Abstract
Blood group antigens are structural variants in surface carbohydrate or amino acid polymorphisms on extracellular domains of membrane proteins. The red cell water channel-forming integral protein (Aquaporin CHIP) is a homotetramer with only one N-glycosylated subunit, however no CHIP-associated blood group antigens have yet been identified. Immunoblotting, monosaccharide composition analysis, and selective glycosidase digestions revealed that the CHIP-associated oligosaccharide contains ABH determinants and resembles a band 3-type glycan that cannot be cleaved from intact membranes by Peptide:N-glycosidase F. The molecular structure of the Colton antigens was previously unknown, but CHIP was selectively immunoprecipitated with anti-Coa or anti-Co(b). The DNA sequence from Colton-typed individuals predicted that residue 45 is alanine in the Co(a+b-) phenotype and valine in the Co(a-b+) phenotype. The nucleotide polymorphism corresponds to a PflMI endonuclease digestion site in the DNA from Co(a-b+) individuals. These studies have defined antigens within two blood group systems on CHIP: (a) an ABH-bearing polylactosaminoglycan attached to a poorly accessible site in the native membrane; and (b) the Colton antigen polymorphism which may permit the identification of rare individuals with defective water channel expression.
Full text
PDF



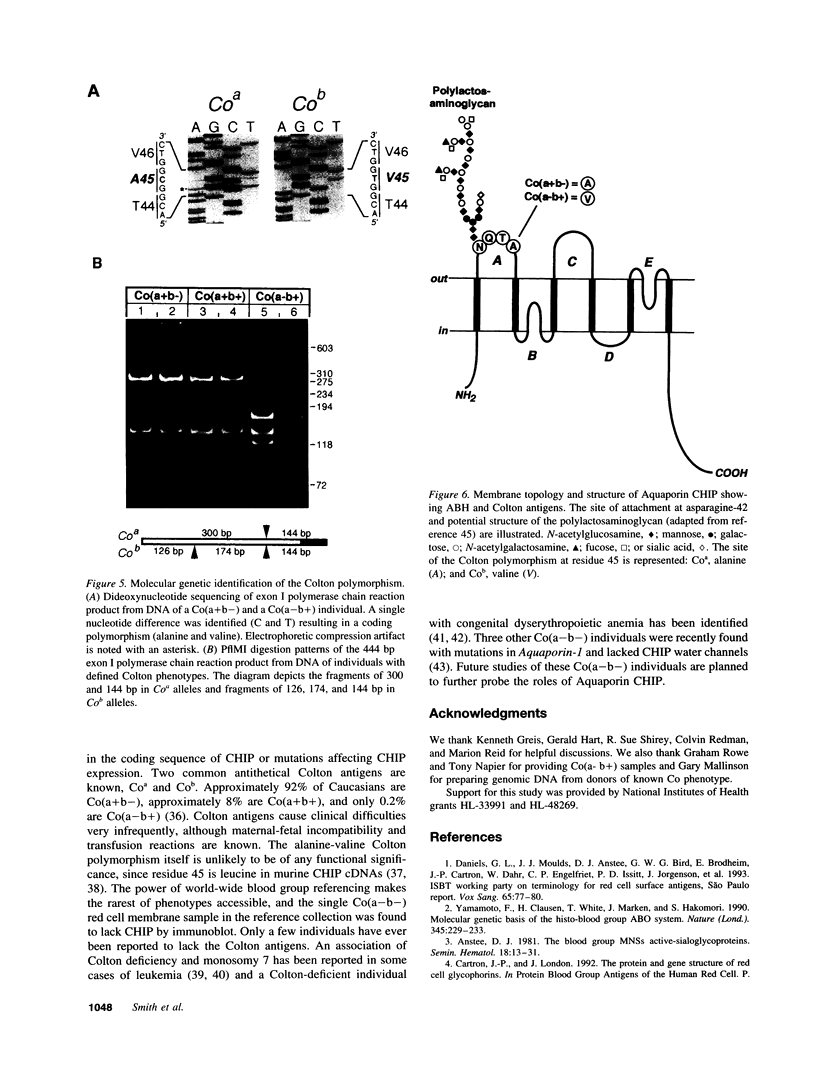

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993 Oct;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Agre P., Saboori A. M., Asimos A., Smith B. L. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem. 1987 Dec 25;262(36):17497–17503. [PubMed] [Google Scholar]
- Agre P., Sasaki S., Chrispeels M. J. Aquaporins: a family of water channel proteins. Am J Physiol. 1993 Sep;265(3 Pt 2):F461–F461. doi: 10.1152/ajprenal.1993.265.3.F461. [DOI] [PubMed] [Google Scholar]
- Agre P., Smith B. L., Baumgarten R., Preston G. M., Pressman E., Wilson P., Illum N., Anstee D. J., Lande M. B., Zeidel M. L. Human red cell Aquaporin CHIP. II. Expression during normal fetal development and in a novel form of congenital dyserythropoietic anemia. J Clin Invest. 1994 Sep;94(3):1050–1058. doi: 10.1172/JCI117419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee D. J. The blood group MNSs-active sialoglycoproteins. Semin Hematol. 1981 Jan;18(1):13–31. [PubMed] [Google Scholar]
- Avent N. D., Ridgwell K., Tanner M. J., Anstee D. J. cDNA cloning of a 30 kDa erythrocyte membrane protein associated with Rh (Rhesus)-blood-group-antigen expression. Biochem J. 1990 Nov 1;271(3):821–825. doi: 10.1042/bj2710821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Polyakova J., Zbrzezna V., Williams K., Gulati S., Pogo A. O. Cloning of glycoprotein D cDNA, which encodes the major subunit of the Duffy blood group system and the receptor for the Plasmodium vivax malaria parasite. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10793–10797. doi: 10.1073/pnas.90.22.10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chérif-Zahar B., Bloy C., Le Van Kim C., Blanchard D., Bailly P., Hermand P., Salmon C., Cartron J. P., Colin Y. Molecular cloning and protein structure of a human blood group Rh polypeptide. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6243–6247. doi: 10.1073/pnas.87.16.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels G. L., Moulds J. J., Anstee D. J., Bird G. W., Brodheim E., Cartron J. P., Dahr W., Engelfriet C. P., Issitt P. D., Jørgensen J. ISBT Working Party on Terminology for Red Cell Surface Antigens. São Paulo report. Vox Sang. 1993;65(1):77–80. doi: 10.1111/j.1423-0410.1993.tb04534.x. [DOI] [PubMed] [Google Scholar]
- De Ca Chapelle A., Vuopio P., Sanger R., Teesdale P. Monosomy-7 and the Colton blood-groups. Lancet. 1975 Oct 25;2(7939):817–817. doi: 10.1016/s0140-6736(75)80042-0. [DOI] [PubMed] [Google Scholar]
- Deen P. M., Dempster J. A., Wieringa B., Van Os C. H. Isolation of a cDNA for rat CHIP28 water channel: high mRNA expression in kidney cortex and inner medulla. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1267–1273. doi: 10.1016/0006-291x(92)91368-z. [DOI] [PubMed] [Google Scholar]
- Denker B. M., Smith B. L., Kuhajda F. P., Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988 Oct 25;263(30):15634–15642. [PubMed] [Google Scholar]
- Fukuda M. N., Masri K. A., Dell A., Thonar E. J., Klier G., Lowenthal R. M. Defective glycosylation of erythrocyte membrane glycoconjugates in a variant of congenital dyserythropoietic anemia type II: association of low level of membrane-bound form of galactosyltransferase. Blood. 1989 Apr;73(5):1331–1339. [PubMed] [Google Scholar]
- Fukuda M., Dell A., Fukuda M. N. Structure of fetal lactosaminoglycan. The carbohydrate moiety of Band 3 isolated from human umbilical cord erythrocytes. J Biol Chem. 1984 Apr 25;259(8):4782–4791. [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Lee Y. C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988 Apr;170(1):54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Jung J. S., Preston G. M., Smith B. L., Guggino W. B., Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994 May 20;269(20):14648–14654. [PubMed] [Google Scholar]
- Lacey P. A., Robinson J., Collins M. L., Bailey D. G., Evans C. C., Moulds J. J., Daniels G. L. Studies on the blood of a Co (a-b-) proposita and her family. Transfusion. 1987 May-Jun;27(3):268–271. doi: 10.1046/j.1537-2995.1987.27387235637.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Zambas E. D., Marsh W. L., Redman C. M. Molecular cloning and primary structure of Kell blood group protein. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6353–6357. doi: 10.1073/pnas.88.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinson G., Martin P. G., Anstee D. J., Tanner M. J., Merry A. H., Tills D., Sonneborn H. H. Identification and partial characterization of the human erythrocyte membrane component(s) that express the antigens of the LW blood-group system. Biochem J. 1986 Mar 15;234(3):649–652. doi: 10.1042/bj2340649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C., Preston G. M., Griffin C. A., Jabs E. W., Agre P. The human aquaporin-CHIP gene. Structure, organization, and chromosomal localization. J Biol Chem. 1993 Jul 25;268(21):15772–15778. [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. F., Jones J., Anstee D. J., Judson P. A., Gardner B., Wiener E., Poole J., Illum N., Wickramasinghe S. N. A novel form of congenital dyserythropoietic anemia associated with deficiency of erythroid CD44 and a unique blood group phenotype [In(a-b-), Co(a-b-)]. Blood. 1994 Feb 1;83(3):860–868. [PubMed] [Google Scholar]
- Pasquali F., Bernasconi P., Casalone R., Fraccaro M., Bernasconi C., Lazzarino M., Morra E., Alessandrino E. P., Marchi M. A., Sanger R. Pathogenetic significance of "pure" monosomy 7 in myeloproliferative disorders. Analysis of 14 cases. Hum Genet. 1982;62(1):40–51. doi: 10.1007/BF00295602. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. Membrane topology of aquaporin CHIP. Analysis of functional epitope-scanning mutants by vectorial proteolysis. J Biol Chem. 1994 Jan 21;269(3):1668–1673. [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993 Jan 5;268(1):17–20. [PubMed] [Google Scholar]
- Ridgwell K., Eyers S. A., Mawby W. J., Anstee D. J., Tanner M. J. Studies on the glycoprotein associated with Rh (rhesus) blood group antigen expression in the human red blood cell membrane. J Biol Chem. 1994 Mar 4;269(9):6410–6416. [PubMed] [Google Scholar]
- Saboori A. M., Smith B. L., Agre P. Polymorphism in the Mr 32,000 Rh protein purified from Rh(D)-positive and -negative erythrocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4042–4045. doi: 10.1073/pnas.85.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. L., Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991 Apr 5;266(10):6407–6415. [PubMed] [Google Scholar]
- Telen M. J., Chasis J. A. Relationship of the human erythrocyte Wrb antigen to an interaction between glycophorin A and band 3. Blood. 1990 Aug 15;76(4):842–848. [PubMed] [Google Scholar]
- Verbavatz J. M., Brown D., Sabolić I., Valenti G., Ausiello D. A., Van Hoek A. N., Ma T., Verkman A. S. Tetrameric assembly of CHIP28 water channels in liposomes and cell membranes: a freeze-fracture study. J Cell Biol. 1993 Nov;123(3):605–618. doi: 10.1083/jcb.123.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz T., Smith B. L., Zeidel M. L., Engel A., Agre P. Biologically active two-dimensional crystals of aquaporin CHIP. J Biol Chem. 1994 Jan 21;269(3):1583–1586. [PubMed] [Google Scholar]
- Yamamoto F., Clausen H., White T., Marken J., Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990 May 17;345(6272):229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Ambudkar S. V., Smith B. L., Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992 Aug 25;31(33):7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Nielsen S., Smith B. L., Ambudkar S. V., Maunsbach A. B., Agre P. Ultrastructure, pharmacologic inhibition, and transport selectivity of aquaporin channel-forming integral protein in proteoliposomes. Biochemistry. 1994 Feb 15;33(6):1606–1615. doi: 10.1021/bi00172a042. [DOI] [PubMed] [Google Scholar]
- Zelinski T., Kaita H., Gilson T., Coghlan G., Philipps S., Lewis M. Linkage between the Colton blood group locus and ASSP11 on chromosome 7. Genomics. 1990 Apr;6(4):623–625. doi: 10.1016/0888-7543(90)90496-h. [DOI] [PubMed] [Google Scholar]