Abstract
People with schizophrenia are more likely to die prematurely than the general population from both suicide and physical ill health. Published studies examining the incidence of cancer in schizophrenia patients report increased, reduced or similar incidence compared with the general population. Older studies tended to report lower incidence rates which fuelled speculation as to the biological and other mechanisms for this protective effect. Furthermore, mortality rates in patients with schizophrenia appear higher than expected. We undertook a non-systematic review of published data to give an overview for these variable findings and illustrate methodological confounders by highlighting a systematic review of breast cancer studies.
Keywords: Cancer, epidemiology, mortality, schizophrenia
Introduction
People with schizophrenia have a life expectancy 15–20 years shorter than the general population in developed countries (Hennekens et al., 2005). This mortality gap also appears to be increasing, suggesting that people with schizophrenia are not benefiting from advances in healthcare to the same extent as the general population. This increased mortality was initially attributed to suicide, but the incidence of suicide cannot account for this mortality gap. Indeed, a recent epidemiological study reported that only one-third of subjects with schizophrenia attempted to harm themselves (Karagianis et al., 2009). In general, this excess mortality is attributable to cardiovascular, neoplastic and respiratory disease (Leucht et al., 2007).
The physical health of schizophrenia patients has received increasing attention (Barnett et al., 2007; Osborn et al., 2007; Smith et al., 2007). Much of that interest has focused on metabolic and cardiovascular disease, with many guidelines being published on the specific management and detection of these disorders in those with serious mental illness (SMI) (Lehman et al., 2004; Taylor et al., 2007). However, the relationship between cancer and schizophrenia is not straightforward and can appear paradoxical. For example, tobacco smoking rates in those with schizophrenia are typically twice those of the background population which would suggest higher rates of lung cancer in those with schizophrenia, but some studies quote a lower incidence of lung cancer in people with schizophrenia (Hippisley-Cox et al., 2007).
Overview of epidemiological studies exploring the relationship between cancer and schizophrenia
Incidence
In 1909 The Board of Control of the Commissioners in Lunacy for England and Wales (1909) noted the possibility of a decreased incidence in cancer among psychiatric patients. Subsequent studies undertaken in the following three decades appeared to confirm these findings, although these results were based upon proportionate mortality ratios which can be misleading because important confounders are not controlled for (Cohen et al., 2002). These early studies were followed by more sophisticated studies which compared selected groups of psychiatric inpatients with the general population in their respective countries and failed to confirm the earlier findings of a decreased cancer incidence.
Mortensen (1989) followed a cohort of 6168 Danish patients with schizophrenia for nearly 30 years and concluded that the overall incidence of cancer was lower for men with schizophrenia than the general population but the same for females with schizophrenia. Mortensen (1994) was able to control for smoking and thereby address suggestions that lower cancer rates in schizophrenia patients were related to prohibitions on smoking in some psychiatric hospitals at certain times. Mortensen (1989) also speculated that psychotropics had a protective effect. Gulbinat et al. (1992) confirmed these findings in Denmark but not in two other study centres, Honolulu and Nagasaki. No attempt was made to control for age, smoking or other risk factors.
Cohen et al. (2002), in a study that controlled for many confounding variables and utilized data from the 1986 US National Mortality Followback Survey, found an odds ratio (OR) of 0.59 (95% confidence interval (CI) 0.38–0.93) for schizophrenia patients developing cancer compared with general population controls. Other modern studies have not replicated these findings of a lower incidence of cancer. Ananth and Burnstein (1977) demonstrated an increase in gastrointestinal tract cancers and breast cancer. Saku et al. (1995) reported similar cancer incidence rates in the general and schizophrenia populations. Lichtermann et al. (2001), using the Finnish Cancer Registry, observed a total of 446,653 subject years and found an increased cancer rate. Nearly half this excess related to lung cancer. However, cancer rates were lower in non-psychotic siblings and parents than in the general population, leading them to suggest that lifestyle factors accounted for the increased cancer rate in those with schizophrenia but that there was a protective effect of schizophrenia risk genes in those not showing the disease.
Catts et al. (2008) were the first to publish a meta-analysis of cancer incidence rates in those with schizophrenia and their relatives. The overall standardized incidence rate (SIR) of cancer in schizophrenia patients was the same as in the general population but was lower in their siblings (SIR = 0.89, CI 0.84–0.94) and parents (SIR = 0.90, CI 0.88–0.93). In a large study in the UK, Goldacre et al. (2005) also found similar incidence rates in those with schizophrenia and the general population. However, in this study they were unable to control for confounders which may have masked differing incidences such as smoking. They did, however, note a lower incidence of skin cancer in those with schizophrenia.
Mortality
Historically, patients with schizophrenia have failed to benefit from the healthcare changes that have benefited the general population, such as improvements in the management of tuberculosis (Odegård, 1952). Tran et al. (2009), in an 11-year prospective follow-up study of a cohort of 3470 patients with schizophrenia, examined cancer-related mortality and predictors. They found a higher standardized mortality rate (SMR) for cancer in those with schizophrenia, and the SMR for the two commonest cancers, namely lung in men and breast in women, was 2.2 (95% CI 1.6–3.3) and 2.8 (95% CI 1.6–4.9), respectively. In the meta-analysis by Saha et al. (2007), the category neoplastic disorder had a median SMR of 1.37 but the authors observed that this was relatively low compared with SMRs for other causes of death. Brown et al. (2010) have reported increased SMRs for patients with schizophrenia and breast or lung cancer in a British cohort.
Assuming an incidence mortality gap exists, there are many potential explanations. These range from factors intrinsic to schizophrenia or its treatment accelerating death from neoplasm, to psychosocial effects such as poor compliance with treatment among patients with comorbid schizophrenia receiving suboptimal treatment due to overt (stigma) or covert (paternalism) discrimination.
Schizophrenia as a protective factor
Given the increased cancer risk factors for patients with schizophrenia, such as smoking, physical inactivity and poor diet, it is perhaps surprising that the literature is confused (Table 1). The very fact that there is not a dramatic increase in cancer rates suggests that schizophrenia may afford some protection. Genetic factors have been advocated to explain the reduced risk, such as the p53 gene producing, through apoptosis, the dual beneficial effects of disrupting neurodevelopment and reducing the risk of cancer (Catts and Catts, 2000; Cui et al., 2005; Park et al., 2004; Yang et al., 2004).
Table 1.
Putative cancer risk and protective factors in schizophrenia
| Risk | Protective | |
|---|---|---|
| Biological | Schizophrenia | Schizophrenia |
| Neuroleptics | Neuroleptics | |
| Hyperprolactinaemia secondary to prolactin elevating antipsychotics | Early death from other causes | |
| Low birth weight | Lower fertility | |
| Lower fertility | ||
| Obesity | ||
| Diabetes/elevated blood sugar | ||
| Inflammatory changes | ||
| Birth order | ||
| Behavioural | Tobacco smoking | Lower exposure to occupational carcinogens |
| Cannabis use | Low exposure to sunlight | |
| Alcohol | ||
| Poor dietary intake | ||
| Lower exercise rates | ||
| Obesity | ||
| Poor self-recognition of physical health problems | ||
| Poor treatment adherence | ||
| Access to healthcare | Stigma | Greater screening in some environments |
| Tenuous engagement with physical health services due to mental state |
Imprinting may also represent an epigenetic factor. Abel et al. (2006) observed that a reduced rate of colorectal cancer in schizophrenia, such as described by Goldacre et al. (2005), may be related to abnormal insulin-like growth factor-2 (IGF-2) imprinting, which is aetiological in the development of colorectal cancer (Jirtle, 2004). Abel et al. (2006) suggested abnormal imprinting (deletion of paternally expressed IGF-2) as a possible mechanism associated with schizophrenia risk (Abel, 2004). Early nutritional influences (prenatal/maternal) may stimulate changes in cytosine methylation to which imprinted genes such as IGF-2 seem susceptible. Early nutrition may also influence susceptibility to schizophrenia as well as to adult obesity, diabetes and cardiovascular disease (Waterland and Jirtle, 2004).
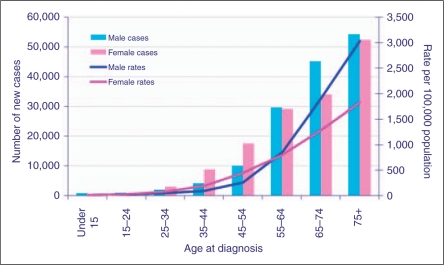
It has been speculated that schizophrenia is a disease of accelerated aging (Kirkpatrick et al., 2008), which seems at odds with the hypothesis that it may be cancer protective. Generally, cancer is a disease of old age and it may be simply that patients are not living long enough to be at significant risk (Figure 1). For example, 80% of cases of breast cancer are diagnosed in women over the age of 50 years. In addition, other factors complicate the interpretation of these studies with regard to accelerated aging. Cancer is not a single disease and has several different aetiological pathways that confluence in the DNA mutation. Cells that have entered senescence from normal aging are not prone to develop cancer because they have reached an irreversible ‘stop’ in the cell cycle (Dimri, 2005).
Figure 1.
Number of new cases and rates, by age and sex, all malignant neoplasms, UK, 2006.
Cancer in the general population
In the UK in 2007 there were 155484 deaths from cancer. Lung, bowel, breast and prostate cancers accounted for 22%, 12%, 8% and 7% of these deaths, respectively (Table 2). Incidence and mortality ratios are higher in men than in women. There is variation in the incidence of cancers between racial groups (Devesa et al., 1999) and countries (Wild et al., 2006), as well as in survival rates (Sant et al., 2001). These persisting regional differences in survival rates suggest there may be corresponding differences in the availability of diagnostic and therapeutic facilities and in the effectiveness of healthcare systems.
Table 2.
The 20 most common cancers in the UK, 2006
| Cancer | Male | Female | Persons | Total |
|---|---|---|---|---|
| Other | 16,380 | 13,948 | 30,328 | 10.33% |
| Mesothelioma | 1942 | 385 | 2327 | 0.79% |
| Cervix | 0 | 2873 | 2873 | 0.98% |
| Liver | 2015 | 1178 | 3193 | 1.09% |
| Multiple myeloma | 2174 | 1813 | 3987 | 1.36% |
| Brain with central nervous system | 1921 | 2611 | 4532 | 1.54% |
| Oral | 3540 | 1785 | 5325 | 1.81% |
| Ovary | 0 | 6596 | 6596 | 2.25% |
| Uterus | 0 | 7045 | 7045 | 2.40% |
| Leukaemias | 4229 | 3008 | 7237 | 2.46% |
| Pancreas | 3731 | 3929 | 7660 | 2.61% |
| Stomach | 4970 | 2743 | 7713 | 2.63% |
| Oesophagus | 5034 | 2790 | 7824 | 2.66% |
| Kidney | 4879 | 2961 | 7840 | 2.67% |
| Bladder | 7307 | 2957 | 10,264 | 3.50% |
| Melanoma | 4803 | 5607 | 10,410 | 3.55% |
| NHL | 5658 | 4911 | 10,569 | 3.60% |
| Prostrate | 35,515 | 0 | 35,515 | 12.10% |
| Colorectal | 20,430 | 17,084 | 37,514 | 12.78% |
| Lung | 22,381 | 16,646 | 39,027 | 13.29% |
| Breast | 314 | 45,508 | 45,822 | 15.61% |
| All | 147,223 | 146,378 | 293,601 |
NHL, non-Hodgkin’s lymphoma.
Numerous other factors influence the incidence of cancers, such as smoking, exposure to other carcinogens, menopausal status and lifestyle factors including exercise level, diet and obesity. For example, in the UK, male lung cancer rates have fallen by 46% from 113 per 100,000 in 1975 to 61 in 2006. Over the same time period, female lung cancer incidence rates have risen by 64% from 23 to 37 per 100,000. These changes are related to smoking rates. The histology of diagnosed lung cancer is also changing, with an increase in adenocarcinoma, which is the most common histological diagnosis in non-smokers. This increase in adenocarcinomas may also be related to the increase use of low-tar cigarettes (Franceschi and Bidoli, 1999).
Many of these factors may be related to observations from many countries showing a socioeconomic effect on cancer survival. In England and Wales, where healthcare is centrally funded, patients from affluent areas have increased survival rates compared with those from disadvantaged areas, and this deprivation gap widened for most cancers between the mid-1980s and the late 1990s (Rachet et al., 2008; Richards, 2008). These deprivation gaps may be avoidable after a time lag, and testicular cancer provides an interesting example of this (Nur et al., 2008; Richards, 2008). Five-year relative survival rates in the most affluent groups were near to reaching the ceiling of 100% by 1996–1999, and survival rates for the most deprived groups seem to have been catching up. In reviewing England and Welsh survival data clinicians tended to explain this survival gradient in terms of comorbidity, but epidemiologists were more likely to argue that late presentation and diagnosis was more relevant (Richards, 2008). A strong socioeconomic effect has also been demonstrated in Denmark, another country where healthcare is centrally funded (Dalton et al., 2008). This national register study of over 26 million patient-years showed poor survival mirrored socioeconomic adversity, including poor housing, unemployment, limited education and the presence of SMI or depression.
Why should cancer rates be different in those with schizophrenia?
Schizophrenia is a devastating illness with a prevalence of around 0.7% and accounts for significant morbidity. The fact that this disease persists has led to speculation that the genes or linkages that predispose to schizophrenia may also confer advantage, and hence the persistence of a disease which apparently confers a reproductive disadvantage. A lower susceptibility to cancer may be one of these genetic advantages. Table 1 describes some putative factors which may affect the incidence and mortality from cancer in the schizophrenia population. The picture is complicated and many of the listed factors can be both protective and confer risk, as well as being inter-related. Just as schizophrenia is not a single illness, cancer is a complex biological phenomenon made up of more than 100 anatomical subtypes, many of which are further divided into variants with differing histology, prognosis and response to treatment. Only some may be modifiable by factors associated with schizophrenia.
Smoking and substance misuse
In general, patients with schizophrenia tend to smoke at twice the rate of the background population (Kelly and McCreadie, 1999). The association between tobacco smoking and cancer is well known, but the use of other noxious substances with potential carcinogenicity is also higher in the schizophrenia population. For example, alcohol is associated with cancers of the oesophagus, and cannabis smoking exposes users to a variety of carcinogens.
Exercise and diet
Exercise and diet are modifiable risk factors for many cancers. While anecdotally patients with schizophrenia are described as having poor diets and being physically inactive, there are few studies investigating these parameters. McCreadie et al. (1998) reported poor dietary habits in a case-control study, with schizophrenia patients consuming significantly less energy, total fibre, retinol, carotene, vitamin C, vitamin E and fruit/vegetables than matched controls. Daumit et al. (2005) surveyed outpatients with schizophrenia and affective disorders at two psychiatric centres in Maryland, United States, and compared physical activity patterns with an age-gender-race-matched national sample of the general population. People with SMI were overall less physically active than the general population, although the proportion with recommended physical activity levels was equal. Participants with SMI were more likely to walk as their sole form of physical activity. Within the SMI group, those without regular social contact and women had higher odds of being inactive. There is strong evidence that physical activity and fitness are cancer protective (Kampert et al., 1996). Ruiz et al. (2008) showed muscular strength is inversely and independently associated with death from all causes and cancer in men, even after adjusting for cardiorespiratory fitness and other potential confounders.
The number of SMI subjects who exercise appropriately is low (Smith et al., 2007), and data in both adolescent and treatment-naïve psychosis populations have confirmed that this reduction in exercise precedes initiation of drug treatments. There are some theoretical reasons, both treatment and illness related, to the interaction of negative symptoms and exercise. There has been little attention on improving exercise rates, but where this has been attempted as part of a lifestyle programme there have been impressive improvements (Smith et al., 2007).
Diet in schizophrenia is also less than ideal as a result of multiple factors ranging from cognition issues, knowledge and money (McCreadie et al., 1998). Research from several sources provides strong evidence that vegetables, fruits, whole grains, dietary fibre, certain micronutrients and some fatty acids protect against some cancers. In contrast, other factors, such as alcohol, some fatty acids and food preparation methods may increase risks (Greenwald, 2001).
Treatment effects
Since the 1950s, effective biological treatments have been developed for schizophrenia and these treatments may have both direct and indirect effects on cancer incidence and mortality (Csatary, 1972). Many antipsychotics appear to have anti-tumour properties in vitro (Carrillo and Benítez, 1999). Pimozide and thioridazine (Strobl and Peterson, 1992) kill cancer cells by blocking the synthesis or movement of cholesterol and lipid in these cells. The addition of mevastatin, a drug that inhibits cholesterol production in cells, augmented this effect. Phenothiazines have also been shown to have anti-tumour properties (Motohashi et al., 2000). However, extrapolating controlled laboratory findings to the real world is difficult given the many potential confounders. Nevertheless, Hippisley-Cox et al. (2007) showed a non-significant statistical increase in the risk of colon cancer with antipsychotics. Harlow et al. (1998) studied the effect of psychotropic medication exposure on epithelial ovarian cancer incidence, and found that any association was largely confined to use of medications that operate through dopaminergic mechanisms (OR 2.9; CI 1.3–6.4) or gabaergic pathways (OR 1.5; CI 0.9–2.5) as opposed to serotoninergic pathways (OR 1.0; CI 0.4–2.1). A case–control study of endometrial cancer after antipsychotics exposure in premenopausal women (Yamazawa et al., 2003) reported that the use of antipsychotics, diabetes mellitus and obesity were identified as independent variables with risk estimates of 5.4, 9.3 and 4.9, respectively.
Hyperprolactinaemia
While antipsychotics may have anti-tumour properties, they also cause side effects which may have the opposite effect. Hyperprolactinaemia is a common side effect for first generation and some second generation antipsychotics such as risperidone and amisulpride (Bushe and Shaw, 2007; Bushe et al., 2008; Kahn et al., 2008). Initially, hyperprolactinaemia was an occult side effect but it is now recognized as a cause of amenorrhoea, galactorrhoea, sexual dysfunction, and osteoporosis and bone fractures (Abel et al., 2008; Howard et al., 2007; Kohen and Wildgust, 2008). While raised prolactin levels are known to promote rodent mammary carcinogenesis and transition to invasive carcinoma, a link between human breast cancer and hyperprolactinaemia was initially discounted (Harvey et al., 2008). In part this was due to the putative protective effect of neuroleptics, differences between rodent and human endocrinology and the failure of human tumours to respond to prolactin-lowering drugs such as bromocriptine (Harvey et al., 2008). However, there is increasing evidence linking elevated prolactin levels to human breast cancer in the general population (Tworoger et al., 2004, 2007). The large-scale epidemiological US nurses’ study reported an increased risk associated with normal prolactin levels when comparing breast cancer incidence in the upper versus lower quartile of normal levels (Tworoger et al., 2004, 2007). Wang et al. (2002) found an increase of 16% in the risk of breast cancer among women who had used antipsychotics and other prolactin-elevating drugs such as some anti-emetics. Furthermore, this relationship showed a dose response. Peveler et al. (2008) stated that hyperprolactinaemia should be considered when starting an antipsychotic in a woman with a personal or family history of breast cancer.
Szarfman et al. (2006) described a disproportionality in reported incidence of hyperprolactinaemia, galactorrhoea and pituitary tumours between seven widely used antipsychotic drugs. The role of hyperprolactinaemia in prostate cancer is unclear, but the effects of prolactin on prostate cancer cells are similar to those in breast cancer cells (Harvey et al., 2008). Compared with breast cancer, few clinical studies have been undertaken and a potential confounder is whether a high prolactin level is a cause or an effect of prostate cancer. One small study of 50 men with metastatic disease did show a relationship (Lissoni et al., 2005), but a prospective case-control study of 144 men with prostate cancer matched with 289 controls revealed no relationship (Stattin et al., 2001).
Obesity
Obesity is described as a modifiable risk factor for cancer and increases the risk of cancers including cancers of the breast (postmenopausal), endometrium, colon, kidney and oesophagus. Research suggests that maintaining a healthy weight conveys the greater protective effect, but weight loss has been shown to be beneficial in reducing breast cancer risk (Harvie et al., 2005; Parker and Folsom, 2003). However, the effects of weight cycling are less well understood, with one small study (Luo et al., 2007) showing an increased kidney cancer risk in women who went through 10 or more episodes of varying their weight by 10 lbs or more.
Patients with schizophrenia may be metabolically obese in spite of a normal body mass index (BMI) (Thakore, 2004). Antipsychotics and antidepressants may predispose patients to weight gain, and patients with schizophrenia are more likely to have centripetal obesity which predisposes to certain cancers such as bowel (Dai et al., 2007) and breast (Connolly et al., 2002). Proposed mechanisms by which obesity may predispose to cancer risk include through glucose metabolism and, especially in the case of breast and endometrial cancer, through hormonal mechanisms. Other mechanisms are also relevant, such as obesity increasing the risk of oesophageal cancer by causing gastric acid reflux (Freeman, 2004) and increasing the risk of gallstones, which in turn increase the risk of gallbladder cancer (Larsson and Wolk, 2007). Obesity may be a consequence of or a cause of physical inactivity and an unhealthy diet.
Obesity is just one feature of metabolic syndrome; some other components including hypertension and diabetes may also confer an increased risk of cancer. Grossman (2002) systematically reviewed the literature and reported that in prospective studies, patients with hypertension experienced an increased rate of cancer mortality (follow-up period 9–20 years), with an age- and smoking-adjusted pooled OR of 1.23 (95% CI 1.11–1.36). In 13 case–control studies, including 6964 cases of renal cell cancer and 9181 controls, the adjusted OR for renal cell cancer among hypertensive patients, relative to normotensive counterparts, was 1.75 (95% CI 1.61–1.90).
Obesity is a risk factor for diabetes, and there is a developing epidemiological literature suggesting an association between history of metabolic syndrome/diabetes and risk of developing a variety of cancers (Zänker, 2008). This suggests that metabolic syndrome/diabetes mellitus type 2 are associated with an increased risk of cancer, or are even independent predictors of mortality from hepatocellular carcinoma and cancer of the colon and pancreas in men and women, as well as cancer of the breast and endometrium in women and liver and bladder in men. However, the association is complex, with some studies showing a lower risk of cancer among patients with diabetes; for example, Waters et al. (2009) found a lower risk of prostate cancer in diabetic than in non-diabetic subjects. An association between diabetes, hyperinsulinemia, insulin resistance, insulin-like growth factors, lipotoxicity, obesity, adipokines, Western-style dietary habits and carcinogenesis appears plausible. Furthermore, increased blood sugars are associated with an increased mortality (Stocks et al., 2009). Stocks et al. (2009) described raised blood glucose as a risk factor for cancer that was independent of obesity and smoking. The increased prevalence of diabetes in patients with schizophrenia in an increasingly obesogenic society should be seen as a public health concern.
Obstetric factors
The risk of schizophrenia is influenced by birth order, although findings are not entirely consistent. Kemppainen et al. (2001) demonstrated increased risk of schizophrenia in male first-borns (OR 1.5; 95% CI 1.0–2.2) and last-born females (OR 1.3; 95% CI 0.9–1.9). Male first-borns are also at higher risk of testicular cancer (Cook et al., 2009). These findings may suggest that patients with schizophrenia may be at increased risk of testicular cancer; Goldacre et al. (2005) have shown higher rates of testicular cancer in schizophrenia (OR 1.30; 95% CI 0.35–3.39), but there are too few cases to draw conclusions.
Decreased birth weight is also a risk factor for schizophrenia, as well as for cardiovascular disease. Furthermore, recent research suggests that low birth weight may be a risk factor for malignancy. Very low birth weight was associated with an increased risk of certain types of paediatric brain cancer, such as some gliomas (Spector et al., 2009). However, if breast cancer is considered, a different conclusion is reached. In a meta-analysis of 18 studies (16,424 breast cancer cases) (Xu et al., 2009), women with a birth weight of less than 4000 g or 8.5 lb had a higher risk for developing breast cancer than those with birth weight less than 2500 g or 3000 g (OR 1.20, 95% CI 1.08, 1.34). These findings were also consistent with a dose-response pattern effect. Breast cancer is described as hormone sensitive, but in another hormone-sensitive cancer, endometrial cancer, there was no evidence of a link between birth weight and incidence (Löf et al., 2007). In addition, this study of 38,566 Swedish women demonstrated the protective effect of low birth weight on breast cancer risk.
Methodological issues
Introduction/overview
Cancer is, in general terms, an illness diagnosed in patients aged over 50 years. Some of the complexity in interpreting dissonant cancer data can be explained by the simple metric that few current studies have been able to include and follow cohorts that are age appropriate. For example, cancer data in first-episode subjects may be of lesser clinical utility and require potentially immense powering to demonstrate significant differences. Often the cancers studied are not the most prevalent in this age group, such as leukaemia. Furthermore, cancer, like schizophrenia, is not a unitary disease. Even in one organ there are many potential cellular types, each with a different aetiology and prognosis.
Breast cancer
Breast cancer is the most common cancer in women, with a lifetime risk of 1 in 9 in the general population. Incidence rates increase markedly with age, with 80% of cases diagnosed in women aged over 50 years (Office for National Statistics, 2007). This high lifetime risk should facilitate epidemiological studies, and a recent systematic review identified over 400 reports (Bushe et al., 2009). However, when preset quality indicators were applied, only 13 studies were judged to be of sufficient quality. One identified quality indicator was the inclusion of more than 100,000 patient-years follow-up (Baldwin et al., 1987). Studies not achieving this threshold tended to report no difference in breast cancer rates between the general population and those with schizophrenia. Studies with greater patient-years, and therefore better powered to account for confounders, generally found a higher incidence of breast cancer in those with schizophrenia (Table 3). The three largest studies, Grinshpoon et al. (2005), Lichtermann et al. (2001) and Dalton et al. (2005), report SIRs (95% CIs) of 1.11 (1.00–1.22), 1.20 (1.05–1.38) and 1.15 (0.98–1.34), respectively.
Table 3.
Systematic review of breast cancer incidence since 1986
| Study | N | Breast cancer cases observed | Study period | Breast cancer outcome |
|---|---|---|---|---|
| Dupont et al. (1986) Denmark | 3196 ♀ | 102 | 1957–1980 | SIR 1.09 (0.89–1.33) |
| Nakane and Ohta (1986) Japan | 1388 ♀ | 6 | 1960–1978 | RR 3.23 (1.16–6.87) p < 0.01. In ♀s < 53 years at study end RR 8.06 (2.62–18.82) p < 0.001 |
| Mortensen (1989) Denmark | 3196 ♀♂ | 125 ♀, 2 ♂ | 1957–1984 | IRR 1.19 (ns) ♀s IRR 1.19 ♀s and ♂s (p < 0.05) |
| Gulbinat et al. (1992) | 2779 ♀ | Not stated | 1962–1980 | SIR 1.6 (0.52–3.74) |
| Mortensen (1994) Denmark | 3498 ♀ | 22 | 1970–1987 | SIR 0.88 (non sig) |
| Lichtermann et al. (2001) Finland | 11,418 ♀ | 152 | 1971–1996 | SIR 1.15 (0.98–1.34) |
| Dalton et al. (2003) Denmark | 7541 ♀ | 74 | 1970–1997 | RR 0.90 (0.71–1.12) |
| Barak (2005) Israel | 1247 ♀ | 22 | 1993–2003 | SIR 0.60 (0.37–0.90) |
| Dalton et al. (2005) Denmark | 9743 ♀ | 215 | 1969–1993 | SIR 1.2 (1.05–1.38) |
| Goldacre et al. (2005) UK | 9649 ♀♂ | 80 | 1963–1999 | Adjusted rate ratio 1.01 (0.80–1.26) |
| Grinshpoon et al. (2005) Israel | 33,372 ♀ | 370 | 1962–2001 | SIR total, 1.11 (1.00–1.22) |
| Hippisley-Cox et al. (2007) UK | 49 | 1995–2005 | Adjusted OR 1.52 (1.10–2.11) | |
| Barak et al. (2008) Israel | 2011 ♀ | 51 | 1960–2005 | SIR 0.63 (0.47–0.83) |
IRR, incidence rate ratio; RR, relative risk; SIR, standardized incidence ratio.
The largest risk is in the cohort with median age 61 years at cancer diagnosis (OR 1.52; 95% CI 1.10–2.11) (Hippisley-Cox, et al., 2007). This was a nested, case-control study and thus differs from all other included studies. In reviewing the literature, Bushe et al. (2009) also highlight the difficulties posed in this area of research. There are many potential confounders to account for, including age, parity, menopausal status, contraceptive history, obesity, smoking history and dietary history. Most published research relies on national disease databases, which are only maintained in a few countries and, as Bushe et al. (2009) identified, there is potential double counting in meta-analysis as published papers may have used subjects from the same population. Even in national cohorts of a common cancer such as breast, the absolute number of actual cases is small. Epidemiological studies cannot generally investigate histological tumour type, so it is unclear as to whether the distribution of breast cancers in women with schizophrenia follows that in the general population.
Access to healthcare
In the UK there is evidence that people with schizophrenia do consult with their general practitioner (Harvey, 1996). However, they may not benefit from these consultations. Observational studies show high rates of hypertension and dyslipidaemias, but also that prescribing rates of anti-hypertensives and statins are far lower than would be expected (Strom et al., 2008). In a Danish cohort study of cardiovascular outcomes, Laursen et al. (2009) observed that patients with SMI were far less likely to receive invasive cardiac procedures than matched controls, which probably explained their lower life expectancy. Kisely et al. (2007) reported similar findings from a study in Novia Scotia, Canada. Two Canadian studies exploring uptake of cervical (Martens et al., 2009) and breast cancer screening (Chochinov et al., 2009) showed lower rates for those with schizophrenia. A study of mammography screening from the UK was slightly more optimistic for those with mental illness in general, but highlighted SMI as a risk factor for lower uptake (Werneke et al., 2006). Kisely et al. (2008) also demonstrated that people with mental illness in Nova Scotia had an increased mortality from cancer, which could not be explained, for example, by an increased incidence. They speculated that this excess mortality was caused by delays in detection or initial presentation leading to more advanced staging at diagnosis, and difficulties in communication or access to healthcare.
Relevance to psychiatric clinical practice
While some psychiatrists will not see the physical health of their patients as their concern, or indeed, within their competence (Kick et al., 1997), the overwhelming evidence of poor physical health in those with schizophrenia makes such a stance difficult to justify (Hodgson and Adeyemo, 2004). Guidance from the UK’s National Institute of Clinical Health (NICE, 2009) places primary care centrally in physical healthcare but outlines what is expected from mental health services in helping patients access these services.
Psychiatrists can play a preventative role and encourage patients to review lifestyle risk factors such as a sedentary lifestyle, poor nutrition, smoking and obesity. Tackling these issues not only reduces cancer risk factors but also those associated with cardiovascular disease. Of course, there are also other tangible benefits to be achieved by attention to lifestyle. Smoking cessation interventions may potentially reap the greatest rewards in improving physical health. While our review would suggest that certain cancers are more prevalent in the schizophrenia population and others less prevalent, this does not mean a complacent attitude can be adopted for the latter.
Mental health professionals need to encourage patients to make use of appropriate screening opportunities and to support them through a healthcare system that may seem daunting if a malignancy is discovered, in order to maximize outcomes. Even though cancer is a common cause of death, it is still primarily a disease of older people. However, while psychiatrists will see fewer patients with cancer than many other non-cancer specialists, this should not dissuade them from advocating for their patients.
Conclusions
This overview has highlighted the complexities of research in this area. There are many methodological considerations for epidemiological studies relating to cancer risk and schizophrenia. The potential number of confounders makes unravelling clinically significant associations difficult unless very large samples are used. Many of these confounders will vary temporally, such as relatively recent trends in obesity. A greater comprehension of the genetics and molecular mechanisms of cancer and schizophrenia may also refine our understanding of any relationship between these disorders, and also potentially focus epidemiological research with a greater understanding of phenotypes and genotypes. The recent unveiling of a cancer genome and the considerations that advances in epigenetic factors underline the complexities of research in this area.
If the risk of certain tumours is found to differ between the general and the schizophrenia population, this should aid targeting at risk populations for screening, as is seen in the Canadian Diabetic Guidelines where schizophrenia is seen as defining an at-risk group. However, some patients with schizophrenia will still need help in accessing appropriate screening and oncology treatment services if a reduction in mortality is to be achieved. If some cancers do have a lower incidence in schizophrenia; this does not mean they can be ignored. Even if there is a decreased risk of cancer, it should be remembered that patients with schizophrenia are still at an increased risk of metabolic and cardiovascular disease, and many of the risk factors for these disorders overlap with those for cancer.
From a pragmatic perspective, the practising clinician should be mindful of the mortality gap in schizophrenia and be vigilant for somatic health problems in their patients.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
Richard Hodgson received educational support and speaker fees from a number of drug companies. Hiram J Wildgust received lecture and consultancy fees from Eli Lilly and Company. Chris Bushe is an employee of Eli Lilly and Company, the manufacturer of olanzapine.
References
- Abel KM. (2004) Foetal origins of schizophrenia: testable hypotheses of genetic and environmental influences. Br J Psychiatry 184: 383–385 [DOI] [PubMed] [Google Scholar]
- Abel KM, Allin MP, Jirtle AL. (2006) Schizophrenia, cancer and imprinting: early nutritional influences. Br J Psychiatry 188: 394. [DOI] [PubMed] [Google Scholar]
- Abel KM, Heatlie HF, Howard LM, Webb R. (2008) Sex and age-specific incidence of fractures in mental illness: an historical population-based cohort study. J Clin Psychiatry 69: 1398–1403 [DOI] [PubMed] [Google Scholar]
- Ananth J, Burnstein M. (1977) Cancer: less common in psychiatric patients? Psychosomatics 18: 44–46 [DOI] [PubMed] [Google Scholar]
- Baldwin J, Acheson ED, Graham WJ. (1987) Textbook of Medical Record Linkage Oxford: Oxford University Press [Google Scholar]
- Barnett AH, Mackin P, Chaudhry I, et al. (2007) Minimising metabolic and cardiovascular risk in schizophrenia: diabetes, obesity and dyslipidaemia. J Psychopharmacol 21: 357–373 [DOI] [PubMed] [Google Scholar]
- Barak Y, Achiron A, Mandel M, Mirecki I, Aizenberg D. (2005) Reduced cancer incidence among patients with schizophrenia. Cancer 104: 2817–2821 [DOI] [PubMed] [Google Scholar]
- Barak Y, Levy T, Achiron A, Aizenberg D. (2008) Breast cancer in women suffering from serious mental illness. Schizophr Res 102: 249–253 [DOI] [PubMed] [Google Scholar]
- Brown S, Kim M, Mitchell C, Inskip H. (2010) Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry 196: 116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushe C, Shaw M. (2007) Prevalence of hyperprolactinaemia in a naturalistic cohort of schizophrenia and bipolar outpatients during treatment with typical and atypical antipsychotics. J Psychopharmacol 21: 768–773 [DOI] [PubMed] [Google Scholar]
- Bushe C, Bradley A, Wildgust H, Hodgson R. (2009) Schizophrenia and breast cancer incidence. A systematic review of clinical studies. Schizophr Res 114: 6–16 [DOI] [PubMed] [Google Scholar]
- Bushe C, Yeomans D, Floyd T, Smith SM. (2008) Categorical prevalence and severity of hyperprolactinaemia in two UK cohorts of patients with severe mental illness during treatment with antipsychotics. J Psychopharmacol 22(2 suppl): 56–62 [DOI] [PubMed] [Google Scholar]
- Carrillo JA, Benítez J. (1999) Are antipsychotic drugs potentially chemopreventive agents for cancer? Eur J Clin Pharmacol 55: 487–488 [DOI] [PubMed] [Google Scholar]
- Catts VS, Catts SV. (2000) Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res 41: 405–415 [DOI] [PubMed] [Google Scholar]
- Catts VS, ` SV, O’Toole BI, Frost ADJ. (2008) Cancer incidence in patients with schizophrenia and their first-degree relatives – a meta-analysis. Acta Psychiatr Scand 117: 323–336 [DOI] [PubMed] [Google Scholar]
- Chochinov HM, Martens PJ, Prior HJ, Fransoo R, Burland E, Need To Know Team (2009) Does a diagnosis of schizophrenia reduce rates of mammography screening? A Manitoba population-based study. Schizophr Res 113: 95–100 [DOI] [PubMed] [Google Scholar]
- Cohen ME, Dembling B, Schorling JB. (2002) The association between schizophrenia and cancer: a population-based mortality study. Schizophr Res 57: 139–146 [DOI] [PubMed] [Google Scholar]
- Commissioners in Lunacy for England and Wales (1909) Annual Report London: HMSO [Google Scholar]
- Cook MB, Akre O, Forman D, et al. (2009) A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer – experiences of the mother. Int J Epidemiol 38: 1532–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly BS, Barnett C, Vogt KN, Li T, Stone J, Boyd NF. (2002) A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer. Nutr Cancer 44: 127–138 [DOI] [PubMed] [Google Scholar]
- Csatary LK. (1972) Chlorpromazines and cancer. Lancet 2: 338–339 [DOI] [PubMed] [Google Scholar]
- Cui DH, Jiang KD, Jiang SD, Xu YF, Yao H. (2005) The tumor suppressor adenomatous polyposis coli gene is associated with susceptibility to schizophrenia. Mol Psychiatry 10: 669–677 [DOI] [PubMed] [Google Scholar]
- Dai Z, Xu YC, Niu L. (2007) Obesity and colorectal cancer risk: A meta-analysis of cohort studies. World J Gastroenterol 13: 4199–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton SO, Laursen TM, Mellemkjaer L, Johansen C, Mortensen PB. (2003) Risk for cancer in parents of patients with schizophrenia. Am J Psychiatry 161: 903–908 [DOI] [PubMed] [Google Scholar]
- Dalton SO, Mellemkjaer L, Thomassen L, et al. (2005) Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969–1993. Schizophr Res 75: 315–324 [DOI] [PubMed] [Google Scholar]
- Dalton S, Steding-Jessen M, Gislum M, et al. (2008) Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: Summary of findings. Eur J Cancer 44: 1938–1949 [DOI] [PubMed] [Google Scholar]
- Daumit GL, Goldberg RW, Anthony C, et al. (2005) Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 193: 641–646 [DOI] [PubMed] [Google Scholar]
- Devesa SS, Grauman DG, Blot WJ, et al. (1999) Atlas of Cancer Mortality in the United States, 1950–94 Washington, DC: US Govt Print Office; NIH Publ No. 99-4564. [Google Scholar]
- Dimri GP. (2005) What has senescence got to do with cancer? Cancer Cell 7: 505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont A, Jensen OM, Strömgren E, Jablensky A. (1986) Incidence of cancer in patients diagnosed as schizophrenic in Denmark. In: Ten Horn GHMM, Giel R, Gulbinat W, Henderson JH. (eds) Psychiatric Case Registries in Public Health Amsterdam: Elsevier Science; 229–239 [Google Scholar]
- Franceschi S, Bidoli E. (1999) The epidemiology of lung cancer. Ann Oncol 10(suppl 5): S3–6 [DOI] [PubMed] [Google Scholar]
- Freeman H. (2004) Risk of gastrointestinal malignancies and mechanisms of cancer development with obesity and its treatment. Best Pract Res Clin Gastroenterol 18: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V. (2005) Schizophrenia and cancer: an epidemiological study. Br J Psychiatry 187: 334–338 [DOI] [PubMed] [Google Scholar]
- Greenwald P. (2001) Diet and cancer prevention. Eur J Cancer 37: 948–965 [DOI] [PubMed] [Google Scholar]
- Grinshpoon A, Barchana M, Ponizovsky A, et al. (2005) Cancer in schizophrenia: is the risk higher or lower. Schizophr Res 73: 333–341 [DOI] [PubMed] [Google Scholar]
- Grossman E. (2002) Is there an association between hypertension and cancer mortality? Am J Med 112: 479–486 [DOI] [PubMed] [Google Scholar]
- Gulbinat W, DuPont A, Jablensky A, et al. (1992) Cancer incidence of schizophrenic patients. Results of record linkage studies in three countries. Br J Psychiatry 161(suppl. 18): 75–85 [PubMed] [Google Scholar]
- Harlow BL, Cramer DW, Baron JA, Titus-Ernstoff L, Greenberg ER. (1998) Psychotropic medication use and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 7: 697–702 [PubMed] [Google Scholar]
- Harvey C. (1996) The Camden schizophrenia surveys. I. The psychiatric, behavioural and social characteristics of the severely mentally ill in an inner London health district. Br J Psychiatry 168: 410–417 [DOI] [PubMed] [Google Scholar]
- Harvey PW, Everett DJ, Springall CJ. (2008) Adverse effects of prolactin in rodents and humans: breast and prostate cancer. J Psychopharmacol 22: S2: 20–27 [DOI] [PubMed] [Google Scholar]
- Harvie M, Howell A, Vierkant RA, et al. (2005) Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev 14: 656–661 [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. (2005) Schizophrenia and increased risks of cardiovascular disease. Am Heart J 150: 1115–1121 [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J, Vinogradova Y, Coupland C, Parker C. (2007) Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case–control study. Arch Gen Psychiatry 64: 1368–1376 [DOI] [PubMed] [Google Scholar]
- Hodgson RE, Adeyemo O. (2004) Too little, too late? Physical examinations performed by trainee psychiatrists on newly admitted psychiatric patients. Int J Psychiat Clin Pract 8: 57–60 [DOI] [PubMed] [Google Scholar]
- Howard L, Kirkwood G, Leese M. (2007) Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry 190: 129–134 [DOI] [PubMed] [Google Scholar]
- Jirtle RL. (2004) IGF2 loss of imprinting: a potential heritable risk factor for colorectal cancer. Gastroenterology 126: 1190–1201 [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H, et al. (2008) Effectiveness of antipsychotic drugs in first episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371: 1085–1097 [DOI] [PubMed] [Google Scholar]
- Kampert JB, Blair SN, Barlow CE. (1996) Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol 6: 452–457 [DOI] [PubMed] [Google Scholar]
- Karagianis J, Novick D, Pecenak J, Haro JM. (2009) Worldwide-Schizophrenia Outpatient Health Outcomes (W-SOHO): Baseline characteristics of pan-regional observational data from more than 17,000 patients. Int J Clin Pract 63: 1578–1588 [DOI] [PubMed] [Google Scholar]
- Kelly C, McCreadie RG. (1999) Smoking habits, current symptoms and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry 156: 1751–1757 [DOI] [PubMed] [Google Scholar]
- Kemppainen L, Veijola J, Jokelainen J, Hartikainen A-L. (2001) Birth order and risk for schizophrenia: a 31-year follow-up of the Northern Finland 1966 Birth Cohort. Acta Psychiatr Scand 104: 148–152 [DOI] [PubMed] [Google Scholar]
- Kick SD, Morrison M, Kathol RG. (1997) Medical training in psychiatry residence. A proposed curriculum. Gen Hosp Psychiatry 19: 259–266 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. (2008) Is schizophrenia a syndrome of accelerated aging. Schizophr Bull 34: 1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisely S, Sadek J, MacKenzie A, Lawrence D, Campbell LA. (2008) Excess cancer mortality in psychiatric patients. Can J Psychiatry 53: 753–761 [DOI] [PubMed] [Google Scholar]
- Kisely S, Smith M, Lawrence D, Cox M, Campbell LA, Maaten S. (2007) Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ 176: 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen D, Wildgust HJ. (2008) The evolution of hyperprolactinaemia as an entity in psychiatric patients. J Psychopharmacol 22(suppl 2): 6–11 [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wolk A. (2007) Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer 96: 1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Mortensen PB. (2009) Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry 66: 713–720 [DOI] [PubMed] [Google Scholar]
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH. (2004) American Psychiatric Association; Steering Committee on Practice Guidelines 2004. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 161(2 suppl): 1–56 [PubMed] [Google Scholar]
- Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. (2007) Physical Illness and Schizophrenia Cambridge: Cambridge University Press; [DOI] [PubMed] [Google Scholar]
- Lichtermann D, Ekelund J, Pukkala E, et al. (2001) Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 58: 573–578 [DOI] [PubMed] [Google Scholar]
- Lissoni P, Bignami A, Frontini L, Manganini V. (2005) Possible involvement of prolactin in endocrine-resistant metastatic prostate cancer. Int J Biol Markers 20: 123–125 [DOI] [PubMed] [Google Scholar]
- Löf M, Sandin S, Hilakivi-Clarke l, Weiderpass E. (2007) Birth weight in relation to endometrial and breast cancer risks in Swedish women. Br J Cancer 96: 134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Margolis KL, Adami HO, et al. (2007) Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: The Women’s Health Initiative (United States). Am J Epidemiol 166: 752–759 [DOI] [PubMed] [Google Scholar]
- McCreadie RG, MacDonald E, Blacklock C, et al. (1998) Dietary intake of schizophrenic patients in Nithsdale, Scotland: case–control study. Br Med J 317: 784–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens PJ, Chochinov H, Prior H, et al. (2009) Are cervical cancer screening rates different for women with schizophrenia? A Manitoba population-based study. Schizophr Res 113: 101–106 [DOI] [PubMed] [Google Scholar]
- Mortensen PB. (1989) The incidence of cancer in schizophrenic patients. J Epidemiol Community Health 43: 43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB. (1994) The occurrence of cancer in first-admitted schizophrenic patients. Schizophr Res 12: 185–194 [DOI] [PubMed] [Google Scholar]
- Motohashi N, Kawase M, Saito S, Sakagami H. (2000) Antitumor potential and possible targets of phenothiazine-related compounds. Curr Drug Targets 1: 237–245 [DOI] [PubMed] [Google Scholar]
- Nakane Y, Ohta Y. (1986) The example of a linkage with a cancer register. In: Ten Horn GHMM, Giel R, Gulbinat WH, Henderson JH. (eds) Psychiatric Case Registers in Public Health Oxford: Elsevier; 240–245 [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) (2009) NICE clinical guideline 82. Schizophrenia: Core interventions in the treatment and management of schizophrenia in adults in primary and secondary care. Update of NICE clinical guideline 1 Available at: (www.nice.org.uk).
- Nur U, Rachet B, Mitry E, Cooper N, Coleman MP. (2008) Survival from testicular cancer in England and Wales up to 2001. Br J Cancer 99: S80–S82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegård O. (1952) The excess mortality of the insane. Acta Psychiatr Neurol Scand 27: 353–367 [PubMed] [Google Scholar]
- Office for National Statistics (2007) Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2004, England. Series MB1 no.35 London: Office for National Statistics [Google Scholar]
- Osborn DJP, Levy G, Nazareth I, Peterson I. (2007) Relative risk of cardiovascular and cancer mortality in people with severe mental illness. From the United Kingdom’s General Practice Research Database. Arch Gen Psychiatry 64: 242–249 [DOI] [PubMed] [Google Scholar]
- Park JK, Lee HJ, Kim JW, et al. (2004) Differences in p53 gene polymorphisms between Korean schizophrenia and lung cancer patients. Schizophr Res 67: 71–74 [DOI] [PubMed] [Google Scholar]
- Parker ED, Folsom AR. (2003) Intentional weight loss and incidence of obesity-related cancers: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord 27: 1447–1452 [DOI] [PubMed] [Google Scholar]
- Peveler RC, Branford D, Citrome L, et al. (2008) Antipsychotic associated hyperprolactinaemia: Clinical recommendations. J Psychopharmacol 22(suppl 2): 98–103 [DOI] [PubMed] [Google Scholar]
- Rachet B, Woods LM, Mitry E, et al. (2008) Cancer survival in England and Wales at the end of the 20th century. Br J Cancer 99: S2–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MA. (2008) Forward. Br J Cancer 99: S1–S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JR, Sui X, Lobelo F, Morrow JR. (2008) Association between muscular strength and mortality in men: prospective cohort study. Br Med J 337: a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. (2007) A systematic review of mortality in schizophrenia. Is the differential mortality gap worsening over time? Arch Gen Psychiatry 64: 1123–1131 [DOI] [PubMed] [Google Scholar]
- Saku M, Tokudome S, Ikeda M, et al. (1995) Mortality in psychiatric patients, with a specific focus on cancer mortality associated with schizophrenia. Int J Epidemiol 24: 366–372 [DOI] [PubMed] [Google Scholar]
- Sant M, Capocaccia R, Coleman MP, et al. (2001) Cancer survival increases in Europe, but international differences remain wide. Eur J Cancer 37: 1659–1667 [DOI] [PubMed] [Google Scholar]
- Smith S, Yeomans D, Bushe CJ, et al. (2007) A well-being programme in severe mental illness. Reducing risk for physical ill-health: A post-programme service evaluation at 2 years. Eur Psychiatry 22: 413–418 [DOI] [PubMed] [Google Scholar]
- Spector LG, Puumala SE, Carozza SE, Chow EJ. (2009) Cancer risk among children with very low birth weights. Pediatrics 124: 96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin P, Rinaldi S, Stenman UH, Riboli E. (2001) Plasma prolactin and prostate cancer risk: a prospective study. Int J Cancer 92: 463–465 [DOI] [PubMed] [Google Scholar]
- Stocks T, Rapp K, Bjørge T, et al. (2009) Blood glucose and risk of incident and fatal cancer in the Metabolic Syndrome and Cancer Project (Me-Can): Analysis of six prospective cohorts. PLoS Med 6: e1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl JS, Peterson VA. (1992) Tamoxifen-resistant human breast cancer cell growth: inhibition by thioridazine, pimozide and the calmodulin antagonist, W-13. J Pharmacol Exp Ther 263: 186–193 [PubMed] [Google Scholar]
- Strom BL, Faich GA, Reynolds RF, et al. (2008) The Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC): design and baseline subject characteristics. J Clin Psychiatry 69: 114–121 [DOI] [PubMed] [Google Scholar]
- Szarfman A, Tonning JM, Levine JG, Doraiswamy PM. (2006) Atypical antipsychotics and pituitary tumours: a pharmacovigilance study. Pharmacotherapy 26: 748–758 [DOI] [PubMed] [Google Scholar]
- Taylor D, Paton C, Kerwin R. (2007) The South London and Maudsley NHS Foundation Trust and Oxleas NHS Foundation Trust Prescribing Guidelines, 9th ed London: Informa Healthcare [Google Scholar]
- Thakore J. (2004) Metabolic disturbance in first-episode schizophrenia. Br J Psychiatry 184: s76–S79 [DOI] [PubMed] [Google Scholar]
- Tran E, Rouillon F, Loze JY, et al. (2009) Cancer mortality in patients with schizophrenia: an 11-year prospective cohort study. Cancer 115: 3555–3562 [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE. (2004) Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res 64: 6814–6819 [DOI] [PubMed] [Google Scholar]
- Tworoger S, Eliassen H, Sluss P, Hankinson S. (2007) A prospective study of plasma prolactin concentrations and the risk of premenopausal and postmenopausal breast cancer. J Clin Oncol 25: 1–7 [DOI] [PubMed] [Google Scholar]
- Wang PS, Walker AM, Tsuang MT, et al. (2002) Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 59: 1147–1154 [DOI] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. (2004) Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 20: 63–68 [DOI] [PubMed] [Google Scholar]
- Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. (2009) Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol 169: 937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke U, Horn O, Maryon-Davis A, Wessely S, Donnan S, McPherson K. (2006) Uptake of screening for breast cancer in patients with mental health problems. J Epidemiol Community Health 60: 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild SH, Fischbacher CM, Brock A, Griffiths C. (2006) Mortality from all cancers and lung, colorectal, breast and prostate cancer by country of birth in England and Wales, 2001–2003. Br J Cancer 94: 1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Dailey AB, Peoples-Sheps M, Talbott EO, Li N, Roth J. (2009) Birth weight as a risk factor for breast cancer: a meta-analysis of 18 epidemiological studies. J Womens Health (Larchmt) 18: 1169–1178 [DOI] [PubMed] [Google Scholar]
- Yamazawa K, Matsui H, Seki K, Sekiya S. (2003) A case–control study of endometrial cancer after antipsychotics exposure in premenopausal women. Oncology 64: 116–123 [DOI] [PubMed] [Google Scholar]
- Yang Y, Xiao Z, Chen W, et al. (2004) Tumor suppressor gene TP53 is genetically associated with schizophrenia in the Chinese population. Neurosci Lett 369: 126–131 [DOI] [PubMed] [Google Scholar]
- Zänker K. (2008) The Epidemiologic Relationship between Diabetes and Cancer. In: Masur K, Thevenod F, Zänker KS. (eds) Diabetes and Cancer (Frontiers in Diabetes. Vol. 19) Basel: Karger; 84–96 [Google Scholar]