Abstract
Augmenting antitumor immunity is a promising way to enhance the potency of oncolytic adenoviral therapy. Granulocyte–macrophage colony–stimulating factor (GMCSF) can mediate antitumor effects by recruiting natural killer cells and by induction of tumor-specific CD8+ cytotoxic T-lymphocytes. Serotype 5 adenoviruses (Ad5) are commonly used in cancer gene therapy. However, expression of the coxsackie-adenovirus receptor is variable in many advanced tumors and preclinical data have demonstrated an advantage for replacing the Ad5 knob with the Ad3 knob. Here, a 5/3 capsid chimeric and p16-Rb pathway selective oncolytic adenovirus coding for GMCSF was engineered and tested preclinically. A total of 21 patients with advanced solid tumors refractory to standard therapies were then treated intratumorally and intravenously with Ad5/3-D24-GMCSF, which was combined with low-dose metronomic cyclophosphamide to reduce regulatory T cells. No severe adverse events occurred. Analysis of pretreatment samples of malignant pleural effusion and ascites confirmed the efficacy of Ad5/3-D24-GMCSF in transduction and cell killing. Evidence of biological activity of the virus was seen in 13/21 patients and 8/12 showed objective clinical benefit as evaluated by radiology with Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Antiadenoviral and antitumoral immune responses were elicited after treatment. Thus, Ad5/3-D24-GMCSF seems safe in treating cancer patients and promising signs of efficacy were seen.
Introduction
Metastatic solid tumors are often incurable by current therapies and thus require novel strategies. Oncolytic adenoviruses present one promising approach; their utility is based on selective replication and lytic effect on cancer cells. In clinical cancer trials, they have been safe and the first randomized trial yielded positive data.1,2 Nevertheless, efficacy of the approach still needs improvements.3
The immune system plays a pivotal role in virotherapy of cancer. Neutralizing antibodies may hinder viral replication and spreading limiting the therapeutic effect.4 Yet, virotherapy can assist in breaking of the immune tolerance acquired by tumors.5 Oncolytic replication is an immunogenic phenomenon and oncolytic efficacy may partly be due to activation of the immune system against virus-infected tumor cells.5 However, the antiviral immune reaction is usually inadequate to result in tumor eradication. Therefore, to boost this effect, arming oncolytic adenoviruses with immunostimulatory molecules is an attractive prospect,6 but not much studied in humans.7,8,9
Granulocyte–macrophage colony–stimulating factor (GMCSF) is a potent inducer of antitumor immunity,10 used in various strategies to create antitumor effects via tumor-reactive cytotoxic CD8+ T-lymphocytes and natural killer cells.11 However, systemic use of recombinant GMCSF is compromised by side effects and induction of potentially harmful myeloid-derived suppressor cells, related to systemic exposure, whereas efficacy may remain limited due to low local concentration in tumors.11,12 Therefore, local GMCSF production by cancer cells could ensure sufficient local concentration while minimizing systemic exposure. Thus, GMCSF is an appealing molecule and possibly particularly useful in the context of oncolytic adenoviruses.9,10
The tumor microenvironment is immunosuppressive, which hinders the attempts of the immune system to eradicate tumors. Reduction of regulatory T cells could be useful for enhancing the efficacy of antitumor T cells. It has been reported in preclinical and clinical studies that metronomic administration of low-dose cyclophosphamide leads to selective reduction of circulating regulatory T cells, which associates with suppression of their inhibitory functions on cytotoxic T cells and natural killer cells.13 This can lead to a restoration of peripheral T-cell proliferation and innate killing activities. Recently, promising data were also reported on using metronomic cyclophosphamide in cancer patients treated with oncolytic adenoviruses.14
Serotype 5 adenoviruses (Ad5) are the only strains used heretofore in adenoviral gene therapy trials. Coxsackie and adenovirus receptor is the primary receptor for serotype 5, but its expression is variable and often low in many human tumors.15,16 Ad3 bind to a non-coxsackie and adenovirus receptor highly expressed on tumor cells.17,18 Placing the Ad3 fiber knob into the Ad5 backbone results in an Ad5/3 chimera that displays improved gene delivery and antitumor efficacy in preclinical assays with cell lines, fresh clinical specimens, and animal models featuring dozens of tumor types.19,20,21,22,23,24,25,26 Gene transfer or toxicity to normal tissues is not increased in preclinical systems.19
In this article, we describe the generation of Ad5/3-D24-GMCSF, a 5/3 chimeric oncolytic adenovirus armed with human GMCSF. Following preclinical testing, we treated 21 patients with advanced solid tumors refractory to standard treatments. Safety, efficacy, virological, immunological, and correlative data are presented. Also a preliminary assessment of pretreatment efficacy prediction, potentially useful as a biomarker for Ad5/3-D24-GMCSF efficacy, was performed.
Results
Construction and characterization of Ad5/3-D24-GMCSF
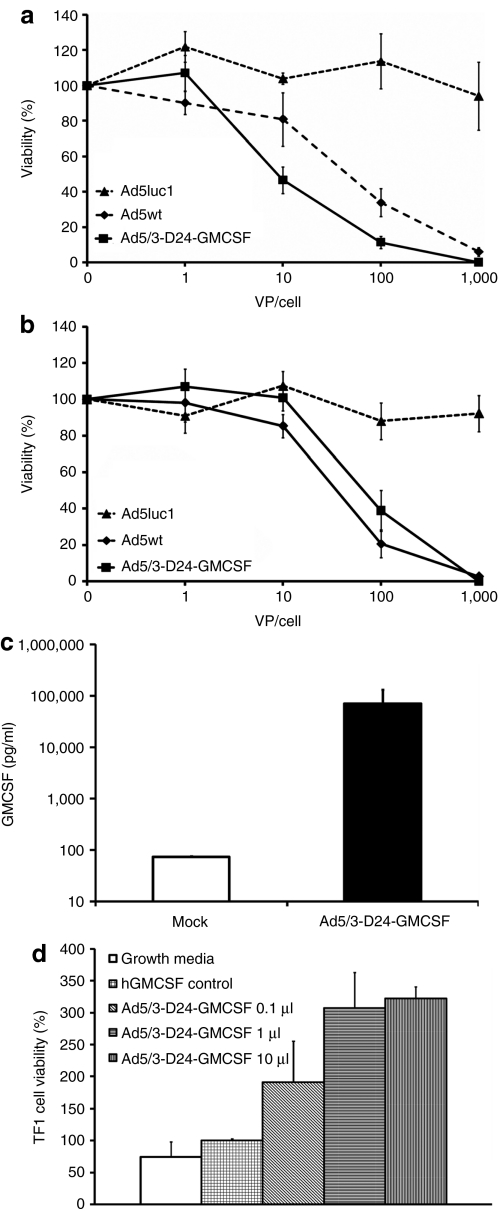
In Ad5/3-D24-GMCSF (Supplementary Figure S1), GMCSF is under endogenous viral E3 control elements, which results in replication-associated transgene expression starting about 8 hours after infection.27 The virus replicates in a tumor-selective manner thus resulting in tumor-restricted production of GMCSF. Tumor specificity is achieved by a 24-bp deletion, which abrogates the Rb-binding site of E1A and, as demonstrated in previous reports, the virus replicates selectively in cells with p16-Rb pathway defects, including most if not all human cancers.20,28,29 The oncolytic potency of Ad5/3-D24-GMCSF was shown to be as effective as the wild-type control virus (Figure 1a,b). GMCSF secretion in vitro was confirmed (Figure 1c). To confirm bioactivity of virus produced GMCSF, we cultured human lymphocyte cell line TF1, whose viability is dependent on functional human GMCSF, with either supernatant from Ad5/3-D24-GMCSF-infected A549 cells or commercially produced GMCSF. Cells cultured without GMCSF started to die at 48 hours, whereas cells cultured with supernatant were growing well and even better than cells cultured with commercial GMCSF (P ≤ 0.005) (Figure 1d).
Figure 1.
Ad5/3-D24-GMCSF induces cell killing and expression of functionally active GMCSF in vitro. Viability of (a) MDA-MB-436 and (b) A549 cells after infection with Ad5luc1, Ad5wt, and Ad5/3-D24-GMCSF. (c) GMCSF secretion by A549 cells after infection with 100 VP/cell Ad5/3-D24-GMCSF. (d) TF1 cells, whose viability is dependent on functional human GMCSF (hGMCSF) were cultured in the presence of 2 ng/ml hGMCSF or indicated amount of filtered supernatant from Ad5/3-D24-GMCSF-infected cells. Normal growth media was used as negative control. Viability of cells was determined after 5 days; viability of cells with commercial hGMSCF was set as 100%. hGMCSF, human granulocyte–macrophage colony–stimulating factor; VP, virus particles.
Ad5/3-D24 is a similar virus without GMCSF and has been widely studied with regard to efficacy, safety, and biodistribution in preclinical models previously.19,20,21,22,23,24,25,26,30 Toxicity studies in rodents with GMCSF-expressing and nonexpressing oncolytic adenoviruses have been performed previously.9,31,32 Safety of GMCSF in humans is well established.11
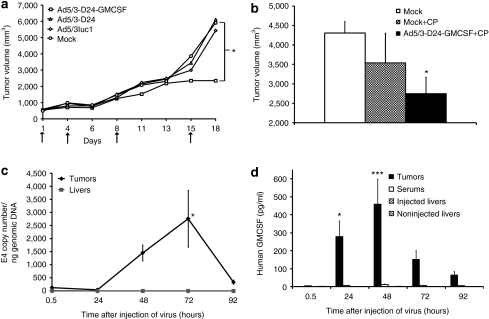
Potency of Ad5/3-D24-GMCSF was characterized in an immune-competent Syrian hamster model reported semipermissive for human adenovirus.33 Human GMCSF has also been suggested to be active in hamsters.8,34 Hamsters were treated exactly as our patients, with 4/5 of the dose given intratumorally and 1/5 intravenously and the dose was chosen to correspond wt/wt to the largest dose used in humans. Ad5/3-D24-GMCSF was capable of hindering tumor growth (P ≤ 0.05), whereas hamsters treated with a nonreplicating control virus or oncolytic virus without GMCSF showed no antitumor effect (Figure 2a). We also studied the effect of simultaneous low-dose cyclophosphamide that did not result in significant antitumor efficacy when used alone although the combination inhibited tumor growth (P ≤ 0.05) (Figure 2b).
Figure 2.
Antitumor efficacy and tumor selectivity of Ad5/3-D24-GMCSF in immune-competent Syrian hamsters. Syrian hamsters were inoculated subcutaneously with HapT1 cells. (a,b) Tumors were injected with 1 × 108 VP/tumor on days indicated by arrows, 1/5 of dose was given intravenously on day 1. NaCl was used as mock treatment. *P ≤ 0.05 against mock. In b the effect of low-dose cyclophosphamide (CP) in conjunction with Ad5/3-D24-GMCSF was tested and found to increase efficacy. Figure indicates tumor volumes recorded 15 days after injection of virus. (c,d) HapT1 tumors were grown and injected once with 1 × 108 VP/tumor Ad5/3-D24-GMCSF. (c) Virus replication was studied with quantitative PCR. To evaluate tumor selectivity of the virus, livers of non-tumor-bearing hamsters were injected and no replication was seen. Viral E4 copy number was normalized to genomic DNA with GAPDH primers. (d) Human GMCSF concentration was measured in virus injected tumors, serum and livers of tumor-bearing hamsters and livers of non-tumor-bearing hamsters injected into the liver. Data are expressed as mean ± SEM, *P ≤ 0.05 and ***P ≤ 0.005 versus the 0.5-hour time point. GMCSF, granulocyte–macrophage colony–stimulating factor; VP, virus particles.
To confirm selective replication and GMCSF secretion by Ad5/3-D24-GMCSF in tumors, we collected tumor and liver samples from HapT1 tumor–bearing Syrian hamsters after injection of virus intratumorally or into the liver. Measuring the amount of virus particles revealed a 23-fold increase in tumors between 0.5 and 72 hours after injection (P ≤ 0.05). In the livers, the amount of virus particle remained low at all time points (Figure 2c). GMCSF concentration was very low in the tumors and serum, as well as injected and noninjected livers, at 0.5 hours after virus injection. It increased significantly in tumors (P = 0.0009), reaching a 437-fold increase at 48 hours after injection. There was no significant increase of GMCSF concentration in the serum or noninjected livers, whereas there was a small twofold increase in injected livers at the 48-hour time point (P = 0.024) (Figure 2d).
Safety of Ad5/3-D24-GMCSF in cancer patients
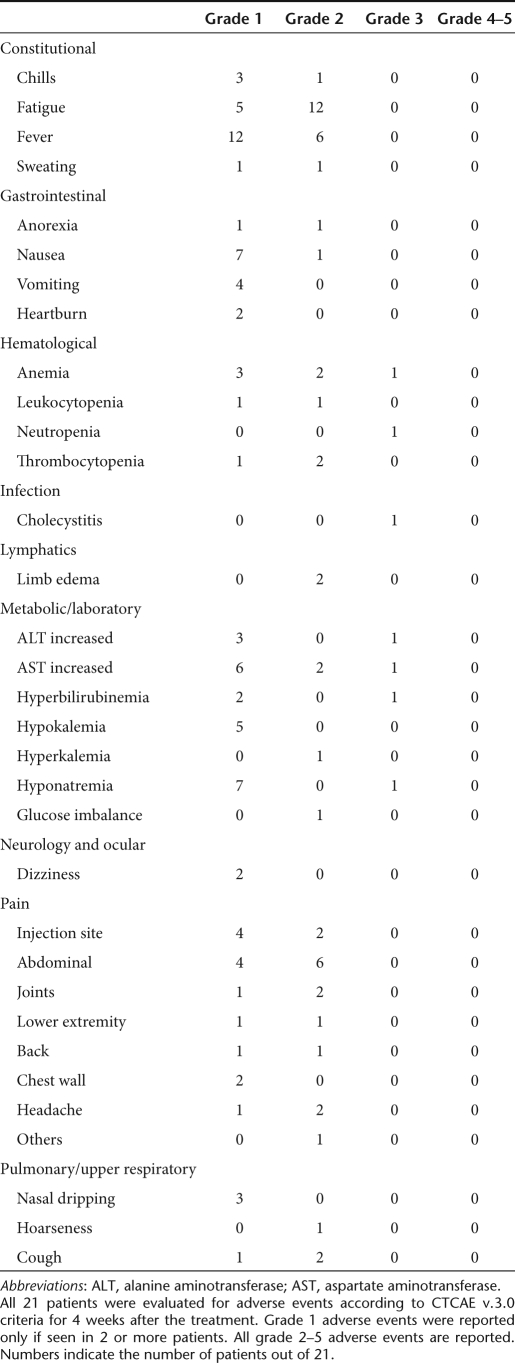
Treatments were well tolerated and no grade 4–5 adverse events were seen. Grade 1–2 flu-like symptoms, injection site and abdominal pain were common as well as liver enzyme elevations and hematological side effects (Table 2). Asymptomatic and self-limiting grade 3 hematological side effects were seen in four patients. The only nonhematological grade 3 side effect was a case of cholecystitis.
High levels of proinflammatory cytokines have been suggested to predict adenovirus mediated toxicity.35 We analyzed serum levels of interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor-α, and no significant elevations were seen (Supplementary Table S2 and Supplementary Figure S2). In parallel with the hamster data, there were no significant changes in systemic levels of GMCSF or total white blood cell counts (Supplementary Figure S3).
Neutralizing antibody titers and presence of Ad5/3-D24-GMCSF in serum after treatment
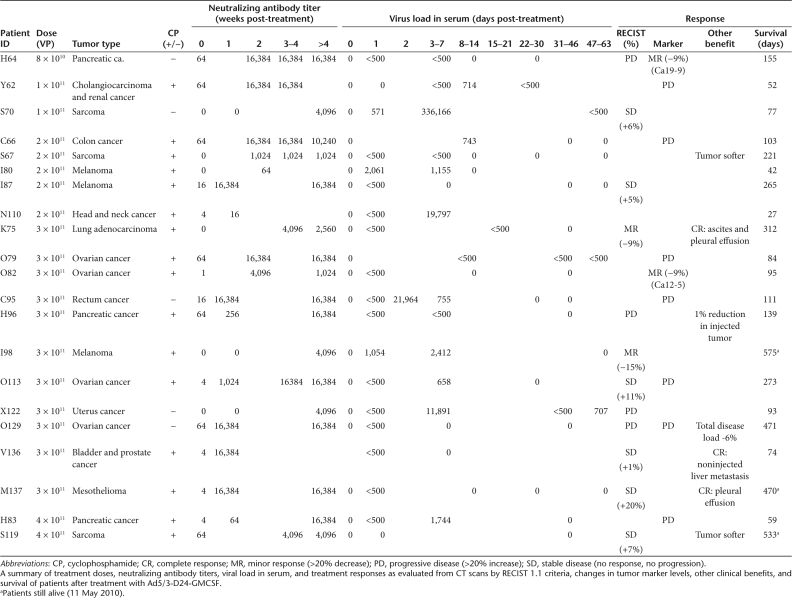
At baseline, 6/21 patients were completely negative for neutralizing antibodies against Ad5/3, 6 patients had barely detectable titers (1–4), and 9 patients had low neutralizing titer (16–64). After treatment the titer increased in all patients (P < 0.005) and the maximum titer (16,384) was reached in 11/21 patients within 3 weeks (Table 3).
Injected virus is rapidly cleared from the bloodstream36 and therefore extended presence or increase in virus genomes in blood has been suggested to be a sign of virus replication.37,38,39 All patients were negative for Ad5/3-D24-GMCSF before treatment (Table 3). On day 1, 17/19 patients had measurable levels in the serum, with the highest titer being 2.06 × 103 virus particles (VP)/ml. Between days 3 and 7, 12/15 patients presented virus in the blood, with a highest titer of 3.36 × 105 VP/ml. Of these cases, 8 had an increase in virus titer compared to day 1 and positive samples were seen up to day 58 after treatment.
Efficacy of Ad5/3-D24-GMCSF
All patients had progressing tumors before treatment. Twelve patients could be assessed for radiological benefit according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 criteria (Table 3); 2 patients had a minor response, 6 patients had stable disease (SD), and 4 patients had progressive disease (PD). Therefore, the radiological clinical benefit (=disease control) rate was 67% (Table 3).
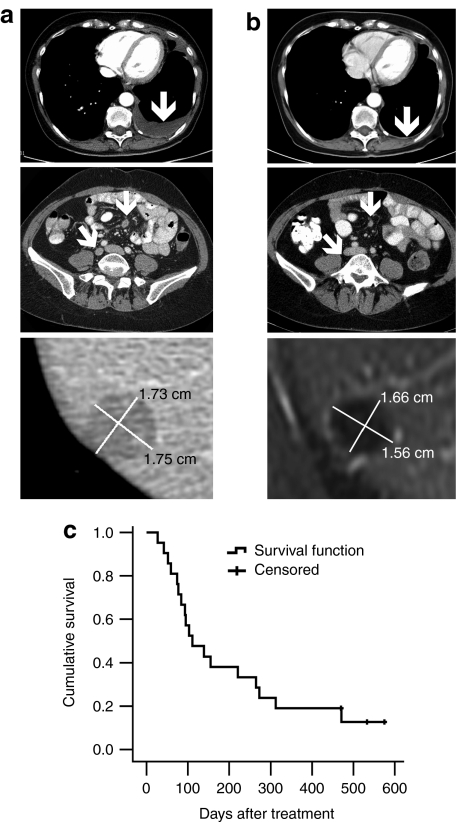
In addition, the rapidly growing pancreatic tumor in H96 stabilized, but a small metastatic lesion appeared in the lungs, and therefore H96 could have been classified as SD if immune-related criteria would have been used.40 Patient O129 had a 6% reduction of the total tumor burden but had a new metastasis. Again, immune-related criteria might have classified this as SD. In patient V136, a noninjected liver lesion disappeared, while total disease remained SD. With regard to tumor markers, 2/9 had a minor decrease in marker levels and 7/9 displayed an increase in marker levels (Table 3). Other clinical and/or subjective benefits included two patients who previously suffered from rapid accumulation of ascites and/or pleural effusion, which resolved completely for several months after virus treatment (Figure 3a,b, Table 3). Overall, signs of antitumor efficacy were seen in 13/21 patients in intent-to-treat analysis (62%, Table 3).
Figure 3.
Radiological responses to treatment with Ad5/3-D24-GMCSF and survival after treatment. (a) Pre- and (b) post-treatment computed tomography scans of K75 with complete resolution of pleural effusion, O129 with reduction of peritoneal tumors, and I98 illustrating reduction of a liver metastases (note: post-treatments scan for this patient is a magnetic resonance image). (c) Kaplan–Meier analysis of the survival of patients. Censored refers to patients who were still alive at the time of submission of the manuscript.
Antitumor activity was seen in patients receiving and not receiving low-dose cyclophosphamide. With regard to the latter, who had contraindications or did not tolerate cyclophosphamide, H64 had minor response in markers, S70 had SD in computed tomography and O129 had a decrease in overall disease burden and long survival. Overall, 38% of patients survived >200 days and the 400 day survival was 19%. At the time of manuscript submission three patients were still alive and well (Figure 3c). The cause of death was cancer progression in all cases where information was available.
Effect of Ad5/3-D24-GMCSF administration on white blood cell compartments
The phenotypic panel of circulating white blood cells was evaluated (Supplementary Table S3). Interestingly, most patients (10/14) showed an increase in the total number of CD8+ T-lymphocytes (Supplementary Figure S4) and for them the average CD8+ T-cell count increased from 0.61 × 109/l to 1.20 × 109/l (P = 0.076). This suggested that in addition to the oncolytic effect, Ad5/3-D24-GMCSF was consistently able to stimulate a T-cell response.
Adenovirus- and tumor-specific T-cell responses
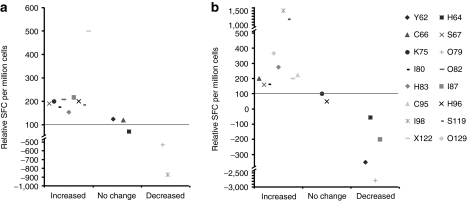
Based on the increase in CD8+ T cells (Supplementary Figure S4), and on preclinical data,41,42 we investigated adenovirus- and tumor-specific T-cell responses. In 9/14 patients, the level of Ad5-specific CD8+ lymphocytes in blood increased (Figure 4). Tumor biopsies were not available for assessment of tumor antigens present in each tumor and thus survivin was chosen for assessment of tumor-specific immunity. Survivin is a classic pan-carcinoma antigen reported to be present in practically all tumors.43,44 In 8/14 patients, the level of survivin-specific CD8+ lymphocytes increased after treatment (Figure 4).
Figure 4.
Ad5/3-D24-GMCSF treatment influences adenovirus- and tumor-specific cytotoxic T-lymphocytes. Total peripheral blood mononuclear cells were isolated from treated patients before and a month after treatment and pulsed with (a) an adenovirus type 5 penton-derived peptide pool and (b) a survivin-derived peptide pool. Interferon-γ ELISPOT was performed. The value before treatment was assigned 100 and the value after treatment is expressed relative to that. Spot forming colonies (SFC) are expressed as a mean of triplicate experiments, and background (SFC without peptide) was subtracted. Patients were grouped depending on the change in SFC after treatment; increased (>50%), no change, and decreased (>50%).
Pretreatment prediction of Ad5/3-D24-GMCSF efficacy and local virus load
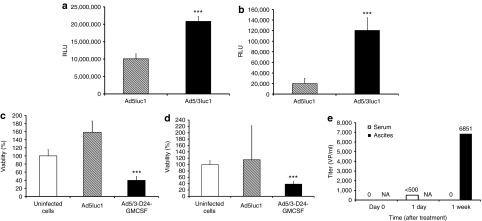
Fresh pretreatment samples of ascites and pleural effusion, from K75 and V136, respectively, were analyzed for efficacy of gene delivery. In both samples, high transduction was seen with Ad5/3luc1, which has a capsid identical to Ad5/3-D24-GMCSF (P < 0.005) (Figure 5a,b). This seemed to correlate with patient benefit as K75 had a ‐9.4% reduction in the sum of tumor diameters and V136 had SD in total tumor burden and CR of a noninjected liver metastasis (Table 3). Pretreatment samples of pleural effusion from V136 and M137 were assessed for oncolytic potency of Ad5/3-D24-GMCSF. Six days after infection there were 62 and 29% less viable cells, respectively, compared to uninfected control sample (P < 0.005), suggesting that Ad5/3-D24-GMCSF was able to kill cells present in the effusion (Figure 5c,d). Ad5/3-D24-GMCSF was also more effective than the nonreplicative control virus. Both patients had SD in post-treatment computed tomography scans (Table 3).
Figure 5.
5/3 chimeric adenoviruses are able to transfect and kill cells in ex vivo ascites and pleural effusion samples. Before treatment cells from (a) pleural effusion of V136 and (b) ascites of K75 were infected with Ad5luc1 (Ad5 capsid) and Ad5/3luc1 (Ad5/3 capsid) at 5,000 VP/cell and analyzed for luciferase expression (expressed as relative light units, RLU). ***P < 0.005 against Ad5luc1. Cells from pretreatment samples of pleural effusion of (c) V136 and (d) M137 were infected with 100 VP/cell Ad5/3-D24-GMCSF or the nonreplicative control. Viability of cells was analyzed 6 days later. ***P < 0.005 against uninfected cells. (e) Copy numbers of Ad5/3-D24-GMCSF were analyzed with quantitative PCR from serum samples and a post-treatment ascites sample of O82. NA, not analyzed; VP, virus particles.
To assess replication of Ad5/3-D24-GMCSF at the tumor, we analyzed an ascites sample obtained from patient O82 at 1 week after treatment. The viral load was 6,851 VP/ml, while the corresponding serum sample was negative (Figure 5e). In this case, it was also possible to analyze the oncolytic and infectious capacity of the virus present in the sample; cytopathic effect was seen in 70% of infected wells (data not shown). Patient O82 had a reduction of tumor markers after treatment (Table 3).
Discussion
A selectively replicating, Rb-p16 binding site defective, 5/3 capsid chimeric oncolytic adenovirus armed with GMCSF was generated. Ad5/3-D24-GMCSF showed good oncolytic potential and production of functionally active human GMCSF in vitro. In immune-competent hamsters, the virus was effective in hindering the growth of aggressive syngeneic pancreatic tumors. Evidence of replication of the virus in tumors was shown by measuring viral copy number. Selectivity of replication was demonstrated as there was no increase in viral copy numbers in directly injected liver tissue. Local replication-linked production of GMCSF in tumors was demonstrated, while there was very little leakage of GMCSF into serum or liver. We also showed that combining low-dose cyclophosphamide with Ad5/3-D24-GMCSF can enhance antitumor effect, whereas cyclophosphamide treatment alone did not result in significant reduction of tumor growth.
A total of 21 patients with advanced cancer refractory to available treatments were treated with Ad5/3-D24-GMCSF. A density of 4 × 1011 VP were administered without grade 4–5 adverse events, while mild flu-like symptoms and local pain were common. There were no elevations of proinflammatory cytokines, previously suggested as sensitive indicators of adenoviral toxicity. The absence of systemic GMCSF or white blood cell count elevations suggests restriction of GMCSF production to local sites of virus replication, as shown in preclinical data (Figure 2d). This is in contrast with a report on oncolytic vaccinia virus coding for GMCSF, where elevations in serum GMCSF and white blood cells were seen.45 One reason for this could be less restricted GMCSF expression; JX-594 expresses GMCSF in all transduced cells, whereas Ad5/3-D24-GMCSF produces GMCSF only in cells allowing replication.
Treatment resulted in disease control (SD or better) in 8/12 (67%) patients by radiological assessment. In addition, two patients had reductions of tumor marker values. Further, there were several patients with qualitative clinical benefits such as resolution of ascites and pleural effusion. Evaluation of efficacy of oncolytic viruses is rather problematic as approaches developed for chemotherapy may be poorly applicable. Standard radiological criteria such as RECIST may not optimally reflect treatment benefits as virus replication causes inflammation and swelling of the tumor which may then be interpreted incorrectly as tumor growth and progression. Also assessment of tumor markers may lack utility as they can increase due to induction of the marker gene promoter, virus replication, and subsequent cell lysis.46 Thus, the assessment of oncolytic efficacy may require development of new imaging tools less sensitive to inflammation.
A similar situation exists for immunotherapies such as anti-CTLA4 antibody ipilimumab, for which immune-related response criteria have been proposed to accommodate for the immune response.40 Evaluating the most relevant end points (quality of life, overall survival) would be a reliable method to assess efficacy. However, these end points do require a randomized trial, which has so far only been achieved with one oncolytic virus.1 The necessity of randomized trials was well demonstrated in the recent US Food and Drug Administration approval of sipuleucel-T, a T-cell therapy that does not give traditional RECIST responses but does increase overall survival.47 In our patient series, a subset of patients survived for an unusually long time given the advanced nature of their disease. Further studies are needed to understand which factors predict long-term survival after oncolytic adenovirus treatment.
Preclinical studies have suggested that 5/3 chimerism might be advantageous over Ad5 in the context of many tumor types.19,20,21,22,23,24,25,26,30 Therefore, it was interesting to see that with 67% radiological clinical benefit, Ad5/3-D24-GMCSF seems to yield at least similar disease control rates as the 50% reported for Ad5-D24-GMCSF, which is an isogenic virus without the capsid modification.8 However, caution should be exercised in nonrandomized comparisons, even though in this case the patient populations and inclusion criteria are well matched.
Although a classic approach, single-agent low-dose metronomic cyclophosphamide has generally proven ineffective and is only rarely used in contemporary oncology. However, the effect on regulatory T cells is well documented.13,14 Because this effect is theoretically highly synergistic with an oncolytic adenovirus coding for GMCSF, we studied the effect in hamsters (Figure 2b) and have subsequently incorporated the approach into our routine treatment scheme.48
Shedding of virus into serum was observed in 12/15 patients on days 3–7 and even up to day 58. Eight patients had an increase in virus titer in blood when compared to day 1. This type of increase of virus titers in blood has been suggested to be a sign of replication.36,37,38,39 Neutralizing antibodies increased in all patients, usually within 1–2 weeks. No clear correlation was seen between neutralizing antibody titers and viral dose, virus shedding in blood, antitumor activity, or toxicity, as supported by previous reports.1,37,38
With regard to the cellular immune response, we saw increases in CD8+ lymphocytes against Ad5. Induction of antiadenoviral T cells seems to support the notion that adaptive cellular responses can be produced even in patients with advanced and refractory tumors. Intriguingly, CD8+ cells against the classic tumor epitope survivin also increased in the majority of patients, which suggests that also an antitumoral immune response may have been elicted. In some patients, the frequency of antiadenoviral and anti-survivin CD8+ cells in blood decreased. We speculate that this might indicate accumulation of such cells at the tumor where the virus replicates and GMCSF is produced. Further studies are needed to demonstrate the role of GMCSF and adenoviral oncolysis, respectively, in breaking tolerance to tumor epitopes.
We had access to some pretreatment ascites and pleural effusion samples that could be analyzed for transductional efficacy and oncolytic potency ex vivo. Ad5/3luc1 was superior to Ad5luc1, in accordance with preclinical reports.20,30 Ad5/3-D24-GMCSF also showed effective cell killing activity in both pleural effusion samples. These findings seemed to correlate with clinical benefits, suggesting that the ex vivo transduction and cell killing assays might perhaps be usable for predicting clinical utility of virotherapy. Formal testing in larger patient series is needed to clarify this. Post-treatment ascites from O82 indicated a high virus titer, while the corresponding serum sample was negative. Also, this sample showed cytopathic effect in cell culture, indicating the presence of functional oncolytic virus. These findings suggest effective local replication of virus, as seen in hamsters (Figure 2), which could be an important factor in the balance between efficacy and safety. However, it is also possible that the virus found in ascites may have been remaining from the initial injected dose.
Overall, treatment of advanced cancer patients with Ad5/3-D24-GMCSF appears to be safe and promising signs of possible efficacy were observed. Furthermore, preliminary correlations between patient responses and ex vivo analysis of treatment efficacy set the stage for formal investigation into biomarkers predictive of oncolytic virus activity. Although virus was present in serum for extended periods even after a single dose, multiple injections are likely to improve tumor transduction and enhance antitumor immunity. A multiple-injection phase 1–2 clinical trial with Ad5/3-D24-GMCSF, combined with low-dose metronomic cyclophosphamide, is ongoing.
Materials and Methods
Adenoviruses. Ad5/3-D24-GMCSF was generated and amplified using standard adenovirus preparation techniques.19,20 A pAdEasy-1-derived plasmid containing a chimeric 5/3 fiber, pAdEasy5/3, was created by homologous recombination in Escherichia coli of Ad5/3luc1 viral genome and BstXI-digested 8.9 kb fragment of pAdEasy-1. Next, a shuttle vector containing a 24-bp deletion in E1A (pShuttleD24) was linearized with PmeI and recombined with pAdEasy5/3 resulting in pAd5/3-D24. In order to insert human GMCSF gene into E3 region, an E3-cloning vector pTHSN was created by inserting SpeI to NdeI fragment from Ad5 genome into the multicloning site of pGEM5Zf+ (Promega, Madison, WI). pTHSN was further digested with SunI/MunI creating a 965-bp deletion in E3 region (6.7K and gp19K deleted). The 432 bp complementary DNA encoding human GMCSF (Invitrogen, Carlsbad, CA) was amplified with primers featuring specific restriction sites SunI/MunI flanking the gene and then inserted into SunI/MunI-digested pTHSN. pAd5/3-D24-GMCSF was generated by homologous recombination in E. coli between FspI-linearized pTHSN-GMCSF and SrfI-linearized pAd5/3-D24. Ad5/3-D24-GMCSF virus genome was released by PacI digestion and transfection to A549 cells for amplification and rescue. All phases of the cloning were confirmed with PCR and multiple restriction digestions. The shuttle plasmid pTHSN-GMCSF was sequenced. Absence of wild-type E1 was confirmed with PCR. The E1 region, transgene, and fiber were checked in the final virus with sequencing and PCR. Virus production was done, according to the principles of cGMP by Oncos Therapeutics (Helsinki, Finland), on A549 cells to avoid the risk of wild-type recombination. Virus stock buffer formulation was 10 mmol/l Trizma base, 75 mmol/l NaCl, 5% (wt/vol) sucrose, 1 mmol/l MgCl, 10 mmol/l (+)histidine, 0.5% (vol/vol) EtOH, 0.02% Tween, 100 µmol/l EDTA; 0.9% (wt/vol) NaCl solution (B. Braun, Melsungen, Germany) was used as a diluent.
Ad5luc1, Ad5/3luc1, and Ad5/3-D24 have been published previously.20,30 Ad5wt is wild-type Ad5 strain Ad300 from American Type Culture Collection (ATCC, Manassas, VA).
Cell lines. MDA-MB-436 human breast cancer cells, A549 human lung adenocarcinoma cells, 293 transformed embryonal kidney cells, and TF1 human erythroleukemic cell line were obtained from ATCC. Hamster pancreatic carcinoma–derived cell line HapT1 was kindly provided by Ruben Hernandez-Alcoceba. Cells were grown and maintained in the recommended conditions.
Animals. Syrian hamsters (Mesocricetus auratus) were obtained from Harlan (Indianapolis, IN) at 11 weeks of age and quarantined for at least 1 week. All animal protocols were reviewed and approved by the Experimental Animal Committee of the University of Helsinki and the Provincial Government of Southern Finland. Health status of the animals was monitored daily. Hamsters were killed according to local animal care rules if tumors grew too large or the condition of the hamsters otherwise deteriorated. Anesthesia for injections and tumor measurements was performed with with Hypnorm (VetaPharma, Leeds, UK) and Dormicum (Roche, Espoo, Finland): fentanyl citrate 0.315 mg/kg, fluanisone 10 mg/kg, and midazolam 5 mg/kg diluted in sterile water.
Oncolytic potency of viruses in vitro. Potency of Ad5/3-D24-GMCSF in vitro, cells were seeded into two 96-well plates at 10,000 cells/well and infected after 24 hours, in 2% Dulbecco's modified Eagle's medium (DMEM). Cells were daily and maintained in 10% DMEM. Six days after infection, (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) cytotoxicity assay was performed (MTS-Assay; Promega). Absorbance was measured using Multiskan Ascent and Ascent Software v2.6 (Thermo Labsystems, Helsinki, Finland) at 490 nm and background absorbance was subtracted.
Functionality of GMCSF. TF1 cells were cultured in suspension in complete growth medium supplemented with 2 ng hGMCSF/ml and kept on a shaker. A549 cells were grown in growth medium with 2% fetal calf serum and infected with 10 VP/cell of Ad5/3-D24-GMCSF. Supernatant was collected 48 hours later and filtered through a 0.02 µm inorganic membrane filter (Whatman, Maidstone, UK). TF1 cells were centrifuged and resuspended with growth medium devoid of hGMCSF, and 1 × 104 cells/well were plated on 96-well plate and kept on a shaker. A volume of 0.1, 1, or 10 µl of filtered supernatant from Ad5/3-D24-GMCSF-infected A549 cells (6 wells each) was applied on TF1 cells. hGMCSF (2 ng/ml) was applied on the positive control cells (36 wells). TF1 cells without hGMCSF supplementation were used as a negative control. Three days later fresh growth medium without hGMCSF was added to the cells. Cell viability was measured with MTS-assay (Promega) after 5 days in culture. The viability of positive control cells, with supplementation of 2 ng/ml hGMCSF, was assigned 100%.
Efficacy of oncolytic adenovirus with and without cyclophosphamide. Hamsters (N = 5–10/group) were injected subcutaneously at four different sites with 1 × 107 HapT1 cells/site. Tumors were allowed to develop until they reached ~600 mm3. To model human treatment as closely as possible, 4/5 of the virus dose was injected intratumorally and 1/5 was given intravenously on day 1. On following days, treatment was given intratumorally only. Each treatment comprised a total of 4 × 108 VP/hamster (i.e., 1 × 108 VP/tumor on most days). NaCl was used as mock treatment. Cyclophosphamide was administered intraperitoneally twice a week at 20 mg/kg. Tumor growth was observed thereafter by measuring the width and height of the tumors and tumor volume was calculated by approximating the shape of the tumors as prolate spheroids.
Tumor selectivity of Ad5/3-D24-GMCSF replication and GMCSF concentration in tissues and serum. Hamsters (n = 2/time point) were injected subcutaneously in four different sites with 2.8 × 106 HapT1 cells/site. Tumors were allowed to develop and 1 × 108 VP/tumor Ad5/3-D24-GMCSF was injected intratumorally when average tumor volume was ~1,000 mm3. Simultaneously non-tumor-bearing hamsters were injected with 4 × 108 VP Ad5/3-D24-GMCSF into the liver. Hamsters were killed at five different time points: 0.5, 24, 48, 72, and 92 hours after virus injection and tumors and livers were collected and stored at ‐80 °C.
For quantitative PCR, tissues were homogenized and total DNA was extracted using the QIAamp Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Quantitative real-time PCR targeting E4 gene of the adenovirus was done using primers E4-forward (5′-GGAGTGCGCCGAGACAAC-3′) and E4-reverse (5′-ACTACGTCCGGCGTTCCAT-3′) and the E4-probe (5′-TGGCATGACACTACGACCAACACGATCT-3′). Hamster GAPDH primers and probe (primerFW: 5′-CACCGAGGACCAGGTTGTCT-3′, PrimerRW: 5′-CATACCAGGAGATGAGCTTTACGA-3′ and probeGAPDH: 6′FAM-CAAGAGTGACCCCACTCTTCCACCTTTGA) were used as an internal control and to normalize viral DNA copies per amount of genomic DNA. The PCR conditions were as described earlier. A regression standard curve for GADPH was established using known amounts of DNA extracted from cultured cells (1,800–0.18 ng).
For GMCSF measurements, tissues were minced with a scalpel and 50 mg was incubated with 5 µl of protease inhibitor (P8340; Sigma-Aldrich, St Louis, MO) and 500 µl of digestion mixture consisting of RPMI 1640 medium with 10 mmol/l HEPES buffer and 1.6 mmol/l phenylmethylsulfonyl fluoride (Sigma-Aldrich), 40 µg/ml gentamycin (Amresco, Solon, OH) 100 µg/ml bovine serum albumin (Sigma-Aldrich), and 100 µg/ml Zwittergent 3-12 (Merck4Biosciences, Darmstadt, Germany). After incubation of 90 minutes at 37 °C under continuous agitation the digestates were subjected to 30 seconds of sonication and centrifuged at 2,000g for 10 minutes at 4 °C. Supernatants were collected and stored in ‐80 °C until used in FACSArray. Human GMCSF concentrations in tissue digestion supernatants and serum samples were measured using BD Cytometrin Bead Array Soluble Protein Master Buffer Kit and BD Cytometrin Bead Array Human GMCSF Flex set according to manufacturer's instructions with BD FACSArray bioanalyzer, BD FACSArray SystemSoftware, and FCAP Array v1.0 software (BD Biosciences, San Diego, CA).
Patients. A total of 21 patients with advanced solid tumors refractory to standard therapies (Table 1) were treated with Ad5/3-D24-GMCSF (Supplementary Table S1). The inclusion criteria were solid tumors refractory to conventional therapies, PD, World Health Organization performance score ≤3 and no major organ function deficiencies. Exclusion criteria were organ transplant, human immunodeficiency virus, hyperbilirubinemia, severe thrombocytopenia, threefold or higher elevated aspartate aminotransferase or alanine aminotransferase levels, other severe disease, or organ malfunction. Written informed consent was required and treatments were administered according to Good Clinical Practice and the Declaration of Helsinki. This Advanced Therapy Access Program is in compliance with EU and Finnish regulations and is regulated by Finnish medicines agency FIMEA.
Table 1. Baseline features of patients.
Treatment protocol. Patients received a single round of treatment on day 0, by ultrasound-guided intratumoral injection and at least one-fifth of the dose was given intravenously. In the case of intrapleural or intraperitoneal disease, intratumoral injection was performed intracavitary. The injection was personalized for each patient according to the location and size of tumors. Typically, a 10 ml volume was injected intratumorally with 10 needle tracts in ultrasound guidance, while the intravenous dose was given as a 2.5 ml bolus after intratumoral injection. The starting dose of 8 × 1010 VP was chosen based on published safety results.49 To reduce regulatory T cells, concurrent low-dose oral metronomic cyclophosphamide 50 mg/day was given in the absence of contraindications.14 Patients were monitored for 24 hours in the hospital and 4 weeks as outpatients. Adverse events were recorded according to CTCAE v3.0 (Table 2). Pre-existing symptoms were not listed unless they worsened. Tumor size was assessed by contrast-enhanced computer tomography scanning before and ~2 months after treatment. Maximum tumor diameters were calculated according to RECIST v1.1,50 including injected and noninjected lesions. These criteria are complete response (CR; tumor completely undetectable after treatment), partial response (PR; ≥30% reduction in the sum of tumor diameters), SD (no reduction/increase), and PD (≥20% increase). Tumor decreases not fulfilling PR were scored as minor responses. Tumor markers were also evaluated when elevated at baseline, and the same percentages were used (Table 3).
Table 2. Adverse events.
Table 3. Responses.
Cytokine measurements. GMCSF production by virus-infected A549 cells and cytokine levels in serum were measured using BD Cytometrin Bead Array Soluble Protein Master Buffer Kit and BD Cytometrin Bead Array Human IL-6, IL-8, IL-10, tumor necrosis factor-α, and GMCSF Flex sets according to manufacturer's instructions. BD FACSArray bioanalyzer, BD FACSArray SystemSoftware, and FCAP Array v1.0 software (BD Biosciences) were used according to manufacturer's instructions for recording the results of the assay.
Neutralizing antibody titer determination and detection of viral DNA in serum and fluid samples. Neutralizing antibody determination was done as described previously,8 using Ad5/3luc1 to evaluate the effect of neutralizing antibodies in the serum of patients treated with Ad5/3-D24-GMCSF. DNA extraction and real-time PCR were performed as described previously.8 The viral loads in fluids were calculated using a regression standard curve based on serial dilutions of pAd5/3-D24-GMCSF DNA (1 × 109 to 1 × 10). Positive samples were confirmed by real-time PCR using LightCycler480 SYBR Green I Master mix (Roche, Mannheim, Germany) and primers specific for adenovirus and GMCSF sequences (forward primer 5′-AAACACCACCCTCCTTACCTG-3′ and reverse primer 5′-TCATTCATCTCAGCAGCAGTG-3′).
ELISPOT analysis. Peripheral blood mononuclear cells were isolated before treatment and 4–8 weeks after treatment by Percoll gradient according to standard protocols. Cells were immediately frozen in CTL-CryoABC serum-free media (Cellular Technology, Cleveland, OH). ELISPOT was performed according to MABtech manufacturer instructions (h-IFN-y ELISPOT PRO 10 plate kit, code 3420-2APT-10). Cells were stimulated with two peptides and also with positive and negative control (no peptide) for 20 hours. For adenovirus ELISPOT, cells were stimulated with the HAdV-5 penton peptide pool. For survivin, BIRC5 PONAB peptide was used.
Ex vivo analysis of ascites and pleural samples. Cells were isolated from fresh ascites/pleural effusion samples by centrifugation (900 r.p.m., 8 minutes, +4 °C). Red blood cells samples were lysed with 25 ml ACK Lysis Buffer. Samples were washed with 2% DMEM and cell suspension in 2% DMEM-fungizone was prepared (50 ml 2% DMEM + 200µl Fungizone; Bristol-Meyers Squibb, Espoo, Finland).
To test transductional efficacy, cells were seeded into 24-well plates, 50,000 cells/well. Twenty-four hours later, cells in triplicates were infected with Ad5luc1 or Ad5/3luc1 5,000 VP/cell in 2% DMEM. Forty-eight hours later luciferase expression was analyzed by Luciferase Assay System (Promega). To test the potency of Ad5/3-D24-GMCSF, cells were seeded into 96-well plates, 10,000 cells/well and 24 hours later infected with 100 VP/cell Ad5/3-D24-GMCSF in 2% DMEM. MTS analysis was carried out as above.
For assessing presence of functional virus in a post-treatment ascites samples, cells were resuspended in 3 ml 2% DMEM after lysing red blood cells and freeze-thawed four times in ‐80 °C. Sample was centrifuged for 15 minutes at 4,000 r.p.m. (+4 °C) and supernatant collected. HEK293 cells were seeded on 96-well plate, 10,000 cells/well, and 24 hours later infected with 100 µl/well of the supernatant. After 10 days of incubation, wells were assessed for cytopathic effect.
Statistical analysis. Statistics were done with SPSS v17.0 (SPSS, Chicago, IL). Two-tailed Student's t-test was used to compare luciferase activity and pre- and post-treatment neutralizing antibody titers, cytokine levels. One-way analysis of variance and two-tailed Dunnett's t-test was used to asses tumor volume and virus load data for hamster experiments. Survival data were processed with Kaplan–Meier analysis.
SUPPLEMENTARY MATERIAL Figure S1. Schematic of Ad5/3-D24-GMCSF genome. The virus bears a 24 bp deletion in E1. gp19k and 6.7k have been replaced by the cDNA of human GMCSF. The Ad5 knob domain has been replaced with knob domain from adenovirus serotype 3. Figure S2. No significant elevations in pro-inflammatory cytokines in patients after treatment with Ad5/3-D24-GMCSF. Average cytokine concentrations in serum after treatment. Expressed as mean + standard error of mean, n=21. Figure S3. No significant increases in serum GMCSF levels or circulating WBC count in patients after viral treatment. A) GMCSF concentration in serum and B) white blood cell (WBC) count in serum after treatment. Figure S4. Ad5/3-D24-GMCSF treatment increases total CD8+ T lymphocyte counts. A-N) Blood CD8+ T lymphocyte counts of all analyzable patients (n=14) before and after treatment. O) Average increase in CD8+ T lymphocytes in patients who displayed an increase in CD8+ T lymphocyte counts (n=10), before and after treatment + standard error of mean. Table S1. Characteristics of patients at baseline and description of treatment. aWHO Performance status at the time of treatment, scale 0-5. bConcurrent metronomic cyclophosphamide 50 mg/d was given orally in the absence of contraindications. cTotal dose; vp=viral particles. Table S2. Cytokine concentrations in serum after treatment. Table S3. Phenotypic panel of circulating lymphocytes.
Acknowledgments
We thank Saila Eksymä-Sillman, Marina Rosliakova, Satu Nikander, Jenni Kylä-Kause, and other Eira/Docrates personnel for help and support. This work was supported by Helsinki Biomedical Graduate School, University of Helsinki, Biocentrum Helsinki. A.H. is K. Albin Johansson Research Professor of the Foundation for the Finnish Cancer Institute. A.H. is a shareholder in Oncos Therapeutics Ltd. T.J. is a partner in International Comprehensive Cancer Center Docrates.
Supplementary Material
Schematic of Ad5/3-D24-GMCSF genome. The virus bears a 24 bp deletion in E1. gp19k and 6.7k have been replaced by the cDNA of human GMCSF. The Ad5 knob domain has been replaced with knob domain from adenovirus serotype 3.
No significant elevations in pro-inflammatory cytokines in patients after treatment with Ad5/3-D24-GMCSF. Average cytokine concentrations in serum after treatment. Expressed as mean + standard error of mean, n=21.
No significant increases in serum GMCSF levels or circulating WBC count in patients after viral treatment. A) GMCSF concentration in serum and B) white blood cell (WBC) count in serum after treatment.
Ad5/3-D24-GMCSF treatment increases total CD8+ T lymphocyte counts. A-N) Blood CD8+ T lymphocyte counts of all analyzable patients (n=14) before and after treatment. O) Average increase in CD8+ T lymphocytes in patients who displayed an increase in CD8+ T lymphocyte counts (n=10), before and after treatment + standard error of mean.
Characteristics of patients at baseline and description of treatment. aWHO Performance status at the time of treatment, scale 0-5. bConcurrent metronomic cyclophosphamide 50 mg/d was given orally in the absence of contraindications. cTotal dose; vp=viral particles.
Cytokine concentrations in serum after treatment.
Phenotypic panel of circulating lymphocytes.
REFERENCES
- Yu W., and, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J. Live viruses in cancer treatment. Oncology (Williston Park, NY) 2002;16:1483–92; discussion 1495. [PubMed] [Google Scholar]
- Jounaidi Y, Doloff JC., and, Waxman DJ. Conditionally replicating adenoviruses for cancer treatment. Curr Cancer Drug Targets. 2007;7:285–301. doi: 10.2174/156800907780618301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK, Machado HB., and, Herschman HR. The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem. 2009;108:778–790. doi: 10.1002/jcb.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105:1169–1172. doi: 10.1172/JCI9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh N, Ge Y, Ennist DL, Zhu M, Mina M, Ganesh S, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor–armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12:305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70:4297–4309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- Chang J, Zhao X, Wu X, Guo Y, Guo H, Cao J, et al. A Phase I study of KH901, a conditionally replicating granulocyte-macrophage colony-stimulating factor: armed oncolytic adenovirus for the treatment of head and neck cancers. Cancer Biol Ther. 2009;8:676–682. doi: 10.4161/cbt.8.8.7913. [DOI] [PubMed] [Google Scholar]
- Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22:3188–3192. doi: 10.1038/sj.onc.1206459. [DOI] [PubMed] [Google Scholar]
- Arellano M., and, Lonial S. Clinical uses of GM-CSF, a critical appraisal and update. Biologics. 2008;2:13–27. doi: 10.2147/btt.s1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan KA, Tan G, Bronte V., and, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Raki M, Ahtiainen L, ASGT Abstract 644 et al. 2009Immunological effects of metronomic cyclophosphamide in cancer patients treated with oncolytic adenoviruses Mol Ther 17S1): S246
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bauerschmitz GJ, Barker SD., and, Hemminki A. Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review) Int J Oncol. 2002;21:1161–1174. [PubMed] [Google Scholar]
- Fleischli C, Sirena D, Lesage G, Havenga MJ, Cattaneo R, Greber UF, et al. Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor. J Gen Virol. 2007;88:2925–2934. doi: 10.1099/vir.0.83142-0. [DOI] [PubMed] [Google Scholar]
- Tuve S, Wang H, Ware C, Liu Y, Gaggar A, Bernt K, et al. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J Virol. 2006;80:12109–12120. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva A., and, Hemminki A. Modified adenoviruses for cancer gene therapy. Int J Cancer. 2004;110:475–480. doi: 10.1002/ijc.20129. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther. 2003;8:449–458. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- Volk AL, Rivera AA, Kanerva A, Bauerschmitz G, Dmitriev I, Nettelbeck DM, et al. Enhanced adenovirus infection of melanoma cells by fiber-modification: incorporation of RGD peptide or Ad5/3 chimerism. Cancer Biol Ther. 2003;2:511–515. doi: 10.4161/cbt.2.5.440. [DOI] [PubMed] [Google Scholar]
- Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB., and, Lesniak MS. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–160. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- Ranki T, Särkioja M, Hakkarainen T, von Smitten K, Kanerva A., and, Hemminki A. Systemic efficacy of oncolytic adenoviruses in imagable orthotopic models of hormone refractory metastatic breast cancer. Int J Cancer. 2007;121:165–174. doi: 10.1002/ijc.22627. [DOI] [PubMed] [Google Scholar]
- Sarkioja M, Kanerva A, Salo J, Kangasniemi L, Eriksson M, Raki M, et al. Noninvasive imaging for evaluation of the systemic delivery of capsid-modified adenoviruses in an orthotopic model of advanced lung cancer. Cancer. 2006;107:1578–1588. doi: 10.1002/cncr.22209. [DOI] [PubMed] [Google Scholar]
- Kangasniemi L, Kiviluoto T, Kanerva A, Raki M, Ranki T, Sarkioja M, et al. Infectivity-enhanced adenoviruses deliver efficacy in clinical samples and orthotopic models of disseminated gastric cancer. Clin Cancer Res. 2006;12:3137–3144. doi: 10.1158/1078-0432.CCR-05-2576. [DOI] [PubMed] [Google Scholar]
- Guse K, Ranki T, Ala-Opas M, Bono P, Särkioja M, Rajecki M, et al. Treatment of metastatic renal cancer with capsid-modified oncolytic adenoviruses. Mol Cancer Ther. 2007;6:2728–2736. doi: 10.1158/1535-7163.MCT-07-0176. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Zinn KR, Peng KW, Ranki T, Kangasniemi L, Chaudhuri TR, et al. Noninvasive dual modality in vivo monitoring of the persistence and potency of a tumor targeted conditionally replicating adenovirus. Gene Ther. 2005;12:87–94. doi: 10.1038/sj.gt.3302387. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–280. [PubMed] [Google Scholar]
- Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer JM, Shashkova EV, et al. 2009An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector Cancer Gene Therepub ahead of print). [DOI] [PMC free article] [PubMed]
- Sonabend AM, Ulasov IV, Han Y, Rolle CE, Nandi S, Cao D, et al. Biodistribution of an oncolytic adenovirus after intracranial injection in permissive animals: a comparative study of Syrian hamsters and cotton rats. Cancer Gene Ther. 2009;16:362–372. doi: 10.1038/cgt.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- Cohen AM, Hines DK, Korach ES., and, Ratzkin BJ. In vivo activation of neutrophil function in hamsters by recombinant human granulocyte colony-stimulating factor. Infect Immun. 1988;56:2861–2865. doi: 10.1128/iai.56.11.2861-2865.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Alemany R, Suzuki K., and, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- Tuve S, Liu Y, Tragoolpua K, Jacobs JD, Yumul RC, Li ZY, et al. In situ adenovirus vaccination engages T effector cells against cancer. Vaccine. 2009;27:4225–4239. doi: 10.1016/j.vaccine.2009.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanOosten RL., and, Griffith TS. Activation of tumor-specific CD8+ T Cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res. 2007;67:11980–11990. doi: 10.1158/0008-5472.CAN-07-1526. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Ryan BM, O'Donovan N., and, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–562. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Turunen TMO, Nokisalmi P, Cerullo V, Pesonen S, Oksanen M, Escutenaire S, ASGT Abstract 280 et al. 2009Effect of oncolytic adenovirus on tumor marker levels in cancer patients and in preclinical test systems Mol Ther 17S1): S110 [Google Scholar]
- Bot A. The landmark approval of Provenge, what it means to immunology and “in this issue”: the complex relation between vaccines and autoimmunity. Int Rev Immunol. 2010;29:235–238. doi: 10.3109/08830185.2010.490777. [DOI] [PubMed] [Google Scholar]
- Nokisalmi P, Pesonen S, Escutenaire S, Särkioja M, Raki M, Cerullo V, et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin Cancer Res. 2010;16:3035–3043. doi: 10.1158/1078-0432.CCR-09-3167. [DOI] [PubMed] [Google Scholar]
- Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned. Gene Ther. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic of Ad5/3-D24-GMCSF genome. The virus bears a 24 bp deletion in E1. gp19k and 6.7k have been replaced by the cDNA of human GMCSF. The Ad5 knob domain has been replaced with knob domain from adenovirus serotype 3.
No significant elevations in pro-inflammatory cytokines in patients after treatment with Ad5/3-D24-GMCSF. Average cytokine concentrations in serum after treatment. Expressed as mean + standard error of mean, n=21.
No significant increases in serum GMCSF levels or circulating WBC count in patients after viral treatment. A) GMCSF concentration in serum and B) white blood cell (WBC) count in serum after treatment.
Ad5/3-D24-GMCSF treatment increases total CD8+ T lymphocyte counts. A-N) Blood CD8+ T lymphocyte counts of all analyzable patients (n=14) before and after treatment. O) Average increase in CD8+ T lymphocytes in patients who displayed an increase in CD8+ T lymphocyte counts (n=10), before and after treatment + standard error of mean.
Characteristics of patients at baseline and description of treatment. aWHO Performance status at the time of treatment, scale 0-5. bConcurrent metronomic cyclophosphamide 50 mg/d was given orally in the absence of contraindications. cTotal dose; vp=viral particles.
Cytokine concentrations in serum after treatment.
Phenotypic panel of circulating lymphocytes.