lesions were examined for GFP+ MSCs. On days 1, 3, and 7 after laser-induced CNV, PB samples from tail veins were collected into heparinized tubes. The mice were then killed and perfused transcardially with 20 ml warm 0.9% saline followed by 50 ml cold 4% paraformaldehyde in 0.1 mol/l phosphate buffer (pH 7.4). BM from femurs and tibiae were collected into heparinized tubes. Red blood cells in PB and BM were schizolysed, and the percentage of cells expressing GFP in the remaining population was analyzed by flow cytometry. Lung, liver, spleen, and heart tissues were excised and post-fixed in the same fixative for 2 hours at 4 °C. Subsequently, the samples were successively treated with 10, 20, and 30% sucrose in 0.1 mol/l phosphate buffer overnight at 4 °C for cryoprotection. Alternate sets of 8 µm thick serial vertical sections of the tissues were obtained on a cryostat (CM1990; Leica, Nussloch, Germany) and mounted on gelatin-coated slides for examination with a fluorescent microscope (BX51; Olympus, Tokyo, Japan). Choroidal flat mount preparations were performed as previously described.43 After the mice were perfused, eyes were enucleated and incubated first in 4% paraformaldehyde and then in phosphate-buffered saline. The corneas and lenses were removed, and the neural retinas were carefully dissected from the eyecups. The remaining RPE–choroid–sclera complexes were flat mounted with six radial cuts. Whole flat mounts were permeabilized in 0.2% Triton X-100 in Tris-buffered saline and incubated with rhodamine-conjugated Ricinus communis agglutinin (1:1000; Vector Laboratories, Burlingame, CA) for 24 hours at room temperature followed by washing in Tris-buffered saline. Finally, the flat mounts were mounted on gelatin-coated slides in aqueous mounting medium (Aquamount; BDH, Poole, UK) with the scleras facing down. Microscopy was conducted using a confocal laser scanning microscope (Fluoview 300; Olympus, Tokyo, Japan).
Analysis of MSC differentiation in CNV. To analyze differentiation of MSCs in CNV, cell markers for the five cell types of CNV (i.e., ECs, VSMCs, macrophages, epithelial cells, and fibroblasts) were examined by immunofluorescence as previously described.42 One week after CNV induction and injection of 4.0 × 106 GFP+ MSCs, mice were killed and perfused. Serial cross sections of eyes were incubated with primary antibodies for CD31 (1:500; Chemicon, Temecula, CA), αSMA (1:80; Neomarkers, Fremont, CA), F4/80 (1:400; Serotec, Raleigh, NC), keratin (1:30; Neomarkers), or vimentin (1:100; Abcam, Cambridge, MA). Following incubation with Texas Red–conjugated secondary antibodies, cell nuclei were stained with 40, 60-diamino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR). Slides were sealed with antifading medium and examined by confocal laser scanning microscopy. Observations were carried out with an ultraviolet-corrected objective lens (UPLAPO ×40). Digital images were captured by Fluoview application software (Olympus).
Adenoviral vectors and transduction of MSCs. Human type 5 AdPEDF from a cytomegalovirus (CMV) immediate-early promoter were generated as previous described.44 The E1 and E3 regions of the viral genome were replaced with a GFP reporter gene. The human, full-length PEDF open-reading frame was cloned into a pAdCMV shuttle plasmid containing the expression cassette and an adjacent portion of the adenoviral genome. By co-transformating Escherichia coli with the shuttle plasmid and a recipient plasmid containing the remaining viral genome, a plasmid construct carrying the complete genome of the vector with the PEDF expression cassette was generated. Adenoviral vectors were generated by transfection of the complementing cells with the plasmid. Virus concentration was determined by the plaque assay. The control vector, AdNull, which does not express PEDF was constructed and produced in parallel.
MSCs were incubated with adenoviruses at a multiplicity of infection of 3,000 for 2 hours. Transduction efficiency was analyzed 24 hours after transduction by flow cytometry and inverted microscopy.
ELISA for PEDF expression in vitro. The concentrations of PEDF released by the transduced MSCs were measured by a human PEDF ELISA kit (USCN Life Science and Technology, Double Lake, MO). The AdPEDF-transduced MSCs were incubated in culture dishes at a density of 3 × 106 cells/dish. Supernatants were collected daily from each dish for 8 days and kept frozen at −80 °C until used for the assays. PEDF secreted from transduced MSCs was measured following the manufacturer's instructions. Supernatants from MSCs transduced with AdNull and nontransduced MSCs were used as controls.
CNV induction and transplantation of transduced MSCs. AdPEDF-transduced MSCs were trypsinized 24 hours after transduction, and the cell concentration was adjusted to 1.0 × 107 cells/ml with saline. 4.0 × 106 cells/0.4 ml/mouse MSCs were injected into C57BL/6 mice tail veins 0.5–1 hour after laser photocoagulation. As controls, mice received injections of AdNull-transduced MSCs, nontransduced GFP-expressing MSCs derived from the BM of GFP-transgenic mice or 0.4 ml phosphate-buffered saline. The three groups were defined as the AdNull group, nontransduction group, and the control group.
Detection of PEDF expression in vivo. Mice were killed on days 1, 3, 5, and 7 after laser photocoagulation. As described above, PEDF expression in or around CNV was identified by immunofluorescence in serial vertical sections of the eyes. Sections of eyes were sequentially incubated with goat antihuman PEDF primary antibodies (1:50; R&D Systems, Minneapolis, MN) and Texas Red–conjugated secondary antibodies. PEDF levels in blood and eyes were determined by using a human PEDF ELISA kit (USCN Life Science and Technology). Blood was collected into heparinized tubes and immediately centrifuged (700g for 5 minutes at 4 °C) to remove cells, and plasma was collected and stored at −80 °C. Eyes were removed and prepared for ELISAs as previously reported.21 Eyes were quick-frozen in 100 µl of phosphate-buffered saline (pH 7.4) with 0.05% phenylmethylsulfonyl fluoride and homogenized manually on ice followed by three freeze–thaw cycles on liquid nitrogen and wet ice. The homogenates were centrifuged in a refrigerated desktop centrifuge to pellet the insoluble material, and the supernatants were collected. ELISAs were performed following the manufacturer's instructions.
Size measurement of laser-induced CNV lesions. The sizes of laser-induced CNV lesions in the four groups of mice were measured 1 week after laser photocoagulation by histopathological examination and choroidal flat mount analyses. To perform histopathological examinations, mice were killed and perfused with 0.9% saline followed by cold 10% paraformaldehyde. Eyes were enucleated and post-fixed. Alternate sets of serial vertical sections of the eye were cut and mounted. Prepared sections were processed for standard hematoxylin and eosin staining. Serial slices were examined, and specimens representing the thickest and/or widest lesions within the examined specimens were evaluated for each CNV. Hematoxylin and eosin–stained sections were digitized using a light microscope (BX51; Olympus) connected to a color video camera and a frame grabber. Using image analysis software (Photoshop CS2; Adobe, San Diego, CA), the maximum thicknesses and diameters of each CNV were calculated from hematoxylin and eosin–stained specimens.43 Morphometric data for different lesions in each eye were averaged to provide one value per eye. The data of each group were then calculated and compared using an LSD t-test (SPSS software; SPSS, Chicago, IL). The area of CNV at each rupture site was measured in choroidal flat mounts. Flat mounts were examined and digitized separately by a confocal laser scanning microscope (Fluoview 300). Images were analyzed by Image-Pro Plus (Media Cybernetics, Carlsbad, CA) to measure the area of red fluorescence associated with each burn. The areas within each eye were averaged to give one experimental value, and mean values were calculated for each treatment group and compared using an LSD t-test (SPSS software).
Coculture of RPE cells with MSCs for proliferation and migration assays. Human RPE cells were cultured and preserved in our laboratory as previously described.45 Experiments were carried out using RPE cells between passages 3 to 8. To test the impact of PEDF secreted by transduced MSCs on RPE proliferation, AdPEDF-transduced MSCs (1.5 × 105 cells/cm2) were plated in a transwell insert (Millipore, Billerica, MA) with 0.4 µm pores and allowed to adhere for 24 hours. The inserts were then placed into 24-well plates, where RPE cells were plated at 2 × 105 cells/cm2 and allowed to adhere overnight. RPE proliferation was evaluated 3 days after coculture using 3, (4,4-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide colorimetric assays. The optical densities were recorded at 490 nm on a Quant Microplate Spectrophotometer (BioTek Instruments, Winooski, VT). RPE migration assays were carried out using the transwell inserts with 8 µm pore sizes. Chemotaxis was induced by the transfected MSCs in the lower compartments. RPE cells were serum-starved overnight and 5 × 104 cells were seeded in the upper compartments. Cells were allowed to migrate for 8 hours. The filters were washed, fixed, and stained with crystal violet (0.5% crystal violet and 20% methanol) for microscopy. The number of cells that migrated to the lower surface of the filters was counted manually. Five randomly chosen fields were counted per filter. RPE cells only, and RPE cells cocultured with AdNull-transduced MSCs or nontransduced MSCs were used as controls in both assays, which were defined as the control group, AdNull group, and nontransduction group. The data from the control group were normalized to 1, and data were compared by the LSD t-test (SPSS software).
Acknowledgments
We thank Guo-Dong Yang (State Key Laboratory of Oncobiology, Department of Biochemistry and Molecular Biology, Fourth Military Medical University, Xi'an, China) and Shu-Guang Yang (State Key Laboratory of Proteomics, Department of Neurobiology, Institute of Basic Medical Sciences, Beijing, China) for thoughtful comments and suggestions during the course of this project. This work was supported by grants from the National Natural Science Foundation of China (No. 30672291, No. 30872818) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China (2004). The project was sponsored partly by an equipment donation from the Alexander Von Humboldt Foundation in Germany (to YSW, V8151/02085). All the authors of this article have no financial or personal relationships with other people or organizations that could inappropriately influence their work.
REFERENCES
- Tong JP., and, Yao YF. Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions. Clin Biochem. 2006;39:267–276. doi: 10.1016/j.clinbiochem.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE., and, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Noël A, Jost M, Lambert V, Lecomte J., and, Rakic JM. Anti-angiogenic therapy of exudative age-related macular degeneration: current progress and emerging concepts. Trends Mol Med. 2007;13:345–352. doi: 10.1016/j.molmed.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Ambati J, Ambati BK, Yoo SH, Ianchulev S., and, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Afzal A, Shaw LC, Ljubimov AV, Boulton ME, Segal MS., and, Grant MB. Retinal and choroidal microangiopathies: therapeutic opportunities. Microvasc Res. 2007;74:131–144. doi: 10.1016/j.mvr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Ball SG, Shuttleworth CA., and, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med. 2007;11:1012–1030. doi: 10.1111/j.1582-4934.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Blevins KS, Hughes CC, George SC., and, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- Liu K, Chi L, Guo L, Liu X, Luo C, Zhang S, et al. The interactions between brain microvascular endothelial cells and mesenchymal stem cells under hypoxic conditions. Microvasc Res. 2008;75:59–67. doi: 10.1016/j.mvr.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ., and, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, et al. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30:121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- Mohr A, Lyons M, Deedigan L, Harte T, Shaw G, Howard L, et al. Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J Cell Mol Med. 2008;12:2628–2643. doi: 10.1111/j.1582-4934.2008.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B, Andreeff M., and, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007;180:263–283. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- Kyriakou CA, Yong KL, Benjamin R, Pizzey A, Dogan A, Singh N, et al. Human mesenchymal stem cells (hMSCs) expressing truncated soluble vascular endothelial growth factor receptor (tsFlk-1) following lentiviral-mediated gene transfer inhibit growth of Burkitt's lymphoma in a murine model. J Gene Med. 2006;8:253–264. doi: 10.1002/jgm.840. [DOI] [PubMed] [Google Scholar]
- Tomita M, Yamada H, Adachi Y, Cui Y, Yamada E, Higuchi A, et al. Choroidal neovascularization is provided by bone marrow cells. Stem Cells. 2004;22:21–26. doi: 10.1634/stemcells.22-1-21. [DOI] [PubMed] [Google Scholar]
- Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW., and, Grant MB. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4908–4913. doi: 10.1167/iovs.03-0342. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Caicedo A, Hernandez EP, Csaky KG., and, Cousins SW. Bone marrow-derived progenitor cells contribute to experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4914–4919. doi: 10.1167/iovs.03-0371. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yanagi Y, Tamaki Y, Muranaka K, Usui T., and, Sata M. Contribution of bone-marrow-derived cells to choroidal neovascularization. Biochem Biophys Res Commun. 2004;320:372–375. doi: 10.1016/j.bbrc.2004.05.177. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Reinoso MA, Pina Y, Csaky KG, Caicedo A., and, Cousins SW. Quantitative enumeration of vascular smooth muscle cells and endothelial cells derived from bone marrow precursors in experimental choroidal neovascularization. Exp Eye Res. 2005;80:369–378. doi: 10.1016/j.exer.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Yamamoto S, Duh E, Zack DJ, Li Q, et al. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1994–2000. [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Ando A, McVey D, Wei L., and, Campochiaro PA. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002;43:2428–2434. [PubMed] [Google Scholar]
- Spaide RF. Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol. 2006;141:149–156. doi: 10.1016/j.ajo.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Kent D., and, Sheridan C. Choroidal neovascularization: a wound healing perspective. Mol Vis. 2003;9:747–755. [PubMed] [Google Scholar]
- Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marce M., and, Semenza GL. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- Csaky KG, Baffi JZ, Byrnes GA, Wolfe JD, Hilmer SC, Flippin J, et al. Recruitment of marrow-derived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exp Eye Res. 2004;78:1107–1116. doi: 10.1016/j.exer.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Docheva D, Popov C, Mutschler W., and, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M., and, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- Bhutto IA, McLeod DS, Hasegawa T, Kim SY, Merges C, Tong P, et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini H, Estrada R, Lu H, Dekova S, Lee MJ, Gray RD, et al. PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. J Cell Biochem. 2008;104:1793–1802. doi: 10.1002/jcb.21748. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Parke K., and, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- Apte RS, Barreiro RA, Duh E, Volpert O., and, Ferguson TA. Stimulation of neovascularization by the anti-angiogenic factor PEDF. Invest Ophthalmol Vis Sci. 2004;45:4491–4497. doi: 10.1167/iovs.04-0172. [DOI] [PubMed] [Google Scholar]
- Becerra SP. Focus on molecules: pigment epithelium-derived factor (PEDF) Exp Eye Res. 2006;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ., and, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Notari L, Baladron V, Aroca-Aguilar JD, Balko N, Heredia R, Meyer C, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- Becerra SP, Fariss RN, Wu YQ, Montuenga LM, Wong P., and, Pfeffer BA. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution. Exp Eye Res. 2004;78:223–234. doi: 10.1016/j.exer.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Grisanti S., and, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res. 2008;27:372–390. doi: 10.1016/j.preteyeres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- Hou HY, Wang YS, Xu JF, Wang YC., and, Liu JP. The dynamic conduct of bone marrow-derived cells in the choroidal neovascularization microenvironment. Curr Eye Res. 2006;31:1051–1061. doi: 10.1080/02713680601100459. [DOI] [PubMed] [Google Scholar]
- Hou HY, Wang YS, Xu JF., and, Wang BR. Nicotine promotes contribution of bone marrow-derived cells to experimental choroidal neovascularization in mice. Exp Eye Res. 2008;86:983–990. doi: 10.1016/j.exer.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- Wang YS, Hui YN., and, Wiedemann P. Role of apoptosis in the cytotoxic effect mediated by daunorubicin in cultured human retinal pigment epithelial cells. J Ocul Pharmacol Ther. 2002;18:377–387. doi: 10.1089/10807680260218542. [DOI] [PubMed] [Google Scholar]