Abstract
Exposure to air pollutants has been associated with adverse health effects. However, analyses of the effects of season and ambient parameters such as ozone have not been fully conducted. Residential indoor and outdoor air levels of polycyclic aromatic hydrocarbons (PAH), black carbon (measured as absorption coefficient [Abs]), and fine particulate matter <2.5 μm (PM)2.5 were measured over two-weeks in a cohort of 5–6 year old children (n=334) living in New York City’s Northern Manhattan and the Bronx between October 2005 and April 2010. The objectives were to: 1) characterize seasonal changes in indoor and outdoor levels and indoor/outdoor (I/O) ratios of PAH (gas + particulate phase; dichotomized into Σ8PAHsemivolatile (MW 178–206), and Σ8PAHnonvolatile (MW 228–278)), Abs, and PM2.5; and 2) assess the relationship between PAH and ozone. Results showed that heating compared to nonheating season was associated with greater Σ8PAHnonvolatile (p<0.001) and Abs (p<0.05), and lower levels of Σ8PAHsemivolatile (p<0.001). In addition, the heating season was associated with lower I/O ratios of Σ8PAHnonvolatile and higher I/O ratios of Σ8PAHsemivolatile (p<0.001) compared to the nonheating season. In outdoor air, Σ8PAHnonvolatile was correlated negatively with community-wide ozone concentration (p<0.001). Seasonal changes in emission sources, air exchanges, meteorological conditions and photochemical/chemical degradation reactions are discussed in relationship to the observed seasonal trends.
Keywords: Nonvolatile PAH, I/O ratio, Heating season, Degradation, Inner-city children
1. Introduction
Exposure to traffic and industry-related sources of airborne particulate matter <2.5 μm (PM)2.5 has been associated with respiratory symptoms, lung function decrements, hospitalizations for cardiorespiratory disease, cancer and mortality (Brunekreef and Holgate, 2002; Pope et al., 2002). The contributions of individual chemical components of the complex PM2.5 mixture to these health outcomes are an area of active investigation. Components of ongoing health concern include nickel (Ni) and zinc (Zn) (Burnett et al., 2000; Patel et al., 2009), iron (Fe) (Burnett et al., 2000), vanadium (V) (Patel et al., 2009), strong acidic aerosols such as nitrates and sulfates (Burnett et al., 2000) and polycyclic aromatic hydrocarbons (PAH). Exposure to each of these has been associated with adverse health effects (Boffetta, 1997; Miller et al., 2004; Perera et al., 2006). A better understanding of the conditions that influence the concentrations of the different contaminants would improve our understanding of health risks associated with airborne pollutants.
In particular, exposure to PAH remains understudied. PAH are emitted readily from multiple sources in the urban environment, including vehicles, industry, cigarette smoking, incense burning, cooking and space heating (Bjorseth and Ramdahl, 1985; Chuang et al., 1991). In U.S. cities, traffic emissions are one of the most abundant sources of outdoor PAH; PAH accounted for 36% of the yearly total in one study in 1985 (Bjorseth and Ramdahl, 1985). Furthermore, comparisons of the variations in indoor PAH concentrations to the morning rush-hour traffic pattern revealed that traffic can be the primary determinant for the indoor PAH exposure as well (Dubowsky et al., 1999). Similar to several other traffic-related air pollutants, exposure to particle-bound PAH has been shown to decrease with increasing distance from the road (Levy et al., 2003), indicating that outdoor exposure differs spatially depending on the proximity to the local sources.
Once PAH enter the atmosphere, they redistribute between the gas and particle phases. This process depends on their vapor pressures as a function of temperature, available particle surface area for adsorption, or affinity of PAH with organic particles. Differences in gas/particle partitioning may affect their toxicity (Baek et al., 1991). Lower molecular weight PAH smaller than pyrene (molecular-weight 202) are present largely in the gas phase whereas heavier ones such as benzo(a)pyrene (molecular-weight 252) are found predominantly in the particle phase. The sources of lower molecular weight PAH such as phenanthrene and methylated phenanthrene include both direct evaporation of crude oils and light petroleum products and incomplete combustion products. The higher molecular weight PAH with 4–6 benzene rings are predominantly generated by incomplete combustion (Page et al., 1999).
Assessment of PAH exposure has been hampered by limitations in exposure monitoring. Data collected at fixed ambient monitoring sites, while useful in characterizing average levels and trends, are not adequate for representing the effects of personal or residential exposures. Personal monitoring has yielded important information on the association between prenatal PAH exposure and multiple adverse health effects in offspring (Miller et al., 2004; Perera et al., 2006). However, personal monitoring can be undertaken only over short durations (Chuang et al., 1991; Naumova et al., 2002) and is difficult to conduct in young children. One important finding to date from micro-environmental monitoring suggests that kindergarten-aged children who play inside and outside their school in a high traffic area may be exposed to inhalatory PAH levels up to six times higher than what may occur in less polluted areas (Fiala et al., 2001). Also, school bus emissions may be large sources of children’s daily exposure to diesel and other combustion emissions such as PM2.5, and elemental carbon (Adar et al., 2008; Sally Liu et al., 2010). Residential monitoring can be conducted for much longer durations and remains another useful option for assessing PAH exposures, especially in young children. Further, the longer sampling time frame permits a more comprehensive and representative assessment of indoor and outdoor PAH exposures and analysis of conditions such as season and residential heating on airborne pollutants levels.
In addition, the importance of photochemical and chemical reactions between PAH and atmospheric oxidants such as ozone, hydroxyl radial, and nitrogen oxides (Albinet et al., 2008; Goriaux et al., 2006; Schauer et al., 2003) still needs to be elucidated. A number of studies have shown that PAH can react readily with ozone and substantial chemical degradation of PAH can occur in the atmosphere and on the filter during sampling (Goriaux et al., 2006; Schauer et al., 2003). High ozone levels in the summer may contribute to lower measured summer levels of certain PAH, such as BaP, benzo(a)anthracene, and pyrene through homogeneous gas-phase reactions or heterogeneous reactions on aerosol particles (Marr et al., 2006).
Our objectives were to characterize seasonal changes in indoor (I) and outdoor (O) levels and I/O ratios of PAH (gas + particulate phase), black carbon (measured as absorption coefficient [Abs]) and PM2.5 levels, on a cohort of inner city children known to be at greater risk for air pollution-related respiratory disease such as asthma (Perera et al., 2002). Another objective was to assess the relationship between PAH and ambient ozone concentrations as an indicator of photochemical activity. Our approach was to measure residential indoor and outdoor PAH, Abs, and PM2.5 levels for a group of 5–6 year old children living in Northern Manhattan and the South Bronx who were participating in a cohort study being conducted by the Columbia Center for Children’s Environmental Health (CCCEH). Two-week residential monitoring with repeated measurement after 6 months was conducted. Comparisons with ambient ozone levels, from a New York State Department of Environmental Conservation monitoring site in New York City (NYC), were conducted as well.
2. Methods
2.1. Study design and location
Children were primarily of African-American and Dominican ethnicity and lived in Northern Manhattan and the South Bronx, geographical areas where exposure to traffic-related air pollution has been implicated in asthma and other diseases (Perera et al., 2002). Three hundred thirty four children were enrolled from the parent CCCEH cohort study (Miller et al., 2004; Perera et al., 2006) if they were age 5–6 years during the periods between October 2005 and April 2010 and resided in Northern Manhattan and the South Bronx during pregnancy and continued to live in Northern Manhattan and the Bronx at enrollment. The catchment area for this study was expanded to include the entire Bronx because many CCCEH study participants had moved since initial enrollment (See Fig. S-1, Supporting Information). Voluntary participation in this study exceeded 98% for those meeting eligibility criteria. The study was approved by the Columbia University Institutional Review Board and informed consent obtained.
2.2. Residential monitoring
For black carbon and PM2.5 measurements, two-week integrated indoor and outdoor monitoring was conducted at each of the first 262 homes between October 2005 and April 2010 for two time points each 6 months (±22 day, SD) apart. For an additional n= 72 homes, only one two-week measure was collected. While black carbon and PM2.5 were measured on filters collected at both time points, a panel of 16 PAH were measured at one time point only. A detailed sampling scheme of this study is presented in Fig. S-2 of the Supporting Information. Indoor air monitors were placed in a room where the child spent most of his or her time (e.g. child’s bedroom or main living area of the apartment) at a height of about 1.2 m and at least 0.3 m from the walls. At one third of homes, selected randomly, simultaneous outdoor sampling was conducted by placing samplers out of windows securely hung 0.9 m from the outside wall with a window unit that was designed so as not to appreciably affect air exchange rates (AERs) of the apartment (i.e., subject can have the window open or closed).
PM2.5 and Abs were analyzed on Teflon filter samples collected in a cassette attached downstream from a cyclone with a 2.5 μm aerodynamic-diameter cut point (model SCC 1.062, BGI, Inc.) that operated at 1.5 L/min for two weeks, leading to an average sampling volume of 30.1 m3. Flows were checked at the beginning and end of sampling. Filters were pre- and post-weighed on a microbalance after being equilibrated under a temperature-humidity controlled environment for at least 24 hours. Details of analytical protocols for PM2.5 and Abs are described in the Supporting Information.
Gas and particulate phase of PAH (≤2.5 μm) were collected on a precleaned quartz microfiber filter and polyurethane foam (PUF) in the downstream from a cyclone (model SCC 1.062, BGI, Inc.) at the same flow rate of 1.5 L/min for two weeks. Sixteen 3-ring to 6-ring PAH were selected as target compounds due to their abundance in traffic emissions and their possible carcinogenicity and mutagenicity (Boffetta, 1997; Tonne et al., 2004). Nine of these PAH were measured previously by our group using 48-hour personal prenatal sampling of the cohort mothers (Miller et al., 2004; Perera et al., 2006; Tonne et al., 2004). The sixteen PAH monitored were: benzo[a]anthracene (BaA), chrysene/iso-chrysene (Chry), benzo[b]fluoranthene (BbFA), benzo[k]fluoranthene (BkFA), benzo[a]pyrene (BaP), indeno[1,2,3-c,d]pyrene (IP), dibenzo[a,h]anthracene (DahA), benzo[g,h,i]perylene (BghiP), pyrene (Pye), phenanthrene (Phe), 1-methylphenanthrene (1Meph), 2-methylphenanthrene (2Meph), 3-methylphenanthrene (3Meph), 9-methylphenanthrene (9Meph), 1,7-dimethylphenanthrene (1,7DMeph), and 3,6-dimethylphenanthrene (3,6DMeph). A single soxhlet extraction of both the filters and PUFs together was analyzed at Southwest Research Institute (San Antonio, TX) as described (Tonne et al., 2004). Two deuterated compounds (anthracene-d10 and p-terphenyl-d14) were used as surrogate standards for recovery and chrysene-d12 and perylene-d12 were used as internal standard for quantification. Mean recovery rates of deuterated surrogate standards exceeded 98% for both d-10 anthracene and d14-p-terphenyl as described previously (Jung et al., 2010). Details of quality controls and data management, including the definition of heating season, are described in the Supporting Information.
Ambient ozone data for NYC were obtained from the New York State Department of Environmental Conservation monitoring site located within the study area (Intermediate School 52). The average ozone concentration at this site over the corresponding two-week sampling period for each subject was used for the analysis because ozone levels in NYC show very little spatial variations (Callaghan et al., 2007)
2.3. Statistical analysis
Unless specified, PAH, Abs, and PM2.5 data were log-transformed to normalize skewed distributions and parametric analyses were conducted. Additional comparisons were made with PAH levels summed according to their relative volatility and gas/particle partitioning i.e. Σ8PAHsemivolatile (sum of 8 low molecular-weight-PAH≤ 206) and Σ8PAHnonvolatile (sum of 8 high molecular-weight-PAH≥ 228). The precision of duplicate measurements was calculated as the standard deviation of the differences between duplicate and corresponding samples divided by . As a tool for evaluating dominance of indoor sources over outdoor concentration, the median I/O ratio was calculated across pollutants. Differences in individual I/O ratios by season were compared using the Mann-Whitney test to avoid overrepresentation of the effect of two outliers. Analyses were conducted using SPSS software (SPSS; Chicago, IL, version 17).
3. Results
3.1. Seasonal variations in PAH, Abs and PM2.5 levels
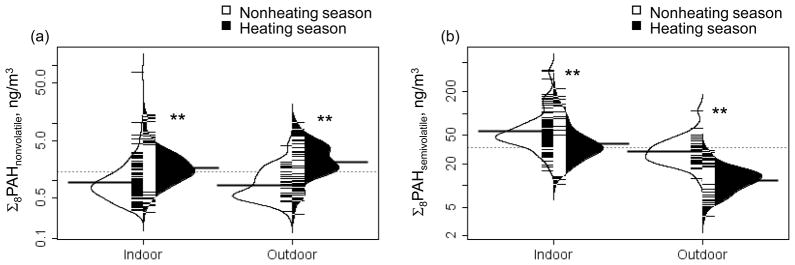
A significant seasonal pattern was detected for the categorized PAH, and Abs, both indoors and outdoors. The heating compared to the nonheating season was associated significantly with elevated levels of Σ8PAHnonvolatile (Fig. 1-a; p<0.001) and Abs (p<0.001 for indoor and p< 0.05 for outdoor, Fig. S-3a), and lower levels of Σ8PAHsemivolatile (Fig. 1-b; p<0.001). Heating season was not associated with altered PM2.5 concentration (Fig. S-3b).
Fig. 1.
Seasonal variations in (a) Σ8PAHnonvolatile and (b) Σ8PAHsemivolatile concentrations. T-tests were performed to compare heating season and nonheating concentrations of log-transformed Σ8PAHnonvolatile and Σ8PAHsemivolatile indoors and outdoors.
The white and black lines show individual observations, while the white and black area show the distribution. The dotted line indicates the overall geometric mean and the thicker solid line shows the geometric mean concentration of indoors and outdoors for each season.
**p<0.001, t test.
Σ8PAHnonvolatile includes benzo(a)anthracene (BaA), chrysene/iso-chrysene (Chry), benzo(b)fluoranthrene (BbFA), benzo(k)fluoranthrene (BkFA), benzo(a)pyrene (BaP), indeno(c,d)pyrene (IP), dibenzo(a,h)anthracene(DahA), and benzo(ghi)perylene(BghiP). Σ8PAHsemivolatile includes pyrene (Pye), phenanthrene (Phe), 1-methylphenanthrene (1Meph), 2-methylphenanthrene (2Meph), 3-methylphenanthrene (3Meph), 9-methylphenanthrene (9Meph), 1,7-dimethylphenanthrene (1,7DMeph), and 3,6-dimethylphenanthrene (3,6DMeph).
3.2. Relationship between indoor and outdoor concentrations
Differences between mean indoor and outdoor Σ8PAHnonvolatile concentrations were significant only in the heating season (p<0.001), whereas the levels of Σ8PAHsemivolatile concentrations were higher in indoor air, compared to outdoor, regardless of season (p<0.001) (Table 1). Indoors and outdoors, the level of Σ8PAHsemivolatile was an order of magnitude larger than that of Σ8PAHnonvolatile, and the largest contribution of individual PAH to the total PAH mass in the samples was made by phenanthrene, followed by its methylated derivatives. Among the nonvolatile PAH, BghiP was the most dominant compound both indoors and outdoors, followed by IP in indoors and BbFA in outdoor samples (Table S-1). Elevated levels of Abs were found in outdoor air, compared to indoor, only during the heating season (p<0.05). In general, the median I/O concentration ratios were close to or lower than 1 for nonvolatile PAH, but considerably greater than 1.0 (range 1.2–3.7) for semivolatile PAH in both the nonheating and heating seasons (Fig. 2). The heating versus nonheating season was associated with lower I/O ratios of Σ8PAHnonvolatile (median ratio of 0.72 vs. 0.99 respectively, p<0.001) and higher I/O ratios of Σ8PAHsemivolatile (median ratio of 3.02 vs. 1.73 respectively, p<0.001). This pattern was apparent when the individual PAH were assessed as well (p<0.05, except BaP, DahA and pyrene). The median I/O ratio for Abs was close to 1.0 for both seasons. Although heating season was not associated with altered I/O ratios for PM2.5, the median I/O ratio exceeded 1.0 in both seasons.
Table 1.
Indoor and outdoor residential exposure levels of air pollutants stratified by seasona.
| Analyte | Indoor | Outdoor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | d Mean | SD | Range | n | Median | d Mean | SD | Range | |
| Heating Season | ||||||||||
| bΣ8PAHnonvolatile | 203 | 1.56 | 2.14 | 2.02 | 0.29–13.9 | 68 | 2.06 | 2.68** | 1.93 | 0.27–10.3 |
| cΣ8PAHsemivolatile | 203 | 34.9 | 45.6 | 32.3 | 10.4–217 | 67 | 12.22 | 12.7** | 5.29 | 3.77–30.5 |
| Phe | 203 | 24.2 | 32.7 | 26.2 | 7.85–174 | 67 | 7.91 | 8.39** | 3.66 | 2.70–22.9 |
| 1Meph | 203 | 1.76 | 2.00 | 1.16 | 0.54–7.30 | 67 | 0.48 | 0.53** | 0.27 | 0.10–1.39 |
| 2Meph | 203 | 2.66 | 3.01 | 1.79 | 0.38–11.7 | 67 | 0.88 | 0.96** | 0.42 | 0.21–2.54 |
| 3Meph | 203 | 3.15 | 3.74 | 2.66 | 0.70–20.8 | 67 | 1.03 | 1.11** | 0.51 | 0.35–2.97 |
| 9Meph | 203 | 1.91 | 2.19 | 1.29 | 0.29–7.87 | 67 | 0.63 | 0.64** | 0.28 | 0.13–1.58 |
| 1,7DMeph | 203 | 0.37 | 0.42 | 0.23 | 0.10–1.41 | 67 | 0.11 | 0.12** | 0.06 | 0.02–0.33 |
| 3,6DMeph | 203 | 0.37 | 0.44 | 0.28 | 0.08–1.99 | 67 | 0.15 | 0.16** | 0.09 | 0.03–0.49 |
| Pye | 203 | 0.95 | 1.09 | 0.58 | 0.27–4.23 | 68 | 0.74 | 0.82** | 0.39 | 0.21–2.15 |
| BaA | 203 | 0.07 | 0.10 | 0.12 | 0.02–1.32 | 68 | 0.10 | 0.13** | 0.10 | 0.01–0.47 |
| Chry | 203 | 0.10 | 0.14 | 0.19 | 0.02–2.12 | 68 | 0.22 | 0.27** | 0.20 | 0.04–0.90 |
| BbFA | 203 | 0.22 | 0.30 | 0.29 | 0.05–2.26 | 68 | 0.49 | 0.61** | 0.47 | 0.06–2.37 |
| BkFA | 203 | 0.08 | 0.11 | 0.14 | 0.02–1.40 | 68 | 0.14 | 0.19** | 0.17 | 0.02–1.14 |
| BaP | 203 | 0.14 | 0.23 | 0.28 | 0.02–2.01 | 68 | 0.12 | 0.17** | 0.13 | 0.02–0.75 |
| IP | 203 | 0.35 | 0.47 | 0.44 | 0.05–3.40 | 68 | 0.33 | 0.43* | 0.32 | 0.04–1.97 |
| DahA | 203 | 0.03 | 0.04 | 0.05 | 0.01–0.35 | 68 | 0.04 | 0.05** | 0.04 | 0.02–0.20 |
| BghiP | 203 | 0.55 | 0.74 | 0.75 | 0.09–6.49 | 68 | 0.64 | 0.83** | 0.69 | 0.05–3.89 |
| Abs | 289 | 0.95 | 0.97 | 0.35 | 0.05–2.20 | 96 | 0.96 | 0.99* | 0.26 | 0.03–1.71 |
| PM2.5 | 286 | 13.64 | 17.8 | 14.9 | 0.58–105 | 95 | 10.5 | 10.9** | 3.13 | 0.22–22.2 |
| Nonheating Season | ||||||||||
| bΣ8PAHnonvolatile | 98 | 0.82 | 1.97 | 7.60 | 0.30–75.2 | 31 | 0.74 | 1.02 | 0.76 | 0.29–4.10 |
| cΣ8PAHsemivolatile | 98 | 49.9 | 72.3 | 70.4 | 16.0–393 | 31 | 30.0 | 32.9** | 17.94 | 12.4–109 |
| Phe | 98 | 34.9 | 56.6 | 64.3 | 10.4–370 | 31 | 21.3 | 25.1** | 15.98 | 8.46–93.3 |
| 1Meph | 98 | 2.17 | 2.39 | 1.27 | 0.49–8.35 | 31 | 1.06 | 1.11** | 0.36 | 0.49–1.92 |
| 2Meph | 98 | 3.13 | 3.62 | 2.16 | 1.04–13.3 | 31 | 1.67 | 1.82** | 0.59 | 0.80–3.57 |
| 3Meph | 98 | 3.81 | 4.46 | 2.76 | 1.18–17.1 | 31 | 1.96 | 2.08** | 0.70 | 1.05–4.10 |
| 9Meph | 98 | 2.47 | 2.67 | 1.58 | 0.56–10.8 | 31 | 1.19 | 1.22** | 0.37 | 0.62–2.09 |
| 1,7DMeph | 98 | 0.45 | 0.51 | 0.27 | 0.02–1.97 | 31 | 0.22 | 0.22** | 0.06 | 0.12–0.34 |
| 3,6DMeph | 98 | 0.46 | 0.49 | 0.24 | 0.15–1.52 | 31 | 0.25 | 0.25** | 0.08 | 0.10–0.42 |
| Pye | 98 | 1.20 | 1.55 | 1.93 | 0.33–19.0 | 31 | 1.03 | 1.14** | 0.67 | 0.32–3.72 |
| BaA | 98 | 0.05 | 0.07 | 0.05 | 0.02–0.38 | 31 | 0.04 | 0.07** | 0.10 | 0.02–0.59 |
| Chry | 98 | 0.06 | 0.09 | 0.10 | 0.02–0.69 | 31 | 0.09 | 0.11** | 0.09 | 0.03–0.57 |
| BbFA | 98 | 0.12 | 0.23 | 0.39 | 0.04–3.46 | 31 | 0.15 | 0.22** | 0.16 | 0.08–0.63 |
| BkFA | 98 | 0.04 | 0.06 | 0.08 | 0.02–0.74 | 31 | 0.04 | 0.06** | 0.06 | 0.02–0.26 |
| BaP | 98 | 0.06 | 0.15 | 0.46 | 0.02–4.49 | 31 | 0.05 | 0.08** | 0.10 | 0.02–0.55 |
| IP | 98 | 0.16 | 0.40 | 1.42 | 0.02–14.0 | 31 | 0.14 | 0.18** | 0.14 | 0.03–0.63 |
| DahA | 98 | 0.02 | 0.03 | 0.04 | 0.01–0.30 | 31 | 0.02 | 0.02** | 0.02 | 0.02–0.11 |
| BghiP | 98 | 0.27 | 0.94 | 5.22 | 0.09–51.7 | 31 | 0.21 | 0.28* | 0.18 | 0.07–0.79 |
| Abs | 227 | 0.82 | 0.88 | 0.24 | 0.39–1.97 | 64 | 0.85 | 0.90 | 0.19 | 0.57–1.69 |
| PM2.5 | 227 | 13.9 | 16.6 | 9.92 | 6.27–75.2 | 61 | 12.77 | 12.56** | 3.29 | 7.04–20.9 |
Heating season was defined as any sampling that was initiated October 1 through April 30
Paired t-test performed between indoor and outdoor concentrations of log-transformed PAH, Abs and PM2.5;
p-value < 0.05;
p-value< 0.001; Unit expressed in ng/m3 for PAH, m−1*10−5 for Abs, μg/m3 for PM2.5
Σ8PAHnonvolatile: BaA, Chry, BbFA, BkFA, BaP, IP, DahA, and BghiP
Σ8PAHsemivolatile: Phe, 1Meph, 2Meph, 3Meph, 9Meph, 1,7DMeph, 3,6DMeph, and Pye
Arithmetic mean presented.
Fig. 2.
Median indoor (I) to outdoor (O) ratio. Median values of I/O ratio for each individual PAH, Abs, and PM2.5 presented separately by season. *p<0.05 and **p<0.001, Mann-Whitney test.
3.3. Seasonal variations across pollutants
During the heating season, Σ8PAHnonvolatile was weakly correlated with Abs and PM2.5 both indoors and outdoors (r>0.265; Table 2); however, significant correlation was not observed outdoors during the nonheating season. While indoor Σ8PAHsemivolatile was not significantly correlated with Abs and PM2.5 in both seasons, outdoor Σ8PAHsemivolatile was moderately correlated with PM2.5 during the nonheating season (r=0.579). Abs was moderately correlated with PM2.5 measured indoors (r > 0.358; p<0.001) and strongly correlated when measured outdoors, especially during the heating season (r= 0.876; p<0.001).
Table 2.
Correlations among pollutants indoors and outdoors, stratified by season.
| Analyte | Location | Heating season | Nonheating season | ||||
|---|---|---|---|---|---|---|---|
| N | Abs | PM2.5 | N | Abs | PM2.5 | ||
| Σ8PAHnonvolatile | Indoor | 201 | 0.472** | 0.381** | 97 | 0.366** | 0.242* |
| Outdoor | 67 | 0.298* | 0.265* | 31 | 0.065 | −0.349 | |
| Σ8PAHsemivolatile | Indoor | 201 | 0.106 | 0.119 | 97 | 0.002 | 0.018 |
| Outdoor | 67 | 0.372** | 0.290* | 31 | 0.331 | 0.579** | |
| Pye | Indoor | 201 | 0.190** | 0.207** | 97 | 0.239* | 0.011 |
| Outdoor | 67 | 0.443** | 0.317* | 31 | 0.452* | 0.201 | |
| PM2.5 | Indoor | 208 | 0.570** | 1 | 100 | 0.358** | 1 |
| Outdoor | 70 | 0.876** | 1 | 32 | 0.501** | 1 | |
Pearson correlation coefficient performed on log-transformed data;
p-value < 0.05;
p-value< 0.001
3.4. Effects of season on the relative abundance of PAH vs. Abs, PM2.5
The differences in the relative PAH contribution to Abs or PM2.5 between the heating and nonheating season were assessed to estimate the effect of season on chemical compositions (Table 3). Ratios of Σ8PAHnonvolatile to Abs or PM2.5. were significantly higher during the heating compared to the nonheating season (p<0.001, Table 3), indoors and outdoors. In contrast, the ratios of Σ8PAHsemivolatile to Abs or PM2.5 were significantly higher during the nonheating season, compared to the heating season (p<0.001 for both indoor and outdoor). Moreover, the ratios of Abs to PM2.5 outdoors were significantly higher during the heating season than the nonheating season (p<0.001). However, indoors, there were no significant differences in Abs to PM2.5 ratios by season (Table 3).
Table 3.
Seasonal variation of median PAH to Abs or PM2.5 ratio, stratified by location.
| Ratio | Indoor | Outdoor | ||
|---|---|---|---|---|
| Heating (n=201) | Nonheating (n=97) | Heating (n=67) | Nonheating (n=31) | |
| Σ8PAHnonvolatile/Abs | 1.867 | 0.975** | 2.239 | 0.853** |
| Σ8PAHsemivolatile/Abs | 39.64 | 59.06** | 12.34 | 28.53** |
| BaP/Abs | 0.167 | 0.072** | 0.142 | 0.055** |
| Σ8PAHnonvolatile/PM2.5 | 0.118 | 0.062** | 0.219 | 0.074** |
| Σ8PAHsemivolatile/PM2.5 | 2.523 | 3.804** | 1.126 | 2.469** |
| BaP/PM2.5 | 0.010 | 0.004** | 0.013 | 0.004** |
| Abs/PM2.5 | 0.066 | 0.063 | 0.090 | 0.076** |
Mann-Whitney test performed;
p<0.05,
p<0.001
3.5. Association between ambient ozone and outdoor PAH levels
In both seasons, Σ8PAHnonvolatile were negatively associated with 2-week average ozone (Fig. 3a, β=−49.44 and −37.49 for the heating and nonheating season, respectively). In contrast, no significant relationship was observed between Σ8PAHsemivolatile and ozone in either season (Fig. 3b). Similarly, all individual nonvolatile PAH were negatively associated with ozone (Table S-2). While most individual semivolatile PAH followed the similar trend as Σ8PAHsemivolatile, pyrene was the exception, showing a significant negative correlation during the heating season (data not shown). No significant relationship was found between PM2.5 levels and ozone concentrations; however, significant but opposite relationships were detected when analyzed seasonally (Fig. S-4). The significant relationships were not found between Abs and ozone (data not shown).
Fig. 3.
Associations between ambient ozone and outdoor (a) S8PAHnonvolatile, and (b) S8PAHsemivolatile. The average ambient ozone data over the corresponding two-week sampling period for each subject were used. The linear regression analysis was performed between ozone concentration and log-transformed outdoor S8PAHnonvolatile and S8PAHsemivolatile concentration.
4. Discussion
Our objective was to characterize the effects of heating season on residential exposure to both indoor and outdoor air pollutants (PAH, Abs and PM2.5) based on residential monitoring conducted over two-weeks on a cohort of inner city children known to be at greater risk for air pollution-related disease. This report describes one of the largest datasets of residential PAH levels to date, and includes values collected over a longer period of time than reported in previously (Chuang et al., 1991; Naumova et al., 2002). Heating season was identified as a key parameter that influences pollutant levels and I/O ratios of PAH as well as the relative abundance of PAH to Abs or PM2.5. Furthermore, outdoor Σ8PAHnonvolatile concentrations also were associated negatively with ambient ozone levels.
Seasonal variations differed across PAH when classified according to their volatility (Σ8PAHnonvolatile vs. Σ8PAHsemivolatile). Several explanations may be responsible, including seasonal variations in emission sources, air exchange rates, PAH transformation through photochemical/chemical reaction, gas/particle partitioning and meteorological conditions such as ambient temperature, mixing height, humidity and wind speeds. For example, several studies reported that PAH emissions from motor vehicles are dominated by volatile and semivolatile PAH, whereas nonvolatile PAH concentrations become elevated both indoors and outdoors by the greater use of fossil fuel combustion for residential heating (Khalili et al., 1995; Schauer et al., 2003). Consistent with these prior studies, higher nonvolatile PAH concentrations were observed in the heating season when there is greater use of residual fuel oil for space-heating in NYC.
In the heating season, there is not only reduced atmospheric dilution resulting from lower mixing height, but also there is reduced potential for atmospheric photochemical/chemical reactions that can convert nonvolatile PAH to oxygenated/nitrated PAH. Consistent with this loss mechanism, lower residential levels of nonvolatile PAH during periods of higher ambient ozone concentrations in the atmosphere were measured (See Table S.2). The chemical degradation of nonvolatile PAH in the atmosphere by ozone reaction has been demonstrated previously, especially for BaP (Goriaux et al., 2006). In comparison, the increase of semivolatile PAH concentration during the nonheating season may be attributable to the temperature dependence of gas/particle partitioning. As temperature increases, gas/particle partitioning of PAH is in favor of the gas phase; thus volatilization of particulate PAH from road surfaces, soil and vegetation would be enhanced. In addition, evaporations from crude oil and petroleum products may contribute to the elevated semivolatile PAH levels during the nonheating season. Ozone may not drive the chemical degradation of particle-bound semivolatile PAH (Schauer et al., 2003). Hence, gas/particle partitioning and petrogenic emissions may be the dominant influence on semivolatile PAH levels, whereas residential heating emissions and photochemical degradation may influence the nonvolatile PAH levels (Ohura et al., 2004a; Ohura et al., 2004b).
Indoor and outdoor PM2.5 levels did not vary by season. Higher PM2.5 concentration in northeastern U.S. cities during summer has been reported, predominantly as a result of higher upwind photochemical production of sulfate and other secondary organic carbons (Cyrys et al., 2003). Supporting these prior studies, PM2.5 during the nonheating season was associated positively with ozone, an indicator of photochemical reactivity (See Fig S.2). However, increased PM2.5 concentrations due to the rise in photochemical secondary products initiated by atmospheric oxidants during the nonheating season may be countered with the increase in PM2.5 concentrations from residential heating source emissions during the NYC heating season. The latter was supported further by the negative correlation between PM2.5 and ozone during the heating season, if one considers that the two-week average levels of ozone can be seen as a proxy for nonuse of heating oil in the heating season. When the ozone level is lower, ambient temperatures are lower, and thus fossil fuel consumption for residential heating tends to be higher, resulting in higher PM2.5 levels. The seasonal variations of both indoor and outdoor Abs concentrations imply that the elevations of Abs levels during the heating season may be due to the increase of primary emissions from fossil fuel combustion for residential heating beyond traffic emissions.
Semivolatile PAH I/O ratios higher than 1.0 (Fig. 2) implicates prominent indoor sources such as space heating (e.g., kerosene heaters), smoking, or burning incense or candles (Naumova et al., 2002; Ohura et al., 2004a; Ohura et al., 2004b). The significantly higher I/O ratios of semivolatile PAH in the heating, compared to the nonheating season, suggest that the indoor-generated semivolatile PAH are better trapped indoors, consistent with lower air exchange rates in the winter. Kinney et al. (2002) measured AERs in NYC apartments and observed significant seasonal differences in AERs, with median rates going from 0.85 to 1.6 during the winter and summer respectively, that impact I/O ratios of a wide suite of particle-associated and gas phase pollutants.
The I/O ratios of PM2.5 were slightly greater than 1.0 across season, indicating the small presence of indoor emission sources of PM2.5, as reported (Kinney et al., 2002). In comparison, the median I/O ratios of Abs nearing 1.0 across season suggest a lack of significant indoor sources and a high penetration efficiency of outdoor-originated black carbon into the indoor environment. Black carbon is a fairly stable contaminant especially during the heating season (Kinney et al., 2002). As such, the Abs I/O ratio is a good benchmark to compare against the nonvolatile PAH that are predominantly associated with particles. The I/O ratios for nonvolatile PAH tend to be below one or near one (see Fig. 2), suggesting that the indoor concentrations of nonvolatile PAH arise predominantly from the transport of outdoor air into the indoor environment, as reported (Dubowsky et al., 1999; Naumova et al., 2002; Ohura et al., 2004a; Ohura et al., 2004b). However, in the heating season the BaA, Chry, BbFA, BkFA have median ratios significantly below that seen for Abs while the BaP, DahA, and IP have I/O ratio very similar to a value of 1 (slightly above that of Abs). Assuming that Abs is benchmark marker of penetration of outdoor-originated PM, then these depressed ratios (<1) for 4–5 ring PAH suggest that outdoor-originated nonvolatile PAH can be redistributed from the particle phase to the gas phase. This may occur once they enter into the relatively warm indoor environments during the heating season, and then the vaporized PAH are adsorbed onto indoor surfaces (e.g, carpets, settled dust, or stationary room surfaces) rather than airborne particles, resulting in loss of measured indoor PAH level (Weschler and Nazaroff, 2008).
Because PAH and Abs form during similar combustion processes (e.g., heating, traffic and cooking, and wood smoke), they are expected to be well-correlated, depending on the sources of emissions (Fischer et al., 2000; Marr et al., 2004). However, outdoors, strong correlations of both semi-and nonvolatile PAH with Abs and PM2.5 were absent in this study. Marr et al. (2004) reported that the correlation between PAH and elemental carbon was relatively low when PAH and elemental carbon were not dominated by fresh emission, suggesting that PAH adsorbed on particles could be diminished over time through evaporation of semivolatile PAH or transformation through heterogeneous reactions of nonvolatile PAH. During residential monitoring, PAH generated from street level traffic sources travel considerably to reach high-rise (most 5–10 stories) apartment buildings, during which time the PAH sample could age. However, the observed moderate correlation between Σ8PAHsemivolatile and PM2.5 outdoors during the nonheating season may be attributable to the temperature dependence of both compounds. In contrast, the moderate correlations of nonvolatile PAH with Abs and PM2.5 indoors, especially during the heating season, could be due to the slower chemical degradation associated with the absence of direct sunlight and lower levels of ozone and OH radical indoors (Weschler and Nazaroff, 2008).
Seasonal variations of emission sources (i.e., residential heating emissions), meteorological conditions (atmospheric mixing, temperature), and photochemical/chemical activity appeared to affect the chemical composition of samples. For example, a significantly lower ratio of Σ8PAHnonvolatile and BaP to Abs or PM2.5 during the nonheating compared to the heating season suggests that Σ8PAHnonvolatile diminished in association with reduced fossil fuel consumption for residential heating and/or enhanced photochemical/chemical degradation rate due to higher ambient ozone. In contrast, the ratio of Σ8PAHsemivolatile to Abs or PM2.5 was much higher during the nonheating season, indicating that the abundance of Σ8PAHsemivolatile may be dominated by evaporation of semivolatile PAHs from particles or crude oils and light petroleum products. The effect of photochemical activity, at least the reaction with ozone, on the semivolatile PAH concentration may be less important. Lower ratio of Abs to PM2.5 during the nonheating season probably reflects a significant fraction of PM2.5 being driven by the secondary products formed through photochemical reactions.
While the study was not designed to compare air pollutant levels over time or across cohorts, differences were noted. For example, the two-week indoor and outdoor PAH levels are comparable or lower to shorter-term averages (24–48 hour) measured in Los Angeles, CA, Houston, TX, and Elizabeth, NJ (Naumova et al., 2002), and considerably lower than those reported for homes impacted by industrial and heavy traffic emissions (Ohura et al., 2004b). Interestingly, they averaged lower than earlier (1998 – 2002) levels measured using 48-hr prenatal personal within the same CCCEH cohort that used similar sampling and the same analytical techniques (Tonne et al., 2004). One explanation is that personal activity (i.e., commuting in heavy traffic, ETS exposure, grill cooking) could contribute to the elevated PAH exposure detected using personal monitors as seen for many personal exposure studies. In addition, two-week longer-term sampling will dampen short-term peaks and also may lead to the greater degradation of nonvolatile PAH by ambient ozone after collection on the filter, compared to short-term period. Another explanation is that true reductions in local diesel or PAH exposure occurred over time, supported by New York policy initiatives to increase use of cleaner fuels and improved engines to reduce diesel emissions since 2000 (Narvaez et al., 2008). The residential outdoor Abs levels averaged lower compared to those reported in Kinney et al. (2002) conducted in a similar area of NYC from 1999 to 2001 for two seasons using 48-hour long samples.
We acknowledge several study limitations. For one, chemical degradation of PAH can occur not only in the atmosphere but also after deposition on filters, underestimating the atmospheric PAH concentrations. The effect can be augmented when sampling for an extended duration, such as the two-week period in this study. Studies have demonstrated that substantial degradation of BaP and other 5–6 ring PAH can occur during filter sampling (Goriaux et al., 2006; Schauer et al., 2003), although these studies used much higher flow rates. It also should be noted that ozone may be serving as an indicator of other ambient parameters that are highly correlated with ozone such as temperature, solar intensity, or other ambient oxidant levels (i.e., hydroxyl radical). Therefore, ozone-induced degradation of PAH needs further investigation. Another limitation is that the PAH were not measured for both seasons in all homes because of budgetary constraints. This information could give us a better understanding of within-home variation.
5. Conclusion
This study highlights the important influence of the heating compared to nonheating season on variations in residential exposure levels and I/O ratios of PAH, Abs, and PM2.5 as well as the composition of PM2.5 particles. Distinct seasonal trends could be associated not only with varying emission sources (i.e. residential heating emissions) but also variations in meteorological conditions (atmospheric mixing, temperature), air exchange rates, and photochemical/chemical degradation. Seasonal variations of PAH, Abs and PM2.5 concentrations and their compositions may be associated with seasonal differences in air pollution-related health risks for children. For example, Mhor et al. (2008) reported that the association between ambient elemental carbon and childhood asthma emergency room visits varies according to season. Our findings have important implication for the design of epidemiologic studies evaluating the effects of the airborne contaminants among urban children.
Supplementary Material
Fig. S-1. Map of the prenatal enrollment area and CCCEH catchment area. White area (Northern Manhattan and South Bronx) indicates the area for full enrollment into CCCEH during the prenatal assessment. White and gray areas (the rest of the Bronx) together denote entire catchment area for the current study.
Fig. S-2. Sampling scheme. *Time 1 and Time 2 samples were recategorized into heating vs. nonheating season samples.
Fig. S-3. Seasonal variations in (a) absorbance coefficient (Abs), and (b) PM2.5 concentrations. The white and black lines show individual observations, while the white and black area show the distribution. The dotted line indicates the overall geometric mean and the thicker solid line shows the geometric mean concentration of indoors and outdoors for each season.
*p<0.05 and **p<0.001, paired t-test.
Fig. S-4. Association between ambient ozone and outdoor PM2.5. The average ambient ozone data over the corresponding two-week sampling period for each subject were used. The linear regression analysis between ozone concentration and outdoor PM2.5 concentration was performed separately by season. (r2=0.13, p<0.001 for heating season and r2=0.40, p<0.01 for nonheating season).
Acknowledgments
This work was supported by NIH R01ES013163, P50ES015905, P01ES09600, R01ES08977, the Educational Foundation of America, the John & Wendy Neu Family Foundation, the New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund.
Abbreviations
- CCCEH
Columbia Center for Children’s Environmental Health
- NYC
New York City
- PAH
Polycyclic Aromatic Hydrocarbons
- Abs
Absorption Coefficient
- PM
Particulate Matter
- PUF
Polyurethane Foam
- Σ8PAHsemivolatile
Sum of 8 low molecular-weight-PAH≤ 206, including pyrene (Pye), phenanthrene (Phe), 1-methylphenanthrene (1Meph), 2-methylphenanthrene (2Meph), 3-methylphenanthrene (3Meph), 9-methylphenanthrene (9Meph), 1,7-dimethylphenanthrene (1,7DMeph), and 3,6-dimethylphenanthrene (3,6DMeph)
- Σ8PAHnonvolatile
Sum of 8 high molecular-weight-PAH≥ 228, including benzo[a]anthracene (BaA), chrysene/iso-chrysene (Chry), benzo[b]fluoranthene (BbFA), benzo[k]fluoranthene (BkFA), benzo[a]pyrene (BaP), indeno[1,2,3-c,d]pyrene (IP), dibenzo[a,h]anthracene (DahA), and benzo[g,h,i]perylene (BghiP)
- I/O ratio
Indoor-to-outdoor Ratio
- AERs
Air Exchange Rates
Footnotes
Conflicts of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adar S, Davey M, Sullivan J, Compher M, Szpiro A, Sally Liu L. Predicting airborne particle levels aboard Washington State school buses. Atmospheric Environment. 2008;42:7590–7599. doi: 10.1016/j.atmosenv.2008.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albinet A, Leoz-Garziandia E, Budzinski H, Villenave E, Jaffrezo J. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys Part 2: Particle size distribution. Atmospheric Environment. 2008;42:55–64. [Google Scholar]
- Baek S, Goldstone M, Kirk P, Lester J, Perry R. Phase distribution and particle size dependency of polycyclic aromatic hydrocarbons in the urban atmosphere. Chemosphere. 1991;22:503–520. [Google Scholar]
- Bjorseth A, Ramdahl T. Handbook of polycyclic aromatic hydrocarbons: Emission sources and recent progress in analytical chemistry. Marcel Dekker 1985 [Google Scholar]
- Boffetta P. Cancer risk from occupational and environmental exposure to polycylic aromatic hydrocarbons. Cancer causes and control. 1997;8:442–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate S. Air pollution and health. The Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Burnett R, Brook J, Dann T, Delocla C, Philips O, Cakmak S, Vincent R, Goldberg M, Krewski D. Association between particulate-and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhalation toxicology. 2000;12:15–39. doi: 10.1080/08958370050164851. [DOI] [PubMed] [Google Scholar]
- Callaghan K, Moors K, Ross J, Narvaez R, Borjas M, Kinney P, Pererra F, Miller R, Chillrud S. Pilot survery of street-side summer ozone in northern Manhattan. 2007. [Google Scholar]
- Chuang J, Mack G, Kuhlman M, Wilson N. Polycyclic aromatic hydrocarbons and their derivatives in indoor and outdoor air in an eight-home study. Atmospheric environment Part B, Urban atmosphere. 1991;25:369–380. [Google Scholar]
- Cyrys J, Heinrich J, Hoek G, Meliefste K, Lewne M, Gehring U, Bellander T, Fischer P, van Vliet P, Brauer M, Wichmann HE, Brunekreef B. Comparison between different traffic-related particle indicators: elemental carbon (EC), PM2.5 mass, and absorbance. J Expo Anal Environ Epidemiol. 2003;13:134–143. doi: 10.1038/sj.jea.7500262. [DOI] [PubMed] [Google Scholar]
- Dubowsky S, Wallace L, Buckley T. The contribution of traffic to indoor concentrations of polycyclic aromatic hydrocarbons. Journal of exposure analysis and environmental epidemiology. 1999;9:312–321. doi: 10.1038/sj.jea.7500034. [DOI] [PubMed] [Google Scholar]
- Fiala Z, Vyskocil A, Krajak V, Viau C, Ettlerova E, Bukac J, Fialova D, Emminger S. Environmental exposure of small children to polycyclic aromatic hydrocarbons. International archives of occupational and environmental health. 2001;74:411–420. doi: 10.1007/s004200100239. [DOI] [PubMed] [Google Scholar]
- Fischer P, Hoek G, Van Reeuwijk H, Briggs D, Lebret E, Van Wijnen J, Kingham S, Elliott P. Traffic-related differences in outdoor and indoor concentrations of particles and volatile organic compounds in Amsterdam. Atmospheric Environment. 2000;34:3713–3722. [Google Scholar]
- Goriaux M, Jourdain B, Temime B, Besombes J, Marchand N, Albinet A, Leoz-Garziandia E, Wortham H. Field comparison of particulate PAH measurements using a low-flow denuder device and conventional sampling systems. Environ Sci Technol. 2006;40:6398–6404. doi: 10.1021/es060544m. [DOI] [PubMed] [Google Scholar]
- Jung KH, Yan B, Chillrud SN, Perera FP, Whyatt R, Camann D, Kinney PL, Miller RL. Assessment of Benzo(a)pyrene-equivalent Carcinogenicity and Mutagenicity of Residential Indoor versus Outdoor Polycyclic Aromatic Hydrocarbons Exposing Young Children in New York City. International Journal of Environmental Research and Public Health. 2010;7:1889–1900. doi: 10.3390/ijerph7051889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili N, Scheff P, Holsen T. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric Environment. 1995;29:533–542. [Google Scholar]
- Kinney PL, Chillrud SN, Ramstrom S, Ross J, Spengler JD. Exposures to multiple air toxics in New York City. Environ Health Perspect. 2002;110(Suppl 4):539–546. doi: 10.1289/ehp.02110s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Bennett D, Melly S, Spengler J. Influence of traffic patterns on particulate matter and polycyclic aromatic hydrocarbon concentrations in Roxbury, Massachusetts. Journal of Exposure Science and Environmental Epidemiology. 2003;13:364–371. doi: 10.1038/sj.jea.7500289. [DOI] [PubMed] [Google Scholar]
- Marr L, Dzepina K, Jimenez J, Reisen F, Bethel H, Arey J, Gaffney J, Marley N, Molina L, Molina M. Sources and transformations of particle-bound polycyclic aromatic hydrocarbons in Mexico City. Atmos Chem Phys. 2006;6:1733–1745. [Google Scholar]
- Marr L, Grogan L, Wohrnschimmel H, Molina L, Molina M, Smith T, Garshick E. Vehicle traffic as a source of particulate polycyclic aromatic hydrocarbon exposure in the Mexico City metropolitan area. Environ Sci Technol. 2004;38:2584–2592. doi: 10.1021/es034962s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Garfinkel R, Horton M, Camann D, Perera F, Whyatt R, Kinney P. Polycyclic Aromatic Hydrocarbons, Environmental Tobacco Smoke, and Respiratory Symptoms in an Inner-city Birth Cohort*. Chest (Am Coll Chest Phys) 2004:1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez RF, Hoepner L, Chillrud SN, Yan B, Garfinkel R, Whyatt R, Camann D, Perera FP, Kinney PL, Miller RL. Spatial and temporal trends of polycyclic aromatic hydrocarbons and other traffic-related airborne pollutants in New York City. Environ Sci Technol. 2008;42:7330–7335. doi: 10.1021/es801273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova Y, Eisenreich S, Turpin B, Weisel C, Morandi M, Colome S, Totten L, Stock T, Winer A, Alimokhtari S. Polycyclic aromatic hydrocarbons in the indoor and outdoor air of three cities in the US. Environ Sci Technol. 2002;36:2552–2559. doi: 10.1021/es015727h. [DOI] [PubMed] [Google Scholar]
- Ohura T, Amagai T, Fusaya M, Matsushita H. Polycyclic aromatic hydrocarbons in indoor and outdoor environments and factors affecting their concentrations. Environ Sci Technol. 2004a;38:77–83. doi: 10.1021/es030512o. [DOI] [PubMed] [Google Scholar]
- Ohura T, Amagai T, Sugiyama T, Fusaya M, Matsushita H. Characteristics of particle matter and associated polycyclic aromatic hydrocarbons in indoor and outdoor air in two cities in Shizuoka, Japan. Atmospheric Environment. 2004b;38:2045–2054. [Google Scholar]
- Page D, Boehm P, Douglas G, Bence A, Burns W, Mankiewicz P. Pyrogenic polycyclic aromatic hydrocarbons in sediments record past human activity: a case study in Prince William Sound, Alaska. Marine Pollution Bulletin. 1999;38:247–260. [Google Scholar]
- Patel M, Hoepner L, Garfinkel R, Chillrud S, Reyes A, Quinn J, Perera F, Miller R. Ambient Metals, Elemental Carbon, and Wheeze and Cough in New York City Children through Age 24 Months. American Journal of Respiratory and Critical Care Medicine. 2009;180:1107–1113. doi: 10.1164/rccm.200901-0122OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Illman S, Kinney P, Whyatt R, Kelvin E, Shepard P, Evans D, Fullilove M, Ford J, Miller R. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environmental Health Perspectives. 2002;110:197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama-Journal of the American Medical Association. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sally Liu L, Phuleria H, Webber W, Davey M, Lawson D, Ireson R, Zielinska B, Ondov J, Weaver C, Lapin C. Quantification of Self Pollution from Two Diesel School Buses using Three Independent Methods. Atmospheric Environment. 2010 doi: 10.1016/j.atmosenv.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer C, Niessner R, Poschl U. Polycyclic aromatic hydrocarbons in urban air particulate matter: decadal and seasonal trends, chemical degradation, and sampling artifacts. Environ Sci Technol. 2003;37:2861–2868. doi: 10.1021/es034059s. [DOI] [PubMed] [Google Scholar]
- Tonne C, Whyatt R, Camann D, Perera F, Kinney P. Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environmental health perspectives. 2004;112:754–760. doi: 10.1289/ehp.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler C, Nazaroff W. Semivolatile organic compounds in indoor environments. Atmospheric Environment. 2008;42:9018–9040. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S-1. Map of the prenatal enrollment area and CCCEH catchment area. White area (Northern Manhattan and South Bronx) indicates the area for full enrollment into CCCEH during the prenatal assessment. White and gray areas (the rest of the Bronx) together denote entire catchment area for the current study.
Fig. S-2. Sampling scheme. *Time 1 and Time 2 samples were recategorized into heating vs. nonheating season samples.
Fig. S-3. Seasonal variations in (a) absorbance coefficient (Abs), and (b) PM2.5 concentrations. The white and black lines show individual observations, while the white and black area show the distribution. The dotted line indicates the overall geometric mean and the thicker solid line shows the geometric mean concentration of indoors and outdoors for each season.
*p<0.05 and **p<0.001, paired t-test.
Fig. S-4. Association between ambient ozone and outdoor PM2.5. The average ambient ozone data over the corresponding two-week sampling period for each subject were used. The linear regression analysis between ozone concentration and outdoor PM2.5 concentration was performed separately by season. (r2=0.13, p<0.001 for heating season and r2=0.40, p<0.01 for nonheating season).