Abstract
Efficient gene transfer into quiescent T and B lymphocytes for gene therapy or immunotherapy purposes may allow the treatment of several genetic dysfunctions of the hematopoietic system, such as immunodeficiencies, and the development of novel therapeutic strategies for cancers and acquired diseases. Lentiviral vectors (LVs) can transduce many types of nonproliferating cells, with the exception of some particular quiescent cell types such as resting T and B cells. In T cells, completion of reverse transcription (RT), nuclear import, and subsequent integration of the vesicular stomatitis virus G protein pseudotyped LV (VSVG-LV) genome does not occur efficiently unless they are activated via the T-cell receptor (TCR) or by survival-cytokines inducing them to enter into the G1b phase of the cell cycle. Lentiviral transduction of B cells is another matter because even B-cell receptor-stimulation inducing proliferation is not sufficient to allow efficient VSVG-LV transduction. Recently, a new LV carrying the glycoproteins of measles virus (MV) at its surface was able to overcome vector restrictions in both quiescent T and B cells. Importantly, naive as well as memory T and B cells were efficiently transduced while no apparent activation, cell-cycle entry, or phenotypic switch were detected, which opens the door to a multitude of gene therapy and immunotherapy applications as reported here.
T and B Cells for Gene Therapy and Immunotherapy
T cells as targets for gene therapy and immunotherapy
Efficient gene transfer into T lymphocytes may allow the treatment of several genetic dysfunctions of the hematopoietic system, like certain severe combined immunodeficiencies,1,2 and the development of novel therapeutic strategies for cancer and acquired diseases such as AIDS.3 Importantly, the naive T-cell subset, which responds to novel antigens, has a long-term lifespan and persists over years in patients. Thus, long-term correction can be envisaged by naive T-cell gene therapy. In addition, T cells most likely have a lower risk of transformation as compared to hematopoietic stem cells, as up to now leukemia has not been observed in T-cell-based gene therapies.1,2,4,5 It was also shown that retroviral vector integration deregulates gene expression in T cells but this had no evident consequences on the function and biology of the transplanted T cells.6
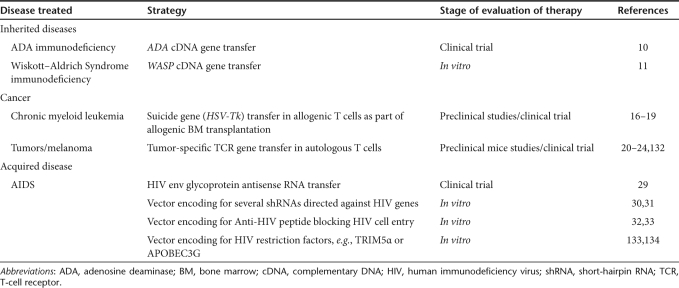
T-cell gene therapy has great potential for multiple target diseases as indicated in Table 1. Adenosine deaminase (ADA)–deficient severe combined immunodeficiency was the first inherited disease investigated for T-cell gene therapy because of a postulated survival advantage for corrected T lymphocytes.7,8,9 Aiuti et al. showed immune reconstitution in ADA–severe combined immunodeficiency patients after T-cell gene therapy, which then led to a selective growth of the infused ADA-expressing lymphocytes, eventually replacing the nontransduced T-cell population.10 These ADA-corrected T cells were capable of responding to novel antigens and represented a new polyclonal T-cell repertoire. For another immunodeficiency, Wiskott–Aldrich Syndrome, T cells were also functionally corrected in vitro by transduction with lentiviral vectors (LVs) encoding the Wiskott–Aldrich Syndrome protein.11,12 However, for both ADA and Wiskott–Aldrich Syndrome disease, T-cell based gene therapy might not be the best solution and hematopoietic stem cell–based gene therapy might represent a better option as these diseases affect multiple blood cell lineages. In the treatment of several blood cancers, T-cell gene therapy has now been proven to correct severe side effects of allogeneic bone marrow transplantation. Allogeneic bone marrow transplantation is widely used as a curative approach to many hematologic malignancies.13,14 Treatment with allogeneic T cells, as a part of an allogeneic bone marrow transplant offers the possibility of cure for patients with chronic myelogenous leukemia.15 The specificity of this therapeutic effect is called “graft-versus-leukemia effect”. Unfortunately, graft-versus-leukemia effect is frequently associated with graft-versus-host disease, mediated by allospecific T cells within the graft. A strategy for the prevention of graft-versus-host disease is the depletion of the donor T cells in vivo in cases where graft-versus-host disease becomes severe. This can be achieved by gene transfer of a suicide gene into the T cells before infusion. Should the need for eradication of these cells arise, administration of a drug will induce specifically apoptotic death of suicide gene transduced T cells.16,17,18,19 A lot of effort is also being made to reprogram T cells by transferring tumor-specific T-cell receptor (TCR) genes into patient cells to redirect their specificity toward autologous tumor cells.20,21,22,23,24 A pivotal clinical trial showed that the TCR gene–modified lymphocytes caused melanoma regression in some patients.21,23
Table 1. T-cell-based gene therapy and immunotherapy.
Also for acquired diseases such as AIDS for which there is a demand for novels therapies, T-cell gene therapy might be an important option as it would allow to protect the major reservoir, the CD4+ T cells, against human immunodeficiency virus (HIV) infection.25,26 An HIV-1-based LV was engineered expressing an HIV envelope antisense that highly protected T cells from infected patients against HIV infection in vitro27,28 and in the clinic.29,30 Similarly, LVs carrying several short-hairpin RNAs directed against HIV allowed protection of the T cells against viral infection.31 For another therapeutic anti-HIV approach, an antiviral gene was developed encoding a membrane-anchored peptide, which inhibits HIV entry at the level of virus-cell fusion with great efficacy.32,33 These kinds of gene therapies may offer a solution for patients who no longer respond any more to antiretroviral therapy.
In summary, it becomes clear that T-cell-based gene therapy offers a valuable alternative for treating primary and acquired immune disorders.
B cells as gene therapy and immunotherapy targets
B-cell gene therapy or immunotherapy has been strongly hampered due to the lack of an efficient LV ensuring stable long-term transgene expression in these cells but, as reported below (see A Novel Lentiviral Pseudotype Allows Quiescent T- and B-Lymphocyte Transduction section), a novel LV pseudotype may now allow many applications (Table 2).
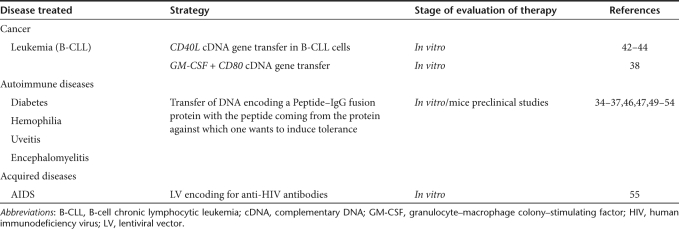
Table 2. B-cell-based gene therapy and immunotherapy.
Transgene expression in B cells is of particular interest as B cells have the potential to induce specific immune activation and tolerance, which could improve genetic vaccination against cancer or autoimmune diseases.34,35,36,37,38 One of the major goals of cancer immunotherapy is to increase the immunogenicity of tumor cells. Although several surface molecules can mediate T–B cell collaborations, the role of the CD40 receptor appears to be crucial.39,40 Thus, to overcome the immunological defect in malignant B cells, several groups induced forced ectopic expression of CD40L or other stimulatory factors (Table 2).38,41,42,43,44 Thus, vaccination strategies using autologous tumor cells manipulated ex vivo might be considered as a new approach for patients with B-cell malignancies, especially for those patients who are not responding to current treatment regimens.45
Autoimmune diseases represent failure of self-tolerance in populations of circulating B and T cells.35,36,37,46 Current treatments include immunosuppression and immune deviation, but they are accompanied by deleterious effects. Thus, novel approaches that can induce tolerance are required. B-cell gene therapy is an important option for induction of potent tolerogenic antigen-presenting cells. Scott et al. have shown that peptide–IgG fusion proteins, delivered via retroviral vectors into mouse B cells, render these cells highly tolerogenic for the epitopes present in the peptide associated with the IgG in vitro and in vivo.36,37,47,48,49,50,51,52 Mouse B cells can in this way efficiently express multiple antigenic epitopes presented in a tolerogenic manner on class II major histocompatibility complex, inducing tolerance in naive and already primed immunocompetent animals. The clinical relevance of this B-cell gene therapy approach has been demonstrated by tolerance induction for targeted antigens in experimental models of several autoimmune diseases (Table 2).36,35,49,53,54 In these models, this gene therapy–induced protection delayed the onset of the disease or even reversed the ongoing clinical course.35,36,49,53,54
Of importance, primary B cells are the most potent antibody-producing cells. Indeed, the programming of B cells to make a predefined protective antibody would provide a continuous supply of antibodies in vivo that might reduce the viral load in patients.55 Recently, a B-cell based anti-HIV strategy was proposed. LVs were used to introduce an anti-HIV antibody coding regions into hematopoietic stem cells and differentiation of the transduced cells into antibody-secreting autologous B cells was achieved with success.55 Alternatively, lentiviral transduction and reinfusion of autologous B cells would allow a quick and continuous supply of HIV-neutralizing antibodies in vivo. Of note, in the context of B-cell immunotherapy, the traditional methods used for generating human monoclonal antibodies include screening Epstein–Barr virus–transformed human B-cell clones56,57 or antibody phage display libraries.58 These methods are often time-consuming and can give low yields of pathogen-specific monoclonal antibodies. A novel strategy requiring efficient B-cell gene transfer may facilitate production and the identification of monoclonal neutralizing antibodies by allowing efficient transduction of primary patient memory B cells with BCL6 and BCL-XL genes inducing immortalization.59
Stable gene transfer in human B cells may thus allow the engineering of improved genetic vaccination strategies against cancer, infectious, or autoimmune diseases.34,43,60,61
Restrictions of LV-Mediated Gene Transfer in Human T Cells and B Cells
Overcoming restrictions of lentiviral transduction of T cells
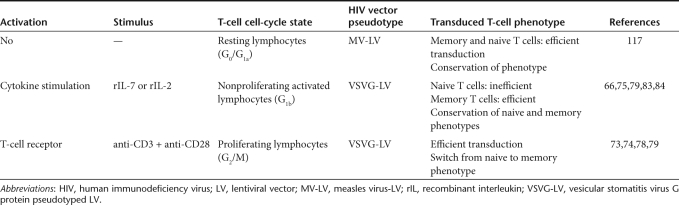
Several studies have now established the capacity of vesicular stomatitis virus G protein (VSVG)-pseudotyped HIV-1-derived vectors to transduce various types of nonproliferating cells both in vitro and in vivo.62 However, some cell types that are important gene therapy targets are refractory to gene transfer with LVs. These include, in particular, early progenitor hematopoietic stem cells in G0,63 monocytes,64,65 and resting T lymphocytes.66 That the parental virus, HIV-1, can enter into resting T lymphocytes but does not replicate67,68,69,70 has been attributed to multiple postentry blocks as well as several cellular restriction factors.71 Focusing on LVs, restrictions encountered include in particular, (i) defects in initiation and completion of the reverse transcription (RT) process,67,69,70 (ii) lack of adenosine triphosphate-dependent nuclear import,72 and (iii) lack of integration of the proviral genome. Indeed, activation of these cells, causing G0 to G1b transition of the cell cycle is required to relieve the blocks in gene delivery66,67,68,69,70,73 (Table 3). It has been reported that inducing the resting T cells to enter into the G1b phase of the cell cycle without triggering cell division could render them permissive to transduction with HIV-1-vectors73,74,75 (Table 3).
Table 3. LV transduction of T cells.
Toward an efficient VSVG-LV transduction of T cells
The population of mature adult T cells can be divided into two different subsets namely memory and naive T cells. Naive T cells are especially important as gene therapy target cells as they maintain the capacity to respond to novel antigens. It is also of the utmost importance that the responses of T cells to antigens are not dramatically altered by the gene transfer protocol. We and others have reported that inducing cell-cycle entry into G1b via stimulation through the TCR allows efficient transduction of adult naive T cells by HIV-1-based vectors.73,74 However, TCR-stimulation of T cells alters their half-life and immune-competence, results in an inversion of the CD4/CD8 ratio, and is associated with loss of naive T-cell subsets and a skewed TCR repertoire76,77,78,79 (Table 3). Of note, up to now T-cell gene therapy trials were based on TCR-mediated stimulation of T cells. However, transduction of naive T cells is a prerequisite for any T-cell mediated gene therapy trial aimed at providing long-lasting immune reconstitution in patients. Therefore, protocols were developed allowing LV transduction of T cells in the absence of TCR triggering. It was shown that interleukin-7 (IL-7), but also IL-2 promoted long-term survival of T lymphocytes.80,81,82 Interestingly, exposure of adult T cells to the cytokines IL-2 and IL-7 renders them permissive to lentiviral transduction in the absence of TCR activation.66,75,79,83,84 These cytokine-treated T cells move out of G0 into the G1b phase of the cell cycle, the phase in which T cells are susceptible to LV transduction, but do not yet proliferate66,79,83,84 (Table 3). Clearly, IL-2 and IL-7 stimulation allowing lentiviral T-cell transduction could preserve a functional T-cell repertoire and maintain an appropriate proportion of naive and memory CD4+ and CD8+ T cells.83 Naive and memory T cells respond differently to recombinant IL-7 stimulation.85 In agreement with this, naive adult T cells need longer IL-7 stimulation than memory cells to allow efficient LV transduction.79,86
In conclusion, LV transduction of IL-2 or IL-7 stimulated T cells overcomes the limitation of TCR-mediated LV gene transfer.
B-cell lentiviral gene transfer restrictions
Until recently, achieving long-term gene transfer into primary human B cells has been notoriously difficult87,88,89 and LV transduction of truly quiescent B cells had not yet been reported. As mentioned above, in the case of T cells, IL-7 stimulation leads to passage from G0/G1a to G1b thus enabling gene delivery with high efficiency.66,79,83 However, this is not the case for human B cells. Serafini et al. showed that conventional VSVG-LVs only achieved low transduction efficiency in anti-CD40-triggered B cells, whereas the same vector doses allowed efficient transduction of phytohemagglutinin-stimulated T cells.89 Both cell types exhibited similar proliferation rates and identical cell-cycle status, with part of the T- and B-cell populations actively dividing. Evidence of differences in LV transduction of resting T and B cells is present at the intracellular steps of the vector cycle. It has been demonstrated that VSVG-LVs can indeed enter into a very small subset of B cells, undergo RT and that proviral integration takes place into the B-cell genome. It is worth pointing out though, that VSVG-LV cell entry, RT, and nuclear entry occur in proliferating B cells but are very inefficient as compared to proliferating T cells.89 However, even if it is evident that cellular factors either facilitate or counteract different steps of lentiviral transduction in T cells, no B-cell-specific putative blocking factors have been yet identified. It has been documented that resting T and B cells could be transduced by a VSVG-coated LV carrying most of the HIV accessory proteins (vif, vpr, vpu, and nef).90 It is clear though, that inclusion of HIV accessory proteins, associated with the pathogenicity of HIV, may raise biosafety concerns if considered in an LV design for therapeutic gene delivery into resting T or B cells.
Culture conditions allowing lentiviral gene transfer into primary B cells
Different B-cell activation/stimulation protocols have been set up in order to achieve stable gene transfer into human B cells (Table 4). One way of inducing B-cell activation is by the addition of antiCD40-crosslinking antibodies and IL-4 that induces proliferation and entry into S/G2/M phase of the cell cycle. Under these conditions, VSVG-LVs allow only very poor transduction of B cells, whereas the same vector doses allow transduction of almost 100% of phytohemagglutinin-stimulated T cells. This was reported in three independent studies87,88,89 (Table 4). As reported by Bovia et al., VSVG-LVs allow efficient transduction of human B cells only upon their activation and proliferation in a coculture system using murine thymoma cells as helper T cells in the presence of a cocktail of cytokines87,91,92,93 (Table 4). Additionally, stimulation of B cells by Epstein–Barr virus or stimulation of cells with CpG DNA and cytokines can lead to efficient transduction.87,88,94,95 More sophisticated approaches use LVs that display an anti-CD20scFv to target gene transfer to B cells96,97,98 (Table 4). However, all these protocols that render the B cells permissive to LV transduction have serious drawbacks. The thymoma coculture system led to proliferation and subsequent plasmoid differentiation of all naive and memory human B-cell subsets91 (Table 4), and Epstein–Barr virus–stimulated cells rapidly become infected by Epstein–Barr virus.99 Even the milder stimulation protocol using anti-CD40/IL-4 is accompanied by B-cell differentiation.100 Finally, binding of anti-CD20-targeted vectors to B cells results most probably in a proliferative stimulus through clustering of the B-cell receptor.101,102,103
Table 4. Lentiviral transduction of B cells.
It is of the utmost importance for many applications and fundamental studies that the primitive characteristics of the target B-cell remain as intact as possible upon transduction, and that the vector does not induce itself any activation and/or differentiation as a secondary effect.
Strategies for targeting LV expression to T and B cells
An important objective in gene therapy is to engineer LVs for T- and B-cell transduction in vivo. This demands specific targeting of expression to these targets to avoid immune responses due to off-target transduction, e.g., of antigen-presenting cells. We can mainly distinguish between two types of strategies to target LV-mediated expression to T or B cells. The first strategy is called transductional targeting and relies on the modification of the vector surface either by the incorporation of foreign envelope glycoproteins that have a natural restricted tropism to T or B cells, or by the inclusion of specific ligands fused to the envelope proteins that will determine the affinity of the vector for T or B cells. The vector specificity is determined at the entry stage and therefore, only the cells that carry the T- or B-cell-specific receptor will be transduced. The second strategy is transcriptional targeting that is often achieved by the insertion of a tissue-specific promoter, or a fragment of this promoter, upstream of the therapeutic transgene.
For transductional targeting, the E2 glycoproteins derived from Sindbis virus and the hemagglutinin of influenza virus have been used with success to redirect the host range of vector particles selectively to cells expressing the target cell-surface molecules.104,105 Because both proteins also efficiently pseudotype LVs,105,106 they offer new possibilities to retarget LVs. A recent report showed a retargeting to human B cells by displaying an anti-CD19scFv together with hemagglutinin or Sindbis glycoprotein on LVs in vitro and transduction of human B cells with these new LV pseudotypes in vivo in an immunodeficient mice model transfused with human white blood cells was efficient.97 Additionally, measles virus (MV) glycoproteins have been used to redirect LV transduction to B cells successfully by insertion of anti-CD20scFv in the H glycoprotein of MV for attachment thereby leaving the Fusion protein (F) untouched.96 Successful attempts have been made to redirect retroviral vectors to T cells by displaying the HIV glycoprotein at their surface, which directs them to the CD4+ T-cell population.107,108,109 Another strategy for LV T-cell targeting uses specific vector-mediated T-cell activation. This targeting strategy consists of an interaction of a T-cell ligand displayed on the surface of the vector, with its specific receptor thereby inducing signaling and stimulation of resting T cells. As a consequence of the specific stimulation, gene transfer into the target T cells significantly enhanced as the cell is induced into the G1b phase of the cell cycle as mentioned above (see section “Toward an efficient VSVG-LV transduction of T cells”). An anti-CD3scFv antibody fragment, which recognizes and activates the TCR, was fused to the murine leukemia virus envelope glycoprotein, which allows efficient LV pseudotyping. Stimulation by this vector was sufficient to allow 100-fold more gene transfer in lymphocytes than unmodified LVs in nonactivated T cells.74 To transduce resting T cells while conserving their phenotype, human IL-7 gene was fused to the amino terminus of the murine leukemia virus envelope glycoprotein. IL-7-displaying LVs allowed efficient transduction, up to 40%, of naive CD4+ T cells as well as memory CD4+ T cells and preserved the functional characteristics of the naive T cells.79
For transcriptional targeting, the design of LVs carrying endogenous promoters takes advantage of the target cell regulatory machinery thus assuring a correct expression of the transgene in the target T and B cell. However, it should be taken into account that with the provirus inserted in a nontarget cell some “off-target” trangene expression could take place due to the transcription of upstream genes, even if the vector contains a tissue-specific promoter. Several authors have made use of the CD19 promoter or the Ig κ-promoter in an LV context to target the transgene expression to human B cells,110,111,112 whereas for T-cell targeted expression the CD4 promoter was used.113
In summary, these targeting strategies could pave the way to target LV transduction/expression to T cells and B cells in vivo.
A Novel Lentiviral Pseudotype Allows Quiescent T- and B-Lymphocyte Transduction
MV glycoprotein displaying LVs transduce quiescent lymphocytes without need for entry into G1b phase of the cell cycle
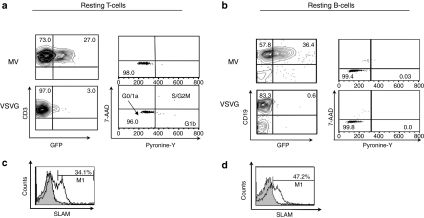
A major limitation of current LVs such as the generally used VSVG-LVs is their inability to govern efficient gene transfer into quiescent cells such as primary T and B cells (Figure 1a,b), which hampers their application for gene therapy and immunotherapy. Very recently, a novel LV pseudotype was generated incorporating MV glycoproteins H and F on their surface (MV-LVs). These allowed efficient transduction through the MV receptors SLAM and CD46, both present on blood T and B lymphocytes.114,115,116 Importantly, a single exposure to these MV-LVs allowed efficient stable gene transfer of quiescent T cells, which are not permissive for classical VSVG-LVs. Indeed, high-level transduction was achieved for resting memory (50%) and naive T cells (11%) with MV-LVs117 (Figure 1a). Especially, the naive T-cell population is of high interest as these are the long-lived cells in vivo that may induce a long-term gene correction. Surprisingly, B cells that are very poorly transduced with VSVG-LVs even when induced to proliferate (Table 4) were transduced up to 50% in their resting state whereas VSVG-LVs remained refractory118 (Figure 1b). Unexpectedly, although classical lentiviral transduction demands at least cell-cycle entry into the G1b phase of the cell cycle (Table 3), MV-LVs did not even induce cell-cycle entry upon transduction in either quiescent T or B cells117,118 (Figure 1a,b). Of importance for gene therapy, MV-LV transduction conserved the naive and memory phenotypes of transduced resting T and B cells. Moreover, in accordance with the lack of cell-cycle entry, MV-LVs did not cause any upregulation of activation markers on T and B cells.
Figure 1.
Efficient transduction of quiescent T and B lymphocytes by measles virus glycoprotein pseudotyped LVs. Adult quiescent (a) T and (b) B cells were isolated from peripheral blood and immediately transduced with MV-LVs or VSVG-LVs at multiplicity of infection = 10 and 30, respectively. GFP expression was analyzed at day 3 after transduction by fluorescent-activated cell sorting (FACS). The percentage of GFP-positive cells is indicated in T cells in a (left plots) and for B cells in b (left plots). In parallel, cell-cycle progression was monitored 3 days post-transduction by simultaneously visualizing the RNA (Pyronine-Y) and DNA (7-AAD) content of the T cells (a, right dot plots) and B cells (b, right dot plots). The percentages of cells in the G0/G1a, G1b, S/G2/M phase of the cell cycle are indicated. Surface SLAM expression was assessed by FACS analysis before transduction by staining with anti-CD150-phycoerythrin. Percentage of SLAM expression is indicated in the histogram for (c) T and (d) B cells. 7-AAD, 7-amino-actinomycin D; GFP, green fluorescent protein; LV, lentiviral vector; MV, measles virus; VSVG, vesicular stomatitis virus G protein.
Of utmost importance, the MV-LVs are the first tools to allow stable transduction of B-cell chronic lymphocytic leukemia cells, one of the most prominent leukemic B-cell cancers arrested in the G0/G1a cell-cycle phase.118 Very recently, it was demonstrated that MV-LVs were in addition able to efficiently transduce unstimulated marginal zone lymphoma B cells, another less recurrent B-cell malignancy.119
Overall this suggests that the MV-LVs may represent a new important tool for quiescent primary lymphocyte transduction.
Unraveling the secret of MV-LVs: how do they allow the transduction of quiescent T and B lymphocytes?
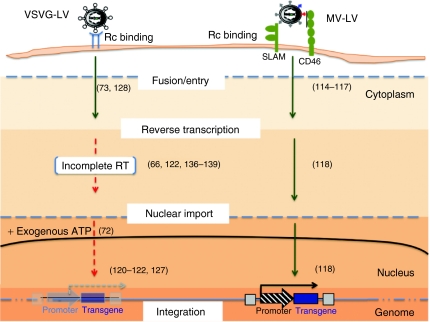
As mentioned above, it is generally accepted that truly quiescent cells in G0/G1a phase are known to be resistant to lentiviral infection, whereas stimulated T cells residing in the G1b phase are susceptible (Table 3). More specifically, it has been shown that RT of HIV vectors was very inefficient in quiescent cells, generating full-length viral transcripts that are very instable120,121 (Figure 2). There is a strong delay in RT (30-fold), and thus subsequently in integration and protein expression in resting cells as compared to TCR-prestimulated cells.122 Low levels of nucleotides in the resting cells do not entirely explain the restricted HIV-1 replication as artificially raising intracellular nucleotide pools increased RT products but not the level of productive infection.123,124 Adenosine triphosphate-treatment of quiescent T cells to increase nuclear import of reverse-transcribed DNA still did not allow efficient transduction72 (Figure 2). VSVG-LVs can overcome all these obstacles in prestimulated T cells, which are induced to enter into G1b phase of the cell cycle and contain a higher total RNA level as compared to quiescent cells (Table 3).
Figure 2.
Schematic presentation of the important restrictive steps in lentiviral transduction of quiescent lymphocytes. VSVG-LVs are able to enter into quiescent T or B cells through a yet unknown ubiquitously expressed receptor. However, they show very low reverse transcription activity and nuclear import resulting in low proviral integration efficiency. This was only partially overcome by addition of nucleosides that increase reverse transcriptase (RT) activity and ATP increase to induce nuclear import. On the other hand, MV-LVs use the CD46 and SLAM receptors for entry into quiescent T and B cells. Upon entry, very rapidly a much more efficient RT activity was detected as compared to VSVG-LVs in these cells without affecting their quiescent state. This was followed by nuclear import and finally efficient proviral integration. LV, lentiviral vector; MV, measles virus; VSVG, vesicular stomatitis virus G protein.
Interestingly, the MV-LVs are superior to the classical late generation VSVG-LVs as they overcome these restrictive steps in quiescent cells resulting in their efficient transduction (Figure 2, Table 3). Several hypotheses can be proposed to explain why MV-LVs transduce completely quiescent cells. Binding of MV-gps to SLAM/CD46 receptors on quiescent cells could activate the uncoating process together with specific requirements for other steps of viral replication. Indeed, upon MV-LV entry RT is rapidly accomplished in a completely quiescent cell, whereas for VSVG-LVs, although they can enter the cell by a yet unknown receptor,125,126 this initial step in the viral life cycle is very inefficient127 (Figure 2). Recently, it was proposed that VSVG-LVs even failed to fuse their membrane with resting T cells. Replacement of VSVG glycoprotein with the HIV envelope glycoprotein on the lentiviral surface permitted efficient fusion with these quiescent cells.128 However, long-term culture of these cells was not performed to confirm stable proviral integration. Interestingly, MV-LV contact with the target B or T cells does not trigger or facilitate VSVG-LV productive transduction, strongly suggesting that the two different vector pseudotypes exploit different entry mechanisms in these cells. This cell entry pathway specific to MV-LVs may also alter trafficking of the particles through cellular compartments, protecting them from proteasome degradation129 or inducing the uncoating process. In addition, MV-LVs might avoid interaction with postentry restriction factors by using alternative cell entry mechanisms, for example through SLAM/CD46.
Importantly, MV-LV transduction of quiescent primary T and B cells suggests that both hCD46 and hSLAM receptors need to be engaged for binding and/or entry to allow efficient transduction (C. Frecha, C. Costa, F.-L. Cosset, and E. Verhoeyen, unpublished results).
Because all lymphocytes express hCD46 at high levels, the limiting factor for LV transduction was the expression of SLAM receptor on T and B lymphocytes.117,118 Indeed, multiple findings show that MV-LV targets gene transfer to a subpopulation of SLAM-expressing quiescent T and B lymphocytes without inducing cell-cycle entry. It will be of importance to elucidate the processes facilitating quiescent cell transduction by MV-LVs.
Conclusion
Most of T and B lymphocytes in the human blood stream are quiescent cells; therefore, efficient quiescent lymphocyte transduction is in itself a goal for many applications. Basic biological studies on cell differentiation and oncogenic onset of lymphocytes as well as the development of gene therapy strategies would benefit from stable quiescent T- and B-cell transduction. This would avoid long-term ex vivo culture and phenotypic loss, which is essential for the long-term persistence of naive cells in the human body. Until recently though, despite the extensive knowledge about the restrictive steps hampering lentiviral transduction of quiescent cells, it was not possible to design VSVG-LVs able to transduce resting lymphocytes in a stable manner.73,127
As reported here, finally, a new LV allows high and stable gene transfer of primary quiescent B cells, T cells, and cancer B cells, which now makes it possible to study with ease gene functions in these cells. These lentiviral tools may open the way to improved genetic vaccination strategies against cancer, infectious or autoimmune diseases as well as gene therapy of genetic diseases.34,43,60 A more challenging step for therapeutic gene transfer will be to optimize these vectors for direct in vivo gene delivery in T and B cells.130,131
Acknowledgments
This work was supported by grants from the “Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales”, the “Agence nationale de la Recherche”, the European Research Council (ERC-2008-AdG-233130-HEPCENT), and the European Community (FP7-HEALTH-2007-B/222878 “PERSIST”).
REFERENCES
- Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- Buchschacher GL., Jr, and, Wong-Staal F. Approaches to gene therapy for human immunodeficiency virus infection. Hum Gene Ther. 2001;12:1013–1019. doi: 10.1089/104303401750214249. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–793. [PubMed] [Google Scholar]
- Recchia A, Bonini C, Magnani Z, Urbinati F, Sartori D, Muraro S, et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc Natl Acad Sci USA. 2006;103:1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R, Yang DR, Puck JM, Huie ML, Jiang CK., and, Kurlandsky LE. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nat Genet. 1996;13:290–295. doi: 10.1038/ng0796-290. [DOI] [PubMed] [Google Scholar]
- Stephan V, Wahn V, Le Deist F, Dirksen U, Broker B, Müller-Fleckenstein I, et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N Engl J Med. 1996;335:1563–1567. doi: 10.1056/NEJM199611213352104. [DOI] [PubMed] [Google Scholar]
- Tjønnfjord GE, Steen R, Veiby OP, Friedrich W., and, Egeland T. Evidence for engraftment of donor-type multipotent CD34+ cells in a patient with selective T-lymphocyte reconstitution after bone marrow transplantation for B-SCID. Blood. 1994;84:3584–3589. [PubMed] [Google Scholar]
- Aiuti A, Vai S, Mortellaro A, Casorati G, Ficara F, Andolfi G, et al. Immune reconstitution in ADA-SCID after PBL gene therapy and discontinuation of enzyme replacement. Nat Med. 2002;8:423–425. doi: 10.1038/nm0502-423. [DOI] [PubMed] [Google Scholar]
- Dupré L, Trifari S, Follenzi A, Marangoni F, Lain de Lera T, Bernad A, et al. Lentiviral vector-mediated gene transfer in T cells from Wiskott-Aldrich syndrome patients leads to functional correction. Mol Ther. 2004;10:903–915. doi: 10.1016/j.ymthe.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Martín F, Toscano MG, Blundell M, Frecha C, Srivastava GK, Santamaría M, et al. Lentiviral vectors transcriptionally targeted to hematopoietic cells by WASP gene proximal promoter sequences. Gene Ther. 2005;12:715–723. doi: 10.1038/sj.gt.3302457. [DOI] [PubMed] [Google Scholar]
- Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- Vigorito AC, Azevedo WM, Marques JF, Azevedo AM, Eid KA, Aranha FJ, et al. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone Marrow Transplant. 1998;22:1145–1151. doi: 10.1038/sj.bmt.1701510. [DOI] [PubMed] [Google Scholar]
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- Qasim W, Mackey T, Sinclair J, Chatziandreou I, Kinnon C, Thrasher AJ, et al. Lentiviral vectors for T-cell suicide gene therapy: preservation of T-cell effector function after cytokine-mediated transduction. Mol Ther. 2007;15:355–360. doi: 10.1038/sj.mt.6300042. [DOI] [PubMed] [Google Scholar]
- Bondanza A, Valtolina V, Magnani Z, Ponzoni M, Fleischhauer K, Bonyhadi M, et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- Tiberghien P, Ferrand C, Lioure B, Milpied N, Angonin R, Deconinck E, et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood. 2001;97:63–72. doi: 10.1182/blood.v97.1.63. [DOI] [PubMed] [Google Scholar]
- Bobisse S, Rondina M, Merlo A, Tisato V, Mandruzzato S, Amendola M, et al. Reprogramming T lymphocytes for melanoma adoptive immunotherapy by T-cell receptor gene transfer with lentiviral vectors. Cancer Res. 2009;69:9385–9394. doi: 10.1158/0008-5472.CAN-09-0494. [DOI] [PubMed] [Google Scholar]
- Burns WR, Zheng Z, Rosenberg SA., and, Morgan RA. Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood. 2009;114:2888–2899. doi: 10.1182/blood-2009-01-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Braun SE, Wong FE, Connole M, Qiu G, Lee L, Gillis J, et al. Inhibition of simian/human immunodeficiency virus replication in CD4+ T cells derived from lentiviral-transduced CD34+ hematopoietic cells. Mol Ther. 2005;12:1157–1167. doi: 10.1016/j.ymthe.2005.07.698. [DOI] [PubMed] [Google Scholar]
- Humeau LM, Binder GK, Lu X, Slepushkin V, Merling R, Echeagaray P, et al. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol Ther. 2004;9:902–913. doi: 10.1016/j.ymthe.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GP, Levine BL, Binder GK, Berry CC, Malani N, McGarrity G, et al. Analysis of lentiviral vector integration in HIV+ study subjects receiving autologous infusions of gene modified CD4+ T cells. Mol Ther. 2009;17:844–850. doi: 10.1038/mt.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Brake O, ‘t Hooft K, Liu YP, Centlivre M, von Eije KJ., and, Berkhout B. Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol Ther. 2008;16:557–564. doi: 10.1038/sj.mt.6300382. [DOI] [PubMed] [Google Scholar]
- Egelhofer M, Brandenburg G, Martinius H, Schult-Dietrich P, Melikyan G, Kunert R, et al. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J Virol. 2004;78:568–575. doi: 10.1128/JVI.78.2.568-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildinger M, Dittmar MT, Schult-Dietrich P, Fehse B, Schnierle BS, Thaler S, et al. Membrane-anchored peptide inhibits human immunodeficiency virus entry. J Virol. 2001;75:3038–3042. doi: 10.1128/JVI.75.6.3038-3042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Rivera A, Ron N, Dougherty JP., and, Ron Y. A gene therapy approach for treating T-cell-mediated autoimmune diseases. Blood. 2001;97:886–894. doi: 10.1182/blood.v97.4.886. [DOI] [PubMed] [Google Scholar]
- Lei TC., and, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105:4865–4870. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo ME, Qian J, El-Amine M, Agarwal RK, Soukhareva N, Kang Y, et al. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J Immunol. 2002;168:4788–4795. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]
- Scott DW, Venkataraman M., and, Jandinski JJ. Multiple pathways of B lymphocyte tolerance. Immunol Rev. 1979;43:241–280. doi: 10.1111/j.1600-065x.1979.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Stripecke R, Cardoso AA, Pepper KA, Skelton DC, Yu XJ, Mascarenhas L, et al. Lentiviral vectors for efficient delivery of CD80 and granulocyte-macrophage-colony-stimulating factor in human acute lymphoblastic leukemia and acute myeloid leukemia cells to induce antileukemic immune responses. Blood. 2000;96:1317–1326. [PubMed] [Google Scholar]
- Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Fuleihan R, Ramesh N, Loh R, Jabara H, Rosen RS, Chatila T, et al. Defective expression of the CD40 ligand in X chromosome-linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci USA. 1993;90:2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell M, Hua T, Pappas J., and, Kipps TJ. Acquired CD40-ligand deficiency in chronic lymphocytic leukemia. Nat Med. 1997;3:984–989. doi: 10.1038/nm0997-984. [DOI] [PubMed] [Google Scholar]
- Cantwell MJ, Sharma S, Friedmann T., and, Kipps TJ. Adenovirus vector infection of chronic lymphocytic leukemia B cells. Blood. 1996;88:4676–4683. [PubMed] [Google Scholar]
- Li LH, Biagi E, Allen C, Shivakumar R, Weiss JM, Feller S, et al. Rapid and efficient nonviral gene delivery of CD154 to primary chronic lymphocytic leukemia cells. Cancer Gene Ther. 2006;13:215–224. doi: 10.1038/sj.cgt.7700883. [DOI] [PubMed] [Google Scholar]
- Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE., and, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- Moskowitz CH. Pretreatment prognostic factors and outcome in patients with relapsed or primary-refractory diffuse large B-cell lymphoma treated with second-line chemotherapy and autologous stem cell transplantation. Ann Oncol. 2006;17 Suppl 4:iv37–iv39. doi: 10.1093/annonc/mdj998. [DOI] [PubMed] [Google Scholar]
- Lei TC, Su Y., and, Scott DW. Tolerance induction via a B-cell delivered gene therapy-based protocol: optimization and role of the Ig scaffold. Cell Immunol. 2005;235:12–20. doi: 10.1016/j.cellimm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- El-Amine M, Melo M, Kang Y, Nguyen H, Qian J., and, Scott DW. Mechanisms of tolerance induction by a gene-transferred peptide-IgG fusion protein expressed in B lineage cells. J Immunol. 2000;165:5631–5636. doi: 10.4049/jimmunol.165.10.5631. [DOI] [PubMed] [Google Scholar]
- Gourley TS, Patel DR, Nickerson K, Hong SC., and, Chang CH. Aberrant expression of Fas ligand in mice deficient for the MHC class II transactivator. J Immunol. 2002;168:4414–4419. doi: 10.4049/jimmunol.168.9.4414. [DOI] [PubMed] [Google Scholar]
- Xu B., and, Scott DW. A novel retroviral gene therapy approach to inhibit specific antibody production and suppress experimental autoimmune encephalomyelitis induced by MOG and MBP. Clin Immunol. 2004;111:47–52. doi: 10.1016/j.clim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Zambidis ET, Barth RK., and, Scott DW. Both resting and activated B lymphocytes expressing engineered peptide-Ig molecules serve as highly efficient tolerogenic vehicles in immunocompetent adult recipients. J Immunol. 1997;158:2174–2182. [PubMed] [Google Scholar]
- Zambidis ET, Kurup A., and, Scott DW. Genetically transferred central and peripheral immune tolerance via retroviral-mediated expression of immunogenic epitopes in hematopoietic progenitors or peripheral B lymphocytes. Mol Med. 1997;3:212–224. [PMC free article] [PubMed] [Google Scholar]
- Zambidis ET., and, Scott DW. Epitope-specific tolerance induction with an engineered immunoglobulin. Proc Natl Acad Sci USA. 1996;93:5019–5024. doi: 10.1073/pnas.93.10.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal RK, Kang Y, Zambidis E, Scott DW, Chan CC., and, Caspi RR. Retroviral gene therapy with an immunoglobulin-antigen fusion construct protects from experimental autoimmune uveitis. J Clin Invest. 2000;106:245–252. doi: 10.1172/JCI9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Wang J, Wang R, Yu M, Sun Y, Han G, et al. Retroviral delivery of GAD-IgG fusion construct induces tolerance and modulates diabetes: a role for CD4+ regulatory T cells and TGF-beta. Gene Ther. 2004;11:1487–1496. doi: 10.1038/sj.gt.3302327. [DOI] [PubMed] [Google Scholar]
- Luo XM, Maarschalk E, O'Connell RM, Wang P, Yang L., and, Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113:1422–1431. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- Cole SP, Campling BG, Atlaw T, Kozbor D., and, Roder JC. Human monoclonal antibodies. Mol Cell Biochem. 1984;62:109–120. doi: 10.1007/BF00223301. [DOI] [PubMed] [Google Scholar]
- Liao HX, Levesque MC, Nagel A, Dixon A, Zhang R, Walter E, et al. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods. 2009;158:171–179. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garet E, Cabado AG, Vieites JM., and, González-Fernández A. Rapid isolation of single-chain antibodies by phage display technology directed against one of the most potent marine toxins: Palytoxin. Toxicon. 2010;55:1519–1526. doi: 10.1016/j.toxicon.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CM, Lukens MV, et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan A, Barnhart B, Shone S, Song C., and, Covey LR. A transcriptional defect underlies B lymphocyte dysfunction in a patient diagnosed with non-X-linked hyper-IgM syndrome. J Immunol. 2000;164:2871–2880. doi: 10.4049/jimmunol.164.6.2871. [DOI] [PubMed] [Google Scholar]
- White H, Thrasher A, Veys P, Kinnon C., and, Gaspar HB. Intrinsic defects of B cell function in X-linked severe combined immunodeficiency. Eur J Immunol. 2000;30:732–737. doi: 10.1002/1521-4141(200003)30:3<732::AID-IMMU732>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vigna E., and, Naldini L. Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med. 2000;2:308–316. doi: 10.1002/1521-2254(200009/10)2:5<308::AID-JGM131>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Reitsma MJ, Uchida N., and, Brown PO. Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent. J Virol. 1999;73:3649–3660. doi: 10.1128/jvi.73.5.3649-3660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kootstra NA, Zwart BM., and, Schuitemaker H. Diminished human immunodeficiency virus type 1 reverse transcription and nuclear transport in primary macrophages arrested in early G(1) phase of the cell cycle. J Virol. 2000;74:1712–1717. doi: 10.1128/jvi.74.4.1712-1717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil S, Martin F, Ikeda Y., and, Collins M. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J Virol. 2001;75:5448–5456. doi: 10.1128/JVI.75.12.5448-5456.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Jaleco S, Kinet S, Herpers B, Steinberg M, Ferrand C, et al. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc Natl Acad Sci USA. 2001;98:9277–9282. doi: 10.1073/pnas.161272698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M, Stanwick TL, Dempsey MP., and, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Pinchuk LM, Agy MB., and, Clark EA. Nuclear import of HIV-1 DNA in resting CD4+ T cells requires a cyclosporin A-sensitive pathway. J Immunol. 1997;158:512–517. [PubMed] [Google Scholar]
- Zack JA. The role of the cell cycle in HIV-1 infection. Adv Exp Med Biol. 1995;374:27–31. doi: 10.1007/978-1-4615-1995-9_3. [DOI] [PubMed] [Google Scholar]
- Zack JA, Haislip AM, Krogstad P., and, Chen IS. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K, Luban J., and, Jeang KT. Human cellular restriction factors that target HIV-1 replication. BMC Med. 2009;7:48. doi: 10.1186/1741-7015-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, et al. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korin YD., and, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice M, Verhoeyen E, Salmon P, Trono D, Russell SJ., and, Cosset FL. Efficient gene transfer into human primary blood lymphocytes by surface-engineered lentiviral vectors that display a T cell-activating polypeptide. Blood. 2002;99:2342–2350. doi: 10.1182/blood.v99.7.2342. [DOI] [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, Marmon S., and, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand C, Robinet E, Contassot E, Certoux JM, Lim A, Hervé P, et al. Retrovirus-mediated gene transfer in primary T lymphocytes: influence of the transduction/selection process and of ex vivo expansion on the T cell receptor beta chain hypervariable region repertoire. Hum Gene Ther. 2000;11:1151–1164. doi: 10.1089/10430340050015202. [DOI] [PubMed] [Google Scholar]
- Marktel S, Magnani Z, Ciceri F, Cazzaniga S, Riddell SR, Traversari C, et al. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood. 2003;101:1290–1298. doi: 10.1182/blood-2002-08-2351. [DOI] [PubMed] [Google Scholar]
- Roth MD. Interleukin 2 induces the expression of CD45RO and the memory phenotype by CD45RA+ peripheral blood lymphocytes. J Exp Med. 1994;179:857–864. doi: 10.1084/jem.179.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeyen E, Dardalhon V, Ducrey-Rundquist O, Trono D, Taylor N., and, Cosset FL. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood. 2003;101:2167–2174. doi: 10.1182/blood-2002-07-2224. [DOI] [PubMed] [Google Scholar]
- Fry TJ., and, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis. Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- Geiselhart LA, Humphries CA, Gregorio TA, Mou S, Subleski J., and, Komschlies KL. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001;166:3019–3027. doi: 10.4049/jimmunol.166.5.3019. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Farkash EA, Gao W., and, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Cavalieri S, Cazzaniga S, Geuna M, Magnani Z, Bordignon C, Naldini L, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- Ducrey-Rundquist O, Guyader M., and, Trono D. Modalities of interleukin-7-induced human immunodeficiency virus permissiveness in quiescent T lymphocytes. J Virol. 2002;76:9103–9111. doi: 10.1128/JVI.76.18.9103-9111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson L, Verhoeyen E, Cosset FL., and, Taylor N. IL-7R alpha gene expression is inversely correlated with cell cycle progression in IL-7-stimulated T lymphocytes. J Immunol. 2006;176:6702–6708. doi: 10.4049/jimmunol.176.11.6702. [DOI] [PubMed] [Google Scholar]
- Verhoeyen E, Costa C., and, Cosset FL.2009Lentiviral vector gene transfer into human T cellsIn: Baum, C (ed). Genetic Modification of Hematopoietic Stem Cells Methods and Protocols, vol. 506. Humana Press: New York; 97–114. [DOI] [PubMed] [Google Scholar]
- Bovia F, Salmon P, Matthes T, Kvell K, Nguyen TH, Werner-Favre C, et al. Efficient transduction of primary human B lymphocytes and nondividing myeloma B cells with HIV-1-derived lentiviral vectors. Blood. 2003;101:1727–1733. doi: 10.1182/blood-2001-12-0249. [DOI] [PubMed] [Google Scholar]
- Janssens W, Chuah MK, Naldini L, Follenzi A, Collen D, Saint-Remy JM, et al. Efficiency of onco-retroviral and lentiviral gene transfer into primary mouse and human B-lymphocytes is pseudotype dependent. Hum Gene Ther. 2003;14:263–276. doi: 10.1089/10430340360535814. [DOI] [PubMed] [Google Scholar]
- Serafini M, Naldini L., and, Introna M. Molecular evidence of inefficient transduction of proliferating human B lymphocytes by VSV-pseudotyped HIV-1-derived lentivectors. Virology. 2004;325:413–424. doi: 10.1016/j.virol.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Chinnasamy D, Chinnasamy N, Enriquez MJ, Otsu M, Morgan RA., and, Candotti F. Lentiviral-mediated gene transfer into human lymphocytes: role of HIV-1 accessory proteins. Blood. 2000;96:1309–1316. [PubMed] [Google Scholar]
- Grimaître M, Werner-Favre C, Kindler V., and, Zubler RH. Human naive B cells cultured with EL-4 T cells mimic a germinal center-related B cell stage before generating plasma cells. Concordant changes in Bcl-2 protein and messenger RNA levels. Eur J Immunol. 1997;27:199–205. doi: 10.1002/eji.1830270130. [DOI] [PubMed] [Google Scholar]
- Matthes T, Werner-Favre C, Tang H, Zhang X, Kindler V., and, Zubler RH. Cytokine mRNA expression during an in vitro response of human B lymphocytes: kinetics of B cell tumor necrosis factor alpha, interleukin (IL)6, IL-10, and transforming growth factor beta 1 mRNAs. J Exp Med. 1993;178:521–528. doi: 10.1084/jem.178.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci A, James H, Chicheportiche R, Bonnefoy JY, Dayer JM., and, Zubler RH. Effects of eleven cytokines and of IL-1 and tumor necrosis factor inhibitors in a human B cell assay. J Immunol. 1992;148:2778–2784. [PubMed] [Google Scholar]
- Kvell K, Nguyen TH, Salmon P, Glauser F, Werner-Favre C, Barnet M, et al. Transduction of CpG DNA-stimulated primary human B cells with bicistronic lentivectors. Mol Ther. 2005;12:892–899. doi: 10.1016/j.ymthe.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Lizée G, Gonzales MI., and, Topalian SL. Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum Gene Ther. 2004;15:393–404. doi: 10.1089/104303404322959542. [DOI] [PubMed] [Google Scholar]
- Funke S, Maisner A, Mühlebach MD, Koehl U, Grez M, Cattaneo R, et al. Targeted cell entry of lentiviral vectors. Mol Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bailey L, Baltimore D., and, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci USA. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler L, Yang L, Joo K, Yang H, Baltimore D., and, Wang P. Targeting lentiviral vectors to antigen-specific immunoglobulins. Hum Gene Ther. 2008;19:861–872. doi: 10.1089/hum.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub C., and, Zubler RH. Immortalization of EBV-infected B cells is not influenced by exogenous signals acting on B cell proliferation. Effects of mutant EL-4 thymoma cells and transforming growth factor-beta. J Immunol. 1989;142:87–93. [PubMed] [Google Scholar]
- Fecteau JF, Roy A., and, Néron S. Peripheral blood CD27+ IgG+ B cells rapidly proliferate and differentiate into immunoglobulin-secreting cells after exposure to low CD154 interaction. Immunology. 2009;128 1 Suppl:e353–e365. doi: 10.1111/j.1365-2567.2008.02976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Walshe CA, Ivanov AO., and, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- Glennie MJ, French RR, Cragg MS., and, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- Buchholz CJ, Mühlebach MD., and, Cichutek K. Lentiviral vectors with measles virus glycoproteins ‐ dream team for gene transfer. Trends Biotechnol. 2009;27:259–265. doi: 10.1016/j.tibtech.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Delahaye E, Martin F, Russell SJ., and, Cosset FL. Retroviral display of functional binding domains fused to the amino terminus of influenza hemagglutinin. Hum Gene Ther. 1999;10:1533–1544. doi: 10.1089/10430349950017860. [DOI] [PubMed] [Google Scholar]
- Morizono K, Bristol G, Xie YM, Kung SK., and, Chen IS. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrin V, Boson B, Salmon P, Gay W, Nègre D, Le Grand R, et al. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100:823–832. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- Liang M, Morizono K, Pariente N, Kamata M, Lee B., and, Chen IS. Targeted transduction via CD4 by a lentiviral vector uses a clathrin-mediated entry pathway. J Virol. 2009;83:13026–13031. doi: 10.1128/JVI.01530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnierle BS, Stitz J, Bosch V, Nocken F, Merget-Millitzer H, Engelstädter M, et al. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc Natl Acad Sci USA. 1997;94:8640–8645. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle S, Steidl S, Panitz S, Coulibaly C, Kalinke U, Cichutek K, et al. Selective gene transfer to T lymphocytes using coreceptor-specific [MLV(HIV)] pseudotype vectors in a transgenic mouse model. Virology. 2006;351:237–247. doi: 10.1016/j.virol.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Moreau T, Bardin F, Imbert J, Chabannon C., and, Tonnelle C. Restriction of transgene expression to the B-lymphoid progeny of human lentivirally transduced CD34+ cells. Mol Ther. 2004;10:45–56. doi: 10.1016/j.ymthe.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Taher TE, Tulone C, Fatah R, D'Acquisto F, Gould DJ., and, Mageed RA. Repopulation of B-lymphocytes with restricted gene expression using haematopoietic stem cells engineered with lentiviral vectors. Gene Ther. 2008;15:998–1006. doi: 10.1038/gt.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie KL, Blundell MP, Baxendale HE, Howe SJ, Sinclair J, Qasim W, et al. Cell-specific and efficient expression in mouse and human B cells by a novel hybrid immunoglobulin promoter in a lentiviral vector. Gene Ther. 2007;14:1623–1631. doi: 10.1038/sj.gt.3303021. [DOI] [PubMed] [Google Scholar]
- Marodon G, Mouly E, Blair EJ, Frisen C, Lemoine FM., and, Klatzmann D. Specific transgene expression in human and mouse CD4+ cells using lentiviral vectors with regulatory sequences from the CD4 gene. Blood. 2003;101:3416–3423. doi: 10.1182/blood-2002-02-0578. [DOI] [PubMed] [Google Scholar]
- Dörig RE, Marcil A, Chopra A., and, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Santiago C, Celma ML, Stehle T., and, Casasnovas JM. Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat Struct Mol Biol. 2010;17:124–129. doi: 10.1038/nsmb.1726. [DOI] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Tanaka K., and, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Frecha C, Costa C, Nègre D, Gauthier E, Russell SJ, Cosset FL, et al. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood. 2008;112:4843–4852. doi: 10.1182/blood-2008-05-155945. [DOI] [PubMed] [Google Scholar]
- Frecha C, Costa C, Lévy C, Nègre D, Russell SJ, Maisner A, et al. Efficient and stable transduction of resting B lymphocytes and primary chronic lymphocyte leukemia cells using measles virus gp displaying lentiviral vectors. Blood. 2009;114:3173–3180. doi: 10.1182/blood-2009-05-220798. [DOI] [PubMed] [Google Scholar]
- Lévy C, Frecha C, Costa C, Rachinel N, Salles G, Cosset FL, et al. Lentiviral vectors and transduction of human cancer B cells. Blood. 2010;116:498–500; author reply 500. doi: 10.1182/blood-2010-03-276014. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C., and, Siliciano RF. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J Virol. 2002;76:8518–8531. doi: 10.1128/JVI.76.17.8518-8531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang H, Siliciano JD., and, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J Virol. 2005;79:2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatakis DN, Bristol G, Wilkinson TA, Chow SA., and, Zack JA. Immediate activation fails to rescue efficient human immunodeficiency virus replication in quiescent CD4+ T cells. J Virol. 2007;81:3574–3582. doi: 10.1128/JVI.02569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korin YD., and, Zack JA. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J Virol. 1999;73:6526–6532. doi: 10.1128/jvi.73.8.6526-6532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesa G, Dai J, Baytop C, Riley JL, June CH., and, O'Doherty U. Addition of deoxynucleosides enhances human immunodeficiency virus type 1 integration and 2LTR formation in resting CD4+ T cells. J Virol. 2007;81:13938–13942. doi: 10.1128/JVI.01745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coil DA., and, Miller AD. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J Virol. 2004;78:10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yau VK, Briggs BJ., and, Whittaker GR. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology. 2005;338:53–60. doi: 10.1016/j.virol.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Vatakis DN, Kim S, Kim N, Chow SA., and, Zack JA. Human immunodeficiency virus integration efficiency and site selection in quiescent CD4+ T cells. J Virol. 2009;83:6222–6233. doi: 10.1128/JVI.00356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto LM, Yu JJ, Liszewski MK, Baytop C, Korokhov N, Humeau LM, et al. The CXCR4-tropic human immunodeficiency virus envelope promotes more-efficient gene delivery to resting CD4+ T cells than the vesicular stomatitis virus glycoprotein G envelope. J Virol. 2009;83:8153–8162. doi: 10.1128/JVI.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni de Sio FR, Cascio, P, Zingale A, Gasparini M., and, Naldini L. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood. 2006;107:4257–4265. doi: 10.1182/blood-2005-10-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche FB, Ertl OT., and, Muller CP. Neutralizing B cell response in measles. Viral Immunol. 2002;15:451–471. doi: 10.1089/088282402760312331. [DOI] [PubMed] [Google Scholar]
- Naniche D. Human immunology of measles virus infection. Curr Top Microbiol Immunol. 2009;330:151–171. doi: 10.1007/978-3-540-70617-5_8. [DOI] [PubMed] [Google Scholar]
- Circosta P, Granziero L, Follenzi A, Vigna E, Stella S, Vallario A, et al. T cell receptor (TCR) gene transfer with lentiviral vectors allows efficient redirection of tumor specificity in naive and memory T cells without prior stimulation of endogenous TCR. Hum Gene Ther. 2009;20:1576–1588. doi: 10.1089/hum.2009.117. [DOI] [PubMed] [Google Scholar]
- Neagu MR, Ziegler P, Pertel T, Strambio-De-Castillia C, Grütter C, Martinetti G, et al. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J Clin Invest. 2009;119:3035–3047. doi: 10.1172/JCI39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma R, Noser JA, Ohmine S., and, Ikeda Y. Inhibition of HIV-1 replication by simian restriction factors, TRIM5alpha and APOBEC3G. Gene Ther. 2007;14:185–189. doi: 10.1038/sj.gt.3302852. [DOI] [PubMed] [Google Scholar]
- Werner-Favre C, Bovia F, Schneider P, Holler N, Barnet M, Kindler V, et al. IgG subclass switch capacity is low in switched and in IgM-only, but high in IgD+IgM+, post-germinal center (CD27+) human B cells. Eur J Immunol. 2001;31:243–249. doi: 10.1002/1521-4141(200101)31:1<243::AID-IMMU243>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Agosto LM, Yu JJ, Dai J, Kaletsky R, Monie D., and, O'Doherty U. HIV-1 integrates into resting CD4+ T cells even at low inoculums as demonstrated with an improved assay for HIV-1 integration. Virology. 2007;368:60–72. doi: 10.1016/j.virol.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Herpers B, Noraz N, Pflumio F, Guetard D, Leveau C, et al. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 2001;8:190–198. doi: 10.1038/sj.gt.3301378. [DOI] [PubMed] [Google Scholar]
- Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R, et al. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol. 2005;79:14179–14188. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiggard WJ, O'Doherty U, McGain D, Jeyakumar D., and, Malim MH. Long HIV type 1 reverse transcripts can accumulate stably within resting CD4+ T cells while short ones are degraded. AIDS Res Hum Retroviruses. 2004;20:285–295. doi: 10.1089/088922204322996527. [DOI] [PubMed] [Google Scholar]