Abstract
Risk assessments are typically conducted on a chemical-by-chemical basis; however, many regulatory bodies are developing frameworks for assessing the cumulative risk of chemical mixtures of chemicals. The current investigation examined how chemicals that disrupt rat sex differentiation via two diverse mechanisms disrupt F1 male rat reproductive development, when administered together orally on days 14–18 of gestation. Experiment 1 used a mixture of 50 mg/kg-d procymidone and 500 mg/kg-d dibutyl phthalate (DBP), whereas experiment 2 used 150 mg/kg-d procymidone and 1125 mg/kg-d DBP (top dose), or 0, 4.17, 8.33, 16.7, 33.3, 50, 66.7, and 83.3% of the top dose. When we compared the dose and response addition predictions to the observed effects we found that dose addition models were more accurate than response addition models, indicating that compounds that act by different mechanisms of toxicity produce cumulative dose-additive effects.
Keywords: Cumulative risk assessment, Male rat sexual differentiation, Di-n-butyl phthalate, Androgen receptor antagonist, Procymidone
1. Introduction
Although risk assessments are typically conducted on a chemical-by-chemical basis, regulatory agencies and the scientific community are actively involved in discussions of how to conduct cumulative risk assessments for mixtures of chemicals. At present, there is no consistent framework for conducting cumulative assessments and experimental data are needed to facilitate the development of such a framework. Concern about mixtures of chemicals has arisen because studies show that humans [1], fish [2–5] and wildlife [6] are exposed to multiple chemicals. As a result, the study of the effects of mixtures of chemicals is an emerging area of increasing scientific and regulatory focus.
The US EPA began considering the cumulative risk of some of the chemicals that act via a common mechanism of toxicity under the 1996 Food Quality Protection Act (FQPA, Public Law 104–170, formerly known as H.R. 1627 at http://www.epa.gov/pesticides/regulating/laws/fqpa/gpogate.pdf). The US EPA’s Offices of Water and Research and Development and the EPA Superfund, Solid Waste and Air Programs have ongoing programs in this area. In this regard, the objective of our research is to provide data that facilitate the development of a guidance framework for assessing cumulative risk to reproduction and development from exposures that occur during pregnancy. Our working hypothesis is that chemicals that disrupt a common system or tissue during development, whether or not they have the same mechanism of toxicity, contribute to cumulative toxicity and should, therefore, be included in cumulative risk assessments. Several investigators have shown that mixtures of chemicals with diverse mechanisms of toxicity produce effects that far exceed those predicted by response addition models, which assume independent action for chemicals with different mechanisms of toxicity [7–10]. Our mixture research has included different combinations of pesticides, phthalates and 2,3,7,8-tetrachlorodibenzodioxin (TCDD) [9–15]. These chemicals disrupt sexual differentiation by acting as androgen receptor (AR) antagonists, inhibitors of fetal testosterone synthesis or as an aryl hydrocarbon receptor (AhR) agonist, respectively. Our results are in agreement with the conclusions of the National Academy of Science (NAS) Report on Phthalates [16] which reviewed the current research on mixtures for the US EPA. This approach represents a fundamental shift from the current cumulative risk assessment frameworks that only include chemicals sharing a narrowly defined mechanism of action in a cumulative assessment.
In the current study, we conducted two mixture experiments to determine how two chemicals with very distinct mechanisms of toxicity interact in a mixture. In these studies, pregnant rats were dosed during sexual differentiation on gestational days (GD) 14–18 with either the individual compounds or a mixture of them, and the postnatal development of the male offspring was monitored through adulthood.
The two chemicals selected for this investigation were chosen because their modes of action have been well characterized and they disrupt fetal male rat reproductive development via different cellular and molecular mechanisms of toxicity. They do not share a “common mechanism of toxicity”. procymidone (PRO) is a fungicide with androgen receptor (AR) antagonist activity, displayed both in vitro and in vivo [17–20]. PRO competitively inhibits the binding of androgens to the AR which leads to an inhibition of androgen-dependent gene expression in vitro and in vivo. In contrast, di-n-butyl phthalate (DBP) does not bind the AR, but rather, it alters male rat sexual differentiation by, in part, disrupting Leydig cell migration, differentiation and function [21–24]. This in turn results in reductions in (1) fetal testis testosterone production, (2) mRNA levels for key proteins in the steroidogenic pathway including StAR and CYP11, and (3) reductions in insl-3 mRNA: insl3 is a peptide that is critical for gubernacular development and normal testis descent [25,26].
The first experiment was a 2 × 2 factorial design with a control group, a PRO group, a DBP group and one mixture group, which combined the pesticide with the phthalate (this experiment has been briefly reviewed in [9,10,15,27] but the detailed results of this experiment have not been presented). We combined 500 mg/kg-d of the fetal testosterone synthesis inhibitor DBP with 50 mg/kg-d of the AR antagonist PRO. These dosage levels were based upon previous dose–response studies on each chemical and the mixture was expected to induce malformations of the external genitalia (hypospadias) in approximately half the male offspring if it behaved in a dose additive fashion.
In the second DBP and PRO mixture experiment we increased the number of dose groups and expanded the dose range with the mixture. This experiment used a fixed ratio design, with a control group and eight dilutions of the mixture of PRO and DBP and was designed to examine the interaction in utero of these two chemicals over a broad dose range. To make predictions the ED50s and Hillslope values from individual chemical dose–response experiments for each chemical were calculated using logistic regression analyses for several androgen-dependent endpoints (Table 1). These values were used in dose addition (DA) and response addition (RA) models to predict the effects of the DBP plus PRO mixture in the second mixture experiment. Then, the ED50s and Hillslope values from this second mixture experiment were calculated using logistic regression analyses and the results were compared to the predictions from the DA and RA models.
Table 1.
A comparison of the effects of the individual chemical dose–response studies with procymidone (PRO) and di-n-butyl phthalate (DBP) on male rat reproductive tract and sex accessory tissues. The table shows the ED50 values, the relative potencies, and Toxicity Equivalence Factors (TEF) based upon the individual PRO and DBP studies. Also shown are the Toxicity Equivalent Quotients (TEQs) that PRO and DBP contribute in mg/kg for the top dose of the second mixture experiment and the relative contribution of PRO and DBP to the expected effects in each dilution group in the second mixture experiment.
| Procymidone dose–response ED50 (mg/kg) |
DBP dose–response ED50 (mg/kg) |
Relative potencya PRO versus DBP |
TEFb PRO/DBP | PRO TEQsc in top dose of the mixture of PRO/DBP in experiment 2 |
% Contributions of PRO/DBP to the total mixture TEQs in experiment 2 |
|
|---|---|---|---|---|---|---|
| PND 13 nips | 90.0 | 694 | 7.7 | 1/0.13 | 150 mg/146 mg | 50%/50% |
| AGD | 177 | 955.0 | 5.4 | 1/0.19 | 150 mg/214 mg | 41%/59% |
| Hypospadias | 191.2 | 1000.0 | 5.2 | 1/0.19 | 150 mg/214 mg | 41%/59% |
| Ventral prostate | 276.1 | 987.4 | 3.6 | 1/0.28 | 150 mg/315 mg | 32%/68% |
| weight | ||||||
| Levator ani | ||||||
| Bulbocavernosus | 3.2 | 1/0.32 | 150 mg/360 mg | 29%/71% | ||
| Muscles weight | 274 | 879 | ||||
| Seminal vesicle weight | 242 | 732 | 3.0 | 1/0.33 | 150 mg/371 mg | 29%/71% |
| Epididymal weight | 220 | 584 | 2.7 | 1/0.37 | 150 mg/416 mg | 27%/73% |
| Total malformed not including nipples | 188 | 316 | 1.7 | 1/0.60 | 150 mg/675 mg | 18%/82% |
| Testis histopathology | >250 | 585 | ND | ND | ND | ND |
| Epididymal agenesis | >250 | 581.6 | ≈1 | ND | ND | ≈100% |
| Ectopic testes | >250 | 538.7 | ≈1 | ND | ND | ≈100% |
| Gubernacular agenesis | Infinity | 536 | 0 | None | Does not exist | 100% |
Relative potency = (DBP ED50)/(PRO ED50).
TEF = the individual potency of DBP relative to PRO in inducing the specific alteration.
PRO TEQs = describes the contributions of PRO and DBP in mg/kg-d in the top dose group in which the TEQ for PRO = 150 mg/kg-d and the TEQ for DBP = (DBP mg/kg-d × TEF for DBP) was calculated for each endpoint. ND: not determined; higher than doses used.
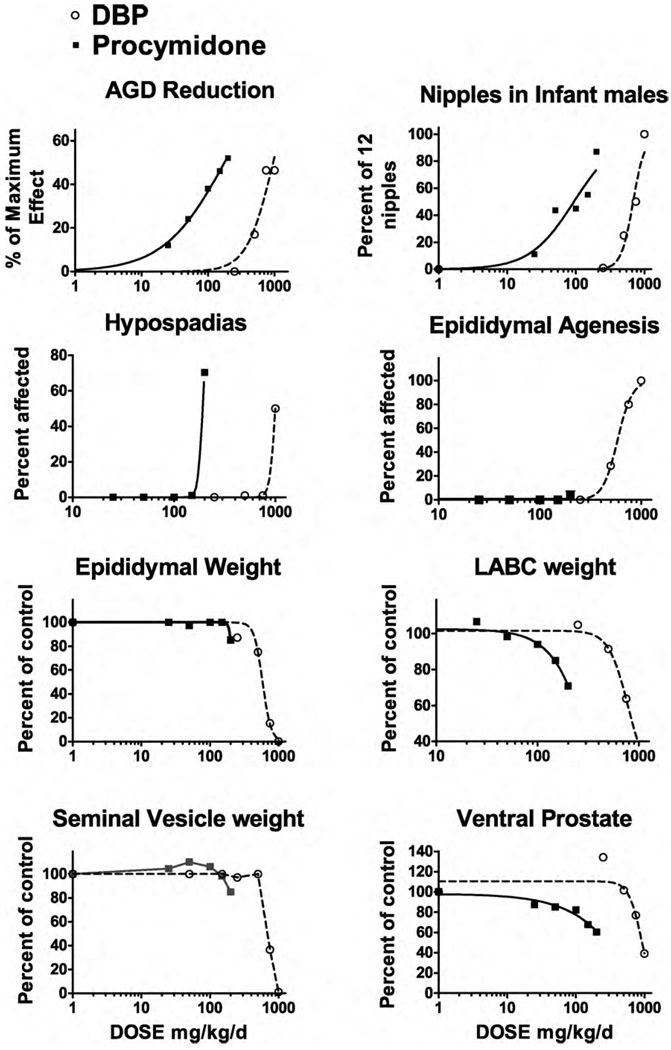
Even though each of these modes of disruption of the androgen signaling pathway results in different phenotypes in male rat offspring, our studies indicate that they both affect some of the same tissues. However, the relative potency factors (RPF) for PRO and DBP vary greatly from tissue to tissue (Table 1 and Fig. 1) [9,10]. Therefore, the contribution of each chemical to the overall mixture response depends upon the specific tissue and is not uniform across the reproductive tract. With this in mind, induction of hypospadias was chosen as the focal endpoint and the ratio of PRO and DBP in the mixture was selected such that each chemical would contribute equally to the induction of hypospadias (Table 1) if they behaved in a dose additive manner. Since this endpoint typically displays a steep threshold, DA and RA models should predict very different values. In contrast, DA and RA predictions for anogenital distance (AGD) and retained nipples in infant male rats are quite similar because the dose–response curves are more linear and, as a consequence, one cannot determine for this endpoint “which is the best model”: DA or RA. In summary, our hypothesis was that DA predictions would provide a more accurate fit than the RA model to the effects of the mixture of PRO and DBP on the induction of hypospadias, and, in addition, that DA models would provide predictions that were as good, or better, than RA models for the other androgen-dependent endpoints.
Fig. 1.
Individual chemical dose–response data for procymidone and di-n-butyl phthalate (DBP) were analyzed using a four parameter logistic regression model with GraphPad Prism 5.0 software. The ED50 and Hillslope values from the logistic regression analyses for anogenital distance in neonatal male rats (AGD), retained areolas/nipples in infant male rats and malformations (hypospadias, epididymal agenesis) and organ weights (epididymal, levator ani plus bulbocavernosus (LABC) muscles, seminal vesicles and ventral prostate) in adult male F1 rats. The X axes are in log10 scale.
2. Materials and methods
2.1. Animals
Timed-pregnant Sprague–Dawley rats were purchased from Charles River Breeding Laboratory (Raleigh, NC) on GD 2. Upon receipt, animals were housed individually in clear polycarbonate cages (20 cm × 25 cm × 47 cm) with a bedding of heat-treated laboratory-grade pine shavings (Northeastern Products, Warrensburg, NY). Animals were fed Purina Rat Chow 5008 (pregnant and lactating females) or Purina Rat Chow 5001 (weanling and adult rats), maintained on a 14:10 h light:dark cycle, and provided with access to filtered municipal drinking water (Durham, NC) ad libitum.
Laboratory-grade corn oil (CAS # 8001-30-7, Sigma C-8267, Lot # 89H0149) was the vehicle chosen to prepare all dosing solutions, which were administered by oral gavage in 2.5 ml of corn oil/g body weight from GD 14 to 18. Dams were randomly assigned to dose groups within each experiment in a manner that provided each group with similar means and variances in initial body weight before dosing was initiated (randomized complete block design with maternal weight as blocking factor (large to small)). In these experiments, the dose administered was adjusted daily based on individual maternal weight changes throughout the dosing period. The current study was conducted under protocols approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee.
2.2. Presentation of individual DBP and PRO chemical studies
Summaries of the individual DBP and PRO studies have been described previously [15,27], however, the results were reanalyzed in order to select the appropriate ratio of DBP and PRO for the second experiment herein and further interpreted to facilitate understanding of the expected behavior of these two toxicants in the two new mixture studies in the current investigation. In the PRO experiment, PRO was administered at 0, 25, 50, 100, 150 and 250 mg/kg-d from GD 14 to 18 to four dams per dose group (n = 17–30 F1 males per dose group). In the DBP experiment, DBP was administered at 0, 250, 500, 750 or 1000 g/kg/d to 3 dams per dose group from GD 14 to 18 (n = 5–15 F1 males per dose group).
First, the ED50 values for the DBP and the PRO studies were determined for male reproductive tract alterations (Table 1). Following this, we calculated the relative potency of DBP relative to PRO [relative potency = (DBP ED50)/(PRO ED50)]. We also calculated a Toxicity Equivalence Factor (TEF) for DBP versus PRO [PRO TEF = 1; DBP TEF = (PRO ED50)/(DBP ED50)] and we calculated the Toxicity Equivalent Quotient (TEQ) for the top dose of the mixture which describes the contributions of PRO and DBP in mg/kg-d in the top dose group [TEQ total = (TEQ PRO) + (TEQ DBP), TEQ for PRO = 150 mg/kg-d; TEQ for DBP = DBP mg/kg-d × TEF].
Comparing the TEQs of PRO to DBP allowed us to determine our mixture ratio in the second experiment in order to administer PRO and DBP in a manner such that each would contribute equally to inducing hypospadias if they behaved in a dose additive manner. Table 1 is sorted such that the endpoints where DBP and PRO should contribute equally to the effect are at the top, and those endpoints for which an effect would be due to the action of a single chemical are at the bottom. The importance of this is that DA and RA models provide more divergent predictions when (1) the two chemicals contribute equally to the induction of the effect as is the case for hypospadias but not for epididymal or gubernaculuar agenesis and (2) the effect has a steep threshold as is the case for hypospadias but not for AGD.
2.3. Experiment 1
The first mixture experiment was a 2 × 2 factorial design (Vehicle control, PRO alone, DBP alone and DBP plus PRO). PRO (Riedel de Haen, Product #34369, Lot # 72480, CAS #32809-16-8) was administered by gavage at 50 mg/kg-d and DBP (Sigma # D2270, Lot #109f0386, CAS #84-74-2) was administered at 500 mg/kg-d. Each of the four dose groups contained 10 dams per group. AGD and the number of retained female-like nipples (out of 12 possible), and malformations were measured in all F1 males and organ weights were measured in 2–3 males per litter (control n = 23, DBP n = 26, PRO n = 24 and DBP plus PRO n = 21 males) with 10 litters per group. F1 Males were necropsied at about 150 days of age.
2.4. Experiment 2
The second experiment was a fixed ratio, dilution experiment with a vehicle control, a top dose, and seven dilutions of the top dose. Pregnant dams were weighed and assigned to 1 of 9 treatment groups (0, 4.17, 8.33, 16.7, 33.3, 50, 66.7, 83.3, and 100% of the top dose (150 mg PRO/kg-d and 1125 mg DBP/kg-d)) with four pregnant rats/group such that each treatment group had a similar group body weight (g) on GD14. The ratio of PRO to DBP in the mixture was constant in each dose group [Ratio = 1 part PRO/7.5 parts DBP] (the contributions of PRO and DBP in each dose group are shown in Table 1).
2.5. Maternal, neonatal and infant F1 male rat data
Maternal weight was recorded daily during dosing and at birth. At birth (postcoital day 23 = postnatal day (PND) 1), litter sizes were counted, while body weight and AGD were measured on offspring at PND 2 (experiment 1) or PND day 3 (experiment 2). At 13 days of age, male offspring were examined (in a blinded manner) for “nipples”. At PND 24 male pups were weaned and housed in groups of 2 or 3 littermates, and the dams were necropsied and the numbers of implantation scars were counted.
Beginning on PND35 and continuing until completion, males were evaluated for timing of preputial separation (PPS), a landmark of puberty in the male rat. At approximately 4 months of age, males were anesthetized with CO2 and decapitated, the animals were shaved and the numbers of permanent nipples counted. Males were examined for the presence of external malformations, including the presence of hypospadias and abnormal glans penis (cleft phallus, exposed os penis and incomplete preputial glans separation) and internal malformations, including: epididymal agenesis, gubernacular malformations (i.e. agenesis and elongated underdevelopment of the gubernaculum), testicular malformations (e.g. testicular atrophy, cryptorchid testes, fluid-filled testes), and prostatic and seminal vesicular agenesis. Gubernacula measuring ≥ 20 mm in length were considered abnormal. The following organs were removed and their weights recorded: glans penis, ventral prostate, seminal vesicles, testes, epididymides, and levator ani/bulbocavernosus (LABC) muscles. Testes were preserved in fixative, imbedded in paraffin, stained with hematoxylin and eosin stain and examined for histopathological lesions by a board-certified pathologist.
3. Mixture modeling
Individual chemical dose–response data can be used in mathematical models to make predictions about the potential effects of mixtures on male reproductive tract development. Predicted mixture responses can then be compared to the observed effects of the mixtures to determine the type of joint action (DA, RA, synergy, or antagonism) exhibited by the mixture.
Using GraphPad Prism 5.0 software (La Jolla, CA), we transformed the individual chemical dose–response data to fit a 0–100% scale. For continuous endpoints (AGD and organ weights), we converted the data to percent change from the control value and Prism input included the mean, standard error and sample size for each group. For malformation data, we presented the data as percent incidence. We then graphed the data on a log-linear scale and fit the data with a sigmoidal (variable slope) equation in GraphPad Prism (see Eq. (1)):
| (1) |
where Y is the response, X is the chemical dose, Top and Bottom refer to the minimum and maximum effect calculated from the data and ED50 is the exposure dose eliciting a 50% response. The parameters (Hillslope and ED50) generated from the logistic fit to the individual chemical data were used in models to make predictions of the mixture responses.
4. Predicted responses from models versus observed responses
We modeled the observed mixture responses (means, standard errors and sample sizes) using Prism software and generated predictions of the mixture effects with DA or RA models (described in more detail in [9]).
The DA equation that we used to calculate predicted responses of mixtures was:
| (2) |
where R is the response to the mixture, Di is the concentration of chemical i in the mixture, ED50i is the concentration of chemical i that causes a 50% response, and ρ′ is the average Hillslope (i.e. slope associated with a logistic fit of the individual chemical dose–response curve) associated with the chemicals.
The RA model has been suggested as the more appropriate model for mixtures of chemicals with different mechanisms of action [28]. The equation for response addition is based on probability theory and is expressed as:
| (3) |
where R is the response of the mixture and Ri is the response of the individual chemicals (i) in the mixture.
The Prism output from the observed mixture responses included the ED50 (with standard errors and 95% confidence limits (CL)), and the goodness of fit (R2) for the “best-fit” model. For statistical purposes, we compared the ED50s of the observed responses from the second experiment with the predictions of the ED50s from the DA and RA models. Predicted ED50s differed significantly from the observed ED50s if the predicted value was outside of the 95% CL of the observed ED50. In addition, we fit the observed effects of the mixture to the logistic regression parameters of the DA and RA logistic regressions and compared how the resulting “goodness of fit” (R2 value) compared to R2 of the “best-fit” for the mixture effects. If either the DA or RA model perfectly predicted the effects seen with the mixture then the R2 value would be equal to the R2 value from the “best-fit” model.
5. Results
5.1. Preliminary experiment with PRO and DBP
The effects of PRO and DBP individually are shown in Fig. 1 and the results of the logistic regression analyses are shown in Table 1.
5.2. Experiment 1: 2 × 2 factorial experiment
In experiment 1, many of the observed effects of the mixture were significantly greater than those predicted by the RA model for several of the androgen-dependent tissues including seminal vesicle, LABC and cauda epididymal weights (Table 2), as well as the incidences of hypospadias, vaginal pouch, malformed ventral prostate and seminal vesicles and the total percent of malformed males (Table 3). The results of this initial experiment indicated that PRO and DBP were not acting independently in the mixture, as described by the RA model.
Table 2.
Cumulative effects in SD rat offspring of procymidone (50 mg/kg-d) and/or DBP (500 mg/kg-d) to the dam on gestational days (GD) 14–18. Neonatal and juvenile and adult male F1 necropsy data were analyzed using litter means. Values (means with standard errors as % different from control) with different letters (a, b, c) differ significantly from one another by LSMEANS test.
| Treatment | Control | Procymidone | DBP | Procymidone and DBP | ANOVA p value |
|---|---|---|---|---|---|
| No. dams | 10 | 10 | 10 | 10 | |
| Maternal body weight gain (g) GD 14–18 | 34.6 g ± 3.3 | 32.4 ± 3.1 | 33.7 ± 2.4 | 29.7 ± 1.8 | NS |
| Litter size at birth | 14.0 ± 1.4 | 14.0 ± 0.7 | 13.3 ± 0.8 | 13.6 ± 0.5 | NS |
| Female pup bodyweight (g) at day 2 | 7.32 ± 0.20 | 7.43 ± 0.22 | 7.44 ± 0.19 | 7.49 ± 0.24 | NS |
| Female pup anogenital distance (mm) at day 2 | 1.50 ± 0.07 | 1.52 ± 0.06 | 1.55 ± 0.07 | 1.53 ± 0.05 | NS |
| Male pup bodyweight (g) at day 2 | 7.83 ± 0.23 | 7.77 ± 0.09 | 7.71 ± 0.26 | 7.95 ± 0.22 | NS |
| Male anogenital distance (mm) at day 2 | 3.50 a ± 0.08 | 2.97 b ± 0.06 | 3.24 c ± 0.08 | 2.59 d ± 0.07 | p < 0.0001 |
| Percent of males with areolas/nipples day 13 | 1.7% ± 1.7 a | 96.6 ± 2.3 b | 41.7 ± 10. c | 97.2 ± 2.8 b | p < 0.0001 |
| Number of areolas/nipples per male day 13 | 0.03 ± 0.03 a | 6.8 ± 0.6 b | 2.1 ± 0.6 c | 9.8 ± 1.0 d | p < 0.0001 |
| Adult body weight (g) | 559 ± 15 | 593 ± 19 | 584 ± 12 | 561 ± 15 | NS |
| Adult glans penis (mg) | 96.7 ± 9.7 | 99.9 ± 2.4 | 101.7 ± 2.1 | 85.6 ± 3.8 | NS |
| Adult ventral prostate (mg) | 687 ± 25 a | 621 ± 33 ab | 586 ± 41 b | 484 ± 34 c | p < 0.003 |
| Adult seminal vesicle (mg)a | 1893 ± 97 a | 1886 ± 33 a (−0%) | 1763 ± 74 ab (−7%) | 1582 ± 86 b (−16%) | p < 0.03 |
| Adult LABC (mg)a | 1238 ± 31 a | 1172 ± 24 ab (−5%) | 1120 ± 33 b (−10%) | 871 ± 56 c (−30%) | p < 0.0001 |
| Adult testes (mg) | 3520 ± 131 | 3498 ± 67 | 3253 ± 139 | 3313 ± 150 | NS |
| Adult kidneys (mg) | 3683 ± 87 | 3798 ± 138 | 3723 ± 119 | 3775 ± 174 | NS |
| Adult nipples | 0 a | 2.8 ± 0.7 b | 1.2 ± 0.4 ab | 5.9 ± 1.1 c | p < 0.0001 |
| Adult epididymides (mg) | 1215 ± 42 | 1227 ± 24 | 1114 ± 65 | 1097 ± 83 | NS |
| Adult Cauda epididymis (mg)a | 300 ± 13 a | 310 ± 5.5 a (+3%) | 272 ± 16.8 a (−9%) | 243 ± 20 b (−19%) | p < 0.02 |
| Adult Caput epididymis (mg) | 302 ± 10 | 304 ± 9 | 295 ± 7 | 274 ± 24 | NS |
Shaded indicates the change in the mixture group exceeds the prediction by response addition.
Table 3.
Cumulative developmental effects in male SD rat offspring of procymidone, an AR antagonist, and the phthalate DBP at 50 and/or 500 mg/kg-d, respectively to the dam on gestational days 14–18. Male offspring reproductive malformation data.
| Treatment | Control n = 36 males | Procymidone n = 66 | DBP n = 58 | Procymidone and DBP n = 45 |
|---|---|---|---|---|
| Percent with nipples | 0% | 68.2 | 22.4 | 91.1 |
| Percent witha hypospadias | 0% | 1.5 | 0 | 48.8a |
| Vaginal poucha | 0% | 0 | 0 | 26.7a |
| Bladder stonesa | 0% | 0 | 0 | 11.1a |
| Ventral prostate malformed (unilateral or complete agenesis)a | 2.8% | 3.0 | 6.9 | 28.9a |
| Seminal vesicle malformeda | 0% | 0 | 10.3 | 31.1a |
| Coagulating gland malformed | 0% | 0 | 0 | 0 |
| Testicular malformations (fluid-filled and flaccid) | 0% | 0 | 24.1 | 28.9 |
| Epididymal malformations | 0% | 0 | 24.1 | 28.9 |
| Percent of males with any malformationa (except permanent nipples) | 0% | 7.5% | 29.3 | 55.6a |
Shaded indicates the percent change in the mixture group exceeds the prediction from the response addition model. n = 10 litters per dose group.
5.3. Experiment 2: fixed ratio, dilution experiment with 9 treatment groups
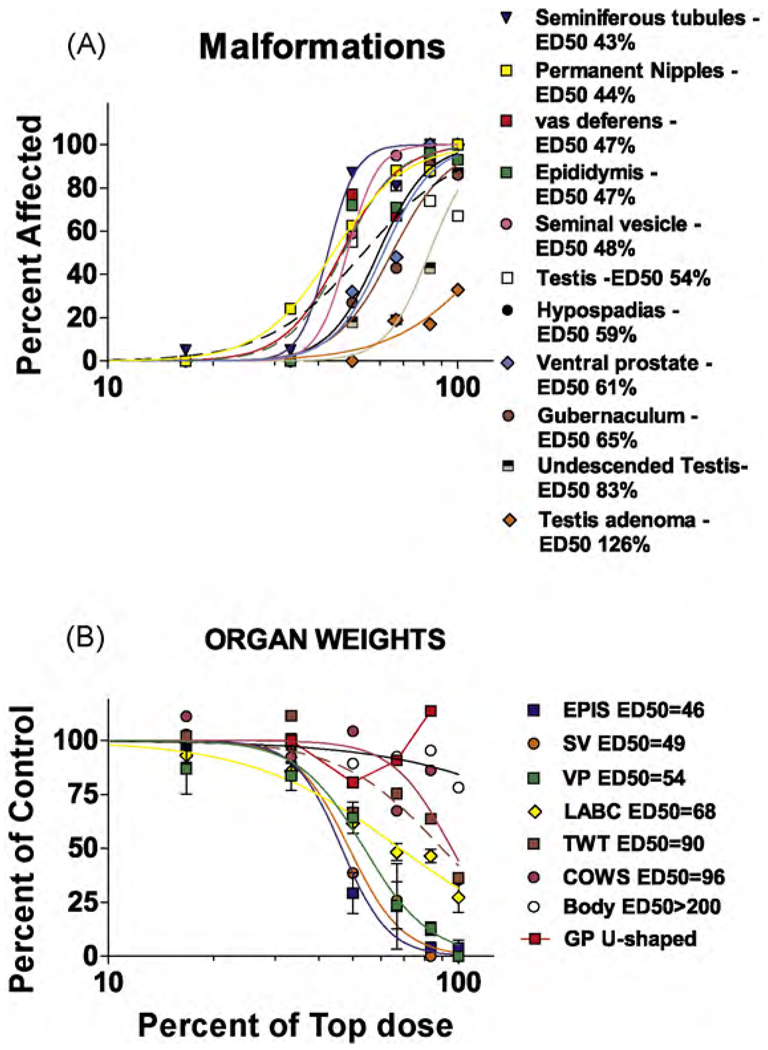
The dose-related effects of the mixture of PRO and DBP are shown in Fig. 2A and the logistic regression with ED50 values is shown in Fig. 2B. The ventral prostate (VP), seminal vesicles (SV) and epididymal (EPI) weight reductions display similar dose-related effects with an ED50 of about 50% of the top dose: LABC weight has an ED50 of about 68% of the top dose and the ED50 for testis and Cowper’s gland weights is about 90% of the top dose. Body weight is relatively less affected and was significantly reduced in only the top dose group. Glans penis (GP) weight displays an apparent inverted U-shaped dose–response because many or all the genitalia in the higher dose groups were too malformed to weigh, but the few that were not malformed were of normal size (Table 4).
Fig. 2.
Logistic regression analyses of the malformation (A) and organ weight (B) data from the second mixture experiment using nine dilutions of a mixture of procymidone and di-n-butyl phthalate (DBP). The list of endpoints at the right of each panel displays the ED50 values for each endpoint at the right. Abbreviations: GP—glans penis, VP—ventral prostate, SV—seminal vesicle, LABC—levator ani bulbocavernosus muscles, COWS—Cowper’s glands, TWT—testis weight, EPIS—epididymis, Body—body weight. The X axes are in log10 scale.
Table 4.
Experiment 2: effects of the mixture of procymidone and DBP on the dams and their male SD rat offspring. Data on the F1 male offspring includes neonatal weight and anogenital distance (AGD), retained female-like nipples at 13 days of age and reproductive organ weights and incidences of reproductive tract malformations taken at full maturity.
| Percent of the top dose (%) | 0 | 4.17 | 8.33 | 16.7 | 33.3 | 50 | 66.7 | 83.3 | 100 |
| Procymidone (mg/kg) | 0 | 6.25 | 12.5 | 25 | 50 | 75 | 100 | 125 | 150 |
| DBP (mg/kg) | 0 | 46.88 | 93.75 | 187.5 | 375 | 562 | 750 | 937.5 | 1125 |
| Number litters/males necropsied | 4/29 | 3/20 | 3/22 | 3/20 | 4/22 | 4/22 | 4/21 | 4/23 | 3/15 |
| Maternal weight gain (g)a | 28 ± 1.4 | 29.3 ± 5.54 | 31.17 ± 4 | 24.8 ± 7.3 | 18.4 ± 3.53 | 9.43 ± 10 | 11.4 ± 5 | 14.4 ± 0.4 | 12.3 ± 2.5 |
| AGD day 3 (mm)a | 3.31 ± 0.11 | 3.48 ± 0.044 | 3.43 ± 0.138 | 3.13 ± 0.048 | 2.72** ± 0.154 | 2.29** ± 0.152 | 2.11** ± 0.06 | 2.105** ± 0.07 | 2.011** ± 0.04 |
| Pup weight day 3 (g)a | 7.65 ± 0.22 | 7.85 ± 0.13 | 7.83 ± 0.36 | 7.97 ± 0.02 | 8.21 ± 0.16 | 7.19 ± 0.58 | 7.59 ± 0.163 | 7.4 ± 0.21 | 6.1** ± 0.45 |
| Number of areolae/nipples day 13a | 0 ± 0 | 0.11 ± 0.11 | 0.17 ± 0.17 | 0.89 ± 0.65 | 6.27** ± 0.97 | 8.1** ± 1.9 | 11.96** ± 0.04 | 11.83** ± 0.17 | 12** ± 0 |
| Age at preputial separation days of agea | 43.9 ± 0.6 | 46.8 ± 2.8 | 43.9 ± 1.1 | 45.1 ± 1.2 | 45.9 ± 0.8 | 55.6** ± 1.5 | 53.0** 1 litter | ND | ND |
| Body weight (g)a at Preputial Separation | 222.7 ± 12.2 | 233.7 ± 6.5 | 226.9 ± 10.6 | 233.6 ± 14.3 | 246.2 ± 9.6 | 288.3** ± 9.6 | 273.4* 1 litter | ND | ND |
| Hypospadias at preputial separationb | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 27.2%** ± 9.7 | 72.7%** ± 9.7 | 78.3%** ± 8.8 | 100%** ± 0 |
| Vaginal pouchb at preputial separation | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 68%** ± 10.1 | 43.5%* ± 10.6 | 100%** ± 0 |
| Undescended testes at preputial separationb | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 0% ± 0 | 4.5% ± 4.5 | 45.5%** ± 10.9 | 39.1%** ± 10.4 | 87.5%** ± 8.5 |
| Adult necropsy data | % of top dose | ||||||||
| 0 | 4.17 | 8.33 | 16.7 | 33.3 | Adult necropsy data | 66.7 | 83.3 | 100 | |
| Body weight at necropsy (g)a | 596 ± 19.3 | 559 ± 33 | 571 ± 25 | 612 ± 21 | 580 ± 18 | 532 ± 19 | 551 ± 15 | 568 ± 3 | 466** ± 66 |
| Number of permanent nipples/male of 12 possiblea | 0 ± 0 | 0 ± 0 | 0.03 ± 0.03 | 0 ± 0 | 2.8** ± 1.0 | 7.6** ± 0.7 | 10.7** ± 0.8 | 10.6** ± 0.6 | 12** ± 0 |
| Glans penis weight (mg)a (not including malformed tissues) | 118 ± 2.4 | 123 ± 4.3 | 112 ± 4.0 | 118 ± 1.9 | 118 ± 2.4 | 95** ± 6.7 | 107 NDc | 134 ± 17 | ND |
| Ventral prostate weight (mg)a | 828 ± 48 | 700 ± 64 | 593 ± 34 | 718 ± 95 | 692 ± 55 | 532** ± 60 | 195** ± 90 | 106** ± 25 | 0** ± 0 |
| Seminal vesicle weight (g)a | 1.81 ± 0.14 | 1.56 ± 0.15 | 1.76 ± 0.06 | 1.77 ± 0.13 | 1.77 ± 0.10 | 0.698** ± 0.218 | 0.469** ± 0.175 | 0** ± 0 | 0** ± 0 |
| Levator ani bulbocavernosus muscles weight (mg)a | 1508 ± 88 | 1403 ± 80 | 1433 ± 49 | 1404 ± 48 | 1293* ± 54 | 929** ± 73 | 728** ± 61 | 701** ± 47 | 410** ± 103 |
| Cowper’s glands (mg)a | 144 ± 18 | 145 ± 16 | 157 ± 9.0 | 160 ± 10 | 133 ± 16 | 150 ± 12 | 97 ± 33 | 124 ± 23 | 51** ± 33 |
| Paired testes weights (g)a | 3.42 ± 0.11 | 3.47 ± 0.19 | 3.54 ± 0.12 | 3.49 ± 0.07 | 3.81 ± 0.23 | 2.28** ± 0.33 | 2.58* ± 0.41 | 2.18** ± 0.15 | 1.24** ± 0.43 |
| Epididymal weight (mg)a | 1260 ± 53 | 1245 ± 47 | 1232 ± 52 | 1229 ± 12 | 1252 ± 47 | 369** ± 120 | 291** ± 249 | 51.5** ± 30 | 47.7** ± 47.7 |
| Number males necropsied | 29 | 20 | 22 | 20 | 22 | 22 | 21 | 23 | 15 |
| Percentage males with malformations | |||||||||
| Hypospadias, cleft phallus or exposed os penisb | 0 | 0 | 0 | 0 | 0 | 27.2++ | 67++ | 87++ | 100++ |
| Malformed vas deferensb | 0 | 0 | 0 | 0 | 0 | 77++ | 67++ | 91++ | 100++ |
| Undescended testisb | 0 | 0 | 0 | 0 | 0 | 18+ | 19+ | 43++ | 87++ |
| Testis with seminiferous tubular degeneration | 7 | 0 | 0 | 5 | 5 | 87++ | 81++ | 100++ | 100++ |
| Testis with interstitial cell Adenoma | 0 | 0 | 0 | 0 | 0 | 0 | 19+ | 17+ | 33++ |
| Malformed gubernaculumb | 0 | 0 | 0 | 0 | 0 | 27++ | 43++ | 91++ | 86++ |
| Malformed testisb | 0 | 0 | 0 | 0 | 0 | 55++ | 81++ | 74++ | 67++ |
| Malformed seminal vesicleb | 0 | 0 | 0 | 0 | 0 | 59++ | 95++ | 96++ | 100++ |
| Malformed ventral prostateb | 0 | 0 | 0 | 0 | 0 | 32++ | 48++ | 100++ | 100++ |
| Malformed epididymis | 0 | 0 | 0 | 0 | 0 | 72++ | 71++ | 96++ | 93++ |
| Any permanent nipples (yes or no) | 0 | 0 | 0 | 0 | 24.2+ | 62.5++ | 88.1++ | 88.0++ | 100++ |
ND—not determined. Shaded areas bolded values differ significantly from control by ANOVA.
Litter means and standard errors.
% individuals affected.
n = 1, the rest were malformed.
p < 0.05.
p < 0.01.
p < 0.05 from control by two tailed Fishers exact test.
p < 0.01 from control by two tailed Fishers exact test.
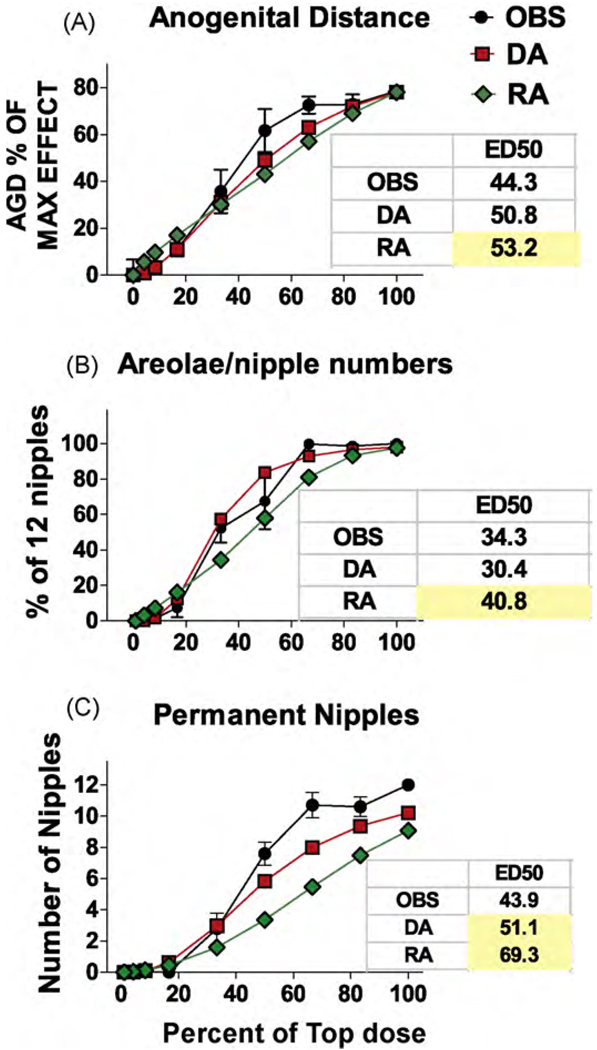
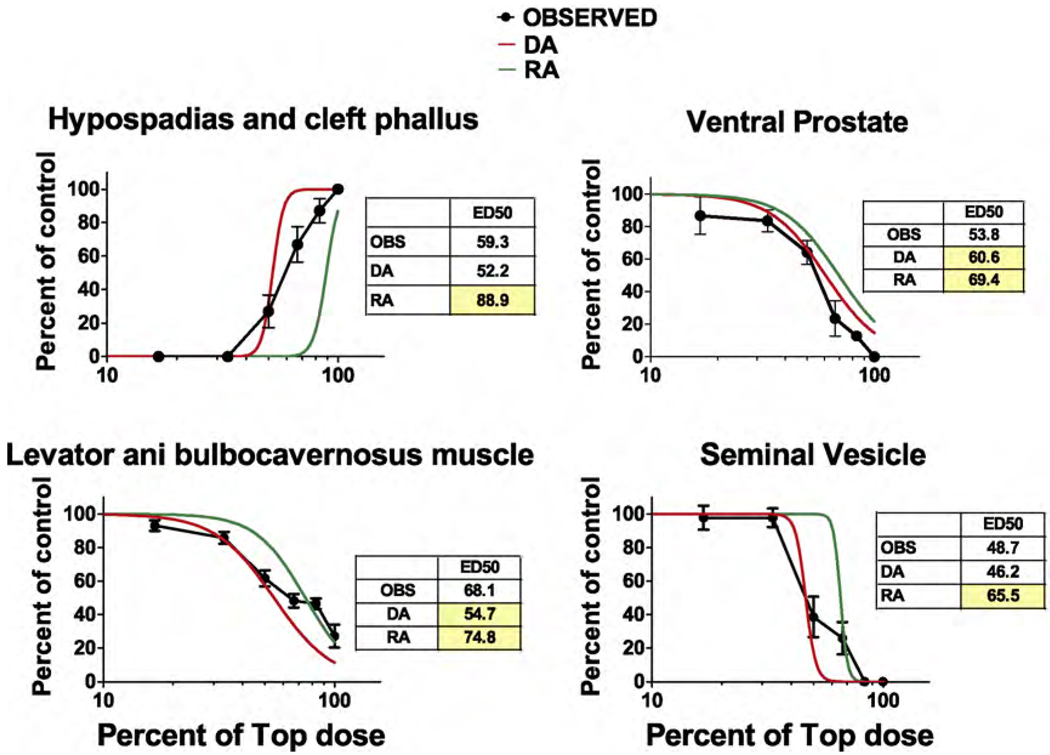
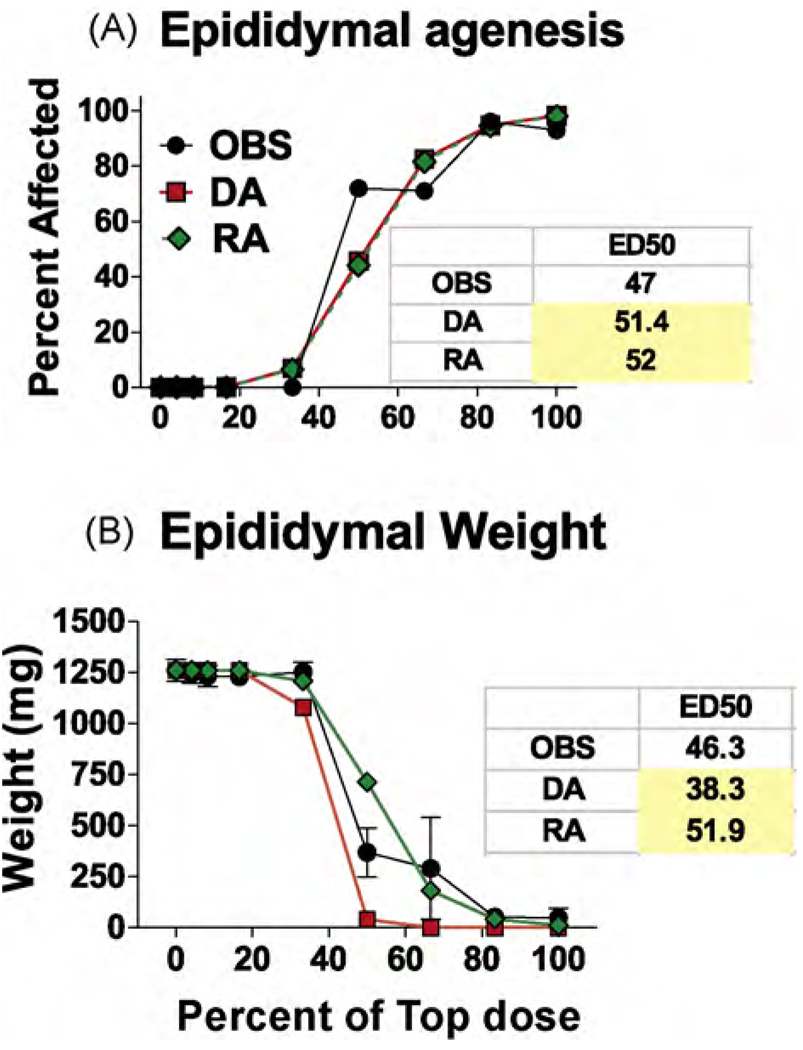
As expected, due to the linear nature of the dose–response curves the DA and RA model predictions for AGD reduction and PND 13 nipple retention were similar (Fig. 3A and B). However, the ED50s for the RA were significantly higher than the ED50s of observed data (as indicated by the 95% CL of the mixture data). The DA model also more accurately predicted the numbers of permanently retained female-like nipples in F1 males than the RA model (Fig. 3C). As hypothesized, the DA model more accurately predicted the effects of the mixture on the induction of hypospadias (Fig. 4A) and also the reductions in ventral prostate weight (Fig. 4B and Table 5) and seminal vesicle weights (Fig. 4D and Table 5). LABC weight reductions were more accurately predicted in the low dose range by the DA than the RA model but the reverse was reverse was true for the two high dosage levels (Fig. 4C). DA and RA model predictions of epididymal agenesis (Fig. 5A) and epididymal weight (Fig. 5B) differed significantly from the observed results, but the overall deviations from the observed effects were not large.
Fig. 3.
Observed (OBS) and dose (DA) and response (RA) addition predictions on the effects of the mixture of procymidone and DBP from experiment two on anogenital distance (AGD) at 3 days of age (A), and retained areolae/nipples in infant (B) and adult male (C) rat offspring. The panels also include the ED50 values from the logistic regression of the observed data and the DA and RA predicted effects. Yellow shaded values differ significantly from the observed ED50s because the values fell outside the 95% confidence limits of the observed ED50s. For AGD, and retained nipples in infants and adult males, DA provided a more accurate prediction than did the RA model. However, for AGD the difference between the ED50 for DA and the ED50 for RA was not great. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 4.
Logistic regression plots of the observed (OBS) and dose- (DA) and response- (RA) addition predictions on the effects of the mixture of procymidone and DBP from experiment two on the induction of hypospadias (A), and reduced ventral prostate (B), levator ani plus bulbocavernosus muscles (C) and seminal vesicle weights (D). The panels also include the ED50 values from the logistic regression of the observed data and the DA and RA predicted effects. Yellow shaded values differ significantly from the observed ED50s. These values fell outside the 95% confidence limits of the observed ED50s. The DA but not RA model provided an accurate prediction of the effects of the mixture on hypospadias and seminal vesicle weight and the DA model was more accurate than the RA model in predicting ventral prostate weight reductions. The X axes are in log10 scale. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Table 5.
A comparison of the goodness of fit (R2) values from logistic regression analyses (GraphPad Prism 5.0 software) obtained for the “best-fit” model versus the R2 values obtained when the data were constrained to the dose addition (DA) and response addition (RA) model parameters. If either the DA or RA model perfectly predicted the effects seen with the mixture then the R2 value would be equal to the R2 value from the “best-fit” model when the observed data were fit to a logistic regression model using DA or RA model parameters. The difference among “best-fit” R2 values and DA and RA R2 values indicates the lack of fit of the observed effects to the DA and RA predictions. In all cases shown in the far right column of the table, DA R2 values were closer than RA R2 values to the actual effects of the mixture. In some cases, the DA model was a considerably better model than was the RA model. For other endpoints like LABC and epididymal weights (data not shown), the DA and RA R2 values are similar, as they are for seminal vesicle weight.
| Endpoint |
R2 “goodness of fit” of the observed data to “best-fit”, DA and RA models |
|||
|---|---|---|---|---|
| Logistic model | R2 | |||
| Best-fit | DA | RA | DA > RA | |
| Hypospadias | 0.94 | 0.85 | 0.25 | 60% |
| Number of permanent nipples | 0.96 | 0.89 | 0.65 | 24% |
| Seminal vesicle weight | 0.89 | 0.83 | 0.65 | 18% |
| Ventral prostate weight | 0.80 | 0.75 | 0.63 | 12% |
| AGD AT day 3 | 0.89 | 0.87 | 0.85 | 2% |
| % of 12 nipples on PND 13 | 0.89 | 0.87 | 0.85 | 2% |
Fig. 5.
Observed (OBS) and dose (DA) and response (RA) addition predictions on the effects of the mixture of procymidone and DBP from experiment two on the induction of epididymal agenesis (A) and reduced epididymal weight (B). The panels also include the ED50 values from the logistic regression of the observed data and the DA and RA predicted effects. Yellow shaded values differ significantly from the observed ED50s. These values fell outside the 95% confidence limits of the observed ED50s. The DA and RA models provided predictions of equivalent accuracy for the effects of the mixture on the epididymis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
In the second mixture experiment, the testes displayed three prominent dose-related lesions including seminiferous tubular degeneration and interstitial cell hyperplasia and adenoma (Fig. 6B). Since DBP induced degeneration of the seminiferous tubules of the testis, whereas PRO did not (Fig. 6A) in our individual chemical dose–response studies, the effects of the mixture on the seminiferous tubules may be attributed to the effect of DBP alone. We also observed interstitial cell hyperplasia and adenoma in the testes of F1 male rats from the high dose groups of the mixture (Fig. 6B); effects that had not been seen in either of our PRO or DBP individual dose–response studies. Given that we do not have any dose–response information for the individual chemicals in the mixture on these Leydig cell alterations, we were unable to calculate DA and RA predictions for these effects.
Fig. 6.
Observed effects of the individual chemicals PRO (procymidone) and di-n-butyl phthalate (DBP) (A) and the mixture of PRO and DBP from experiment two (B) on the induction of histopathological lesions of the testis. Abbreviations: ST degen.—seminiferous tubule degeneration in the testis, IC—interstitial cell (Leydig cell). The panels also include the ED50 values from the logistic regression of the observed data.
6. Discussion
The current investigation was designed to determine if a mixture of two endocrine disrupting reproductive toxicants with disparate mechanisms of toxicity, behaved in a response additive or dose additive manner. While our earlier studies used mixtures of seven chemicals [9] and 10 chemicals [10] in which there are potentially a large number of complex interactions, in the current study there is only a single chemical-to-chemical interaction of PRO with DBP; a pesticide that acts as an AR antagonist, and a phthalate that disrupts fetal testis hormone production, respectively.
The RA model has typically been associated with mixtures like PRO and DBP, based upon the assumption that chemicals that have different mechanisms of action will act independently of one another [28] and is the general approach used by the USEPA’s OPPTS in their assessment of the cumulative effects of food use pesticides; to date, regulatory agencies have not included chemicals like PRO and DBP in cumulative risk assessments. The results of the current experiments clearly indicate that DA models more accurately predict the effects of the mixture of an AR antagonist (PRO) with a chemical that acts by inhibiting fetal testis hormone production (DBP). Not only do these two chemicals have few structural similarities and act via different modes of action, they directly target different fetal tissues; PRO acts on AR in the reproductive tract and accessory tissues whereas DBP acts on the fetal testis by disrupting Leydig cell function.
Administration of the mixture of PRO and DBP produces a wide range of effects on hormone-dependent tissues (Fig. 2A and B). DA models always provided predictions that were equivalent to, or better than, those provided by RA models. DA and RA models produce similar predictions if (1) the effect has a linear dose–response and (2) if the effect results primarily from one of the two chemicals in the mixture. For example, the incidence of elongation or agenesis of the gubernaculum was likely induced solely by DBP in the mixture as phthalates reduce fetal insl3 mRNA hormone levels whereas AR antagonists do not. Although AR antagonists do induce testis nondescent when administered during sexual differentiation, this is due to ectopic attachment of the gubernaculum in the abdominal or suprainguinal regions rather than agenesis or elongation of this tissue [29,30]. In addition, the induction of vas deferens and epididymal agenesis and degeneration of the seminiferous tubules of the testis were induced primarily by DBP in the mixture (Table 1).
We were unable to generate predictions for the induction of interstitial cell hyperplasia and adenoma in the testes in the second experiment (Fig. 6B) because the lesions were not seen in either the PRO or DBP individual dose–response studies. However, other investigators have reported testis interstitial cell hyperplasia and adenomas in male rat offspring after in utero exposure to DBP [31]. This observation, coupled with the absence of Leydig cell adenomas in F1 male rats after in utero exposure to relatively high doses of flutamide [30], a potent AR antagonist, suggests that these testis lesions were primarily induced by DBP or the interaction of DBP with PRO. Given that we do not have any dose–response information for the individual chemicals in the mixture on these Leydig cell alterations, we cannot determine if the induction of interstitial cell hyperplasia or adenoma in the testes by the PRO and DBP mixture represent DA, RA or true synergism.
For a significant percentage of these effects in the second experiment we did not calculate DA or RA predictions since we do not have dose–response data for one or both of the chemicals at the high end of the dose–response curve (Figs. 1 and 6). While it would be desirable to obtain such data on each individual chemical, we do not want to induce overt maternal or fetal toxicity and there also are issues of solubility of a chemical and limits to the volume of the vehicle administered by gavage.
The evidence from the two mixture studies described herein clearly indicates that chemicals that affect the same tissue, regardless of their specific mechanism of action, display cumulative, dose-additive effects when present in combination. This conclusion is supported by all of our previous studies with mixtures of chemicals with diverse mechanisms of toxicity as well as work from other laboratories. For example, the effects of a mixture of seven chemicals (four pesticides and three phthalates) [9] and a mixture of 10 chemicals (four pesticides and six phthalates) [10] were more accurately predicted by DA models than either RA or Integrated Addition models. Similar results have been reported for the in utero effects of antiandrogens by several other investigators [7,32–34]. Furthermore, the reproductive anomalies induced by in utero exposure of male rats to DBP are exacerbated by treatment during sexual differentiation with chemicals like dexamethasone [35] and 2,3,7,8-tetrachlorodibenzodioxin [10].
Taken together, the results of the current study suggest that a modification of the approach for cumulative risk assessments is necessary from one based upon “common mechanism of toxicity” to one that includes the cumulative assessment of chemicals that disrupt development of common tissues.
Acknowledgments
AKH, CVR and CRB were supported in part by USEPA/NCSU Cooperative Training agreement (CT826512010). Support from the NTP, NIEHS/EPA Interagency Cooperative Research Agreement HHS Y1-ES-8014-01; EPA RW75922855-01-0.
Footnotes
The research described in the article has been reviewed by the National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107 Suppl. 3:409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankley GT, Brooks BW, Huggett DB, Sumpter JP. Repeating history: pharmaceuticals in the environment. Environ Sci Technol. 2007;41(24):8211–8217. doi: 10.1021/es072658j. [DOI] [PubMed] [Google Scholar]
- 3.Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Technol. 1998;32(17):2498–2506. [Google Scholar]
- 4.Jobling S, Tyler CR. Introduction: the ecological relevance of chemically induced endocrine disruption in wildlife. Environ Health Perspect. 2006;114 Suppl. 1:7–8. doi: 10.1289/ehp.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, et al. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ Health Perspect. 2006;114 Suppl. 1:32–39. doi: 10.1289/ehp.8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall AJ, Thomas GO. Polychlorinated biphenyls, DDT, polybrominated diphenyl ethers, and organic pesticides in United Kingdom harbor seals (Phoca vitulina)—mixed exposures and thyroid homeostasis. Environ Toxicol Chem. 2007;26(5):851–861. doi: 10.1897/06-310r.1. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, et al. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ Health Perspect. 2009;117(12):1839–1846. doi: 10.1289/ehp.0900689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, et al. A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod. 2004;71(6):1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- 9.Rider CV, Furr J, Wilson VS, Gray LE., Jr Amixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl. 2008;31(2):249–262. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 10.Rider CV, Furr JR, Wilson VS, Gray LE., Jr Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33:443–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray LE, Ostby J, Furr J, Price M, Wolf CJ, Lambright C, et al. Antiandrogenic Effects of Environmental Endocrine Disruptors. In: Metzler DM, editor. Handbook for Environmental Chemistry. New York: Springer-Verlag; 2001. [Google Scholar]
- 13.Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, Gray LE., Jr Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol Sci. 2007;99(1):190–202. doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- 14.Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague–Dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105(1):153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 15.Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, et al. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicol Pathol. 2009;37(1):100–113. doi: 10.1177/0192623308329478. [DOI] [PubMed] [Google Scholar]
- 16.Academies TN, editor. Phthalates and Cumulative Risk Assessment The Task Ahead, in Phthalates and Cumulative Risk Assessment The Task Ahead. Washington, DC 20001: The National Academies Press; 2008. pp. 1–209. [Google Scholar]
- 17.Hosokawa S, Murakami M, Ineyama M, Yamada T, Koyama Y, Okuno Y, et al. Effects of procymidone on reproductive organs and serum gonadotropins in male rats. J Toxicol Sci. 1993;18(2):111–124. doi: 10.2131/jts.18.111. [DOI] [PubMed] [Google Scholar]
- 18.Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol Sci. 2003;71(2):251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- 19.Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE. The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol Ind Health. 1999;15(1–2):80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- 20.Vinggaard AM, Joergensen EC, Larsen JC. Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic effects of environmental chemicals. Toxicol Appl Pharmacol. 1999;155(2):150–160. doi: 10.1006/taap.1998.8598. [DOI] [PubMed] [Google Scholar]
- 21.Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58(2):339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 22.Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, et al. Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and Leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect. 2007;115(3):390–396. doi: 10.1289/ehp.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146(2):613–623. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- 24.Mahood IK, McKinnell C, Walker M, Hallmark N, Scott H, Fisher JS, et al. Cellular origins of testicular dysgenesis in rats exposed in utero to di(n-butyl) phthalate. Int J Androl. 2006;29(1):148–154. doi: 10.1111/j.1365-2605.2005.00574.x. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- 25.Hughes IA, Acerini CL. Factors controlling testis descent. Eur J Endocrinol. 2008;159 Suppl. 1:S75–S82. doi: 10.1530/EJE-08-0458. [DOI] [PubMed] [Google Scholar]
- 26.Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, et al. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem. 2002;277(35):31283–31286. doi: 10.1074/jbc.C200398200. [DOI] [PubMed] [Google Scholar]
- 27.Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008;108(2):168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Greco W, Unkelbach HD, Pöch G, Sühnel J, Kundi M, Bödeker W. Consensus on concepts and terminology for combined-action assessment: the Saariselkä agreement. Arch Complex Environ Stud. 1992;4 65–60. [Google Scholar]
- 29.Gray LE, Jr, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health. 1999;15(1–2):48–64. doi: 10.1177/074823379901500106. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre BS, Barlow NJ, Foster PM. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol Sci. 2001;62(2):236–249. doi: 10.1093/toxsci/62.2.236. [DOI] [PubMed] [Google Scholar]
- 31.Barlow NJ, McIntyre BS, Foster PM. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol. 2004;32(1):79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- 32.Kortenkamp A. Ten years of mixing cocktails - a review of combination effects of endocrine disrupting chemicals. Environ Health Perspect. 2007 doi: 10.1289/ehp.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metzdorff SB, Dalgaard M, Christiansen S, Axelstad M, Hass U, Kiersgaard MK, et al. Dysgenesis and histological changes of genitals and perturbations of gene expression in male rats after in utero exposure to antiandrogen mixtures. Toxicol Sci. 2007;98(1):87–98. doi: 10.1093/toxsci/kfm079. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, et al. Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int J Androl. 2008;31(2):241–248. doi: 10.1111/j.1365-2605.2008.00866.x. [DOI] [PubMed] [Google Scholar]
- 35.Drake AJ, van den Driesche S, Scott HM, Hutchison GR, Seckl JR, Sharpe RM. Glucocorticoids amplify dibutyl phthalate-induced disruption of testosterone production and male reproductive development. Endocrinology. 2009;150(11):5055–5064. doi: 10.1210/en.2009-0700. [DOI] [PubMed] [Google Scholar]