The parasite Trypanosoma brucei (Figure 1) is a major problem for human health and agriculture in regions of Africa (Figure 2). The expression of a trypanosome resistance gene, ApoL1, in transgenic livestock will soon be tested with the aim of reducing the susceptibility of a major reservoir for this parasite. The success of such a project will invariably lead some to consider such an approach in human patients. Indeed, the recent success of a number of somatic gene therapy approaches to treat a variety of human diseases suggests that we may soon possess the technology necessary for such an undertaking. Although the idea of prophylactic genetic modification in humans may seem like science fiction today and is certainly fraught with seemingly insurmountable ethical and technical hurdles, the biomedical and medical ethics communities should at least be prepared for such an idea and its ramifications.
Figure 1.
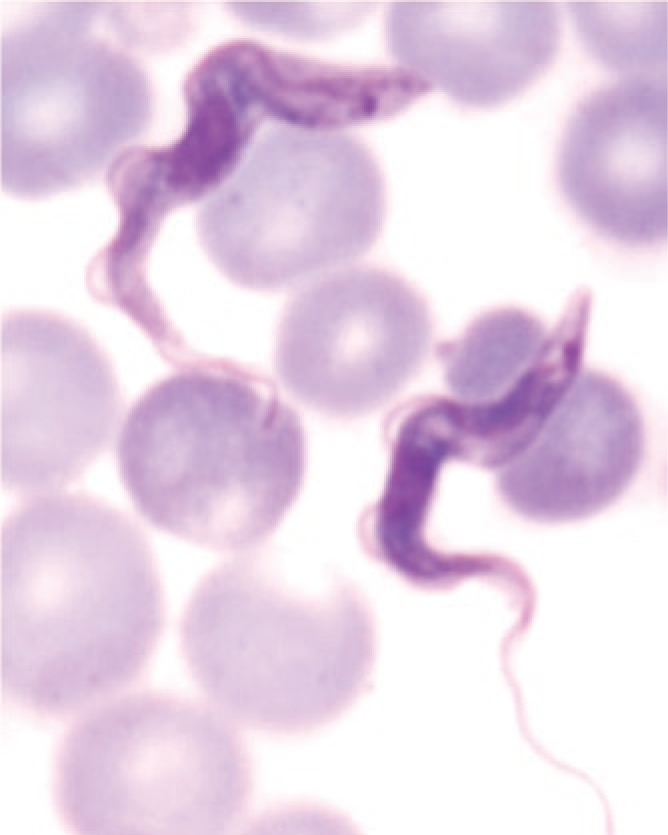
Giemsa stained bloodstream stages of Trypanosoma brucei.
Figure 2.
Bread: Chuma—the Strong Cow. Sub-Saharan Africa is infested with tsetse flies (green zone) that infect cattle and other animals such as cattle with African trypanosomes (see the long slender cell in the image of the dead cow), resulting in a wasting disease that is fatal. Some African primates are naturally resistant to these parasites. A baboon gene has been identified that, when given transiently to mice, completely protects them from infection with trypanosomes. The goal of this project is to determine whether this baboon gene can protect cattle, thereby allowing the raising of cattle in the tsetse belt and thus aiding the smallholder farmer.
The genetic systems that define the relationship between a host organism and its parasite can be strikingly simple. For example, the presence of a single gene encoding primate apolipoprotein L1 (ApoL1) gives rise to resistance of a potential host to infection by T. brucei brucei. Uptake by the trypanosome of the ApoL1 gene product as part of a high-density lipoprotein particle leads to lysis of the invading parasite.1 The presence of primate ApoL1 is both sufficient and necessary for this resistance. Accordingly, transgenic mice expressing human ApoL1 acquire resistance to the parasite,2 and a human patient who exhibited unexpected susceptibility to T. b. brucei was found to express a frameshift mutation in both ApoL1 alleles.3 The ability of a different subspecies, T. b. rhodesiense, to resist lysis by ApoL1 in humans is also dependent on the expression of a single gene, encoding the serum resistance–associated protein (SRA). Again, the presence of the SRA gene is both sufficient and necessary for growth of T. b. rhodesiense in the presence of human serum. SRA binds and neutralizes ApoL1.4 Interestingly, it was shown recently that mutations and insertions introduced into the ApoL1 gene can prevent this interaction between SRA and ApoL1, thereby conferring upon the host trypanolytic activity toward both T. b. brucei and T. b. rhodesiense.5
It has been known for some time that certain species of baboon in East Africa are resistant to infection by the T. brucei subspecies. Thomson and colleagues recently demonstrated that the presence of a baboon ApoL1 transgene is sufficient for survival of mice when challenged with either T. b. brucei or T. b. rhodesiense. Unlike human APOL1, which confers resistance only to T. b. brucei, baboon ApoL1 does not bind SRA, thereby allowing the clearance of both subspecies.6 Very recently, an African-American population was identified with mutations in both alleles of the human APOL1 gene that are correlated with kidney disease in the fourth decade of life. The mutations make human APOL1 more baboon-like, and as predicted, plasma from these patients is able to kill T. b. rhodesiense parasites.7,8 These mutations occur quite frequently in populations in West Africa and suggest that the molecular “arms race” between human APOL1 and trypanosomes continues to evolve. Importantly, heterozygotes for the mutant APOL1 are protected from trypanosomiasis but do not develop kidney disease, thereby providing a selective advantage.
In the case of human African trypanosomiasis, the development of a vaccine in the immediate or near future is highly unlikely because of the sophisticated antigenic variation of the surface proteins of T. brucei.9 Moreover, as C.C. Wang, the editor of Eukaryotic Cell, lamented at the Kinetoplastid Molecular Cell Biology conference in Woods Hole, Massachusetts, no potent therapeutic drug has emerged for this disease during the last several decades despite the development of T. brucei as a model organism and the publication of thousands of studies. There are numerous promising molecular targets, such as N-myristoyltransferase and proteins involved in RNA editing that are unique to trypanosomes,10,11 and yet the design of effective drugs remains early in its development.
The expression of a primate ApoL1 transgene in mice provided protection against the parasite,2 and so it seems possible that the expression of a baboon ApoL1 in transgenic livestock would protect against infection with T. b. rhodesiense and T. b. brucei.6 This idea will be put to the test very soon in the Chuma Cow project (Chuma is Swahili for “strong”) (http://www.genomics.liv.ac.uk/tryps/index.html) (Figure 2). The goal of this study is to determine whether expression of a primate ApoL1 transgene in cattle will confer resistance to trypanosome infection. The transgenic cows will be screened for production of ApoL1 and will be challenged with Trypanosoma congolense, which is closely related to T. brucei. The long-term goal is to replace cows in the endemic regions (~10 million square miles) of Africa with transgenic cattle through extensive breeding programs that maintain the diversity of the existing breeds. T. b. rhodesiense is zoonotic with two main reservoirs: domestic cattle and large game animals. The reduction or elimination of the main domestic reservoir of this parasite in cattle would represent a major advance against a disease that continues to claim so many human lives and to cause huge economic hardship owing to the limitations it imposes on pastoral agriculture in the region.
As noted above, the human APOL1 protects against T. b. brucei but not T. b. rhodesiense. Therefore, as a logical extension of the transgenic program in cattle, could transgenic expression of the baboon ApoL1 in humans provide the basis for counteracting T. b. rhodesiense infection? As a proof of principle, one of us (J.R.) showed recently that plasma transferred from a mouse expressing baboon ApoL1 cured wild-type mice that had been infected with T. b. rhodesiense (unpublished data). Of course, the creation of “transgenic humans” is pure science fiction at this point in time, for a host of technical and ethical reasons. However, recent successes in a number of gene therapy trials treating a variety of human disorders, such as X-linked and adenosine deaminase deficiency–associated severe combined immunodeficiency,12 a genetic form of retinal degeneration,13 some forms of cancer,14 and even a severe neurodegenerative disease15 demonstrate that expression of transgenes in humans can provide therapeutic benefit in some patients.
Even if expression of a baboon ApoL1 transgene in a human were to be shown to provide protection against infection in individual patients, applications of gene transfer methods are limited at the present time to the somatic cells of individual patients and are not feasible for wide-scale use in populations for disease prevention. The imprecision of all current methods of gene transfer and the possibility of genomic damage, even tumor development, from the delivery tools themselves or from incorrectly regulated transgene expression make this approach unfeasible at this time. Until vastly improved gene transfer methods become available, there will be a continuing need for more traditional drug approaches to the prevention of infection or symptomatic treatment of patients already infected with trypanosomes.
Even further in the future is the possibility that humans could be made resistant to parasitic disease through human germline modification and expression of a transgene such as the baboon ApoL1. In fact, positive selection for ApoL1 genotypes conferring such a resistance appears to already occur in Africans.7,8 The possibility of human germline manipulation has been widely debated, and arguments in favor of such applications have usually been found wanting, both technically and ethically.16 In contrast, the Chuma cow study described above provides a mechanism based on transgene expression inherited through the germ line that has the potential to alleviate livestock infection.
In his recent examination of the development of personalized medicine,17 Francis S. Collins noted that his own predictions for the development of such new approaches in medicine have all been surpassed. With that in mind, one cannot help wondering whether genetic methods derived from gene therapy concepts might eventually be applied to broad programs of population-wide disease prevention, such as for human African trypanosomiasis and other devastating parasitic diseases. For that to occur, the introduction of a prophylactic disease-resistance gene in the foreseeable future would become possible only through improved, targeted, and safer methods of wide-scale gene transfer. Such improvements will almost surely come, and therefore broad public health applications toward parasitic disease prevention may soon be considered to be closer to reality than the pure science fiction that it may seem at the time of this writing. Until such a time, it is crucial to continue vigorous drug development programs, with emphasis on drugs that affect the expression or functions of the genes that are known to affect trypanosome infection susceptibility, such as ApoL1 and SRA.
Acknowledgments
The authors thank Theodore Friedmann and Robert Frederickson for critical reading and editorial support during the preparation and review of the manuscript. J.L. was supported by the grants LC07032 and 6007665801 and the Praemium Academiae award.
REFERENCES
- Pérez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, Homblé F, et al. Apolipoprotein L-1 promotes trypanosome lysis by forming pores in lysosomal membrane. Science. 2005;309:469–472. doi: 10.1126/science.1114566. [DOI] [PubMed] [Google Scholar]
- Molina-Portela MP, Samanovic M., and, Raper J. Distinct roles of apolipoprotein components within the trypanosome lytic factor complex revealed in a novel transgenic mouse model. J Exp Med. 2008;205:1721–1728. doi: 10.1084/jem.20071463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B, Truc P, Poelvoorde P, Pays A, Joshi PP, Katti R, et al. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-1. N Engl J Med. 2006;355:2752–2756. doi: 10.1056/NEJMoa063265. [DOI] [PubMed] [Google Scholar]
- Oli MW, Cotlin LF, Shiflett AM., and, Hajduk SL. Serum resistance-associated protein blocks lysosomal targeting of trypanosome lytic factor in Trypanosoma brucei. Eukaryot Cell. 2006;5:132–139. doi: 10.1128/EC.5.1.132-139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecordier L, Vanhollebeke B, Poelvoorde P, Tebabi P, Paturiaux-Hanocq F, Andris F, et al. C-terminal mutants of apolipoprotein L-I efficiently kill both Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. PLoS Pathogens. 2009;5:e1000685. doi: 10.1371/journal.ppat.1000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson R, Molina-Portela P, Mott H, Carrington M., and, Raper J. Hydrodynamic gene delivery of baboon trypanosome lytic factor eliminates both animal and human-infective African trypanosomes. Proc Natl Acad Sci USA. 2009;106:19509–19514. doi: 10.1073/pnas.0905669106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI.et al. (2010Association of trypanolytic ApoL1 variants with kidney disease in African-Americans Science 329841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE., and, Rudenko G. Switching trypanosome coats: what's in the wardrobe. Trends Genet. 2006;22:614–620. doi: 10.1016/j.tig.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Stuart K, Brun R, Croft S, Fairlamb A, Gürtler RE, McKerrow J, et al. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–1310. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson JA, Brand S, McElroy SP, Cleghorn LA, Smid O, Stojanovski L, et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature. 2010;464:728–732. doi: 10.1038/nature08893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Frankel M. Inheritable genetic modification and a brave New World: did Huxley have it wrong. Hastings Cent Rep. 2003;33:31–36. [PubMed] [Google Scholar]
- Collins FS. The Language of Life: DNA and the Revolution in Personalized Medicine. HarperCollins: New York 2010.



