Abstract
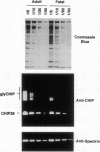
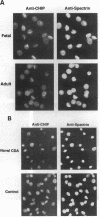
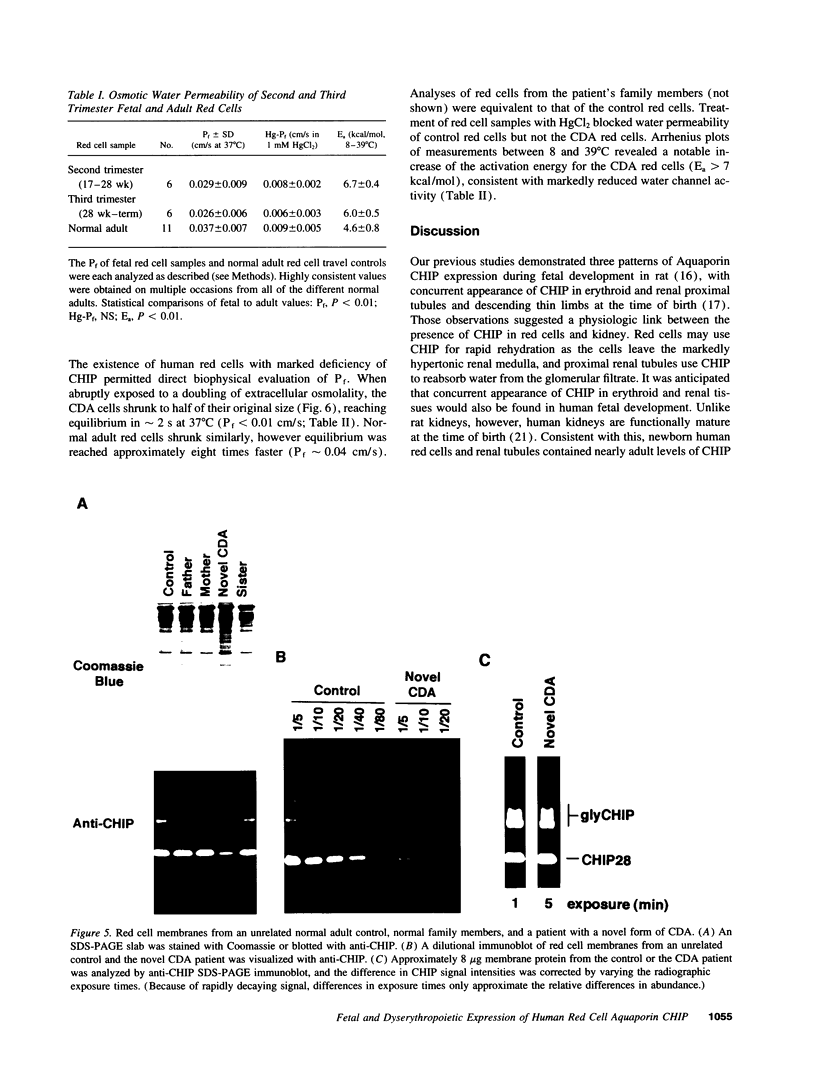
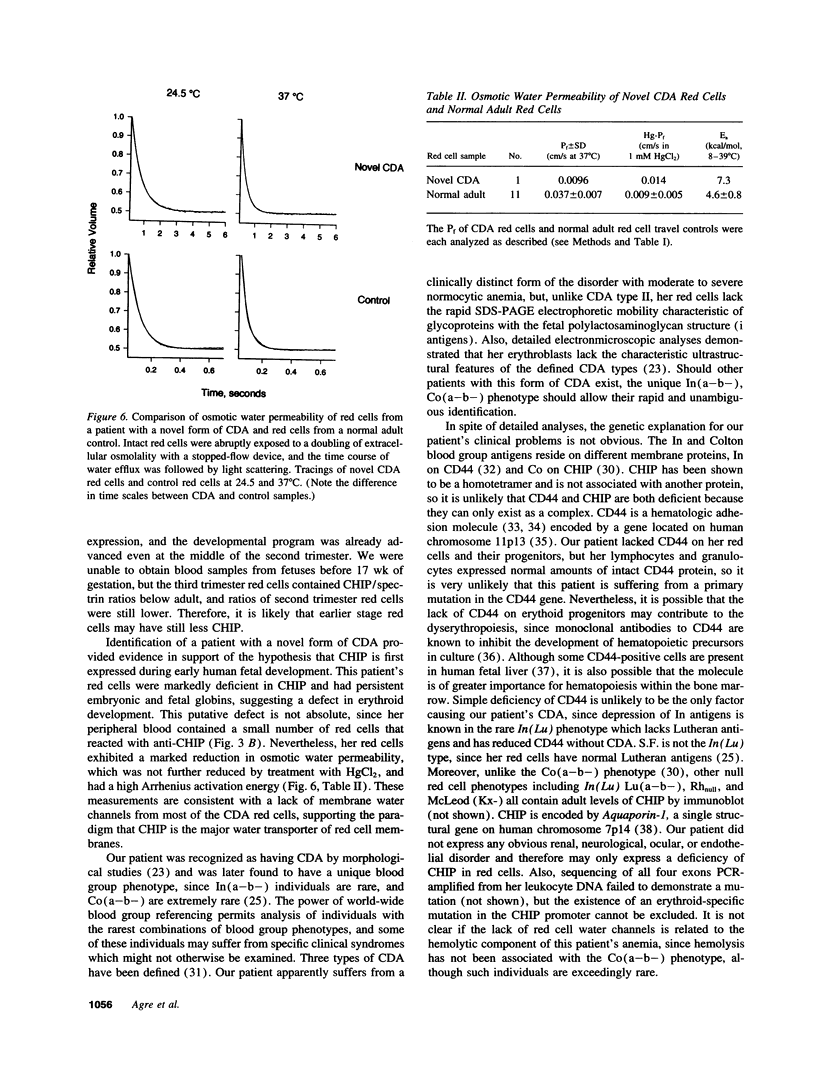
Channel-forming integral protein (CHIP) is the archetypal member of the Aquaporin family of water channels. Delayed CHIP expression was shown recently in perinatal rat (Smith, B. L., R. Baumgarten, S. Nielsen, D. Raben, M. L. Zeidel, and P. Agre. 1993. J. Clin. Invest. 92:2035-2041); here we delineate the human patterns. Compared with adult, second and third trimester human fetal red cells had lower CHIP/spectrin ratios (0.72 +/- 0.12, 0.94 +/- 0.22 vs 1.18 +/- 0.11) and reduced osmotic water permeability (0.029, 0.026 vs 0.037 cm/s); CHIP was already present in human renal tubules by the second trimester. A patient with a novel form of congenital dyserythropoietic anemia (CDA) with persistent embryonic and fetal globins and absent red cell CD44 protein was studied because of reduced CHIP-associated Colton antigens. Novel CDA red cells contained < 10% of the normal level of CHIP and had remarkably low osmotic water permeability (< 0.01 cm/s), but no mutation was identified in Aquaporin-1, the gene encoding CHIP. These studies demonstrate: (a) unlike rat, human CHIP expression occurs early in fetal development; (b) red cell water channels are greatly reduced in a rare phenotype; and (c) disrupted expression of red cell CHIP and CD44 suggests an approach to the molecular defect in a novel form of CDA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993 Oct;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Agre P., Sasaki S., Chrispeels M. J. Aquaporins: a family of water channel proteins. Am J Physiol. 1993 Sep;265(3 Pt 2):F461–F461. doi: 10.1152/ajprenal.1993.265.3.F461. [DOI] [PubMed] [Google Scholar]
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- Bondy C., Chin E., Smith B. L., Preston G. M., Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Verbavatz J. M., Valenti G., Lui B., Sabolić I. Localization of the CHIP28 water channel in reabsorptive segments of the rat male reproductive tract. Eur J Cell Biol. 1993 Aug;61(2):264–273. [PubMed] [Google Scholar]
- De Ca Chapelle A., Vuopio P., Sanger R., Teesdale P. Monosomy-7 and the Colton blood-groups. Lancet. 1975 Oct 25;2(7939):817–817. doi: 10.1016/s0140-6736(75)80042-0. [DOI] [PubMed] [Google Scholar]
- Denker B. M., Smith B. L., Kuhajda F. P., Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988 Oct 25;263(30):15634–15642. [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Wiles M. V., Tunnacliffe A., Parkar M., Solomon E., Dalchau R., Fabre J. W. The gene, MIC4, which controls expression of the antigen defined by monoclonal antibody F10.44.2, is on human chromosome 11. Eur J Immunol. 1982 Aug;12(8):659–663. doi: 10.1002/eji.1830120807. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Strange K., Zeidel M. L. Current understanding of the cellular biology and molecular structure of the antidiuretic hormone-stimulated water transport pathway. J Clin Invest. 1991 Jul;88(1):1–8. doi: 10.1172/JCI115263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C., Preston G. M., Griffin C. A., Jabs E. W., Agre P. The human aquaporin-CHIP gene. Structure, organization, and chromosomal localization. J Biol Chem. 1993 Jul 25;268(21):15772–15778. [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Anagnou N. P., Chui D., Dow L., Sanders J. Expression of embryonic globins by erythroid cells in juvenile chronic myelocytic leukemia. Blood. 1991 Jun 15;77(12):2569–2576. [PubMed] [Google Scholar]
- Parsons S. F., Jones J., Anstee D. J., Judson P. A., Gardner B., Wiener E., Poole J., Illum N., Wickramasinghe S. N. A novel form of congenital dyserythropoietic anemia associated with deficiency of erythroid CD44 and a unique blood group phenotype [In(a-b-), Co(a-b-)]. Blood. 1994 Feb 1;83(3):860–868. [PubMed] [Google Scholar]
- Pasquali F., Bernasconi P., Casalone R., Fraccaro M., Bernasconi C., Lazzarino M., Morra E., Alessandrino E. P., Marchi M. A., Sanger R. Pathogenetic significance of "pure" monosomy 7 in myeloproliferative disorders. Analysis of 14 cases. Hum Genet. 1982;62(1):40–51. doi: 10.1007/BF00295602. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Rane S., Aperia A., Eneroth P., Lundin S. Development of urinary concentrating capacity in weaning rats. Pediatr Res. 1985 May;19(5):472–475. doi: 10.1203/00006450-198505000-00013. [DOI] [PubMed] [Google Scholar]
- Shanahan C. M., Weissberg P. L., Metcalfe J. C. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993 Jul;73(1):193–204. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- Smith B. L., Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991 Apr 5;266(10):6407–6415. [PubMed] [Google Scholar]
- Smith B. L., Baumgarten R., Nielsen S., Raben D., Zeidel M. L., Agre P. Concurrent expression of erythroid and renal aquaporin CHIP and appearance of water channel activity in perinatal rats. J Clin Invest. 1993 Oct;92(4):2035–2041. doi: 10.1172/JCI116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. L., Preston G. M., Spring F. A., Anstee D. J., Agre P. Human red cell aquaporin CHIP. I. Molecular characterization of ABH and Colton blood group antigens. J Clin Invest. 1994 Sep;94(3):1043–1049. doi: 10.1172/JCI117418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring F. A., Dalchau R., Daniels G. L., Mallinson G., Judson P. A., Parsons S. F., Fabre J. W., Anstee D. J. The Ina and Inb blood group antigens are located on a glycoprotein of 80,000 MW (the CDw44 glycoprotein) whose expression is influenced by the In(Lu) gene. Immunology. 1988 May;64(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Tang W., Cai S. P., Eng B., Poon M. C., Waye J. S., Illum N., Chui D. H. Expression of embryonic zeta-globin and epsilon-globin chains in a 10-year-old girl with congenital anemia. Blood. 1993 Mar 15;81(6):1636–1640. [PubMed] [Google Scholar]
- Verkman A. S. Water channels in cell membranes. Annu Rev Physiol. 1992;54:97–108. doi: 10.1146/annurev.ph.54.030192.000525. [DOI] [PubMed] [Google Scholar]
- Weinberg R. S., Leibowitz D., Weinblatt M. E., Kochen J., Alter B. P. Juvenile chronic myelogenous leukaemia: the only example of truly fetal (not fetal-like) erythropoiesis. Br J Haematol. 1990 Oct;76(2):307–310. doi: 10.1111/j.1365-2141.1990.tb07891.x. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N., Illum N., Wimberley P. D. Congenital dyserythropoietic anaemia with novel intra-erythroblastic and intra-erythrocytic inclusions. Br J Haematol. 1991 Oct;79(2):322–330. doi: 10.1111/j.1365-2141.1991.tb04541.x. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Ambudkar S. V., Smith B. L., Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992 Aug 25;31(33):7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Nielsen S., Smith B. L., Ambudkar S. V., Maunsbach A. B., Agre P. Ultrastructure, pharmacologic inhibition, and transport selectivity of aquaporin channel-forming integral protein in proteoliposomes. Biochemistry. 1994 Feb 15;33(6):1606–1615. doi: 10.1021/bi00172a042. [DOI] [PubMed] [Google Scholar]
- van Hoek A. N., Verkman A. S. Functional reconstitution of the isolated erythrocyte water channel CHIP28. J Biol Chem. 1992 Sep 15;267(26):18267–18269. [PubMed] [Google Scholar]