Abstract
Nma/BAMBI is a novel pseudoreceptor with homology to a TGFβ type I receptor that lacks a serine/threonine kinase domain. Nma/BAMBI functions as a dominant-negative protein that regulates reciprocal epithelial-mesenchymal interactions during organogenesis. Therefore, we hypothesized that Nma/BAMBI regulates TGFβ signaling and downstream gene expression during dentinogenesis. To test this hypothesis, we examined the downstream gene expression profiles of major dentin extracellular matrix proteins in response to Nma/BAMBI, and we examined the roles of Nma/BAMBI and TGFβ-1 during dentinogenesis. Overexpression of Nma/BAMBI in the mouse odontoblast-like cell line MD10-A2 down-regulated expression of DSPP by 66% and up-regulated expression of DMP1 four-fold. TGFβ treatment reversed Nma/BAMBI’s negative effect on DSPP expression. Furthermore, we demonstrated that TGFβ negatively regulates Nma/BAMBI’s expression levels in MD10-A2 odontoblast-like cells. Analysis of these data, together, indicates that TGFβ and Nma/BAMBI are inversely regulated and that the sequence of expression determines the net effect on downstream gene expression.
Keywords: bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI), non-metastatic gene A (Nma), TGFβ type I receptor
Introduction
We isolated the mouse Nma/BAMBI (Non-metastatic gene A/Bone Morphogenetic Protein and Membrane Bound Inhibitor) orthologue from a mouse tooth cDNA library and noted its high homology to Transforming Growth Factor Beta type I receptor (TGFβRI) (Knight et al., 2001). TGFβ regulates organogenesis and mineralization events in tooth and bone by binding to a TGFβ type II receptor (TGFβRII) (Padgett et al., 1998; Massagué and Chen, 2000). The TGFβ/TGFβRII complex then recruits and phosphorylates the TGFβRI, thereby activating it. Smad2 and Smad3 are transducer proteins found within the cytoplasm that become phosphorylated by the activated TGFβRI (Padgett et al., 1998; Massagué and Chen, 2000). Hence, Smad2 and Smad3 are termed R-Smads, for receptor-regulated Smads. Activated R-Smads complex with Smad4, also termed Co-Smad, for common mediator Smad. The R-Smad/Co-Smad complex enters the nucleus and interacts with other transcription factors to activate or repress target gene transcription (Nakao et al., 1997).
Nma/BAMBI has homology to TGFβRI and consists of an extracellular amino terminal, a short transmembrane domain, and a truncated carboxyl terminus. However, Nma/BAMBI lacks the serine/threonine kinase domain found in type I receptor molecules, suggesting a dominant-negative function. Analysis of the Xenopus homologue, BAMBI, demonstrated that BAMBI complexes with all TGFβ type I receptors, except for Alk2, thereby inhibiting signal transduction due to its lack of a serine/threonine kinase domain (Onichtchouk et al., 1999). Furthermore, BAMBI’s extracellular amino domain has high homology to TGFβ type I receptors, but it is not capable of binding independently to the ligands TGFβ1 or BMP2. If TGFβ1 or BMP2 is already in a complex with the TGFβ type II receptor, then BAMBI will interact with the complex (Onichtchouk et al., 1999; Tsang et al., 2000). Hence, Nma/BAMBI may function as a dominant-negative molecule through two mechanisms: (1) by physically inhibiting dimerization of TGFβ type I and II receptors, and (2) by failure to transduce phosphorylation of R-Smads.
Expression of Nma/BAMBI orthologues is tightly correlated with BMP4 expression in Xenopus, mouse, and chicken (Onichtchouk et al., 1999; Grotewold et al., 2001; Zuzarte-Luis et al., 2004). In addition, BMP4 activates and maintains expression of BAMBI (Onichtchouk et al., 1999). In theory, the dominant-negative function of BAMBI would enable BAMBI to reduce its own expression levels by interfering with BMP4 signaling. However, studies performed in developing chick embryos demonstrated that both Retinoic Acid (RA) and BMP7 up-regulate BAMBI expression, and Fibroblast Growth Factor-2 (FGF2) down-regulates BAMBI (Higashihori et al., 2008). Interestingly, BMP7 functions through the Alk2 receptor, which is a receptor with which BAMBI does not interact (Onichtchouk et al., 1999). Therefore, BMP7 may serve an important role in maintaining BAMBI expression during development.
The reciprocal nature of BMPs and TGFβ signaling has long been described; however, there are no studies examining the regulation of Nma/BAMBI by TGFβ during dentinogenesis. This study evaluated the competing roles of Nma/BAMBI and TGFβ during this process.
Materials & Methods
Nma/BAMBI-V5-His Expression Construct
The cloning region for Nma/BAMBI was amplified from a mouse tooth cDNA library (primers listed in the Table) and subcloned into the pcDNA 3.1/V5-His-TOPO vector (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol in forward (Nma F) and reverse (Nma R) orientations. Clones were isolated, plasmid purified, and orientation of insert determined via DNA dideoxy sequencing. Clones with Nma/BAMBI insert in the forward and reverse orientation were utilized in stable and transient transfection studies.
Table.
Primer Sets for Semi-quantitative and Quantitative RT-PCR
| Gene | Primer Name | Exons | Sequence (5′-3′) | Size | Temp°C |
|---|---|---|---|---|---|
| DSPP | RT mDSPP-S | 4/5 | agcatgtccttctgggaaga | 208 bp | 60 |
| DSPP | RT mDSPP-AS | 4/5 | actgtgggcagctgatttct | 60 | |
| DMP-1 | RT mDMP-1-S | 5/6 | aggaatcgcatcccaatatg | 221 bp | 60 |
| DMP-1 | RT mDMP-1-AS | 5/6 | gctgtgcgtgtggtcactat | 60 | |
| NMA/BAMBI | NMA-S | 2/3 | cgcaatggatcgccactcc | 500 bp | 60 |
| NMA/BAMBI | NMA-AS | 2/3 | cgcaatggatcgccactcc | 60 | |
| GAPDH | GAPDH-S | 5/7 | caaagttgtcatggatgacc | 407 bp | 60 |
| GAPDH | GAPDH-AS | 5/7 | ccatggagaaggctgggg | 60 |
MD10-A2 Cells
The MD10-A2 cell line is an established mouse odontoblast-like cell line derived from a previously characterized odontoblast monolayer cell culture system (MacDougall et al., 1995). MD10-A2 cells were immortalized as described (MacDougall et al., 1995) and characterized by RT-PCR for their ability to express type I collagen, osteopontin, osteocalcin, bone sialoprotein, dentin matrix protein 1 (DMP1), and dentin sialophosphoprotein (DSPP) (data not shown). The potential for MD10-A2 cells to produce a mineralized matrix in osteogenic media was evaluated by intense alkaline phosphatase (ALP) staining and Von Kossa staining (data not shown).
Transient Co-transfections
Nma/BAMBI-V5-His expression constructs and the DSPP-Luc reporter construct were transiently co-transfected into MD10-A2 cells with Lipofectamine reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Controls included co-transfections of DSPP-Luc with Nma/BAMBI-V5-His in the reverse orientation (Nma R) and empty vector (EV). Each group was cultured with and without TGFβ-1 supplementation (5 ng/mL) for 24 hrs. Cells were either harvested for total RNA isolation or fixed for immunohistochemical analysis to confirm expression of the Nma/BAMBI-V5-His fusion protein (data not shown).
cDNA Synthesis and Semi-quantitative RT-PCR (SQ-RTPCR)
Total RNA was isolated with RNA Stat 60 (Tel-Test, Friendswood, TX, USA) according to the manufacturer’s protocol. cDNA synthesis was performed on 500 ng of total RNA from each sample group with random primers and reverse-transcriptase DNA polymerase. SQ-RTPCR was performed with primers for the housekeeping gene GADPH (Table) and primers for Nma/BAMBI (Table) for 25 cycles. Samples were run on 2% agarose gels, and pixel density analysis was performed with Alpha Imager 2.0 software (Kodak, Rochester, NY, USA).
DSPP Promoter Luciferase Constructs
The DSPP promoter luciferase construct (DSPP-Luc) used in this study was previously generated and characterized by our laboratory (Unterbrink et al., 2002). Briefly, the DSPP promoter fragment, -1243 bp DSPP (Bgl II/HindIII), cloned into the pGL-3 luciferase (LUC) basic expression vector, was used for transient co-transfections and luciferase assays.
Luciferase Assays
Nma/BAMBI-V5-His expression constructs and the DSPP-Luc reporter constructs were transiently co-transfected into MD10-A2 cells, followed by luciferase assays as previously described (Unterbrink et al., 2002). All experiments were performed 3 times independently, each with triplicate samples. Controls included cells co-transfected with Nma/BAMBI-V5-His construct in the reverse orientation (Nma R) and the DSPP-Luc reporter construct, cells transfected with EV and DSPP-Luc reporter construct, and non-transfected MD10-A2cells.
Stable Transfections
MD10-A2 cells were co-transfected with Nma F expression construct and a Green Fluorescent Protein (GFP) construct that contains the Hygromycin B resistance gene as a selectable marker (9:1), with Lipofectamine reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Controls included co-transfections of Nma R and GFP constructs or co-transfection of EV and GFP constructs. Stable transfectants were selected with media containing 50 mg/mL of Hygromycin B.
Alkaline Phosphatase (ALP) Activity Assay
ALP activity was determined with the Alkaline Phosphatase Conjugate Substrate Kit (BioRad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol.
Quantitative RT-PCR (QRT-PCR)
Total RNA was isolated from MD10-A2 cells stably transfected with Nma/BAMBI expression constructs as described above. First-strand synthesis of cDNA was performed with an enhanced avian RT-PCR kit (Sigma, St. Louis, MO, USA). QRT-PCR was performed with Applied Biosystems’ ABI Prism 7000 Sequence Detection System and reagents and primers for Nma/BAMBI, DSPP, and DMP1 (Table).
Statistical Analysis
Data are presented as mean ± SEM or ± SD. Groups were compared by ANOVA with the Bonferroni post hoc test. A p < 0.05 was considered statistically significant.
Results
TGFβ-1 Negatively Regulates Endogenous NMA/BAMBI Expression in vitro
We have previously shown that TGFβ-1 negatively regulates DSPP expression in odontoblast-like cells (Unterbrink et al., 2002). To evaluate the role of Nma/BAMBI in TGFβ signaling, we transiently co-transfected MD10-A2 cells with the Nma/BAMBI-V5-His expression construct (Nma F) and the DSPP-Luciferase reporter construct, followed by treatment with TGFβ-1.
Over-expression of Nma/BAMBI (500-bp amplicon) was confirmed in MD10-A2 cells transiently co-transfected with Nma F and the DSPP-Luc reporter construct. Relative levels of expression, normalized to the housekeeping gene GAPDH (407-bp amplicon), indicated that Nma/BAMBI expression was increased approximately two-fold compared with non-transfected cells (Figs. 1A, 1B [lanes 2, 3], 1C; Appendix A).
Figure 1.
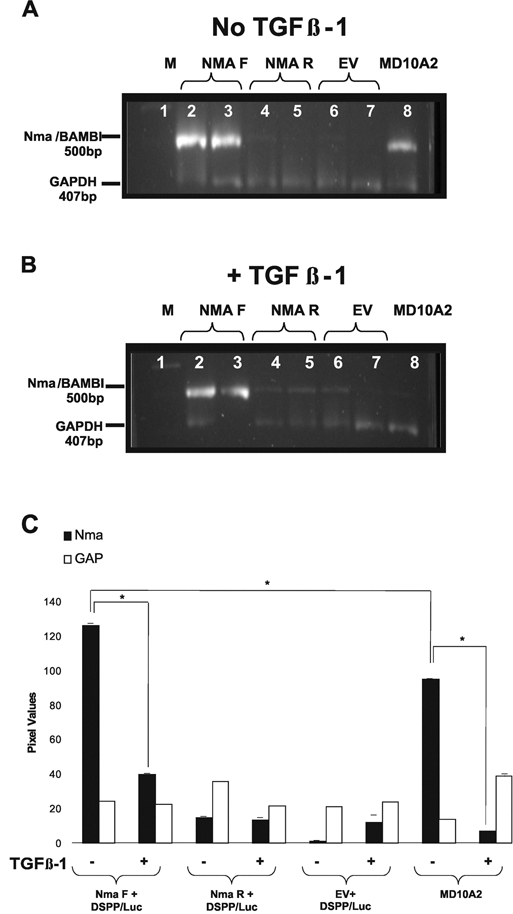
RT-PCR amplification of Nma/BAMBI expression (500 bp) vs. GAPDH (407 bp) transcripts from MD10-A2 cells transiently co-transfected with an Nma/BAMBI-V5-His expression construct and a DSPP-Luc reporter construct. Amplification of transfected MD10-A2 mRNA from cells grown in control media (A) or supplemented with TGFß-1 (B). Lane 1: 100-bp marker. Lanes 2 & 3: transfection with NMA/BAMBI-V5-His in the forward orientation (Nma F) and DSPP promoter-luciferase construct (DSPP-Luc). Lanes 4 & 5: transfection with NMA/BAMBI-V5-His in the reverse orientation (Nma R) and DSPP-Luc. Lanes 6 & 7: transfection with the empty vector (EV) and DSPP-Luc. Lane 8: non-transfected MD10-A2 cells. Increased Nma/BAMBI expression (lanes 2 & 3) compared with non-transfected MD10-A2 cells (lanes 8) is evident regardless of TGFβ supplementation (A, B). (C) Semi-quantitative analysis of RT-PCR Nma/BAMBI expression data presented in panels A & B. Cells treated with TGFβ-1 show a statistically significant reduction in Nma/BAMBI expression compared with untreated cells within the Nma F+DSPP-Luc transfected group and within the MD10-A2 control group (n = 3; p = 0.05; ± SD). Nma F+DSPP/Luc transfected cells had statistically significantly increased Nma/BAMBI expression compared with MD10-A2 control cells (n = 3; p = 0.05; ± SD).
Interestingly, expression of endogenous Nma/BAMBI was down-regulated by the addition of TGFß-1, as indicated by the control non-transfected MD10-A2 cells treated with TGFβ-1 or cultured in media alone (Figs. 1A, 1B, lane 8; Fig. 1C; Appendix B). TGFβ’s negative regulation of Nma/BAMBI was also seen in transfected cells that overexpress Nma/BAMBI (Fig. 1C; Appendix C). Controls consisted of MD10-A2 cells co-transfected with Nma/BAMBI-V5-His in the reverse orientation (Nma R) and the DSPP-Luc construct, MD10-A2 cells co-transfected with EV and DSPP-Luc construct, and non- transfected MD10-A2 cells. Endogenous Nma/BAMBI expression levels, in all controls, were at least 50% less than Nma/BAMBI levels in MD10-A2 cells transfected with Nma F and DSPP-Luc (Fig. 1C).
Overexpression of NMA/BAMBI Negatively Regulates DSPP Expression
Control luciferase assays were performed in MD10-A2 cells transiently transfected with the DSPP-Luc reporter construct alone and treated with TGFβ-1 for confirmation of our previous findings that TGFß-1 down-regulates DSPP promoter activity (Unterbrink et al., 2002). Transiently transfected cells cultured in plain media demonstrated a significant increase in luciferase activity compared with those treated with TGFß-1 (p = 0.05) (Fig. 2A).
Figure 2.
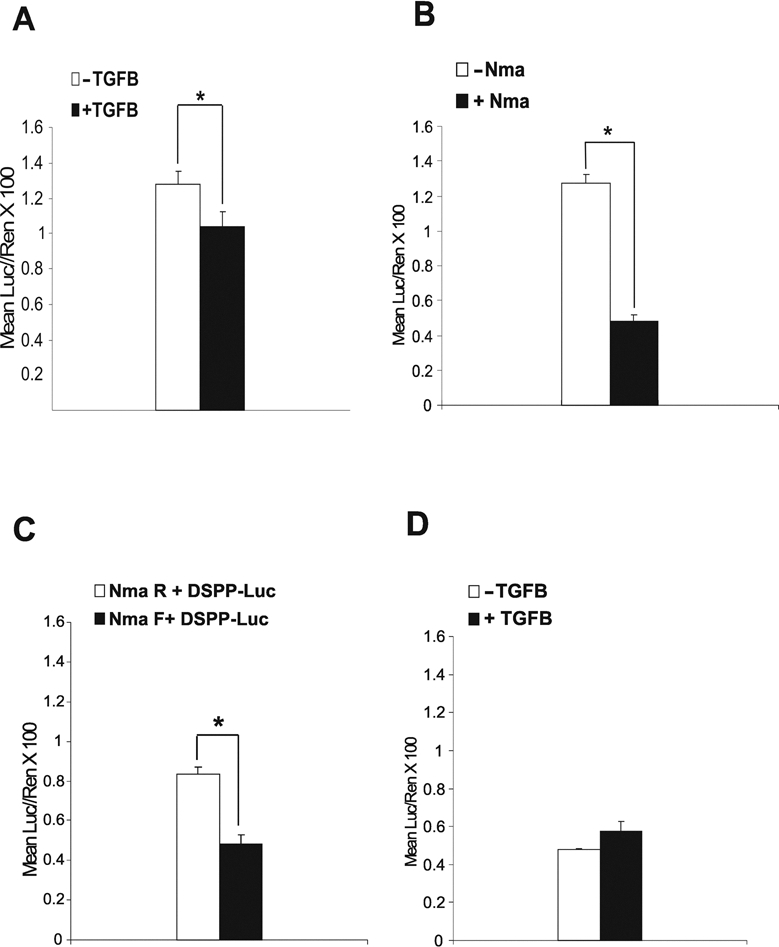
Luciferase assays performed on MD10-A2 cells transiently co-transfected with NMA/BAMBI-V5-His expression construct and the DSPP-Luc reporter construct. (A) Luciferase assay demonstrating the effects of TGFβ on DSPP expression in MD10-A2 cells. TGFβ significantly reduced expression of DSPP, as indicated by the asterisk (*) (n = 6; p = 0.05; ± SEM). (B) Luciferase assay demonstrating the effects of Nma/BAMBI on DSPP expression in MD10-A2 cells. Nma/BAMBI significantly reduced expression of DSPP, as indicated by the asterisk (*) (n = 6; p = 0.05; ± SEM). (C) Luciferase assay demonstrating that overexpression of Nma/BAMBI had a negative effect on DSPP promoter activity compared with the reverse-oriented control construct Nma R. While double transfections appeared to decrease the intensity of the reporter construct, expression of Nma/BAMBI-V5-His (Nma F) significantly down-regulated DSPP promoter activity compared with Nma R Control Vector (n = 6; p = 0.05; ± SEM). (D) Luciferase assay demonstrating the effects of TGFβ on MD10-A2 cells transfected with Nma/BAMBI-V5-His expression construct. TGFβ lifted Nma/BAMBI’s negative effect on DSPP expression, but not at a statistically significant level (n = 6; p = 0.05; ± SEM).
Luciferase assays of MD10-A2 cells overexpressing Nma/BAMBI-V5-His fusion protein (Nma F) in the absence of TGFß supplementation showed a reduction of luciferase activity, indicating that overexpression of Nma/BAMBI significantly negatively regulated DSPP promoter activity (p = 0.05) (Fig. 2B). Additionally, overexpression of Nma/BAMBI (Nma F) significantly down-regulated DSPP promoter activity compared with the Nma R control vector (p = 0.05) (Fig. 2C).
Last, cells overexpressing Nma/BAMBI and treated with TGFß-1 had a slight increase in DSPP-Luc activity, but the difference was not statistically significant at p = 0.05 (Fig. 2D). It may be that the relative concentrations of Nma/BAMBI-V5-His fusion protein and TGFß-1 need to be more carefully titered to show TGFβ’s ability to reverse Nma/BAMBI’s action on DSPP expression definitively at a statistically significant level. However, the trend remains that Nma/BAMBI alone decreased DSPP promoter activity and that TGFß may reverse the negative effects of Nma/BAMBI on DSPP promoter activity as well.
Overexpression of NMA/BAMBI Decreased DSPP and Increased DMP1 Expression
The effects of overexpression of Nma/BAMBI on downstream gene expression were also confirmed, with MD10-A2 cells stably overexpressing Nma/BAMBI (Fig. 3A). QRT-PCR demonstrated that Nma/BAMBI-V5-His fusion protein was overexpressed four-fold in MD10-A2 cells (Fig. 3A). Over-expression of Nma/BAMBI resulted in a 66% decrease in DSPP expression levels and a four-fold increase in DMP1 expression (Fig. 3A). No TGFß-1 supplementation was utilized in these experiments.
Figure 3.
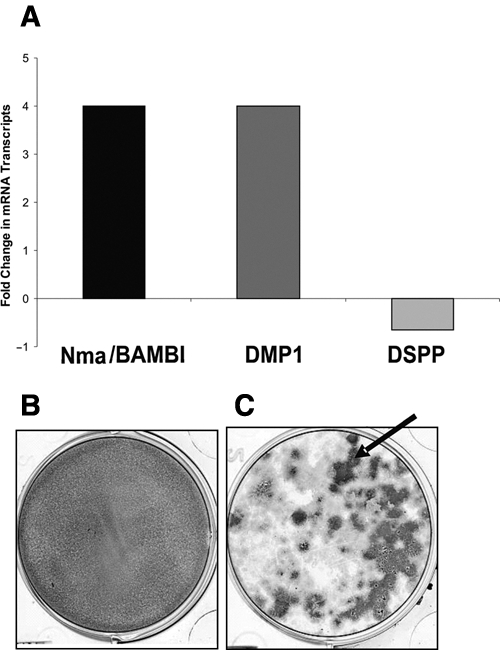
Quantitative real-time PCR (QRT-PCR) and ALP staining of MD10-A2 cells stably transfected with NMA/BAMBI-V5-His expression construct in the forward orientation vs. non-transfected MD10-A2 cells cultured in control media. (A) The average fold change in expression of Nma/BAMBI, DMP1, and DSPP in MD-10A2 cells overexpressing Nma/BAMBI is shown. Nma/BAMBI was expressed four-fold compared with non-transfected control cells. Additionally, DMP1 expression was up-regulated four-fold, while DSPP expression was down-regulated 66%. (B) Control non-transfected MD10-A2 cells showing diffuse uniform ALP staining. (C) MD10-A2 cells overexpressing Nma/BAMBI show positive ALP staining (black arrow) associated with the formation of large multi-layered cell nodules.
Overexpression of NMA/BAMBI Increased ALP Activity
To evaluate the effects of Nma/BAMBI expression on MD10-A2 related to early mineralization events, we performed in situ ALP histochemistry staining on cells stably overexpressing Nma/BAMBI. Transfected cells demonstrated intense ALP staining associated with large multi-layered nodules, evident by 3 days of culture (Fig. 3C). In these cultures, areas that were not multi-layered did not demonstrate detectable ALP activity. In contrast, non-transfected MD10-A2 cells showed a very uniform ALP staining throughout the confluent single cell layer (Fig. 3B).
Discussion
Nma/BAMBI is a potential dominant-negative regulator of TGFβ. To dissect the role of Nma/BAMBI during dentinogenesis, we performed gain-of-function studies in the odontoblast-like cell line MD10-A2. Overexpression of Nma/BAMBI resulted in a four-fold increase in mRNA levels of the dentin extracellular matrix protein, DMP1, and a 66% reduction in DSPP mRNA expression levels. Importantly, we demonstrated that TGFβ down-regulated both endogenous and exogenous levels of Nma/BAMBI, with as much as a 98% reduction of transcripts detected via SQ-RTPCR techniques. Furthermore, TGFβ reversed Nma/BAMBI’s effect on DSPP expression, indicating that Nma/BAMBI and TGFβ compete during dentinogenesis.
These findings are in contrast to those of others, who demonstrated that expression of the human BAMBI orthologue is up-regulated by TGFβ in the human hepatocellular liver cell line, HepG2 (Sekiya et al., 2004). It is important to note that TGFβ plays opposing roles in tumor development (Wieser, 2001; Freeman et al., 2004). TGFβ acts as a tumor suppressor molecule by inhibiting cellular proliferation during the initiation of tumor development, via the Smad pathway, and enhances metastatic potential during later stages of tumor progression and metastases through a pathway that has yet to be fully delineated (Dumont et al., 2003). Hence, TGFβ’s ability to increase BAMBI expression in HepG2 cells may not necessarily be extrapolated to explain its role in normal development. Our findings indicate that TGFβ down-regulates Nma/BAMBI expression during dentinogenesis in odontoblast-like cells.
While we have shown that TGFβ negatively regulated Nma/BAMBI expression in MD10-A2 cells, we hypothesized that when Nma/BAMBI is present, it serves as a dominant-negative regulator of TGFβ signaling, thereby maintaining its own expression. Therefore, it may be that the dominant molecule in this system is the one which is expressed first or at higher levels. These results provide insight into possible mechanisms for regulating odontoblast cytodifferentiation and subsequent dentin mineralization.
Interestingly, luciferase assays, aimed at evaluating the roles of Nma/BAMBI and TGFβ on DSPP promoter activity, also demonstrated that Nma/BAMBI overexpression alone down-regulates DSPP promoter activity, as does TGFß-1 supplementation. Since both Nma/BAMBI and TGFβ down-regulate DSPP expression, we hypothesized that they will work synergistically to inhibit DSPP-Luc luciferase activity. However, our results revealed that overexpression of Nma/BAMBI first, followed by treatment with TGFß-1, demonstrated a slight increase in DSPP promoter activity relative to the level when down-regulated by Nma/BAMBI overexpression alone, but not at a statistically significant level. This finding indicates that TGFβ signaling competes with Nma/BAMBI in this system.
Overexpression of Nma/BAMBI affected downstream gene expression in the absence of TGFβ; thus, other ligands may be involved in this regulation. Our laboratory previously reported that MD10-A2 cells express BMP2 and BMP7, but not BMP4, and they express Alk2 and Alk3 receptors (Dodds et al., 2001). The Alk2 receptor binds BMP7, whereas the Alk3 receptor binds both BMP2 and BMP4. However, Onichtchouk et al. (1999) determined that the BAMBI homologue does not complex with Alk2; thus, in MD10-A2 cells, Nma/BAMBI is probably functioning within an Alk3/BMP2 complex. From these data, we deduce that overexpression of Nma/BAMBI may be affecting the BMP2 signal transduction pathway within our MD10-A2 cells.
In summary, we have shown that Nma/BAMBI regulates the expression of tooth matrix proteins involved in dentin mineralization in odontoblast-like cells (MD10-A2). Furthermore, TGFß-1 down-regulates Nma/BAMBI expression levels in this cell type. However, once Nma/BAMBI is expressed, it inhibits TGFß signaling. Hence, the order of expression or the relative concentrations of these two molecules may ultimately determine which pathway will have dominance within the differentiated cells.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by an NIDCR F30 award DE05749 and an NIDCR T32-CO-STAR award DE014318.
This paper is based upon preliminary work previously published: Knight C, Simmons D, Gu TT, Gluhak-Heinrich J, Zeichner-David M, MacDougall M (2001). Cloning, characterization, and tissue expression pattern of mouse Nma/BAMBI. J Dent Res 80(10):1895-1902.
References
- Dodds A. (2001). Bone morphogenetic proteins and bone morphogenetic protein type I receptors in dentinogenesis (PhD thesis). San Antonio: University of Texas Health Science Center at San Antonio [Google Scholar]
- Dumont N, Bakin AV, Arteaga CL. (2003). Autocrine transforming growth factor-β signaling mediates Smad-independent motility in human cancer cells. J Biol Chem 278:3275-3285 [DOI] [PubMed] [Google Scholar]
- Freeman JW, DeArmond D, Lake M, Huang W, Venkatasubbarao K, Zhao S. (2004). Alterations of cell signaling pathways in pancreatic cancer. Front Biosci 9:1889-1898 [DOI] [PubMed] [Google Scholar]
- Grotewold L, Plum M, Dildrop R, Peters T, Ruther U. (2001). Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mech Dev 100:327-330 [DOI] [PubMed] [Google Scholar]
- Higashihori N, Song Y, Richman JM. (2008). Expression and regulation of the decoy bone morphogenetic protein receptor BAMBI in the developing avian face. Dev Dyn 237:1500-1508 [DOI] [PubMed] [Google Scholar]
- Knight C, Simmons D, Gu TT, Gluhak-Heinrich J, Zeichner-David M, MacDougall M. (2001). Cloning, characterization and tissue expression pattern of mouse Nma/BAMBI. J Dent Res 80:1895-1902 [DOI] [PubMed] [Google Scholar]
- MacDougall M, Thiemann F, Ta H, Hsu P, Chen L, Snead M. (1995). Temperature sensitive simian virus 40 large T antigen immortalization of murine odontoblast cell cultures: establishment of clonal odontoblast cell line. Connect Tissue Res 33:97-103 [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen Y. (2000). Controlling TGFβ signaling. Genes Dev 14:627-644 [PubMed] [Google Scholar]
- Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, et al. (1997). TGF-β receptor mediated signaling through Smad2, Smad3, and Smad4. EMBO J 16:5353-5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onichtchouk D, Chen Y, Dosch R, Gawantka V, Delius H, Massagué J, et al. (1999). Silencing of TGF-β by the pseudoreceptor BAMBI. Nature 401:480-484 [DOI] [PubMed] [Google Scholar]
- Padgett RW, Das P, Krishna S. (1998). TGF-beta signaling, Smads, and tumor suppressors. Bioessays 20:382-390 [DOI] [PubMed] [Google Scholar]
- Sekiya T, Oda T, Matsuura K, Akiyama T. (2004). Transcriptional regulation of the TGF-beta pseudoreceptor BAMBI by TGF-beta signaling. Biochem Biophys Res Commun 320:680-684 [DOI] [PubMed] [Google Scholar]
- Tsang M, Kim R, de Caestecker MP, Kudoh T, Roberts AB, Dawid IB. (2000). Zebrafish nma is involved in TGFβ family signaling. Genesis 28:47-57 [DOI] [PubMed] [Google Scholar]
- Unterbrink A, O’Sullivan M, Chen S, MacDougall M. (2002). TGF beta-1 downregulates DMP-1 and DSPP in odontoblasts. Connect Tissue Res 43:354-358 [DOI] [PubMed] [Google Scholar]
- Wieser R. (2001). The transforming growth factor-[beta] signaling pathway in tumorigenesis. Curr Opin Oncol 13:70-77 [DOI] [PubMed] [Google Scholar]
- Zuzarte-Luís V, Montero JA, Rodriquez-Léon J, Merino R, Rodríguez-Rey JC, Hurlé JM. (2004). A new role for BMP5 during limb development acting through the synergic activation of Smad and MAPK pathways. Dev Biol 272:39-52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


