Abstract
Cardiorespiratory fitness (CRF) is not only an objective measure of habitual physical activity, but also a useful diagnostic and prognostic health indicator for patients in clinical settings. Although compelling evidence has shown that CRF is a strong and independent predictor of all-cause and cardiovascular disease mortality, the importance of CRF is often overlooked from a clinical perspective compared with other risk factors such as hypertension, diabetes, smoking, or obesity. Several prospective studies indicate that CRF is at least as important as the traditional risk factors, and is often more strongly associated with mortality. In addition, previous studies report that CRF appears to attenuate the increased risk of death associated with obesity. Most individuals can improve their CRF through regular physical activity. Several biological mechanisms suggest that CRF improves insulin sensitivity, blood lipid profile, body composition, inflammation, and blood pressure. Based on the evidence, health professionals should encourage their patients to improve CRF through regular physical activity.
Keywords: Biological mechanism, cardiorespiratory fitness, lifestyle, mortality, obesity, physical activity
Introduction
Cardiorespiratory fitness (CRF) is a health-related component of physical fitness defined as the ability of the circulatory, respiratory, and muscular systems to supply oxygen during sustained physical activity. CRF is usually expressed in metabolic equivalents (METs) or maximal oxygen uptake (VO2 max) measured by exercise tests such as treadmill or cycle ergometer. CRF is not only a sensitive and reliable measure of habitual physical activity (American College of Sports Medicine, 1998; Church et al., 2007; Jackson et al., 2009; Wang et al., 2010), but also a relatively low-cost and useful health indicator for both symptomatic and asymptomatic patients in clinical practice (Gibbons et al., 2002; Gulati et al., 2005; Myers et al., 2002). According to a recent World Health Organization (WHO) report, high blood pressure, tobacco use, high blood glucose, physical inactivity, and obesity (in that order) explain 38% of total global deaths (WHO, 2009). The American Heart Association (AHA) stated that ideal cardiovascular disease (CVD) health, a newly defined concept, comprises of four health behaviors (non-smoking, body mass index [BMI] <25 kg/m2, physical activity at goal levels, and pursuit of a recommended diet) and three health factors (untreated total cholesterol <200 mg/dL, untreated blood pressure <120/80 mmHg, and fasting blood glucose <100 mg/dL) (Lloyd-Jones et al., 2010). Scientific evidence shows that such behaviors and factors reported by the WHO and AHA are directly or indirectly associated with CRF (American College of Sports Medicine, 1998; Fletcher et al., 2001; Jackson et al., 2009; Wang et al., 2010).
Although there is convincing evidence that CRF is associated with morbidity and mortality in both men and women independently of other risk factors (Carnethon et al., 2003; Chase et al., 2009; Kodama et al., 2009; Lee et al., 2009b), the importance of CRF is still often ignored from a clinical perspective compared with other risk factors such as smoking, obesity, high blood pressure, or high blood glucose. In this review, we provide recent evidence from prospective studies testing the hypothesis that risks of all-cause and CVD mortality are different between different levels of baseline CRF and change in CRF over time. Also, we address the controversial issue of the relative contributions of CRF and obesity with mortality, and possible mechanisms linking CRF with mortality risk. Finally we describe the measurement issue of CRF in clinical settings and the major determinants of CRF. This review on CRF with mortality may be important in patients with schizophrenia where CRF levels are likely to be poor.
Cardiorespiratory fitness and mortality
There is convincing evidence that a moderate or high level of CRF reduces the risk of all-cause and CVD mortality in both men and women (Blair et al., 1989; Gulati et al., 2005; Kokkinos et al., 2008; Mora et al., 2003; Myers et al., 2002; Sandvik et al., 1993). The protective effect of CRF on mortality is independent of age, ethnicity, adiposity, smoking status, alcohol intake, and health conditions.
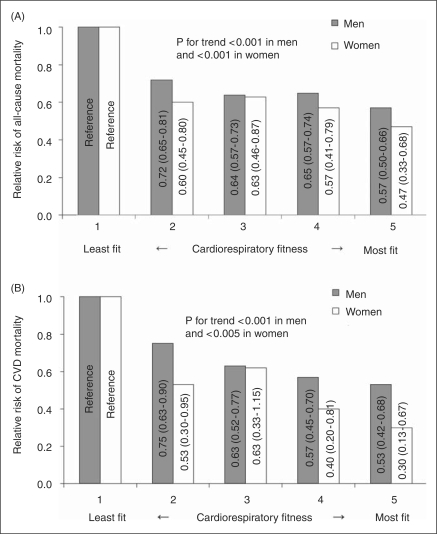
In the Aerobics Center Longitudinal Study (ACLS), compared with the least fit men and women, the most fit men and women had 43% and 53% lower risk for all-cause mortality, and 47% and 70% lower risk of CVD mortality, respectively (Figure 1). The total duration of the maximal treadmill test used in the CRF classification is highly correlated with measured maximal oxygen uptake (r ≥ 0.92) in men (Pollock et al., 1976) and women (Pollock et al., 1982). Recently, the first meta-analysis has been published on the association of CRF with all-cause mortality and CVD events (including fatal and nonfatal CVD) in healthy individuals (Kodama et al., 2009). This review selected 33 studies comprising 102,980 participants with 6910 all-cause deaths, and 84,323 participants with 4485 CVD events in men and women. In dose–response analyses, each 1-MET increment in CRF (corresponding to approximately 1 km/h higher running/jogging speed) was associated with a 13% and 15% risk reduction from all-cause mortality and CVD events, respectively. The authors explained that a 1-MET higher level of CRF is comparable to a 7-cm, 5-mmHg, 1-mmol/L (88 mg/dL), and 1-mmol/L (18 mg/dL) decrement in waist circumference, systolic blood pressure, triglyceride level (in men), and fasting plasma glucose, respectively, and a 0.2-mmol/L (8 mg/dL) increment in high-density lipoprotein cholesterol based on other studies (Coutinho et al., 1999; de Koning et al., 2007; Gordon et al., 1989; Hokanson and Austin, 1996; Lewington et al., 2002). In addition, individuals with low CRF had a substantially higher risk of all-cause mortality and CVD events compared with individuals with moderate and high CRF after adjustment for heterogeneity of study characteristics. Low CRF values for men and women are approximately 9 and 7 METs in those 40 years old, 8 and 6 METs in 50 years old, and 7 and 5 METs in 60 years or older, respectively. Data from the ACLS also supported similar MET values for low CRF level as a mortality predictor in each age group (Blair et al., 1995).
Figure 1.
Relative risks of (A) all-cause and (B) cardiovascular disease (CVD) mortality by cardiorespiratory fitness quintiles for 40,451 (2657 all-cause and 943 CVD deaths) men and 12,831 (375 all-cause and 90 CVD deaths) women aged 20–100 years without CVD or cancer in the Aerobics Center Longitudinal Study. Cardiorespiratory fitness is measured by total duration during a maximal treadmill exercise test. Relative risks (95% confidence intervals) are shown inside the bars and adjusted for age, year of examination, body mass index, smoking status, abnormal electrocardiogram, hypertension, diabetes, hypercholesterolemia, and family history of CVD.
Kodama et al. conducted stratified analyses by some selected confounders in their review (Kodama et al., 2009). The risk reduction for all-cause mortality and CVD events per 1-MET increment in CRF was consistently significant, regardless of age, sex, smoking, coronary risk factors, abnormal exercise electrocardiogram, follow-up period, instrument for assessing CRF, and exercise testing method. Although a publication bias was suggested by statistical assessment in this review, adjustment for this bias did not change the general findings. Based on the above review, we believe that CRF is a strong independent predictor of all-cause mortality and CVD morbidity and mortality. Therefore, along with other well-established mortality risk factors such as smoking, obesity, hypertension, and diabetes, including CRF in risk stratification is recommended. Clinicians should act on this information to promote regular physical activity in order to reduce premature deaths from CVD and all causes.
Change in cardiorespiratory fitness and mortality
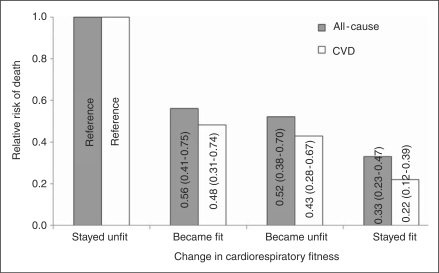
Most studies use a single baseline CRF measurement with subsequent mortality follow-up. However, individual levels of fitness may change over time due to changes in physical activity habits or other factors. Two prospective studies in men have shown an inverse association between change in CRF and mortality risk. We followed 9777 men (aged 20–82 at baseline) who had two CRF assessments over an average period of 4.9 years between examinations. These men were then followed an average of 5.1 years for mortality to test the hypothesis that change in CRF produce change in mortality risk. Men who were unfit at both visits had the highest death risk, men who were fit at both visits had the lowest death risk, and men who changed fitness status had intermediate risks (Figure 2) (Blair et al., 1995). Unfit was defined as quintile 1 and fit was defined as quintiles 2–5 of age-specific maximal exercise duration based on the measurements from the first examination. Investigators from Norway followed 2014 healthy men (aged 40–60 at baseline) and reported that improvements in CRF within 7 years were associated with a significantly lower risk of all-cause mortality during up to 15 years of follow-up, irrespective of CRF level at baseline. Changes in body weight over time had no effect on these results (Erikssen et al., 1998).
Figure 2.
Age-adjusted relative risks of all-cause and cardiovascular disease (CVD) mortality by change in cardiorespiratory fitness for 9777 (223 all-cause and 87 CVD deaths) men aged 20–82 in the Aerobics Center Longitudinal Study. Cardiorespiratory fitness is measured by total duration during a maximal treadmill exercise test. Unfit is defined as quintile 1 (20%) and fit is defined as quintiles 2–5 (80%) of age-specific maximal exercise duration. Relative risks (95% confidence intervals) are shown inside the bars. (Adapted from Blair et al. (1995)).
Health status at baseline may have an influence on the association between CRF change and mortality, and worsening health status at follow-up examinations may lead to early deaths irrespective of CRF change. Therefore, it is important to control the effects of disease conditions at each examination if possible. Controlling disease conditions can be performed by adjusting for them in multivariable modeling, conducting stratified analyses in categories of the disease, or excluding those unhealthy individuals from the analysis. Both studies mentioned here used at least one of these approaches and found that the findings were unlikely to be biased by underlying disease.
These two studies on change in CRF and mortality provide evidence that improvement in CRF is associated with lower risk of all-cause and CVD death in men. To the best of the authors’ knowledge, currently no studies have been conducted in women on CRF change and mortality. Future studies in women are warranted to determine whether the findings in men can be replicated in women.
Cardiorespiratory fitness, obesity, and mortality
Approximately two-thirds of Americans are overweight or obese (Flegal et al., 2010) and obesity is the fifth leading cause of global mortality (WHO, 2009). CRF is a strong and independent predictor of all-cause and CVD mortality (Blair et al., 1995; Wei et al., 1999) and physical inactivity ranks fourth on the WHO list of causes of death (WHO, 2009). Given the difficulties of losing weight and maintaining a reduced weight over the long term (Hainer et al., 2008; Wing and Phelan, 2005), it is important to identify methods other than weight loss for risk reduction among obese individuals. Thus, studying the independent and combined effects of CRF and obesity with mortality may indicate benefits for those who are obese and at increased risk for obesity-related complications such as hypertension, diabetes, or CVD, leading to early death.
The relative contributions of CRF and obesity to health outcomes are controversial (Fogelholm, 2010; Lee et al., 2009a). We have found that a moderate to high level of CRF eliminates the higher risk of mortality associated with obesity (Lee et al., 1999). We observed similar findings among older adults aged 60 years or older (Sui et al., 2007). In these ACLS studies, obese individuals (BMI ≥30) who were fit had comparable mortality risk to normal-weight individuals who were unfit. These findings were consistent when using percentage body fat or waist circumference instead of BMI. Recently, the Veterans Exercise Testing Study in men aged 40–70 years also found that overweight and obese men had higher risk of all-cause mortality only if they had a low fitness level (McAuley et al., 2010).
In contrast, Stevens et al. report that both CRF and obesity are independent predictors of mortality in the Lipid Research Clinics (LRC) study. High fitness substantially ameliorated the risk of obesity, but did not eliminate it (Stevens et al., 2002). Another study from the same research group on the associations of fitness and fatness with mortality reported that both fitness and fatness had similar associations with all-cause and CVD mortality in American men (Stevens et al., 2004). Both LRC studies show that fitness attenuates the detrimental effects of obesity on mortality.
As we concluded earlier (Lee et al., 2009a), there is currently no consensus on whether CRF eliminates the higher risk of obesity for mortality. It does seem likely that individuals can reduce their risk of mortality by improving CRF, regardless of their level of adiposity. The highest risk of mortality is observed in those who are both obese and unfit, therefore, health professionals should encourage these patients to engage in regular physical activity to develop and maintain CRF, whether or not it makes them thin.
Mechanisms linking cardiorespiratory fitness with mortality
There are several possible biological mechanisms for the risk reduction of all-cause and CVD mortality in individuals with higher CRF. CRF improves insulin sensitivity, blood lipid and lipoprotein profile, body composition, inflammation, and blood pressure and the autonomic nervous system. Insulin resistance is a major determinant of CVD, especially in overweight or obese individuals (Reaven, 2005). Emerging evidence suggests that CRF plays an important role in relation to insulin resistance and sensitivity. In a large cross-sectional study, lower CRF was correlated with impaired insulin sensitivity measured by homeostasis model assessment of insulin resistance (HOMA-IR) (Leite et al., 2009). There are similar findings in older adults (Racette et al., 2006), postmenopausal women (Messier et al., 2008), and youths (Lee et al., 2006). Most of these studies also indicate that adiposity is another key factor on insulin sensitivity. Some (Lee et al., 2006; Messier et al., 2008), but not all (Leite et al., 2009; Nyholm et al., 2004; Racette et al., 2006), indicate that the effects of CRF on insulin sensitivity are attenuated after controlling for adiposity, suggesting that the reduction in insulin resistance from higher CRF may be partly mediated through adiposity. However, the relative contributions of adiposity and CRF to insulin sensitivity are still inconclusive. Because most of the evidence is based on cross-sectional analyses, well-conducted prospective studies and randomized controlled trials are needed.
Lipid and lipoprotein concentrations are accepted independent risk factors for coronary heart disease (CHD) (National Cholesterol Education Program Expert Panel on Detection, 2002). In a cross-sectional study of healthy men without diabetes, those with low CRF, in comparison with those with high CRF, had higher triglyceride, apolipoprotein B (a strong predictor of CHD events), and total cholesterol–HDL cholesterol ratio after matching individuals with similar BMI (Arsenault et al., 2007). There are similar findings from another cross-sectional study of 297 healthy men, for a given level of waist circumference, visceral fat, or subcutaneous fat (Lee et al., 2005). A recent randomized controlled trial of 217 men and women with exercise training and dietary instruction evaluated associations among lifestyle improvements, in particular increased CRF, and changes in the blood lipid profile (Kawano et al., 2009). When all participants were divided into three subgroups according to the degree of improvement in CRF, apolipoprotein B decreased and LDL cholesterol–apolipoprotein B ratio increased as CRF increased. In 127 middle-aged Finnish men, plasma saturated fatty acids (positively correlated with triglyceride and insulin concentration) were lower and polyunsaturated fatty acids (negatively correlated with triglyceride) were higher in the most-fit tertile compared with those in the least-fit tertile (Konig et al., 2003).
Obesity is the fifth leading global risk factor for mortality (WHO, 2009), and decreased muscle mass and excess adiposity, especially visceral adiposity, are independently related to mortality (Taylor et al., 2010; Wannamethee et al., 2007). Two cross-sectional studies revealed that fit men had lower visceral adipose tissue compared with unfit men for a given BMI, suggesting that the effect of CRF to ameliorate the health risks associated with BMI may be, in part, mediated through a less abdominal adiposity (Arsenault et al., 2007; Wong et al., 2004). In a longitudinal study, higher CRF at baseline resulted in a lower fat mass gain over 4 years, independent of change in lean tissue mass among overweight Hispanic boys (Byrd-Williams et al., 2008). Another longitudinal study of 459 adults from the Canadian Physical Activity Longitudinal Study showed that higher CRF at baseline was associated with lower future risk of obesity over the 20-year follow-up period, independent of baseline age, physical activity, BMI, sex, smoking status, and alcohol consumption (Brien et al., 2007). There is also evidence that change in CRF is associated with subsequent change in weight gain. In a cohort of healthy middle-aged men (n = 4599) and women (n = 724), each 1 minute improvement in treadmill time reduced the risk of ≥5 kg gain by 14% in men and by 9% in women (DiPietro et al., 1998). Thus, CRF appears to be an important predictor of future adiposity, weight gain, and obesity.
Several population-based cross-sectional studies have found an inverse association between CRF and C-reactive protein, an inflammatory marker as a predictor of CVD (Aronson et al., 2004; Church et al., 2002a; Kuo et al., 2007; Williams et al., 2005). This inverse association was seen in both men and women and held after controlling for BMI and other CVD risk factors. Another study also showed similar results for the association of other inflammatory markers, plasma fibrinogen, white blood cell count, and uric acid, with CRF (Church et al., 2002b). In addition, these results are consistent within strata of body composition assessed by BMI, percentage body fat, or waist circumference. Thus, CRF may partly decrease the risk of all-cause and CVD mortality through less inflammation, regardless of body composition. High blood pressure is a strong predictor of CVD events and the number one global risk factor for mortality responsible for nearly 13% of total deaths in the world (WHO, 2009). Two large prospective studies, the ACLS and the Coronary Artery Risk Development in Young Adults (CARDIA) study, have shown a significant inverse association between CRF and incident hypertension in both men and women after adjustment for potential confounders including BMI (Barlow et al., 2006; Carnethon et al., 2003; Chase et al., 2009). Some studies found that fit individuals had enhanced cardiac autonomic nervous system activity as assessed by analysis of heart rate variability, compared with unfit individuals (Buchheit and Gindre, 2006; Ueno et al., 2002). Higher epicardial fat thickness (measured by echocardiography), an index of cardiac adiposity that may affect autonomic nervous system, is associated with poor CRF independent of body weight in overweight or obese men (Kim et al., 2010). Increasing evidence suggests that oxidative stress may play an important role in hypertension (de Champlain et al., 2004). Another recent study reported a negative association between CRF and oxidative stress and positive association between CRF and antioxidant enzyme activity, demonstrating that CRF may mediate against oxidative stress by maintaining antioxidant enzyme efficiency (Pialoux et al., 2009).
A cluster of several metabolic factors associated with higher risk of diabetes, CVD, and all-cause mortality (Gami et al., 2007; Ford, 2005) may explain an additional proportion of the mechanisms linking CRF to risk of mortality. Several reports demonstrate an inverse association between CRF and risk of developing metabolic syndrome (Hassinen et al., 2008; Janssen and Cramp, 2007; LaMonte et al., 2005). In a randomized controlled trial of 365 men and women from the ProActive study, increased CRF was associated with reduced clustered metabolic risk variables (waist circumference, fasting triacylglycerol, insulin and glucose, blood pressure, and HDL-cholesterol) over 1-year follow-up, independent of age, sex, smoking status, socioeconomic status, and baseline metabolic score (Simmons et al., 2008).
Assessment of cardiorespiratory fitness
CRF can be measured directly from expired gas analysis or estimated through various maximal or submaximal exercise tests generally using a treadmill or cycle ergometer. Because directly measured CRF (VO2 max) is more precise than other methods, it is recommended for most research studies. CRF is typically expressed as multiples of resting metabolic rate (METs), where a score of 10 METs indicates that a person can increase resting metabolism 10-fold. However, if direct measurement of CRF is not feasible due to cost, space, or equipment, CRF can be estimated from heart rate or exercise time to exhaustion in various exercise tests. Submaximal exercise tests are less difficult and more convenient in terms of time, effort, and cost, yet still provide adequate estimates of CRF. A review study indicates that submaximal testing appears to have greater applicability with high correlation between maximal and submaximal testing (r = 0.7–0.9) in various submaximal tests such as submaximal treadmill and cycle ergometer tests, 12-minute run test, and 1-mile walk test (Noonan and Dean, 2000). The American College of Sports Medicine provides guidelines for exercise testing including various test modes and protocols, general procedures, test termination criteria, and normative values for CRF by sex and age (American College of Sports Medicine, 2009). The American Heart Association also provides recommendations for clinical exercise testing laboratories (Fletcher et al., 2001; Myers et al., 2009), and a scientific statement highlighting the major clinical and research applications of CRF assessment (Arena et al., 2007).
Although exercise testing is generally safe, major complications such as myocardial infarction or serious arrhythmias have been reported at a rate of up to 5 per 10,000 tests, and sudden cardiac death has occurred in up to 0.5 per 10,000 tests (Arena et al., 2007; Gordon and Kohl, 1993). The American College of Cardiology/American Heart Association provides guidelines for exercise testing in patients with known or suspected CVD (Gibbons et al., 1997, 2002).
The terms CRF and physical activity are sometimes used interchangeably. However, physical activity is self-reported in many studies and is subject to major misclassification (Walsh et al., 2004), leading to underestimation of the association between physical activity and health outcomes. We recently reported that CRF is more strongly associated with all-cause mortality than self-reported physical activity in 42,373 men and women (Lee et al., 2010). Therefore, using objectively measured CRF rather than self-reported physical activity may provide less biased data on the association between habitual physical activity and risk of mortality.
Many reports have recommended that CRF assessment should be included in the clinical setting based on the importance of CRF to morbidity and mortality prevention (Gibbons et al., 2002; Gulati et al., 2005; Kodama et al., 2009; Myers et al., 2002). CRF is recognized as a stronger predictor of mortality than established risk factors such as hypertension, smoking, and diabetes in both healthy individuals and those with CVD (Myers et al., 2002). In addition, each 1 MET increase in CRF change was associated with a reduction of approximately 16% in mortality risk (Blair et al., 1995).
We believe clinicians should also use CRF from routine exercise test to stratify risk, classify patients, and make clinical recommendations providing important additional information.
Determinants of cardiorespiratory fitness
CRF is a surrogate measure of functional status of respiratory, cardiovascular, and skeletal muscle systems. Individual level of CRF depends on those modifiable (physical activity, smoking, obesity, and medical condition) and non-modifiable (age, gender, and genotype) factors, but is primarily determined by physical activity (Table 1). After reaching the maximal value of CRF between age of 20–30 years, CRF starts to decline with age and the rate of decline accelerates markedly with advancing age in healthy populations according to two longitudinal studies (Fleg et al., 2005; Jackson et al., 2009). In these studies, the pattern of decline with age is accelerated by reducing physical activity and weight gain.
Table 1.
Determinants of cardiorespiratory fitness
| Non-modifiable | Modifiable |
|---|---|
| Age | Physical activity |
| Gender | Smoking |
| Genotype | Obesity |
| Medical condition |
Women have approximately 2 METs lower CRF capacity than men, attributed to their smaller muscle mass, lower hemoglobin and blood volume, and smaller stroke volume compared with men (Fletcher et al., 2001). Based on the US National Health and Nutrition Examination Surveys, the estimated mean CRF level was 12 METs for men and 10 METs for women aged 20–49 years (Wang et al., 2010).
Genetic factors may also contribute to individual variation in CRF level. The most well-known findings on the role of genotype in CRF were derived from the HERITAGE Family Study in more than 700 healthy but sedentary men and women (Bouchard et al., 1999; Bouchard and Rankinen, 2001; Rankinen et al., 2010). After 20 weeks of exercise training, although the mean CRF increased approximately 15–18% in all four sex and generation groups (fathers, mothers, sons, and daughters), there was 2.5 times more variance between families than within families in the CRF response, and the maximal heritability estimate of the CRF response to training reached 47%. Although little is known about the role of specific genes on CRF, familial and genetic factors clearly contribute to CRF.
Medical conditions related to respiratory, cardiovascular, or skeletal muscle function can have an influence on CRF. Data from several studies show that average METs in individuals with CVD, diabetes, or hypertension are roughly 10–25% lower than those in relatively healthy individuals (Blair et al., 1995; Gulati et al., 2005; Myers et al., 2002). In a cross-sectional US national data study, hypertension, hypercholesterolemia, and low HDL-cholesterol levels were more prevalent among adults with low CRF compared with individuals with moderate or high CRF (Carnethon et al., 2005).
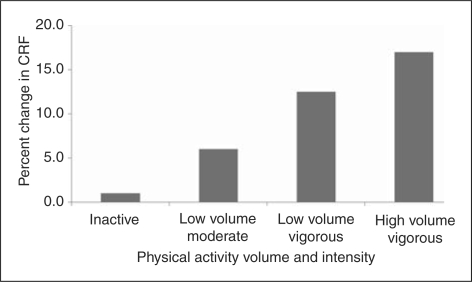
Among lifestyle factors, physical activity is a principal determinant of CRF (American College of Sports Medicine, 1998; Physical Activity Guidelines Advisory Committee, 2008). Evidence from randomized controlled trials demonstrate a dose–response relationship between physical activity and improvement in CRF, suggesting that increasing either intensity or volume of physical activity appears to have additional effects on CRF after controlling for each other (Church et al., 2007; Duscha et al., 2005; O'Donovan et al., 2005), as seen in Figure 3. Even moderate intensity physical activity at 40–55% of peak VO2 intensity is sufficient to improve CRF (Church et al., 2007; Duscha et al., 2005). Also, findings from the prospective studies on CRF change and mortality showed that men who improved CRF increased their physical activity over the same period (Blair et al., 1995; Erikssen et al., 1998).
Figure 3.
Change in cardiorespiratory fitness (CRF) by physical activity. (Adapted from Physical Activity Guidelines Advisory Committee (2008)).
In addition to regular physical activity, the US national survey indicated that individuals who were obese had approximately 10–15% lower CRF than non-obese individuals (Wang et al., 2010). Recent findings from the ACLS show that engaging in physical activity, maintaining a normal weight, and not smoking were associated with substantially higher levels of CRF across the adult life span in both men and women (Jackson et al., 2009).
Conclusion
Moderate to high levels of CRF and improvement in CRF are associated with a lower risk of mortality from all-causes and CVD in both men and women regardless of age, smoking status, body composition, other risk factors, method of CRF assessment, and study design. CRF appears to attenuate the higher risk of death associated with obesity although it is not yet clear whether CRF completely eliminates mortality risk in obese individuals. We believe that including CRF in clinical examinations along with traditional evaluations such as blood pressure measurement and blood chemistry analyses may contribute to chronic disease prevention and longer life span.
Funding
This study was supported by an unrestricted research grant from The Coca-Cola Company.
References
- American College of Sports Medicine (1998) American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 30: 975–991 [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine (2009) ACSM's Guidelines for Exercise Testing and Prescription Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ, et al. (2007) Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 116: 329–343 [DOI] [PubMed] [Google Scholar]
- Aronson D, Sheikh-Ahmad M, Avizohar O, Kerner A, Sella R, Bartha P, et al. (2004) Creactive protein is inversely related to physical fitness in middle-aged subjects. Atherosclerosis 176: 173–179 [DOI] [PubMed] [Google Scholar]
- Arsenault BJ, Lachance D, Lemieux I, Almeras N, Tremblay A, Bouchard C, et al. (2007) Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med 167: 1518–1525 [DOI] [PubMed] [Google Scholar]
- Barlow CE, LaMonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. (2006) Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol 163: 142–150 [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl III HW, Barlow CE, Paffenbarger Jr RS, Gibbons LW and Macera CA (1995) Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 273: 1093–1098. [PubMed]
- Blair SN, Kohl III HW, Paffenbarger Jr RS, Clark DG, Cooper KH and Gibbons LW (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401. [DOI] [PubMed] [Google Scholar]
- Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, et al. (1999) Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 87: 1003–1008 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Rankinen T. (2001) Individual differences in response to regular physical activity. Med Sci Sports Exerc 33: S446–S451 [DOI] [PubMed] [Google Scholar]
- Brien SE, Katzmarzyk PT, Craig CL, Gauvin L. (2007) Physical activity, cardiorespiratory fitness and body mass index as predictors of substantial weight gain and obesity: the Canadian physical activity longitudinal study. Can J Public Health 98: 121–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheit M, Gindre C. (2006) Cardiac parasympathetic regulation: respective associations with cardiorespiratory fitness and training load. Am J Physiol Heart Circ Physiol 291: H451–H458 [DOI] [PubMed] [Google Scholar]
- Byrd-Williams CE, Shaibi GQ, Sun P, Lane CJ, Ventura EE, Davis JN, et al. (2008) Cardiorespiratory fitness predicts changes in adiposity in overweight Hispanic boys. Obesity (Silver Spring) 16: 1072–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs Jr, DR, Liu K. (2003) Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA 290: 3092–3100 [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Gulati M, Greenland P. (2005) Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA 294: 2981–2988 [DOI] [PubMed] [Google Scholar]
- Chase NL, Sui X, Lee DC, Blair SN. (2009) The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am J Hypertens 22: 417–424 [DOI] [PubMed] [Google Scholar]
- Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. (2002a) Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol 22: 1869–1876 [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. (2007) Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA 297: 2081–2091 [DOI] [PubMed] [Google Scholar]
- Church TS, Finley CE, Earnest CP, Kampert JB, Gibbons LW, Blair SN. (2002b) Relative associations of fitness and fatness to fibrinogen, white blood cell count, uric acid and metabolic syndrome. Int J Obes Relat Metab Disord 26: 805–813 [DOI] [PubMed] [Google Scholar]
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22: 233–240 [DOI] [PubMed] [Google Scholar]
- de Champlain J, Wu R, Girouard H, Karas M, El MA, Laplante MA, et al. (2004) Oxidative stress in hypertension. Clin Exp Hypertens 26: 593–601 [DOI] [PubMed] [Google Scholar]
- de Koning L, Merchant AT, Pogue J, Anand SS. (2007) Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 28: 850–856 [DOI] [PubMed] [Google Scholar]
- DiPietro L, Kohl III HW, Barlow CE and Blair SN (1998) Improvements in cardiorespiratory fitness attenuate age-related weight gain in healthy men and women: the Aerobics Center Longitudinal Study. Int J Obes Relat Metab Disord 22: 55–62. [DOI] [PubMed]
- Duscha BD, Slentz CA, Johnson JL, Houmard JA, Bensimhon DR, Knetzger KJ, et al. (2005) Effects of exercise training amount and intensity on peak oxygen consumption in middleage men and women at risk for cardiovascular disease. Chest 128: 2788–2793 [DOI] [PubMed] [Google Scholar]
- Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J. (1998) Changes in physical fitness and changes in mortality. Lancet 352: 759–762 [DOI] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, et al. (2005) Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112: 674–682 [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241 [DOI] [PubMed] [Google Scholar]
- Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, et al. (2001) Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 104: 1694–1740 [DOI] [PubMed] [Google Scholar]
- Fogelholm M. (2010) Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev 11(3): 202–221 [DOI] [PubMed] [Google Scholar]
- Ford ES. (2005) Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28: 1769–1778 [DOI] [PubMed] [Google Scholar]
- Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. (2007) Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and metaanalysis of longitudinal studies. J Am Coll Cardiol 49: 403–414 [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, et al. (1997) ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol 30: 260–311 [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. (2002) ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 40: 1531–1540 [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. (1989) High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79: 8–15 [DOI] [PubMed] [Google Scholar]
- Gordon NF, Kohl HW. (1993) Exercise Testing and sudden cardiac death. J Cardiopulm Rehabil 13: 381–386 [Google Scholar]
- Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, et al. (2005) The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 353: 468–475 [DOI] [PubMed] [Google Scholar]
- Hainer V, Toplak H, Mitrakou A. (2008) Treatment modalities of obesity: what fits whom? Diabetes Care 31(Suppl 2): S269–S277 [DOI] [PubMed] [Google Scholar]
- Hassinen M, Lakka TA, Savonen K, Litmanen H, Kiviaho L, Laaksonen DE, et al. (2008) Cardiorespiratory fitness as a feature of metabolic syndrome in older men and women: the Dose–Responses to Exercise Training study (DR's EXTRA). Diabetes Care 31: 1242–1247 [DOI] [PubMed] [Google Scholar]
- Hokanson JE, Austin MA. (1996) Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population based prospective studies. J Cardiovasc Risk 3: 213–219 [PubMed] [Google Scholar]
- Jackson AS, Sui X, Hebert JR, Church TS, Blair SN. (2009) Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med 169: 1781–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Cramp WC. (2007) Cardiorespiratory fitness is strongly related to the metabolic syndrome in adolescents. Diabetes Care 30: 2143–2144 [DOI] [PubMed] [Google Scholar]
- Kawano M, Shono N, Yoshimura T, Yamaguchi M, Hirano T, Hisatomi A. (2009) Improved cardio-respiratory fitness correlates with changes in the number and size of small dense LDL: randomized controlled trial with exercise training and dietary instruction. Intern Med 48: 25–32 [DOI] [PubMed] [Google Scholar]
- Kim MK, Tanaka K, Kim MJ, Matsuo T, Tomita T, Ohkubo H, et al. (2010) Epicardial fat tissue: relationship with cardiorespiratory fitness in men. Med Sci Sports Exerc 42: 463–469 [DOI] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035 [DOI] [PubMed] [Google Scholar]
- Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, et al. (2008) Exercise capacity and mortality in black and white men. Circulation 117: 614–622 [DOI] [PubMed] [Google Scholar]
- Konig D, Vaisanen SB, Bouchard C, Halle M, Lakka TA, Baumstark MW, et al. (2003) Cardiorespiratory fitness modifies the association between dietary fat intake and plasma fatty acids. Eur J Clin Nutr 57: 810–815 [DOI] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chen JH, Yu YH, Bean JF. (2007) Association of cardiorespiratory fitness and levels of C-reactive protein: data from the National Health and Nutrition Examination Survey 1999–2002. Int J Cardiol 114: 28–33 [DOI] [PubMed] [Google Scholar]
- LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. (2005) Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 112: 505–512 [DOI] [PubMed] [Google Scholar]
- Lee CD, Blair SN, Jackson AS. (1999) Cardiorespiratory fitness, body composition, and allcause and cardiovascular disease mortality in men. Am J Clin Nutr 69: 373–380 [DOI] [PubMed] [Google Scholar]
- Lee DC, Sui X, Blair SN. (2009a) Does physical activity ameliorate the health hazards of obesity? Br J Sports Med 43: 49–51 [DOI] [PubMed] [Google Scholar]
- Lee DC, Sui X, Church TS, Lee IM, Blair SN. (2009b) Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care 32: 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Sui X, Ortega FB, Kim YS, Church TS, Winett RA, et al. (2010) Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med in press. [DOI] [PubMed] [Google Scholar]
- Lee S, Bacha F, Gungor N, Arslanian SA. (2006) Cardiorespiratory fitness in youth: relationship to insulin sensitivity and beta-cell function. Obesity (Silver Spring) 14: 1579–1585 [DOI] [PubMed] [Google Scholar]
- Lee S, Kuk JL, Katzmarzyk PT, Blair SN, Church TS, Ross R. (2005) Cardiorespiratory fitness attenuates metabolic risk independent of abdominal subcutaneous and visceral fat in men. Diabetes Care 28: 895–901 [DOI] [PubMed] [Google Scholar]
- Leite SA, Monk AM, Upham PA, Bergenstal RM. (2009) Low cardiorespiratory fitness in people at risk for type 2 diabetes: early marker for insulin resistance. Diabetol Metab Syndr 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective SC. (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913 [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van HL, et al. (2010) Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 121: 586–613 [DOI] [PubMed] [Google Scholar]
- McAuley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN. (2010) Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years. Mayo Clin Proc 85: 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier V, Malita FM, Rabasa-Lhoret R, Brochu M, Karelis AD. (2008) Association of cardiorespiratory fitness with insulin sensitivity in overweight and obese postmenopausal women: a Montreal Ottawa New Emerging Team study. Metabolism 57: 1293–1298 [DOI] [PubMed] [Google Scholar]
- Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, et al. (2003) Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA 290: 1600–1607 [DOI] [PubMed] [Google Scholar]
- Myers J, Arena R, Franklin B, Pina I, Kraus WE, McInnis K, et al. (2009) Recommendations for clinical exercise laboratories: a scientific statement from the American Heart Association. Circulation 119: 3144–3161 [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. (2002) Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801 [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program Expert Panel on Detection (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421 [PubMed] [Google Scholar]
- Noonan V, Dean E. (2000) Submaximal exercise testing: clinical application and interpretation 2. Phys Ther 80: 782–807 [PubMed] [Google Scholar]
- Nyholm B, Nielsen MF, Kristensen K, Nielsen S, Ostergard T, Pedersen SB, et al. (2004) Evidence of increased visceral obesity and reduced physical fitness in healthy insulin-resistant first-degree relatives of type 2 diabetic patients. Eur J Endocrinol 150: 207–214 [DOI] [PubMed] [Google Scholar]
- O'Donovan G, Owen A, Bird SR, Kearney EM, Nevill AM, Jones DW, et al. (2005) Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol 98: 1619–1625 [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee (2008) Physical Activity Guidelines Advisory Committee Report Washington, DC: US Department of Health and Human Services [Google Scholar]
- Pialoux V, Brown AD, Leigh R, Friedenreich CM, Poulin MJ. (2009) Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension 54: 1014–1020 [DOI] [PubMed] [Google Scholar]
- Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, et al. (1976) A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J 92: 39–46 [DOI] [PubMed] [Google Scholar]
- Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. (1982) Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J 103: 363–373 [DOI] [PubMed] [Google Scholar]
- Racette SB, Evans EM, Weiss EP, Hagberg JM, Holloszy JO. (2006) Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50–95 year olds. Diabetes Care 29: 673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankinen T, Roth SM, Bray MS, Loos R, Perusse L, Wolfarth B, et al. (2010) Advances in exercise, fitness, and performance genomics 1. Med Sci Sports Exerc 42: 835–846 [DOI] [PubMed] [Google Scholar]
- Reaven G. (2005) All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diab Vasc Dis Res 2: 105–112 [DOI] [PubMed] [Google Scholar]
- Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. (1993) Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med 328: 533–537 [DOI] [PubMed] [Google Scholar]
- Simmons RK, Griffin SJ, Steele R, Wareham NJ, Ekelund U. for the ProActive research team (2008) Increasing overall physical activity and aerobic fitness is associated with improvements in metabolic risk: cohort analysis of the ProActive trial. Diabetologia 51: 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Cai J, Evenson KR, Thomas R. (2002) Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol 156: 832–841 [DOI] [PubMed] [Google Scholar]
- Stevens J, Evenson KR, Thomas O, Cai J, Thomas R. (2004) Associations of fitness and fatness with mortality in Russian and American men in the lipids research clinics study. Int J Obes Relat Metab Disord 28: 1463–1470 [DOI] [PubMed] [Google Scholar]
- Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, et al. (2007) Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 298: 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Ebrahim S, Ben-Shlomo Y, Martin RM, Whincup PH, Yarnell JW, et al. (2010) Comparison of the associations of body mass index and measures of central adiposity and fat mass with coronary heart disease, diabetes, and all-cause mortality: a study using data from 4 UK cohorts. Am J Clin Nutr 91: 547–556 [DOI] [PubMed] [Google Scholar]
- Ueno LM, Hamada T, Moritani T. (2002) Cardiac autonomic nervous activities and cardiorespiratory fitness in older men. J Gerontol A Biol Sci Med Sci 57: M605–M610 [DOI] [PubMed] [Google Scholar]
- Walsh MC, Hunter GR, Sirikul B, Gower BA. (2004) Comparison of self-reported with objectively assessed energy expenditure in black and white women before and after weight loss. Am J Clin Nutr 79: 1013–1019 [DOI] [PubMed] [Google Scholar]
- Wang CY, Haskell WL, Farrell SW, LaMonte MJ, Blair SN, Curtin LR, et al. (2010) Cardiorespiratory fitness levels among US adults 20–49 years of age: findings from the 1999–2004 National Health and Nutrition Examination Survey. Am J Epidemiol 171: 426–435 [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Lennon L, Whincup PH. (2007) Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr 86: 1339–1346 [DOI] [PubMed] [Google Scholar]
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger Jr, RS, et al. (1999) Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282: 1547–1553 [DOI] [PubMed] [Google Scholar]
- Williams MJ, Milne BJ, Hancox RJ, Poulton R. (2005) C-reactive protein and cardiorespiratory fitness in young adults. Eur J Cardiovasc Prev Rehabil 12: 216–220 [DOI] [PubMed] [Google Scholar]
- Wing RR, Phelan S. (2005) Long-term weight loss maintenance. Am J Clin Nutr 82: 222S–225S [DOI] [PubMed] [Google Scholar]
- Wong SL, Katzmarzyk P, Nichaman MZ, Church TS, Blair SN, Ross R. (2004) Cardiorespiratory fitness is associated with lower abdominal fat independent of body mass index. Med Sci Sports Exerc 36: 286–291 [DOI] [PubMed] [Google Scholar]
- WHO (2009) Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks Geneva: World Health Organization [Google Scholar]