Abstract
Excessive systemic inflammation following trauma, sepsis, or burn could lead to distant organ damage. The transplantation of bone marrow stromal cells or mesenchymal stem cells (MSCs) has been reported to be an effective treatment for several immune disorders by modulating the inflammatory response to injury. We hypothesized that MSCs can dynamically secrete systemic factors that can neutralize the activity of inflammatory cytokines. In this study, we showed that cocultured MSCs are able to decrease nuclear factor κ-B (NFκB) activation in target epithelial cells incubated in inflammatory serum conditions. Proteomic screening revealed a responsive secretion of soluble tumor necrosis factor (TNF) receptor 1 (sTNFR1) when MSCs were exposed to lipopolysaccharide (LPS)-stimulated rat serum. The responsive effect was eliminated when NFκB activation was blocked in MSCs. Intramuscular transplantation of MSCs in LPS-endotoxic rats decreased a panel of inflammatory cytokines and inflammatory infiltration of macrophages and neutrophils in lung, kidney, and liver when compared to controls. These results suggest that improvements of inflammatory responses in animal models after local transplantation of MSCs are at least, in part, explained by the NFκB-dependent secretion of sTNFR1 by MSCs.
Introduction
Trauma, sepsis, and burn-related syndromes are among the leading causes of death for all age-groups.1 These syndromes are characterized by a generalized, dynamic inflammatory state2 that increases the risk of serious complications beyond the underlying injury.3 There have been clinical attempts in these indications to control the inflammatory response using cytokine modulation therapy by either neutralizing circulating cytokines by monoclonal antibodies or blocking the cognate receptor for an inflammatory cytokine.4,5 Among the cytokine targets, tumor necrosis factor (TNF)-α is an acute phase reactant that invokes an inflammatory reaction that begins with the innate immune system.6 The administration of TNFα causes shock, hypotension, and intravascular coagulopathy, which results in hemorrhagic necrosis and tissue injury by increasing the production of other cytokines and chemokines, reactive oxygen intermediates, nitric oxide, and prostaglandins. The downstream, intracellular effects of TNFα are controlled by the activation of a transcription factor, nuclear factor κ-B (NFκB).7,8 However, current therapeutic approaches involving the modulation of TNFα have demonstrated limited clinical benefits in trauma and sepsis.9,10
Cell therapy is being explored as a new approach to modulate the immune response. In particular, the administration of bone marrow stromal cells, commonly referred to as mesenchymal stem cells (MSCs), has been evaluated in several immune-mediated diseases. A therapeutic response to MSC transplants have been reported in preclinical and/or clinical studies of graft versus host disease,11 ischemic heart disease,12 ischemic kidney injury,13 type 2 diabetes mellitus,14 and Crohn's disease.15 Most recently, several reports clearly demonstrated the therapeutic efficacy of MSC transplantation in animal models of sepsis.16,17,18 These reports revealed that MSCs could reprogram macrophages by releasing prostaglandin E2 in a short-range interaction between the two cell types in the lung.18 Furthermore, signaling by lipopolysaccharide (LPS) or TNFα to MSCs was found to be essential for the induction of macrophage reprogramming. These data suggest that the activation of NFκB by TNFα in MSCs has a crucial role in their anti-inflammatory potential.
Recently, we discovered that the administration of concentrated human MSC–secreted molecules can independently modulate the immune system in an animal model of multiple organ injury.19,20 Herein, we hypothesized that human MSCs could attenuate generalized inflammation by secreting systemic agents that neutralize proinflammatory mediators and consequently prevent end-organ dysfunction. In this study, we demonstrated that human MSCs can neutralize TNFα by producing significant quantities of soluble TNF receptor 1 (sTNFR1). The secretion of sTNFR1 was enhanced when human MSCs were stimulated with serum from animals with endotoxemia induced by LPS. In addition, the release of sTNFR1 was dynamic and dependent on the surrounding, chemical environment. Finally, the therapeutic effect of an intramuscular injection of human MSCs in endotoxemic animals was dependent on the secretion of sTNFR1. These results together indicate that human MSCs can secrete cytokine modulators, such as sTNFR1, in a dynamic and responsive way that contribute to the anti-inflammatory effect of MSCs in diseases of generalized inflammation.
Results
Human MSCs inhibit NFκB activation in reporter cells exposed to inflammatory conditions
Prior work showed that NFκB activation results in inflammatory organ damage and a lower mortality during zymosan-induced organ dysfunction in rats.21 We used a NFκB reporter cell line that expressed green fluorescent protein (GFP) as a function of NFκB activity to evaluate the effect of human MSCs on target epithelial cells during inflammation in vitro. To validate the sensitivity of the cell line, we first measured GFP fluorescence in NFκB reporter cells as a function of TNFα concentration. Reporter cells displayed a saturating, exponential rise in GFP signal with an approximated linear range of 1–15 ng of TNFα and a maximal response found at a TNFα concentration of 100 ng/ml (Figure 1a). We then cocultured MSCs with NFκB reporters in medium supplemented with 20% inflammatory rat serum-derived from LPS-exposed animals as a proxy for endotoxic conditions in vivo. Using fluorescence microscopy, we visualized the number of NFκB-GFP reporter cells after 1 day of culture in inflammatory conditions and compared our results to reporter cells stimulated with no treatment or TNFα as negative and positive controls, respectively (Figure 1b). MSCs prevented the activation of NFκB in target epithelial cells following incubation with LPS-inflamed serum (Figure 1b). After 1 day during stimulation with inflammatory serum, about 25% of the NFκB reporter cells expressed GFP, reaching 30% of the maximal NFκB response (Figure 1c). However, when NFκB reporter cells were cocultured with hMSCs, only an average of 0.7 ± 0.2% of the cells were positive to GFP (Figure 1d). These results clearly demonstrated that human MSCs have the capacity to inhibit the activation of NFκB under inflammatory conditions
Figure 1.
Human mesenchymal stem cells (hMSCs) reduce NFκB activation in a reporter cell line. Fluorescence-activated cell sorting (FACS) analysis was performed to analyze GFP+ cell ratio in cocultures of hMSCs and the NFκB-GFP cell line (1:5 ratio) after 24 hours. (a) Different doses of TNFα (0, 1, 5, 10, 15, 20, 50, and 100 ng/ml) were used to validate the reporter cell line (NFκB-GFP). (b) Quantification of FACS analysis for GFP+ cells when cultured in inflammatory serum with or without hMSCs as well as no treatment as a control. Error bars represent mean ± SD (*P = 0.0003). (c) Results were corroborated by fluorescent microscopy. DAPI was used to counterstain nuclei. c, Bar = 100 µm. (d) Representative data were analyzed by flow cytometry. GFP, green fluorescent protein; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
NFκB complex and responsive genes are activated in human MSCs exposed to inflammatory conditions
To evaluate the response of human MSCs exposed to inflammatory conditions, we profiled MSC gene expression in response to inflammatory conditions. Human MSCs were exposed for 24 hours to inflammatory rat serum or a 100 ng/ml of TNFα. An RT2 Profiler PCR array was used to profile the expression of 84 genes encoding inflammatory chemokines and cognate receptors, transcription factors, and kinases (Supplementary Figure S1a and Supplementary Table S1). Major changes in gene expression related to the NFκB pathway were confirmed using qRT-PCR. Heat map revealed there were several upregulation of genes related to NFκB pathway in both LPS- and TNFα-exposed groups. Supplementary Figure S1b shows that inflammatory rat serum induced the NFκB pathway in human MSC in a similar manner to TNFα. NFκB1, interleukin-1 (IL1) receptor–associated kinase 2 (ref. 22), and NFκB responsive genes (IL1β, IL8)7,23 were all elevated under both conditions compared to nonstimulated controls. These results demonstrate that exposure of different inflammatory stimuli to human MSCs results in a similar pattern of activation that converges on the activation of NFκB transcription factor and subsequent DNA transcription (Supplementary Figure S1c). This gene activation may be part of the responsive machinery within human MSCs, providing strong motivation to analyze the consequences of NFκB inhibition in human MSCs.
Human MSCs secrete natural inflammatory modulators in response to stimulus
Our previous results clearly show that human MSCs could inhibit NFκB activation under inflammatory conditions. Based on our previous studies, and others, demonstrating the ability of human MSCs to secrete anti-inflammatory factors, we next evaluated cellular secretion under normal and inflammatory conditions. We focused our analysis of the MSC secretome to known secreted cytokine modulators. sTNFR1, soluble TNF receptor 2 (sTNFR2), soluble CD40 (sCD40), soluble CD40 ligand (sCD40L), and soluble IL1 receptor 2 (sIL1R2) as well as anti-inflammatory cytokine IL10 were analyzed by enzyme-linked immunosorbent assay (ELISA) after 1 day of stimulation with inflamed serum. Figure 2a demonstrates that human MSCs produce significant quantities of sTNFR1 and that the secretion of sTNFR1 increased fourfold when cells were exposed to inflammatory serum. Similar experiments were performed using human skin fibroblast as control, and the absence of the secretion of these cytokine modulators was confirmed (data not shown). To examine whether human MSCs secrete sTNFR1 in response to NFκB activation, we exposed human MSCs to either inflammatory or normal rat serum for 2 hours with an NFκB inhibitor peptide or control peptide (Figure 2b). Figure 2c demonstrates that inflammatory conditions transiently stimulate human MSC secretion of sTNFR1 by twofold (P = 0.02), a response which is rapidly muted upon return to noninflammatory condition. However, inhibition of NFκB activation blocks the transient increase in sTNFR1 secretion under inflammatory conditions (Figure 2d). These results suggest that human MSCs dynamically respond to inflammatory conditions through an NFκB-dependent secretion of anti-inflammatory factors, such as sTNFR1.
Figure 2.
Characterization of soluble receptor secretion and inflammatory serum-responding secretion by human mesenchymal stem cells (hMSCs). (a) Human sTNFR1 and 2, sCD40, sCD40L, and sIL1R2 as well as IL10 were measured in the culture medium of 0.5 × 106 of hMSCs after 24 hours of exposure to culture media containing 20% normal serum or serum derived from endotoxemic animals (*P = 0.0135). (b) hMSCs were exposed to 20% of serum harvested from healthy rats (normal serum) or 6 hours after rats were treated with LPS (LPS serum). sTNFR1 levels in each medium were analyzed after 2 hours with (c) control peptide and with (d) cell-permeable NFκB inhibitor peptide (*P = 0.0226, **P = 0.0114). N/S, no significant difference. Error bars represent mean ± SD. LPS, lipopolysaccharide.
In addition, to demonstrate the notion that NFκB activation in hMSCs is regulated by multiple cytokine-receptor interaction in inflammatory condition, the secretion of sTNFR1 was analyzed under the influence of sTNFR1 antibody (Supplementary Materials and Methods). Even TNFR on the cell surface could have been binded by the excess amount of sTNFR1 antibodies, the reactive secretion of sTNFR1 is present when hMSCs were stimulated by inflammatory serum (Supplementary Figure S2b–e).
Intramuscular transplantation of human MSCs decrease inflammatory cytokines in LPS-exposed rats
Our results suggest that human MSCs secrete sTNFR1 in response to inflammation and may be of therapeutic value during an inflammatory response to injury. To test this hypothesis, we used LPS intoxication as a model of generalized inflammation. This model resembles the clinical situation of endotoxemia.24,25 Animals exhibit a massive cytokine storm soon after the treatment with LPS,26 thus providing a feasible model to test the therapeutic potential of MSC secreted factors in vivo.
Rats were intraperitoneally administered one injection of 10 mg/kg of LPS, followed by localized, intramuscular transplantation of human MSCs (2 × 106 cells). This cell mass was selected based on a previous dose–response study of concentrated human MSC conditioned medium as an intravenous treatment in rats undergoing organ injury.27 As shown in Figure 3a–c, MSCs were locally detected by one of their membrane proteins, CD105 (ref. 28) 1 day after the cells were transplanted in muscle. Immunostaining of human CD105 in different organs demonstrated that MSCs did not migrate away from the muscle for at least 24 hours (Figure 3d–f). To quantify the potential systemic effects of a local cell graft, we performed an ELISA to detect the presence of human sTNFR1 in the serum of animals transplanted with human MSCs. Significant levels of human sTNFR1 were detected when compared to control animals especially at early time points after LPS administration (Figure 3g).
Figure 3.
Transplantation of mesenchymal stem cells (MSCs) ameliorates proinflammatory cytokines in endotoxemic animals. (a–c) Hematoxylin and eosin staining (a) and immunohistochemical analysis (b) ×40 and (c) ×20 were performed using CD105 for the detection of hMSCs in the muscle where MSCs (2 × 106 cells) were transplanted 24 hours before. (d–f) CD105 staining was performed in three vital organs, namely, (d) kidney, (e) liver, and (f) lung from animals 24 hours after receiving hMSC transplantation intramuscularly. DAPI was used to counterstain nuclei. a–f, Bar = 100 µm. Magnifications are ×20. (g) Data represent human sTNFR1 levels in serum of rats exposed to intraperitoneal injection of LPS treated with or without MSC transplantation. Error bars represent mean ± SD (*P = 0.0135). (h) Cytokine levels in serum of rats exposed to intraperitoneal injection of LPS. Alterations of TNFα, IL6, and IFN-γ were observed in animals with intramuscular transplantation of hMSCs. Data representative of three independent trials with a total of N = 5 per group. Serum was analyzed for cytokines at 0, 6, 12, 24, and 48 hours after treatment by enzyme-linked immunosorbent assay. *P < 0.05: between serum from control animals and animals that were transplanted hMSCs. LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
Inflammatory cytokine levels (TNFα, IFN-γ, and IL6) in serum of LPS-treated animals were significantly reduced in animals that received intramuscular transplantation of MSCs (Figure 3h). We then blocked human sTNFR1 by treating MSC transplanted with a neutralizing antibody and observed an intermediate effect on levels of inflammatory cytokines (Figure 3h). These data suggest a localized MSC graft can modulate systemic levels of cytokines and invoke an anti-inflammatory response that is due, in part, to secreted sTNFR1.
Human MSCs attenuate multiple organ injury in LPS rats
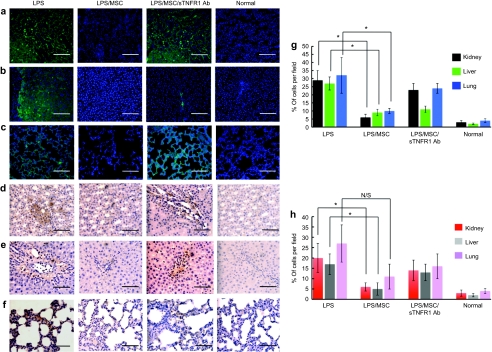
Generalized inflammation produces a cellular inflammatory response in which neutrophils and macrophages infiltrate different organs.29 To evaluate the effect of localized MSC transplantation in end-organ cellular inflammatory infiltration, we performed immunohistochemical evaluation of lung, kidney, and liver tissue 24 hours after LPS intoxication.30 Organ injury after the treatment was quantified by counting positive cell numbers in myeloperoxidase staining for neutrophils (Figure 4a–c) and CD163 staining for macrophages (Figure 4d–f). These histological analyses demonstrated significant reduction of inflammatory cell infiltrations in tissues from rats receiving intramuscular transplantation of MSCs compared to rats without MSC transplantation (Figure 4g,h). Finally, when human MSCs were transplanted in the presence of a specific neutralizing antibody against human sTNFR1, the number of inflammatory cell infiltrates was increased in these three vital organs. These studies indicate that the secretion of sTNFR1 from human MSCs contributes to the reduction of multiple organ injury in endotoxemic animals. Taken together, these data demonstrate that local transplantation of MSCs could effectively reduce inflammatory cell infiltration and alter multiple organ injury.
Figure 4.
Histological appearance of mesenchymal stem cell (MSC)–treated animals after LPS injection. (a–c) Myeloperoxidase staining for detecting neutrophil infiltration of three vital organs; (a) kidney, (b) liver, and (c) lung, and (d–f) CD163 staining for macrophage infiltration of three vital organs; (d) kidney, (e) liver, and (f) lung were performed on the tissues from MSC transplanted animals 24 hours after LPS injection, whereas the samples from animals without cell transplantation after LPS, animals with cell transplantation treated with sTNFR1 antibody, and normal animals were served as controls. DAPI was used to counterstain nuclei in picture a–c. Magnifications are ×40. (g) Neutrophil infiltration of three vital organs in hMSC-treated rats was evaluated comparing to tissues from nontreated rats with LPS intoxication based on myeloperoxidase staining by the cell count in a field of ×40. (h) Macrophage infiltration of three vital organs in hMSC-treated rats was evaluated comparing to tissues from nontreated rats with LPS intoxication based on CD163 staining by the cell count in a field of ×40 (*P < 0.05). N/S, no significant difference. Error bars represent mean ± SD. a–f, Bar = 100 µm. LPS, lipopolysaccharide.
Discussion
These results suggest that local transplantation of MSCs can modulate the response of the host system to generalized inflammation and prevent organ injury. MSCs have been reported to have immunosuppressive and immunomodulatory effects in humans and animal models,31 but their mechanism of action has been unclear. In the present study, we show that MSCs can be used to attenuate generalized inflammation via the reactive secretion of sTNFR1. We suggest that locally transplanted MSCs react to systemic stimulus and that the reactivity secretion is linked to the activation of NFκB complex.
sTNFR1 (55 kd) is one of the proteolytic shedding soluble extracellular domains of the TNF receptors modulating the activity of TNFα,32 which is secreted primarily by mononuclear cells.33 Administration of TNFα or LPS to human MSC increases the concentration of soluble TNF receptors in the media suggesting that soluble receptors may be part of a negative feedback mechanism to inhibit the biological effects of TNFα.34 Thus, the upregulation of the human sTNFR1 by MSCs was of particular interest because of the known anti-inflammatory effects of the protein35,36 and because excessive inflammatory responses contribute to the pathological changes produced by systemic inflammation.37
Our results indicated that the MSCs that were transplanted intramuscularly were activated to secrete sTNFR1 and the sTNFR1 suppressed the excessive inflammatory response to LPS so as to decrease the proteolytic damage to multiple organs. The upregulation of sTNFR1 was detected by the cross-species strategy of human MSCs transplanted into rats. Similar strategies of using human MSCs in animal models previously proved useful because the human MSCs provided numerous endogenous markers for the cells, and no obvious cross-species artifacts were encountered apparently due to the immunomodulatory privilege effects of MSCs.38 Although several reports demonstrated the positive therapeutic effect of human-derived MSCs on a mice model by systemic administration17,39 as a xenogeneic cell transplantation, it has been reported that MSC can express major histocompatibility complex molecules including major histocompatibility complex class II.40 To determine whether there was acute rejection of transplanted cells, we evaluated the local inflammatory response 24 hours after the transplantation of human MSCs by immunohistochemistry. Although we found lymphocyte infiltrates in response to these transplanted cells in 24 hours, further investigation of the long-term response of the host is required to determine semiacute or chronic rejection of transplanted xenogeneic MSCs; however, the capacity of MSCs to recruit lymphocytes and moderate lymphocyte function has been previously described.41
When MSCs were transplanted with a specific neutralizing antibody against human sTNFR1, levels of inflammatory cytokines or infiltrating cells in vital organs were partially but not fully restored. These experiments indicated that although sTNFR1 has an important role in the reduction of inflammation in intoxicated animals, it may not be the sole mediator of this effect. There is likely a combinatorial effect of MSC transplantation that involves other purported mechanisms of MSC immunomodulation such as IL6 (ref. 42), transforming growth factor-β,43 interferon-γ (IFN-γ),44 and prostaglandin E2 (refs. 18,31). We previously evaluated the therapeutic effect of MSCs in a different model, namely a 30% total-body to surface-area burn injury model. Although MSCs attenuated multiple organ injury,20 the inhibition of sTNFR1 by specific antibodies was not sufficient to significantly reduce its therapeutic effect. Thus, our results do not exclude the possibility that there are other factors regulating the anti-inflammatory effects of MSCs.
Interestingly, the secretion of sTNFR1 was regulated dynamically by the surrounding environment, as simulated by incubating the cells with inflammatory serum harvested from intoxicated rats. Clinically, patients in a septic condition show a very characteristic clinical appearance at different time points throughout the illness. According to the “two-hit theory,”45 after first exposure to massive stress such as surgical procedure, burn, trauma, viral/bacterial infection, or organ infarction, the inflammatory response of the body is upregulated to handle this condition, and if this response continues unchecked, it can cause further serious systemic complications (e.g., acute respiratory distress syndrome and multiple organ failure). Systemic inflammation in humans has a dynamic shift from the inflamed state to immunosuppressed. Therefore, the type of therapy needed is the one that can alter its therapeutic potential depending on the patient's immune status at the time treatment is initiated. Here, we demonstrated that MSCs underwent major changes in their patterns of gene expression in response to inflammatory cytokines as well as inflammatory serum from endotoxemic animals. Taken together, our approach using cells as a modality to control generalized inflammation would be applicable to several clinical entities because of their capacity to react to the surrounding environment.
A recent study described that the stimulation of MSCs with LPS as a toll-like receptor agonist led to the activation of downstream signaling pathways, including NFκB.46 Consequently, activation of the signaling pathway triggered the induction and secretion of cytokines and chemokines. These results demonstrated that the activation of NFκB is critical in the reactivity of immunoregulation observed in vivo when MSCs are transplanted.46,47 In the present study, the contribution of NFκB complex to the reactive secretion of sTNFR1 from MSCs was demonstrated in the alteration of the protein secretion. Moreover, the blockage of the reactive secretion of sTNFR1 when using a specific inhibitor peptide of NFκB suggests that the anti-inflammatory ability of MSC is not innate but rather is induced by the surrounding inflammatory cytokines. Interestingly, when hMSCs were stimulated by inflammatory serum under the presence of sTNFR1 antibody, the reactive secretion of sTNFR1 was restored even TNFR1 on the cell surface could be bounded by this antibody, which can bind both soluble and surface receptors. These results suggested that the secretion of sTNFR1 and activation of NFκB is regulated not only by TNF–TNFR interaction on the cell surface but other mediators that can stimulate different surface receptors on hMSCs.
In summary, this study elucidated the potential of human MSCs to control inflammation dynamically in response to the surrounding environment. Because MSCs are relatively easy to obtain and grow in the laboratory, we anticipate that this study will hopefully lead to new approaches to use dynamic cell therapy to control systemic or even local inflammation. Such therapy would help to avoid serious injury and complications and potentially would have an enormous positive clinical impact.
Materials and Methods
Animals. Male Sprague–Dawley rats weighing 270–320 g (Charles River Laboratories, Boston, MA) were used for LPS induction. The animals were cared for in accordance with the guidelines set forth by the Committee on Laboratory Resources, National Institutes of Health, and Subcommittee on Research Animal Care and Laboratory Animal Resources of Massachusetts General Hospital. All animals had free access to food and water, both before and after the procedure.
Endotoxemia induction of animals with LPS. LPS (extracted from Escherichia coli 0111: B4) was purchased from Sigma (St Louis, MO). To induce endotoxemia in animals, 10 mg/kg of LPS was injected intraperitoneally.
Serum collection from endotoxemic animals for cell culture study. Carotid arteries of the rats were cannulated under general anesthesia by intraperitoneal injections of ketamine and xylazine at 110 and 0.4 mg/kg body weight. The blood of injured animals was collected from the carotid artery at 6 hours after the induction, and the serum was stored at −80 °C until using for cell culture studies.
Culture and expansion of MSCs. Human MSCs were kindly provided by the Tulane Center for Gene Therapy (New Orleans, LA). MSCs were cultured and characterized for surface marker expression, and adipocytic and osteogenic differentiation capacity as previously reported.48 Cell culture and expansion of human MSC was performed using Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mmol/l nonessential amino acids, and 1 ng/ml of basic fibroblast growth factor (Life Technologies, Rockville, MD). MSCs were maintained in a humidified tissue culture incubator at 37 °C with 5% carbon dioxide. Cells were used for experiments during passages 3–7. To measure the soluble factors as well as anti-inflammatory cytokines, we cultured 0.5 × 106 cells in 6-well plates in medium containing 20% of serum harvested from healthy rats or 6 hours after rats were treated with LPS, and ELISA was used for the quantification of human sTNFR1 and 2, sCD40, sCD40L, sIL1R2, and IL10 (R&D Systems, Minneapolis, MN).
Measurement of sTNFR1 by MSCs after stimulation with inflammatory serum. Human MSCs were cultured in medium containing 20% of serum harvested from healthy rats or 6 hours after rats were treated with LPS. The first 2 hours, human MSCs were cultured with normal serum, then for 2 hours with inflammatory serum, followed by normal serum culture for an additional 2 hours. Cells were washed three times between each culture condition. In certain experiments, MSCs were incubated with 100 mg/ml of a cell-permeable NFκB inhibitor peptide (NFκB SN50: Calbiochem, La Jolla, CA) or its inactivated control during their 2-hour culture in inflammatory serum. These studies were designed to block NFκB in MSCs using protocols previously reported for this inhibitory peptide.49 At the end of the time points, culture media were collected for quantification of human sTNFR1 measurement using sTNFR1 ELISA (R&D Systems) according to the manufacturer's protocol. Three different independent experiments were performed, and three different human MSC batches were tested for the secretion of soluble receptors.
Coculture of human MSCs and NFκB-GFP reporter cells. The NFκB reporter cell line is a clonal H35 rat hepatoma line, in which a plasmid containing multiple binding sites of the NFκB response element upstream of a minimal cytomegalovirus promoter driving the expression of a destabilized GFP has been stably integrated.50 NFκB activation is recorded as an increase in GFP intensity following TNFα stimulation. The destabilized GFP has a 2-hour half-life allowing the measurement of NFκB inhibition.50 We used different doses of TNFα (0, 1, 5, 10, 15, 20, 50, and 100 ng/ml) to validate the reporter cell line as assessed by flow cytometry analysis using FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). Moreover, we measured the extent of NFκB activation in the reporter cells after 24 hours of coculture of human MSCs (0.1 × 106 and 0.5 × 106 cells per well, 1:5 ratio of MSCs: reporter cells) using a 0.4 mm pore size Transwell membrane (Becton Dickinson) in 6-well culture plate.
Quantitative real-time RT-PCR. Human MSCs were exposed for 24 hours to culture medium containing either 20% inflammatory rat serum from endotoxemic animals or a high dose of TNFα (100 ng/ml), and RNA was extracted using a total RNA isolation kit (SABiosciences, Frederick, MD) according to the manufacturer's protocol. Human MSCs cultured with 20% of normal rat serum served as a control. Quantitative real-time RT-PCR was performed in Stratagene MXPro 3005p (Stratagene, La Jolla, CA) using human NFκB signaling pathway PCR arrays (SABiosciences) following manufacturer's instructions. The results were analyzed using a color clustergram and a scatter plot using RT2 Profiler PCR Array Data Analysis (SABiosciences).
Intramuscular transplantation of human MSCs and quantification of cytokine levels in animals. MSCs (2 × 106) were suspended in 500 µl of phosphate-buffered saline with 2.5 mg/ml of rat type 1 collagen (Trevigen, Gaithersburg, MD) and administered to the left thigh muscle of the animals (n = 7 per group) immediately after the induction of LPS. Specific neutralizing antibody (10 mg/animal) against human sTNFR1 (R&D Systems) was administered intravenously in one of the animal groups, whereas the injection of phosphate-buffered saline with 2.5 mg/ml of rat type 1 collagen without cells served as a negative control. Alterations of TNFα, IL6, and IFN-γ were quantified in each animal group using ELISA kits (R&D Systems) according to the manufacturer's protocol. Data representative of three independent experiments were collected at different time points (0, 6, 12, 24, and 48 hours after treatment).
Histological studies. Muscle, kidney, liver, and lung from each experimental group (control, LPS rats, and MSC-treated LPS rats) were collected at 24 hours after the induction of LPS. Formalin-fixed, paraffin-embedded samples were sectioned at 4 mm thickness. For immunohistochemistry, heat-induced antigen retrieval was performed after sections were deparaffinized. Endogenous peroxidase activity was blocked by incubation in H2O2/methanol. Nonspecific binding was blocked with 10% donkey serum or 5% goat serum. Sections were incubated with the following primary antibodies (at indicated dilution) for 2 hours at room temperature or overnight at 4 °C: rabbit polyclonal prediluted antihuman CD105 antibody (Abcam, Cambridge, MA), rabbit polyclonal anti-rat myeloperoxidase antibody (1:50) (Abcam), and mouse monoclonal anti-rat CD163 antibody (1:50) (Santa Cruz Biotechnology, Santa Cruz, CA). For CD105 and myeloperoxidase staining, sections were incubated with donkey polyclonal horseradish peroxidase–conjugated anti-rabbit secondary antibody (Abcam). For CD163, staining samples were processed using mouse ImmunoCruz staining system containing goat biotinylated secondary antibody (Santa Cruz Biotechnology) according to the manufacturer's protocol. Quantification of cell numbers in stained tissue sections was performed in 10 random ×40 images per animal using the public software ImageJ (http://rsb.info.nih.gov/ij/). Inflammatory cell infiltration in samples was quantified by counting myeloperoxidase and CD163+ cells as neutrophils and macrophages, respectively, by using appropriate criteria for a specific threshold of staining intensity as well as corresponding sizes of the nuclei. Blind analysis of the histological samples was performed by two independent observers.
Statistical analysis. Data represent the mean of each experiment ± SD. Statistical significance was determined by a Student's t-test analysis, in which each value was compared with the control values performed with Prism (GraphPad Software, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. Human PCR array analysis of hMSCs under inflammatory conditions. (a) The human signaling pathway PCR Array was used that profiles the expression of 84 key genes related to inflammatory signal transduction. Relative mRNA expression was represented in a clustergram (Green=gene down-regulation, red=gene up-regulation). (b) Single gene real-time RT-PCR analysis of hMSCs under two different inflammatory conditions (exposed to 100ng/ml of TNFα or serum derived from endotoxemic animals) demonstrated the relative mRNA expression of responsive genes (IL1b and IL8) and genes related to the activation of NFκB pathway (NFκB1 and IRAK2). Data represents fold changes compared to that of normal human MSCs control. (c) Total RNA from normal human MSCs and human MSCs subjected to two different inflammatory conditions, and the relative expression levels for each gene in the four samples were plotted against each other in the Scatter Plot. Similar pattern of gene expressions of human MSCs in two different conditions are observed. Figure S2. Inflammatory serum-responding secretion of sTNFR1 by hMSCs under the influence of sTNFR1 antibody. (a) hMSCs were exposed to 20% of serum harvested from healthy rats (normal serum) or LPS-treated rats serum (LPS serum). sTNFR1 levels in each medium were analyzed after 2h (b) without or with (c) 0.5μg/ml, (d) 5μg/ml or (e) 50μg/ml of sTNFR1 antibody which can bind not only to sTNFR1 but to TNFR1 on the cell surface. After washing out the excess amount of antibodies, part of which had already expected to bind to the receptors on the cell surface, hMSCs were exposed to LPS serum again. Error bars represent mean ± SD. (*P< 0.05). Table S1. List of 84 genes in PCR array. Materials and Methods.
Acknowledgments
We acknowledge the technical assistance of Bob Crowther for preparing histological samples. This work was supported by grants from the National Institutes of Health (1K99DK083556-01 to A.S.-G., 5R01DK059766-06 to M.L.Y.), the Broad Medical Research Foundation (BMRP498382 to B.P.), the Shriners Hospitals for Children and the American Liver Foundation support to A.S.-G. All authors have no conflict of interest.
Supplementary Material
Human PCR array analysis of hMSCs under inflammatory conditions. (a) The human signaling pathway PCR Array was used that profiles the expression of 84 key genes related to inflammatory signal transduction. Relative mRNA expression was represented in a clustergram (Green=gene down-regulation, red=gene up-regulation). (b) Single gene real-time RT-PCR analysis of hMSCs under two different inflammatory conditions (exposed to 100ng/ml of TNFα or serum derived from endotoxemic animals) demonstrated the relative mRNA expression of responsive genes (IL1b and IL8) and genes related to the activation of NFκB pathway (NFκB1 and IRAK2). Data represents fold changes compared to that of normal human MSCs control. (c) Total RNA from normal human MSCs and human MSCs subjected to two different inflammatory conditions, and the relative expression levels for each gene in the four samples were plotted against each other in the Scatter Plot. Similar pattern of gene expressions of human MSCs in two different conditions are observed.
Inflammatory serum-responding secretion of sTNFR1 by hMSCs under the influence of sTNFR1 antibody. (a) hMSCs were exposed to 20% of serum harvested from healthy rats (normal serum) or LPS-treated rats serum (LPS serum). sTNFR1 levels in each medium were analyzed after 2h (b) without or with (c) 0.5μg/ml, (d) 5μg/ml or (e) 50μg/ml of sTNFR1 antibody which can bind not only to sTNFR1 but to TNFR1 on the cell surface. After washing out the excess amount of antibodies, part of which had already expected to bind to the receptors on the cell surface, hMSCs were exposed to LPS serum again. Error bars represent mean ± SD. (*P< 0.05).
List of 84 genes in PCR array.
REFERENCES
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD., and, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- Robertson CM., and, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006;8:1382–1389. doi: 10.1016/j.micinf.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, et al. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–292. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Deans KJ, Haley M, Natanson C, Eichacker PQ., and, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58:867–874. doi: 10.1097/01.ta.0000158244.69179.94. [DOI] [PubMed] [Google Scholar]
- Ulloa L., and, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Lakshmanan U., and, Porter AG. Caspase-4 interacts with TNF receptor-associated factor 6 and mediates lipopolysaccharide-induced NF-kappaB-dependent production of IL-8 and CC chemokine ligand 4 (macrophage-inflammatory protein-1) J Immunol. 2007;179:8480–8490. doi: 10.4049/jimmunol.179.12.8480. [DOI] [PubMed] [Google Scholar]
- Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- Abraham E, Laterre PF, Garbino J, Pingleton S, Butler T, Dugernier T, Lenercept Study Group et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29:503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, et al. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–979. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, Echevarria L, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008;17:1295–1304. doi: 10.3727/096368908787648119. [DOI] [PubMed] [Google Scholar]
- García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C., and, Rodríguez Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D., and, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Büscher D., and, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG., and, Yarmush ML.2010Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn Cell Transplantepub ahead of print). [DOI] [PMC free article] [PubMed]
- Liu SF., and, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Wan Y, Xiao H, Affolter J, Kim TW, Bulek K, Chaudhuri S, et al. Interleukin-1 receptor-associated kinase 2 is critical for lipopolysaccharide-mediated post-transcriptional control. J Biol Chem. 2009;284:10367–10375. doi: 10.1074/jbc.M807822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW., and, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- Frank S, Zacharowski K, Wray GM, Thiemermann C., and, Pfeilschifter J. Identification of copper/zinc superoxide dismutase as a novel nitric oxide-regulated gene in rat glomerular mesangial cells and kidneys of endotoxemic rats. FASEB J. 1999;13:869–882. doi: 10.1096/fasebj.13.8.869. [DOI] [PubMed] [Google Scholar]
- Fischer E, Marano MA, Van Zee KJ, Rock CS, Hawes AS, Thompson WA, et al. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 1992;89:1551–1557. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YM, Redl H, Bahrami S., and, Schlag G. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm Res. 1998;47:201–210. doi: 10.1007/s000110050318. [DOI] [PubMed] [Google Scholar]
- Baue AE, Durham R., and, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle. Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Aggarwal S., and, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Hale KK, Smith CG, Baker SL, Vanderslice RW, Squires CH, Gleason TM, et al. Multifunctional regulation of the biological effects of TNF-alpha by the soluble type I and type II TNF receptors. Cytokine. 1995;7:26–38. doi: 10.1006/cyto.1995.1004. [DOI] [PubMed] [Google Scholar]
- Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H., and, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Lantz M, Malik S, Slevin ML., and, Olsson I. Infusion of tumor necrosis factor (TNF) causes an increase in circulating TNF-binding protein in humans. Cytokine. 1990;2:402–406. doi: 10.1016/1043-4666(90)90048-x. [DOI] [PubMed] [Google Scholar]
- Wallis RS. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect Dis. 2008;8:601–611. doi: 10.1016/S1473-3099(08)70227-5. [DOI] [PubMed] [Google Scholar]
- Leroy S, Guigonis V, Bruckner D, Emal-Aglae V, Deschênes G, Bensman A, et al. Successful anti-TNFalpha treatment in a child with posttransplant recurrent focal segmental glomerulosclerosis. Am J Transplant. 2009;9:858–861. doi: 10.1111/j.1600-6143.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Chatterjee PK, Mazzon E, Serraino I, Dugo L, Centorrino T, et al. Effects of calpain inhibitor I on multiple organ failure induced by zymosan in the rat. Crit Care Med. 2002;30:2284–2294. doi: 10.1097/00003246-200210000-00017. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L., and, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58:570–581. doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry F, Murphy JM., and, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JM, Barry FP, Murphy JM., and, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer JA, Rodrick ML., and, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES., and, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ., and, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through toll-like receptor 4. J Immunol. 2004;172:20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247–252. doi: 10.1016/j.bbrc.2007.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR., and, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- King KR, Wang S, Irimia D, Jayaraman A, Toner M., and, Yarmush ML. A high-throughput microfluidic real-time gene expression living cell array. Lab Chip. 2007;7:77–85. doi: 10.1039/b612516f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human PCR array analysis of hMSCs under inflammatory conditions. (a) The human signaling pathway PCR Array was used that profiles the expression of 84 key genes related to inflammatory signal transduction. Relative mRNA expression was represented in a clustergram (Green=gene down-regulation, red=gene up-regulation). (b) Single gene real-time RT-PCR analysis of hMSCs under two different inflammatory conditions (exposed to 100ng/ml of TNFα or serum derived from endotoxemic animals) demonstrated the relative mRNA expression of responsive genes (IL1b and IL8) and genes related to the activation of NFκB pathway (NFκB1 and IRAK2). Data represents fold changes compared to that of normal human MSCs control. (c) Total RNA from normal human MSCs and human MSCs subjected to two different inflammatory conditions, and the relative expression levels for each gene in the four samples were plotted against each other in the Scatter Plot. Similar pattern of gene expressions of human MSCs in two different conditions are observed.
Inflammatory serum-responding secretion of sTNFR1 by hMSCs under the influence of sTNFR1 antibody. (a) hMSCs were exposed to 20% of serum harvested from healthy rats (normal serum) or LPS-treated rats serum (LPS serum). sTNFR1 levels in each medium were analyzed after 2h (b) without or with (c) 0.5μg/ml, (d) 5μg/ml or (e) 50μg/ml of sTNFR1 antibody which can bind not only to sTNFR1 but to TNFR1 on the cell surface. After washing out the excess amount of antibodies, part of which had already expected to bind to the receptors on the cell surface, hMSCs were exposed to LPS serum again. Error bars represent mean ± SD. (*P< 0.05).
List of 84 genes in PCR array.