Abstract
This longitudinal study used a representative community sample of same-sex twins (485 MZ pairs, 271 DZ pairs) to study longitudinal changes in genetic and environmental influences on nicotine dependence (NicD) symptoms and major depressive disorder (MDD) symptoms and the longitudinal relationships between NicD and MDD symptoms at three relatively discrete ages spanning middle adolescence to early adulthood (ages 15, 18, and 21). Clinical interviews were used to assess NicD and MDD symptoms lifetime at age 15 and during the previous three years at the two subsequent assessments. Biometric models revealed similar patterns of findings for NicD and MDD. Heritability increased with age, particularly between ages 15 and 18. Shared environmental influences were small, and the proportion of variance attributed to shared environmental influences decreased with age. Nonshared environmental influences were moderate to large in magnitude and were entirely age-specific. Both NicD and MDD symptoms showed considerable stability from age 15 to 21, and at each age those with one disorder showed elevated rates of the other. However, a cross-lagged model revealed no longitudinal predictive relationships between MDD symptoms and NicD symptoms after accounting for stability of symptoms within disorders. In summary, the transition between middle and late adolescence is a critical period for developmental shifts in the magnitudes of genetic and environmental influences on both MDD and NicD symptoms. Despite similarities in the development of genetic and environmental influences for the two phenotypes, the association between NicD and MDD reflects concurrent covariation rather than one phenotype being an antecedent influence on the subsequent development of the other.
Keywords: depression, nicotine dependence, genes, adolescent, young adulthood
Adolescence and early adulthood are developmental periods characterized by many transitions as youth become more independent, spend less time with parents and more time with peers, and engage in a variety of risk behaviors (e.g., drugs, sexual relationships). This is also a period of risk and peak incidence for many psychiatric disorders, such as depression and nicotine dependence (Chassin, Presson, Rose, & Sherman, 1996; Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Lewinsohn, Rohde, & Seeley, 1998), two disorders with major public health consequences. Depression is the leading cause of disability worldwide (Ustun & Kessler, 2002), and since it exacerbates the course and outcome of many health problems, its morbidity is comparable to the most serious medical disorders (Cassano & Fava, 2002; Greden, 2001). Nicotine use is the leading avoidable cause of morbidity and premature mortality worldwide (Mathers et al., 2000; Services, 1994). Moreover, these health risks and societal burdens are increased when depression and nicotine use co-occur (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Lopez, 2006). Given that these disorders typically begin to emerge during adolescence and tend to be chronic, reducing the significant health and economic burdens associated with these disorders requires intervening and minimizing early risks to thwart the cascade of events leading to impairing psychopathology.
A cascade of genetic influences and environmental exposures unfolds over time creating variability in how successfully youth adapt to the changing demands of this developmental period and escape or succumb to risks for depression and nicotine dependence. The emergence of symptoms during middle adolescence may be triggered by cumulative environmental risks that have reached a threshold, the introduction age-specific environmental influences (e.g., more time spent with deviant and victimizing peers), and/or genetic influences that come about during this period. The purpose of the present study was to investigate developmental changes in genetic and environmental influences on nicotine dependence symptoms and major depressive disorder symptoms at three discrete ages across the high-risk period spanning middle adolescence, when symptoms of these disorders begin to emerge for many at-risk youths, to early adulthood, when rates and severity of these disorders have reached adult, impairing levels. In light of the added burden associated with the co-occurrence of these disorders, the present study also investigates the extent to which these disorders influence one another during this period.
Cascades of Interacting Genetic and Environmental Effects Shape the Development of Psychopathology
Psychopathology develops through various dynamic processes that unfold throughout development (Cicchetti, 1984; Sroufe & Rutter, 1984) and involve genes, the environment, and their interplay (Michael Rutter, Moffitt, & Caspi, 2006). Genetic and environmental influences underlie personal characteristics (e.g., maladaptive coping skills, negative affect) and experiences (e.g., stressful events, parental psychopathology) that create individual patterns of adaptation to salient developmental challenges and thus create variability in psychopathology (Sroufe & Rutter, 1984). For example, genotype-environment interactions (i.e., individuals with different genotypes responding differently to the same environment) contribute to resilience among some individuals exposed to stressful situations and the development of psychopathology among others exposed to the same stressors.
At the same time, experiences shared among individuals at given developmental periods (i.e., autonomy from parents) likely result in general trends for genetic and environmental mechanisms to have greater or lesser influences at various points during development. For instance, genotype-environment correlations, or tendencies for individuals to experience environments that are correlated with their genotype, may result in increased genetic influences with age. Genotype-environment correlations occur for three main reasons: (a) parents provide both genes and early environments to their children, i.e. passive genetic-environment correlations, (b) individuals actively seek environments that reinforce their genetically-influenced dispositions, i.e., active genetic-environment correlations, and (c) genetically-influenced behaviors tend to elicit certain environmental responses, i.e., evocative/reactive genetic-environment correlation (Plomin, DeFries, & Loehlin, 1977; Scarr & McCartney, 1983). Passive gene-environment correlations have their greatest influence when children are young and parents exert considerable influence on their environments. Active gene-environment correlations become more important during adolescence and early adulthood as individuals gain more independence and thus are freer to seek out their environments. Heritability estimates will increase with age with the growth of active genotype-environment correlations (i.e., niche-fitting as autonomy increases during adolescence) and the decline of passive genetic-environment correlations on a given trait. This idea is supported by a review and meta-analysis that found age-related increases in heritability estimates for various behavioral and psychiatric phenotypes (Bergen, Gardner, & Kendler, 2007). Undoubtedly, the processes through which genes and the environment influence psychopathology are complicated. Age-specific genetic and environmental effects may exert their influences at discrete points during development, cumulative effects of various genetic and environmental factors may alter the relative importance of these factors across development, or both cumulative and age-specific effects may interact in ways that result in fluctuations in the enhancement and overshadowing of these factors across development.
The progressive and bidirectional influences of genes and environmental factors on psychopathology can be exemplified by research on smoking behavior. Adolescents of parents who smoke are more likely to smoke cigarettes and develop nicotine dependence (Clark, Kirisci, & Moss, 1998; Kandela, Hua, Grieslerb, & Schaffranc, 2007; Kardia, Pomerleau, Rozek, & Marks, 2003; Lieb, Schreier, Pfister, & Wittchen, 2003; Vogel, Hurford, Smith, & Cole, 2003). The transmission of risk is in part due to genes that contribute to smoking initiation and persistence in adolescents (Li, Cheng, Ma, & Swan, 2003; Keyes, Legrand, Iacono, & McGue, 2008). At the same time, low parental monitoring and parental approval of smoking increase the likelihood of adolescents smoking tobacco (Biglan, Duncanm, Ary, & Smolkowski, 1995), largely because deficits in parental monitoring lead to affiliation with deviant peers (Patterson, 1982) which in turn increase the likelihood of tobacco use, abuse of other substances, deviant behavior, and a myriad of other problems (Biglan, et al., 1995; Hu, et al., 2006; Kandela, et al., 2007; McGue, lacono, & Krueger, 2006). These parenting risk mechanisms, though, are not solely environmentally mediated, as both peer groups (Baker & Daniels, 1990; Manke, McGuire, Reiss, Hetherington, & Plomin, 1995; Pike, McGuire, Hetherington, Reiss, & Plomin, 1996) and characteristics of parent-child relationships (Elkins, McGue, & Iacono, 1997; Plomin, Reiss, Hetherington, & Howe, 1994) have been shown to be genetically influenced. Research suggests both that the association between peer affiliation and smoking initiation is genetically influenced (White, Hopper, Wearing, & Hill, 2003) and the associations among peer deviance, parent–child relationship problems, and early substance use is mediated by environmental influences shared among members of the same family (Walden, McGue, Iacono, Burt, & Elkins, 2004). In other words, mutual interacting genetic and environmental influences over the course of development lead to a cascade of lasting and interacting effects on adolescents' relationships and social environment, nicotine dependence, and comorbid psychopathology.
Genetic and Environmental Influences on Depression and Nicotine Dependence
Twin studies provide a method for estimating genetic and environmental influences on depression and nicotine dependence symptoms by comparing similarity in symptoms between monozygotic (MZ) twins, who share 100% of their genes, to similarity in symptoms between dizygotic (DZ) twins, who share on average 50% of their genes. Biometric modeling of data from twin studies provides estimates of additive genetic effects, shared environmental effects (i.e., environmental effects shared by reared-together relatives that are sources of behavioral similarity), and nonshared environmental effects (i.e., environmental effects that differ for reared-together relatives and are sources of behavioral dissimilarity). Thus, twin studies can be used to separate variability in depression and nicotine dependence into the proportions of variability attributed to genetic influences (i.e., heritability), shared environmental influences, and nonshared environmental influences.
Various measures of tobacco use (e.g., smoking initiation, regular/daily smoking, nicotine dependence) have been found to be highly heritable in adults (Carmelli, Swan, Robinette, & Fabsitz, 1990; Heath, Madden, & Martin,1998; Kaprio, et al., 1982; Kendler, et al., 1999) and adolescents (Maes, et al., 1999; McGue, Elkins, & Iacono, 2000; Slomkowski, Rende, Lloyd-Richardson, & Niaura, 2005). Meta-analytic reviews of the heritability of tobacco use report that 37–60% of the variance in smoking initiation and 46–59% of the variance smoking persistence can be accounted for by genetic factors (Hall, Madden, & Lynskey, 2002; Li, et al., 2003; Sullivan & Kendler, 1999), with smaller heritabilities more common in samples with younger mean ages. The shared environment also appears to play an important role in smoking during adolescence (Boomsma, Koopmans, van Doornen, & Orlebeke, 1994), although estimates of shared environmental effects vary considerably across studies. Some studies find that shared environmental influences account for more variation than genetic influences (Koopmans, Slutske, Heath, Neale, & Boomsma, 1999), while other studies find no significant shared environmental influences (Maes, et al., 1999).
Inconsistencies in estimates of these influences are likely a function of different ages and age ranges of samples as well as the diverse measures of smoking and tobacco use. Shared environmental influences are often stronger for smoking initiation, which typically occurs during early adolescence, than for daily and persistent smoking and nicotine dependence (Stallings, Hewitt, Beresford, Heath, & Eaves, 1999; Sullivan & Kendler, 1999), which develop later. While these findings suggest the possibility of developmental changes in genetic and environmental influences on tobacco use, almost no published studies have tested longitudinal models for changes in these measures across adolescent development. A meta-analysis of age-related changes in the heritability of smoking initiation (and other behavioral phenotypes) across adolescence and early adulthood reported a very small and nonsignificant overall effect size for an increase in heritability (Bergen, Gardner, & Kendler, 2007). The small effect size, though, reflected considerable variability in estimates across studies with the only two longitudinal studies supporting slight increases in genetic influences (Slomkowski, et al., 2005; White, et al., 2003). Finally, gender is another potential source of variation in the estimates with some evidence supporting gender differences in the proportions of genetic and environmental influences (Kendler, Thornton, & Pedersen, 2000; Li, et al., 2003; McGue, et al., 2000); however, gender differences are typically small and nonsignificant and support both larger (White, et al., 2003) and smaller (Boomsma, et al., 1994) shared environmental estimates for male compared to female adolescents.
The literature on genetic and environmental influences on depression in youth is also limited by few longitudinal studies, differences in methodology, and inconsistent findings. A review and meta-analysis on the genetic epidemiology of depression in adults reported that variability in depression is approximately 40% due to additive genetic factors and 60% due to nonshared environmental factors with no evidence of shared environmental influences (Sullivan, Neale, & Kendler, 2000). Twin studies typically find that depression in adolescents is also heritable (Eaves, et al., 1997; Eley & Plomin, 1997; Eley & Stevenson, 1999; Hewitt, Silberg, Neale, Eaves, & Erickson, 1992; Rende, Plomin, Reiss, & Hetherington, 1993; Schmitz, Fulker, & Mrazek, 1995), but the proportions of variance in depression attributed to genetic and environmental influences are highly inconsistent across studies and likely depend on several factors, such as age and sex compositions of the samples and measurement instrument. Comparisons of heritability estimates across studies with samples of varying mean ages suggest that developmental changes in the magnitude and proportion of genetic and environmental influences are likely. The studies generally find significant shared environmental influences on depressive and internalizing symptoms in child samples but not in adolescent samples (Rice, Harold, & Thaper, 2002a; Schmitz, et al., 1995; Thapar & McGuffin, 1994), and genetic influences have been found to account for larger proportions of variance in adolescents' depression symptoms than children's depression symptoms (Rice, Harold, & Thaper, 2002b; Scourfield, et al., 2003). A few studies of children and adolescents, though, found age-related declines in heritability estimates of depression symptoms (O'Connor, McGuire, Reiss, Hetherington, & Plomin, 1998; O'Connor, Neiderhiser, Reiss, Hetherington, & Plomin, 1998) and internalizing symptoms (Gjone, Stevenson, Sundet, & Eilertsen, 1996).
Most of these twin studies use paper-and-pencil measures of current (e.g., during the previous two weeks or three months) depression symptoms (e.g., Center for Epidemiologic Studies-Depression Scale, Radloff, 1977; Child Depression Inventory, Kovacs, 1985) or current (i.e., during the previous six months) internalizing symptoms (e.g., Child Behavior Checklist, Achenbach, 1991). Only a few studies have used clinical or diagnostic interviews to obtain measures of major depressive disorder symptoms and diagnoses over longer periods of time, and given that self-report instruments have been found to overestimate rates of depression compared to clinical interviews of depression (Schepis & Rao, 2005), estimates may depend on the measurement instrument used. Glowinski, Madden, Bucholz, Lynskey, and Heath (2003) studied female youth aged twelve to twenty-three and found that similar to findings in adults the variance in lifetime diagnoses of major depressive disorder was accounted for by genetic influences (40%) and non-shared environmental influences (60%), but shared environmental influences did not account for a significant portion of the variance (Glowinski, Madden, Bucholz, Lynskey, & Heath, 2003). Ehringer, Rhee, Young, Corley, and Hewitt (2006) used diagnostic interviews to measure major depressive disorder symptoms in male and female adolescents aged twelve to nineteen years. Contrary to the Glowinski et al. (2003) study, they found significant shared and non-shared environmental influences but nonsignificant genetic influences (10–16%) on both past year and lifetime major depressive disorder diagnoses (Ehringer, Rhee, Young, Corley, & Hewitt, 2006). The larger genetic influences in the Glowinski et al. (2003) study may be explained by the use of an exclusively female sample with an age range that included young adults and use of diagnoses rather than symptom counts.
In summary, existing research suggests the possibility of age-related changes in the genetic epidemiology of depression and nicotine dependence, but integration of findings across studies to answer developmental questions is limited by few longitudinal studies and diversity of measurement instruments. Most notably, the majority of these studies estimated genetic and environmental influences in samples of youth with wide age ranges that span multiple developmental levels, thus potentially masking important developmental changes in these influences, and most studies do not use diagnostic interviews to assess clinically-relevant symptoms of depression or nicotine dependence. In the current study, longitudinal twin data are used to answer developmental questions about changes in the nature of genetic and environmental influences on depression and nicotine dependence assessed through clinical interviews at three developmentally important, discrete ages. More specifically, the longitudinal data allow us to quantify developmental changes in the magnitude of genetic and environmental influences on depression and nicotine dependence symptoms, identify developmental periods when genetic influences on these phenotypes become more predominant, and examine the extent to which the same genetic and environmental factors influence the phenotypes across different ages or have developmental specificity and influence the phenotypes during a particular developmental period only.
Comorbidity and Longitudinal Relationship between Depression and Nicotine Dependence in Youth
Adolescence and early adulthood are key periods not only for the emergence of nicotine dependence and depression but also for the onset of depression and smoking co-occurrence. Tobacco use and depressed mood tend to co-occur concurrently and when lifetime measurements are used in samples of adolescents and young adults, although the magnitudes of the associations vary across studies (Fergusson, Lynskey, & Horwood, 1996; Hu, et al., 2006; Pedersen & von Soest, 2009). Studies of longitudinal associations between depression and nicotine use in adolescents also revealed mixed findings regarding both the size and the direction of the associations, with some studies suggesting that depressed mood predicts later nicotine dependence (Griesler, et al., 2008; Patton, Coffey, Carlin, Sawyer, & Wakefield, 2006), other studies suggesting that smoking initiation predicts later depressive episodes (Brown, Lewinsohn, Seeley, & Wagner, 1996), and a recent meta-analysis concluding that the associations are bidirectional (Chaiton, Cohen, O'Loughlin, & Rehm, 2009). Again, differences in methodology, such as variability in time intervals between assessments, differences in ages and age ranges of participants, inclusion of various covariates in the analyses, and wide variability in instruments used to assess depression and tobacco use, likely contributed to the discrepant findings.
Another significant limitation of the existing research is the failure of most studies to adequately account for the effect of stability of depression and tobacco use on longitudinal relationships between these variables, for example by using cross-lag analyses. Previous depressive episodes predict future depressive episodes (Fombonne, Wostear, Cooper, Harrington, & Rutter, 2001; Harrington, Fudge, Rutter, Pickles, & Hill, 1990; Rao, Hammen, & Daley, 1999) and early tobacco use predicts later tobacco use and nicotine dependence (Eissenberg & Balster, 2000; Riley, et al., 1996; Russell, 1990). Thus, analyses used to quantify the temporal relationships between these two constructs should account for history of depression and tobacco use. Wang and colleagues (1996) ran a cross-lagged analysis on a four-item self-report of depressive symptoms and self-reported frequency of smoking and found evidence supporting reciprocal associations between these constructs over approximately a four-year period in a sample of adolescents and young adults (Wang, Fitzhugh, Turner, Fu., & Westerfield, 1996). This study had significant limitations, including a six-year age range of participants and measures that assessed depression and smoking during only a portion of the time elapsing between measurements (last year for depression, last 30 days for smoking).
In summary, research supports continuity within disorders across time and concurrent associations across disorders, but questions remain about the extent to which one disorder influences the other disorder in an enduring way. Both genetic and nonshared environmental factors have been found to account for associations between various measures of nicotine use (e.g., daily smoking, nicotine dependence) and depression (e.g., major depressive disorder diagnoses, self-reported current symptoms) in adults (Kendler, et al., 1993; Lyons, et al., 2008; McCaffery, Niaura, Swan, & Carmelli, 2003). However, little is known about these associations during the formative years of adolescence and early adulthood when use of cigarettes and MDD emerge and approach adult levels. To the extent that there are developmental changes in genetic and environmental influences on depression and nicotine dependence, associations between nicotine dependence and depression may also vary with age. In this study, we used a cross-lagged panel model to study the effects of nicotine dependence symptoms on subsequent levels of depression symptoms and depression symptoms on the subsequent levels of nicotine dependence symptoms while accounting for the stability of both disorders at multiple time points. In addition, clinical interviews were used to assess symptoms of major depressive disorder (MDD) and nicotine dependence (NicD).
Hypotheses
Longitudinal twin and cross-lagged models were used to address ten hypotheses about developmental changes in genetic and environmental influences on MDD and NicD symptoms and longitudinal associations between MDD and NicD symptoms. The twins were studied at three discrete ages (15, 18, and 21 years) within narrow age ranges. The ages correspond to three important developmental periods: (a) middle adolescence, when depressed mood and smoking initiation and experimentation are beginning to rise, (b) late adolescence, when symptoms become more impairing and clinically significant, and (c) early adulthood, when point prevalence rates of both disorders have reached their peaks. These developmental periods are marked by great change in the lives of youths as they spend more time away from their parents and eventually leave their families and learn to function independently (Roisman, Masten, Coatsworth, & Tellegen, 2004), making them critical ages for changes in genetic and environmental influences as well. The clinical interviews used to assess symptoms of MDD and NicD provided the opportunity for guided, standardized assessments during the entire period between time points and for obtaining valid measures of both disorders.
First, given that the strongest predictor of each disorder is a history of the disorder, we hypothesized that NicD symptoms and MDD symptoms would be relatively stable (i.e., correlated within disorders) across assessments. Second, we predicted that the universal and consequential growth in independence and niche-fitting in adolescence and early adulthood would result in age-related increases in the heritability of both NicD and MDD. Third, given the stability of both NicD and MDD, we predicted that some of the same genetic factors would contribute to these phenotypes across the three discrete ages. Nevertheless, we expected that new genetic influences would emerge at older ages as exposure to environmental and genetic cues change with age. Fourth, shared environmental influences were expected to decrease with age and become trivial in early adulthood, but to the extent there were small shared environmental influences at older ages we expected they would be shared with earlier ages. In other words, our fifth prediction was that new environmental influences shared among members of the same family would not emerge and have influence in early adulthood. Sixth, we expected nonshared environmental influences on both MDD and NicD symptoms to increase with age as the twins become more independent from their parents and each other and risk exposures unique to each twin (e.g., separate groups of friends, distinct stressful events) accumulate and become more influential over time. Seventh, we predicted that these unique environmental risk factors would largely differ depending on developmental level and thus the nonshared environmental factors would contribute little to stability in MDD and NicD across the three ages.
Eighth, given findings of co-occurrence of depression and nicotine use in samples of adolescents and young adults, we predicted that symptom counts for the two disorders would be correlated concurrently at all three ages. Ninth, we hypothesized that symptoms of one disorder would be correlated with symptoms of the other disorder across assessments, although the discrepant effect sizes in existing research provided no indication about the expected size of the associations. Finally, we evaluated the degree to which the occurrence of NicD symptoms at an early age influenced the subsequent development of MDD and early MDD symptoms influenced subsequent NicD symptoms after accounting for the stability of each disorder over time. However, we made no specific predictions given the limitations of existing research on longitudinal associations between depression and nicotine use, especially given that there are few studies of clinical phenotypes in adolescents and young adults and almost no studies that account for stability of the disorders across time.
Method
Sample
The sample consisted of 756 same-sex twin pairs (50.3% female; 64% MZ) from the Minnesota Twin Family Study (MTFS), which is a large epidemiological and longitudinal study of twins who were identified from birth records using a population-based method. The participation rate was 82.7% for participants who met inclusion criteria (i.e., lived within than a one-day drive to Minneapolis, were not adopted by non-relatives, and were free from mental or physical handicap that precluded their participation). The MTFS sample was demographically reflective of the population of the state of Minnesota at the time of the twins' births, with approximately 98% Caucasian, mean occupational status (Hollingshead) for both fathers and mothers corresponding to clerical, sales, technician (3.9 and 3.7, respectively), and minimal differences on indicators of socioeconomic status between families who participated and families who did not participate (Iacono et al., 1999). All participants provided written informed consent or assent as appropriate. Additional information about recruitment and the sample can be found elsewhere (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Iacono, McGue, & Krueger, 2006).
Participants were assessed at uniform, discrete ages. Participants were first assessed when the twins were 11 years old (M=11.72, SD=.43), and they returned for three follow-up assessments approximately every three years. Given extremely low lifetime rates of NicD diagnoses (0.2%) and MDD diagnoses (0.7%) and small symptom counts for NicD (M=.01, SD=.20) and MDD (M=.10, SD=.64) at age 11, this paper includes data from only the three follow-up assessments, mean ages 14.80 (.51), 18.16 (.65), and 21.46 (.77). We refer to the three assessments by the rounded mean age of the participants at each assessment: 15 years (middle adolescence), 18 years (late adolescence), and 21 years (early adulthood).
Measures
At each time point, structured interviews were administered to the twins by trained interviewers to assess DSM-IIIR (the DSM in use at the commencement of the study) symptoms of NicD and MDD. The Substance Abuse Module (Robins, Baber, & Cottler, 1987), a supplement to the Composite International Diagnostic Interview (CIDI; Robins, et al., 1988), was used to assess NicD diagnoses and symptom counts, the Diagnostic Interview for Children and Adolescents, Revised (DICA-R; Reich & Welner, 1988) was used to assess MDD diagnoses and symptoms at age 15, and Structured Clinical Interview for DSM-IIIR (SCID; Spitzer, Williams, & Gibbon, 1987) was used to assess MDD diagnoses and symptoms at ages 18 and 21. According to DSM-IIIR criteria for MDD, individuals with symptom counts greater than zero experienced depressed mood or anhedonia for a period of at least two weeks. Reliability for diagnoses of MDD and NicD, established on a subset of 600 participants, was good (Cohen's Kappa ≥.82 for both disorders). Diagnoses and symptom counts at 15 years were assessed lifetime until age 15, and diagnoses and symptom counts at ages 18 and 21 years were assessed since the previous assessment (approximately three years). Symptom counts rather than the presence or absence of a diagnosis were selected for the main analysis because symptom counts are more sensitive to the progression of disorder development during this period when symptoms of these disorders are just beginning to emerge. Symptom counts were regressed on age and the resulting residuals were log transformed and used in the twin analyses.
Zygosity was determined on the basis of agreement among three separate estimates: (a) a standard parent-report zygosity questionnaire, (b) subjective evaluations of twins' physical similarity by experienced MTFS staff members, and (c) anthropometric measures (ponderal index, cephalic index, and fingerprint ridge count). When determination of zygosity was inconsistent across the three measures, serological analyses were performed. Accuracy of this method was evaluated in a subsample of 50 twin pairs for which agreement among the three measures was always confirmed by the serological analysis.
Statistical Analyses
Biometric model-fitting
The size of genetic and environmental effects at each age, the magnitude of age differences in these effects, and the degree of age overlap versus specificity in the effects were examined using 3-factor Cholesky decomposition models. Cholesky decomposition is a multivariate technique based on the principles of factor analysis that was used to estimate genetic and environmental contributions to variance in symptoms at each age and covariance between symptoms across time. This model provided estimates of additive genetic effects (A), shared environmental effects (C, environmental effects that are shared by reared-together relatives and are sources of behavioral similarity), and nonshared environmental effects (E, environmental effects that differ for reared-together relatives and are a source of behavioral dissimilarity). One Cholesky model was calculated for MDD symptoms, and a separate model was calculated for NicD symptoms.
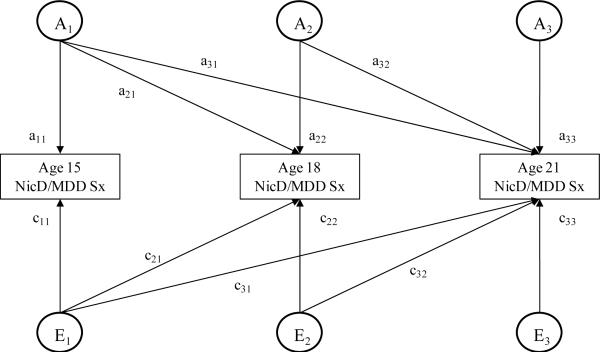
Figure 1 includes the 3-factor ACE Cholesky model used in this study. Genetic and environmental contributions to variance in age 15 phenotypes are obtained by squaring the respective path-coefficients (a11, c11, e11). Variance in the phenotypes at age 18 is divided into components attributable to genetic and environmental influences present at age 15 (a21, c21, e21) and residual (new) components that are independent of the genetic and environmental variance at age 15 (a22, c22, e22). Variance in the phenotypes at age 21 is divided into components attributable to genetic and environmental influences present at age 15 (a31, c31, e31) and present at age 18 but not 15 (a32, c32, e32) and residual (new) components that are independent of the genetic and environmental influences present at age 15 and 18 (a33, c33, e33). To analyze changes in genetic and environmental influences across the ages, fit statistics for a series of Cholesky models in which the A, C, and/or E estimates were constrained to be equal across age were compared to fit statistics for models in which the estimates were free to vary.
Figure 1.
This figure depicts the 3-factor Cholesky decomposition model used in this study. The model decomposes the variance in liability to nicotine dependence symptoms or major depressive disorder symptoms into components attributable to additive genetic effects (Ai), nonshared environmental effects (Ei), and shared environmental effects (Ci; not depicted for ease of presentation) at each of the three assessments (i = 1, 2, 3). The individual paths, which are represented by lowercase letters followed by numerals, when squared estimate the proportion of variance accounted for by the genetic and environmental influences. This figure represents only one twin, and identical model is also calculated for the co-twin. NicD=nicotine dependence. MDD=major depressive disorder.
Both biometric models were fit to variance-covariance matrices in the Mx statistical software system (Neale, Boker, Xie, & Maes, 1999). Variance-covariance matrices were calculated using an expectation-maximization algorithm, which uses an iterative procedure to compute maximum-likelihood estimates thus allowing the use of all relevant twin data regardless of the number of assessments completed. In this procedure, missing data are assumed to be missing at random after accounting for the relationship with available data at other assessments. For both models, separate variance-covariance matrices were calculated for males and females to account for gender differences in mean levels, variance, and covariance of symptoms. Then, parameters were constrained to be equal across sexes.
The models were fit using the maximum likelihood option in Mx, with parameters estimated to minimize two times the natural logarithm of the multivariate normal likelihood (−;2lnL). Differences in the minimized value of −2lnL between the baseline and more restrictive models yield a likelihood χ2 test that is used to test the significance of the model with constraints. A nonsignificant χ2 difference test indicates that the more restrictive model provides an appropriate fit to the data, and in general this more parsimonious model is preferred. The Akaike Information Criteria (AIC = χ2 − 2Δdf) is also reported. It is an alternative to the χ2 goodness-of-fit test statistic that is less likely to result in rejection of the more restrictive model when deviations between the baseline and restricted model are relatively small but sample sizes are large. Models that minimize AIC are preferred.
Cross-lagged model fitting
Cross-lagged panel models were run to analyze the relationship between NicD symptoms and MDD symptoms over time at ages 15, 18, and 21. In these models, symptoms are a function of: (a) symptoms of both disorders at the previous point in time and (b) innovations, which are new sources of influence that account for variance not due to the effects of either disorder at the previous time point. The innovations are disorder-specific but correlations across disorders within time points were included in the model. Latent variable models were fit to the raw symptom count data using SEM software (Mplus 5.2; Muthén & Muthén, 1998–2007). Maximum likelihood estimation with robust standard errors was used, allowing for the use of all available data regardless of missing data at particular time points. The nonindependent nature of the twin data was accounted for by nesting individuals within twin pairs using the survey sampling method for estimating standard errors in clustered data. To account for sex differences in symptom counts, a two-group model was used first to estimate parameters separately for males and females and second to constrain all paths to be equal across the sex cohorts. Means were not constrained to be equal across sexes, though, which allowed for gender differences in mean symptom counts at each age. A model constraining all cross paths between NicD symptoms and MDD symptoms to zero was also estimated to evaluate the importance of the cross paths for model fit. Comparisons of the model fits were made using χ2 difference tests that were calculated based on a formula supplied in Satorra (2000) that accounts for clustered data and incorporates the Satorra-Bentler's (SB) scaling corrections provided by Mplus. The AIC is also reported for these models.
Results
Descriptive Statistics, Phenotypic Correlations, and Twin Correlations
Rates of MDD and NicD diagnoses and means and standard deviations for MDD and NicD symptom counts at each assessment and separately for males and females are presented in Table 1. As expected, for the combined sample, rates of both disorders increased considerably with age. The pattern was similar for males and females, except that the largest increase in rates of MDD for males occurred between ages 18 and 21 years, which is consistent with later rises in rates of depression for males compared to females (Hankin et al., 1998). Sex differences in rates of disorders were tested using generalized estimating equations to account for the correlated nature of the twin data. At age 15, there were no significant sex differences in rates of MDD diagnoses (Wald χ2= 0.42, p = .52), NicD diagnoses (Wald χ2= 0.12, p = .73), MDD symptom counts (Wald χ2= 3.39, p = .07), or NicD symptom counts (Wald χ2= 0.00, p = 1.00). Rates of MDD diagnoses (Wald χ2= 21.15, p < .001) and MDD symptom counts (Wald χ2= 24.44, p < .001) were significantly higher in females than males at age 18, and rates of NicD diagnoses (Wald χ2= 21.27, p < .001) and NicD symptom counts (Wald χ2= 16.77, p < .001) were significantly higher in males than females at age 18. At age 21, diagnoses (Wald χ2= 3.09, p = .08) and symptom counts (Wald χ2= 2.94, p = .09) for MDD did not differ significantly by gender, but rates (Wald χ2= 45.24, p < .001) and symptom counts (Wald χ2= 60.83, p < .001) for NicD were significantly higher in males than females.
Table 1.
Rates for Major Depressive Disorder and Nicotine Dependence at 15, 18, and 21 Years by Gender and Diagnostic Status of Other Disorder and Means and Standard Deviations by Gender
| % Diagnoses |
% Dx for Youth with Other Dx / % Dx for Youth without Other Dx |
Symptom Counts M (SD) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Males & Females | Male | Female | Males & Females | Male | Female | Males & Females | Male | Female | |
| NicD | |||||||||
| 15 years | 7.4 | 7.6 | 7.2 | 23.4 / 6.9 | 23.8 / 7.2 | 23.1 / 6.6 | .31 (1.08) | .31 (1.05) | .31 (1.10) |
| 18 years | 23.0 | 28.4 | 17.8 | 34.8 / 21.4 | 50.0 / 26.8 | 30.0 / 16.0 | .88 (1.65) | 1.07 (1.78) | .70 (1.51) |
| 21 years | 28.1 | 37.0 | 20.2 | 38.5 / 26.7 | 52.3 / 34.9 | 28.6 / 18.8 | 1.13 (1.77) | 1.53 (1.95) | .78 (1.50) |
| MDD | |||||||||
| 15 years | 3.4 | 3.0 | 3.7 | 10.6 / 2.8 | 9.4 / 2.5 | 11.8 / 3.0 | .30 (1.13) | .24 (1.05) | .35 (1.21) |
| 18 years | 7.3 | 3.6 | 10.7 | 11.3 / 6.1 | 6.5 / 2.5 | 18.3 / 9.1 | .53 (1.61) | .31 (1.20) | .74 (1.88) |
| 21 years | 12.3 | 10.6 | 13.9 | 16.8 / 10.5 | 15.1 / 8.0 | 19.7 / 12.4 | .81 (2.04) | .71 (1.89) | .90 (2.16) |
Notes. Rates and symptom counts at 15 years are lifetime until age 15 and at 18 and 21 years are since the pervious assessment (approximately the preceding 3 years). Dx = diagnoses. NicD=nicotine dependence. MDD=major depressive disorder. Columns under the center heading give the rate of the indicated disorder in individuals with the other disorder compared to the rate in those without the other disorder. For instance, 23.4% of depressed 15-year-olds were nicotine dependent, and 6.9% of non-depressed 15-year-olds were nicotine dependent.
Phenotypic correlations for MDD and NicD symptoms counts at all three assessments by gender are presented in Table 2. Correlations were estimated using weighted least squares with robust standard errors in Mplus using all available data and treating twins as clusters. Stability correlations across time within disorder were positive and significant and were moderate in magnitude for MDD symptoms and moderate to high in magnitude for NicD symptoms. Consistent with comorbidity between these disorders, all contemporaneous correlations between MDD and nicotine were positive and significant but were generally small in magnitude and decreased slightly with age. Correlations between MDD symptoms and subsequent NicD symptoms and between NicD symptoms and subsequent MDD symptoms were also positive and significant but small in magnitude. Stability correlations across time within disorder were generally similar in magnitude for males and females. Correlations of MDD symptoms with NicD symptoms tended to be slightly higher for females than males.
Table 2.
Phenotypic Correlations among Major Depressive Disorder (MDD) symptoms and Nicotine Dependence (NicD) Symptoms for Combined Sample and Male and Female Samples
| Combined sample | NicD | MDD | |||||
|---|---|---|---|---|---|---|---|
| 15 years | 18 years | 21 years | 15 years | 18 years | 21 years | ||
| NicD | 15 years | ||||||
| 18 years | .40*** | ||||||
| 21 years | .28*** | .56*** | |||||
| MDD | 15 years | .17*** | .08* | .07* | |||
| 18 years | .07 | .11** | .08* | .24*** | |||
| 21 years | .09** | .08* | .11** | .14*** | .26*** | ||
| Male/Female samples | NicD | MDD | |||||
|---|---|---|---|---|---|---|---|
| 15 years | 18 years | 21 years | 15 years | 18 years | 21 years | ||
| NicD | 15 years | .37*** | .26*** | .13* | .10 | .04 | |
| 18 years | .49*** | .54*** | .03 | .06 | .03 | ||
| 21 years | .32*** | .67*** | .04 | .06 | .10** | ||
| MDD | 15 years | .20** | .15** | .13** | .18* | .07 | |
| 18 years | .06 | .18*** | .12* | .27*** | .28*** | ||
| 21 years | .12* | .12** | .12* | .18*** | .25*** | ||
Notes. Male twins are above diagonal, and female twins are below diagonal. MDD=major depressive disorder. NicD=nicotine dependence.
< .05,
<.01,
<.001.
Table 1 also presents rates of each disorder among individuals with and without the other disorder at each age, which provides additional information about comorbidity. Rates of MDD diagnoses were higher among individuals with NicD diagnoses at all three ages for males and females, and rates of NicD diagnoses were also higher among individuals with MDD diagnoses at all three ages for males and females. Logistic regression analyses with generalized estimating equations to account for the nonindependent twin data indicate that individuals with one disorder were more likely to have the other disorder than not to have the disorder at age 15 (Wald χ2= 17.15, p < .001), age 18 (Wald χ2= 7.82, p= .005), and age 21 (Wald χ2= 9.23, p =.002). This evidence of comorbidity was found for males at each age (Wald χ2> 4.63, p > .03) and females at each age (Wald χ2> 4.80, p > .03).
Twin intraclass within-trait correlations by zygosity and gender are presented in Table 3. These correlations provide information about genetic and environmental influences on NicD and MDD symptoms at each age. The correlations for NicD symptoms were significant, approximately equal, and moderately large for monozygotic (MZ) and dizygotic (DZ) twins at age 15, indicating little to no genetic effects but some shared environmental effects. This was true for male and female twin pairs. At age 18, the magnitude of the correlations for NicD symptoms was significant for both MZ and DZ twin pairs, but the magnitude of the correlation was nearly twice as large for MZ twin pairs as for DZ twin pairs. From age 15 to 18, the MZ twin correlations increased slightly and the DZ correlations decreased slightly. Overall, these correlations indicate increasing genetic influences and decreasing shared environmental influences on NicD symptoms during late adolescence. At age 21, the MZ NicD symptom correlations remained relatively stable while the DZ correlations continued to decrease, suggesting continued declines in shared environmental influences. These patterns are generally similar for male and female twin pairs, with the exception that at age 21, the DZ correlation was not significant and small in magnitude for males but remained significant and moderate in magnitude for females. Altogether, these correlations provide preliminary evidence of age-related increases in heritability.
Table 3.
Twin Intraclass Correlations for Nicotine Dependence (NicD) and Major Depressive Disorder (MDD) Symptoms by Zygosity and Age
| Male |
Female |
||||||
|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | ||
| NicD | 15 years | .51*** | .49*** | .53*** | .45*** | .48*** | .51*** |
| 18 years | .60*** | .37*** | .58*** | .38*** | .60*** | .38*** | |
| 21 years | .55*** | .21** | .51*** | .11 | .62*** | .32*** | |
| MDD | 15 years | .02 | .23*** | .12* | .13 | −.08 | .28** |
| 18 years | .28*** | .20** | .35*** | .12 | .24*** | .22** | |
| 21 years | .28*** | .14* | .28*** | .06 | .25*** | .15* | |
Notes. Male twins are above diagonal, and female twins are below diagonal. MDD=major depressive disorder. NicD=nicotine dependence. MZ=monozygotic. DZ=dizygotic.
< .05,
<.01,
<.001.
Overall, the intraclass correlations are smaller for MDD symptoms than for the NicD symptoms, which suggest smaller genetic and shared environmental influences and larger nonshared environmental influences on MDD symptoms than NicD symptoms. For MDD at age 15, the MZ correlation is nonsignificant while the DZ correlation is significant. This unexpected pattern of correlations is present only among females, and may stem from the fact that depression is not common at this age, thus reducing our ability to reliably estimate twin similarity at this age. At ages 18 and 21, the MZ correlations were higher than the DZ correlation. This pattern is true for both male and female twin pairs, although the pattern is somewhat more pronounced for males than females. The MZ correlations were relatively stable between age 18 and 21, while the DZ correlations decreased slightly from age 18 to age 21. Overall, these correlations suggest that both the genetic and shared environmental influences on MDD will be small, but the genetic influences will increase slightly with age.
Biometric Model Fitting
Nicotine dependence symptoms
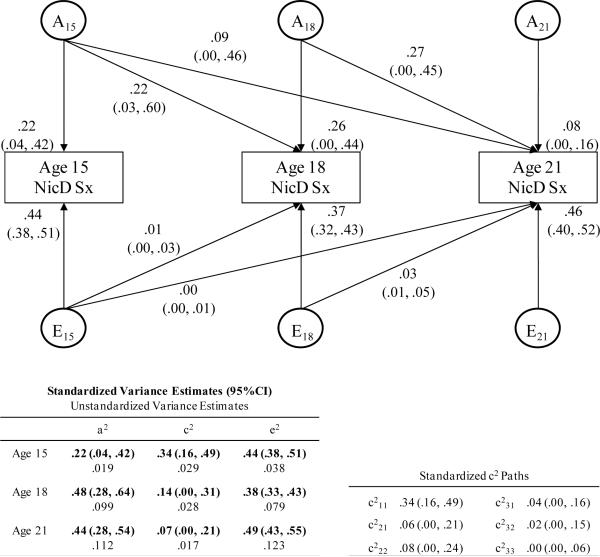
Findings from the Cholesky model for NicD symptoms (Figure 2) were consistent with the twin correlations in that heritability and nonshared environmental influences tended to increase with age. The unstandardized variance estimates (presented in the table in the lower left portion of the figure) indicate that as expected the total variance in NicD increased with age as did genetic variance (which increased considerably between ages 15 and 18 and only slightly between ages 18 and 21) and nonshared environmental variance. By contrast, the shared environmental variance is small at all three ages and decreased slightly with age. Table 4 displays test statistics for comparisons of model fits. Worsening of model fit between the fully age-constrained model (model 1) and age unconstrained model (model 2) indicate that A, C, and E could not be constrained to be equal across all ages without significantly reducing the fit of the model. A more specific nested model with A constrained to be equal across the three ages also worsened the fit of the model (model 3), suggesting that estimates of genetic influence could not be constrained to be equal across ages. Since additive genetic estimates were similar at ages 18 and 21, a model with A constrained to be equal at ages 18 and 21 but free to vary between age 15 and the two older ages (model 4) was also compared to the age unconstrained model. This model provided a better fit to the data and the AIC indicates that this model is preferred to the model with A constrained across all three ages. The small shared environmental influences could be constrained to be equal across all three ages (model 5). The nonshared environmental influences, which increased with age, could not be constrained to be equal across the three ages (model 6). Thus, the best-fitting model (model 7) is one in which genetic influences are free to vary between age 15 and ages 18 and 21 but constrained to be equal at ages 18 and 21, shared environmental influences are constrained to be equal across all ages, and nonshared environmental influences are free to vary at each age. With the exception of the small and relatively stable shared environmental variance, these findings were consistent with expectation and aid the interpretation of the standardized estimates which are also presented in Figure 2
Figure 2.
This figure is a standardized path diagram of the full Cholesky decomposition model for Nicotine Dependence symptoms (NicD) with 95% confidence intervals for the parameters shown in the model. Path coefficient estimates have been squared and represent the proportion of variance in NicD accounted for by the components. The tables at the bottom of the figure include: (a) the overall proportions of variance in NicD attributed to (and the unstandardized variance estimates for) genetic, shared environmental, and nonshared environmental influences at each age and (b) the standardized and squared shared environmental path coefficients. Ai = variance attributable to additive genetic effects; Ei = variance attributable to nonshared environmental effects; a2, c2, e2 = squared path coefficients for genetic, shared environmental, and nonshared environmental influences, respectively.
Table 4.
Test Statistics for Cholesky Decomposition Model for Nicotine Dependence Symptoms with Constraints across Ages
| Model | −2lnL | df | Δ−2lnL (df) | p value | AIC |
|---|---|---|---|---|---|
| 1. Age unconstrained model | 281.54 | 66 | 149.54 | ||
| 2. Fully age-constrained | 780.43 | 72 | 498.98(6) | <.001 | 636.43 |
| 3. Constrain A across age | 303.73 | 68 | 22.19(2) | <.001 | 167.73 |
| 4. Constrain: A: ages 18 and 21 years |
281.94 | 67 | 0.38(1) | .54 | 145.94 |
| 5. Constrain C across age | 282.12 | 68 | 0.58(2) | .75 | 146.12 |
| 6. Constrain E across age | 471.46 | 68 | 189.92(2) | <.001 | 335.46 |
|
7. Constrain:
A: ages 18 and 21 years C: all ages |
282.16 | 69 | 0.62(3) | .89 | 144.16 |
Notes. Separate variance-covariance matrices were used for males and females. The unconstrained model is unconstrained across ages but is constrained across sexes. Abbreviations: A=additive genetic effects. C=shared environmental effects. E=nonshared environmental effects. −2lnL= −2 times the log likelihood. AIC=Akaike Information Criteria. Δ−2lnL=differences in 2lnL values between the sex-constrained but age unconstrained model (model 1). Best fitting model is bolded.
Standardized estimates, which provide a comparison of relative proportions of variance in NicD symptoms attributed each of the three sources of variance, are also shown in Figure 2. These standardized variance estimates also indicate that the proportions of variance in NicD symptoms accounted for by genetic influences increased between age 15 and 18 and were relatively stable between ages 18 and 21. The proportion of variance accounted for by shared environmental influences decreased from age 15 to 18 and from age 18 to 21, decreasing by about 80% between ages 15 and 21. While the magnitude of shared environmental influences was larger than the magnitude of genetic influences at age 15, the reverse was true at ages 18 and 21 when the shared environmental estimates were small and nonsignificant. The proportions of the variance attributed to nonshared environmental influences were relatively stable with the largest proportion at age 21. Despite changes in the relative magnitude of genetic effects across ages, there was considerable overlap in genetic influences across ages. Almost half of the genetic influences on NicD symptoms present at age 18 were present at age 15, and 81% of the genetic influences present at age 21 were present at an earlier age (20% at age 15, 61% at 18). Nonshared environmental influences were almost entirely age-specific. Only 3% nonshared environmental effects at age 18 were present at age 15, and 6% of the nonshared environmental effects at age 21 were present at age 18 (0% at age 15).
In summary, genetic contributions to the variance in NicD symptoms increased with age while the proportion of variance attributed to shared environmental contributions decreased with age and the proportion of variance attributed to nonshared environmental contributions remained relatively stable across the three ages. Some genetic influences were present at all ages, which contributed to the stability of NicD symptoms between ages 15 and 21. Although nonshared environmental influences were significant and moderate in magnitude at each age, these nonshared influences contributed little to the stability in NicD symptoms across the three ages but rather reflected age-specific (i.e., new or unique) environmental influences that were not present at earlier ages.
Major depressive disorder symptoms
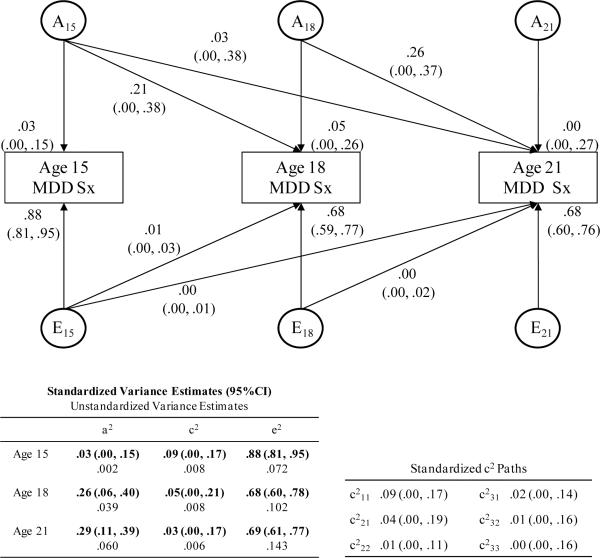
Estimates from the Cholesky model for MDD symptoms were also consistent with the twin correlations (Figure 3) in that heritability effects became stronger with age. As was expected and was the case for NicD, total variance in MDD increased with age, as did genetic variance (which increased more between ages 15 and 18 than between ages 18 and 21) and nonshared environmental variance. Shared environmental variance was generally small and remained stable between ages 15 and 18 and then decreased slightly between ages 18 and 21. Test statistics for models of MDD symptoms are presented in Table 5. Comparison of the age unconstrained (model 1) and fully age-constrained (model 2) models indicated significant heterogeneity in the parameter estimates across the ages. Constraining A across all three ages (model 3) worsened the fit of the model. The more specific model constraining A at ages 18 and 21 while allowing A to vary at age 15 (model 4) improved the fit of the model. Constraining C across all three ages (model 5) also improved the fit of the model, while constraining E across ages (model 6) worsened the fit of the model. As follows, consistent with most predictions (except the prediction of decreasing shared environmental influences), in the best-fitting model (model 7), genetic influences were constrained to be equal at ages 18 and 21 and free to vary at age 15, shared environmental influences were constrained to be equal at all three ages, and nonshared environmental influences were free to vary across all three ages. Thus, the same model fit best for MDD symptoms and NicD symptoms.
Figure 3.
This figure is a standardized path diagram of the full Cholesky decomposition model for Major Depressive Disorder symptoms (MDD) with 95% confidence intervals for the parameters shown in the model. Path coefficient estimates have been squared and represent the proportion of variance in MDD accounted for by the components. The tables at the bottom of the figure include: (a) the overall proportions of variance in MDD attributed to (and the unstandardized variance estimates for) genetic, shared environmental, and nonshared environmental influences at each age and (b) the standardized and squared shared environmental path coefficients. Ai = variance attributable to additive genetic effects; Ei = variance attributable to nonshared environmental effects; a2, c2, e2 = squared path coefficients for genetic, shared environmental, and nonshared environmental influences, respectively.
Table 5.
Test Statistics for Cholesky Decomposition Model for Major Depressive Disorder Symptoms with Constraints across Ages
| Model | −2lnL | df | Δ−2lnL (df) | p value | AIC |
|---|---|---|---|---|---|
| 1. Age unconstrained model | 370.61 | 66 | <.001 | 238.61 | |
| 2. Fully age-constrained | 684.42 | 72 | 313.81(6) | <.001 | 540.42 |
| 3. Constrain A across age | 384.93 | 68 | 14.32(2) | <.001 | 248.93 |
| 4. Constrain: A: ages 18 and 21 years |
371.58 | 67 | 0.97(1) | .32 | 237.58 |
| 5. Constrain C across age | 370.64 | 68 | 0.03(2) | .99 | 234.64 |
| 6. Constrain E across age | 451.71 | 68 | 81.10(2) | <.001 | 315.71 |
|
7. Constrain:
A: ages 18 and 21 years C: all ages |
373.15 | 69 | 2.54(3) | .47 | 235.15 |
Notes. Separate variance-covariance matrices were used for males and females. The unconstrained model is unconstrained across ages but is constrained across sexes. Abbreviations: A=additive genetic effects. C=shared environmental effects. E=nonshared environmental effects. −2lnL= −2 times the log likelihood. AIC=Akaike Information Criteria. Δ−2lnL=differences in 2lnL values between the sex-constrained but age unconstrained model (model 1). Best fitting model is bolded.
The standardized estimates in Figure 3 indicate that almost all variance in MDD symptoms at age 15 can be attributed to nonshared environmental effects, but again it is important to interpret these findings with caution given the low variability in symptom counts and unexpected higher twin correlations for DZ than MZ twins. The proportion of variance attributed to genetic influences increased between age 15 and 18 and then for the most part leveled off between ages 18 and 21. Shared environmental contributions were generally small at each age but decreased from age 15 to 18 and from age 18 to 21. The proportion of variance attributed to nonshared environmental influences continued to predominate at all ages. Most of the genetic effect at ages 18 and 21 was present at the previous ages, indicating few age-specific or new genetic influences with age. Notably, approximately 80% of the genetic influence at age 18 was present at age 15, and 100% of the genetic influence at age 21 was present at an earlier age. Nonshared environmental influences were almost entirely age specific and therefore did not contribute to the stability of MDD symptoms across ages. Shared environmental effects were small and contributed little to the stability of MDD.
Overall, the Cholesky model of MDD symptoms indicated increasing genetic contributions, small and decreasing shared environmental contributions, and very large nonshared environmental influences across the three ages. Most of the genetic influences were common to all three ages, with little evidence of age-specificity of these influences. Nonshared environmental influences were virtually entirely age-specific. Thus, similar to the findings for NicD symptoms, stability of symptoms was explained largely by genetic influences.
Cross-lagged Model Fitting
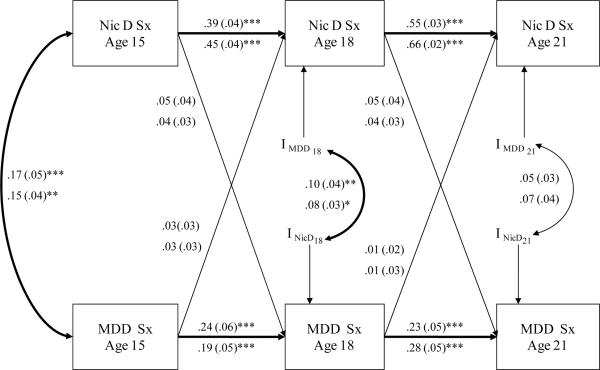
As previously presented, NicD symptoms and MDD symptoms were stable within disorders across time and were correlated with one another concurrently and across ages (see Table 2). Figure 4 displays the cross-lagged model with parameters constrained to be equal across sexes with mean levels of symptoms free to vary between the sexes, which results in different standardized estimates for males and females. Stability correlations within disorders between ages 15 and 18 and between 18 and 21 were significant and stronger for NicD symptoms than MDD symptoms. This indicates that each disorder predicted increased risk for the same problem over time. Parameters for all cross-lag paths connecting one disorder at an earlier time point to the other disorder at a later time point were small in magnitude and nonsignificant. A χ2 difference test revealed a nonsignificant decrease in the fit for the model with all cross paths between the two disorders fixed to zero [Δχ2(4)=4.36, p =.36]. MDD symptoms at an earlier point in time did not predict NicD symptoms at a later point in time, and NicD symptoms at an earlier point in time did not predict MDD symptoms at a later point in time. Correlations between the innovations were small in magnitude and significant at age 18 but nonsignificant at age 21. In other words, there is a small but significant association between new sources of variance in NicD symptoms at age 18 and new sources of variance in MDD symptom at age 18 that are not accounted for by either disorder at age 15.1
Figure 4.
This figure displays a cross-lag model with parameters constrained to be equal across sexes, mean levels of symptoms free to vary between the sexes, and thus different standardized estimates for males and females. Standardized parameter estimates (with standard errors) are displayed and can be interpreted in standard deviation units. Parameters for males are displayed above the parameters for females. The letter “I” denotes innovations, and curved arrows connecting two innovations represent correlations between the innovations. Values next to the curved arrows represent Pearson correlations. NicD Sx=nicotine dependence symptoms. MDD Sx = major depressive disorder symptoms. * < .05, ** <.01, ***<.001.
Discussion
The purpose of this study was to use longitudinal models to examine developmental shifts in the genetic and environmental influences underlying the emergence of NicD symptoms and MDD symptoms across the high-risk period of middle adolescence, when depressed mood and smoking initiation are emerging, to early adulthood, when point prevalence rates of both disorders have reached their peaks, and to examine the longitudinal associations between NicD and MDD symptoms after accounting for developmental continuity of these phenotypes. The findings support active, dynamic processes involving developmental changes in the magnitude of genetic and environmental influences on both NicD and MDD symptoms but no evidence supporting longitudinally predictive relationships between MDD and NicD symptoms after accounting for the stability and concurrent associations of these disorders.
As hypothesized, genetic influences on NicD and MDD symptoms increased between age 15 and age 18, a developmental period when independence and niche-fitting are likely to accelerate, but were relatively stable between ages 18 and 21. The rise in the influence of genetic factors demonstrated in this study likely occurred within the context of a cascade of interacting genes and environmental exposures that unfold over time and begin to emerge as symptoms of NicD and MDD during middle adolescence. Consistent with this idea, the present study findings also support the importance of environmental influences on MDD and NicD during this period, particularly environmental factors unique to individual twins rather than shared among family members. The increase in variance attributed to nonshared environmental influences may reflect key developmental tasks occurring during adolescence, such as growing independence, greater involvement with peers, and more time spent in extracurricular activities away from the family. Indeed, depression and nicotine use during adolescence have been associated with many such social risk factors, for example affiliation with peers who smoke and engage in deviant behavior (Hu, et al., 2006), peer rejection and victimization (Prinstein, Cheah, & Guyer, 2005), and peer relationships characterized by conflict and lack of trust (Eberhart & Hammen, 2006). These cascades of interacting genetic and environmental influences on NicD and MDD occur within the context of other developmentally relevant domains of functioning (e.g., academic achievements, career successes and failures, interpersonal relationship problems), which may also influence the developmental course of NicD and MDD. Future research may investigate these moderating influences, for instance by testing hypotheses about larger genetic effects or stronger age-related increases in genetic influences for individuals who have high levels of affiliation with deviant peers, have experienced romantic relationship break-ups, or fail to complete schooling and/or obtain employment.
For both NicD and MDD, genetic influences on symptoms overlapped considerably across the three ages, suggesting that genetic factors are a significant part of the processes underlying the continuity of symptoms across development. Nonshared environmental factors, on the other hand, were almost entirely age-specific and thus contributed little to the stability of NicD and MDD symptoms across time. Instead, different nonshared environmental factors influenced MDD and NicD symptoms at different points in development. Changes in environmental risk factors are consistent with the major developmental transitions that occur during this period. For example, conflict with parents is an important predictor of psychopathology and substance use during adolescence (McGue, Elkins, Walden, & Iacono, 2005) but is not likely to be as influental after young adults have moved out of their parents' homes.
The small shared environmental variance for both MDD and NicD across all three ages was somewhat surprising and may help explain inconsistencies in estimates of shared environmental influences across previous twin studies. Consistent with our summary of existing twin research on depression and smoking in samples of youth, at younger ages shared environmental influences accounted for a larger proportion of variance in symptoms than genetic influences for both phenotypes (but especially for NicD), and at older ages the reverse was true for both disorders. In fact, the proportions of variability accounted for by shared environmental influences diminished with age to nearly zero by early adulthood. It is important to note, though, that the decline in the relative proportion of shared environmental influence compared to genetic and nonshared environmental influences is almost entirely a reflection of the rather dramatic increase in both genetic and nonshared environmental variance during this period, rather than the slight (and nonsignificant) decline in shared environmental variance.
Since symptoms of NicD were more heritable than symptoms of MDD and environmental factors shared among family members (though minimally influential overall) were more important for NicD than MDD, it follows that environmental influences unique to each twin were more important sources of variability in MDD symptoms than NicD symptoms. In fact, 66–88% of the variability in MDD symptoms could be attributed to these influences. Characteristics of the family environment, such as family stress (Fendrich, Warner, & Weissman, 1990), parent-child conflict (Shiner & Marmorstein, 1998), and interparental conflict (Fergusson, Horwood, & Lynskey, 1995) have consistently been found to be important correlates of elevated risk for depression in adolescents. Integrating this research with our findings suggests that the role of these family factors in the development of depression is via individual processes not shared among family members (i.e., diverse experiences and responses to family stress and conflict for different family members), an idea consistent with developmental psychopathology principles that emphasize individual trajectories of risk (Rutter & Sroufe, 2000) and the tendency for individuals to experience and perceive family characteristics in different ways (Rutter, 1999).
Finally, as expected, there was considerable developmental continuity of MDD and NicD symptoms across adolescence and into early adulthood. Within disorder stability was stronger for NicD than MDD, which may be related to the episodic nature of depression and to stronger genetic influences on NicD symptoms than MDD symptoms, since genetic effects were shown to contribute to stability of symptoms over time. Consistent with research supporting the co-occurrence of smoking and depressed mood in adolescents and young adults (Fergusson et al., 1996), NicD symptoms were associated concurrently with MDD symptoms at all three ages, and diagnoses of MDD were more common among individuals with NicD and vice versa. These associations were relatively small in magnitude, which is not unexpected given variability in effect sizes for associations between smoking and depression in the literature and the paucity of comparable research employing interviews to measure clinically-relevant constructs in adolescents and young adults. Although we identified small, significant associations between symptoms of the disorders across ages, after accounting for stability within disorders over time, associations between symptoms of one disorder at an earlier point in time did not predict symptoms of the other disorder at a later point in time. The cross-lagged model, though, supported correlated age-specific sources of variance for NicD and MDD symptoms at age 18, indicating that the two disorders have correlated risk factors and perhaps share common nonshared environmental risk factors, possibly risks related to peer affiliation and relationships. Since most previous studies did not adequately account for stability within disorders across time or use clinically-relevant measures, these findings provide important new information about the longitudinal relationships between MDD and NicD.
Strengths and Limitations
A few study limitations should be noted. First, the measures of depression and nicotine use selected for this study had clinical relevance but were limited in their developmental sensitivity to the emergence of problems and ability to detect variability in symptoms at age 15, perhaps contributing to the small magnitude MZ twin correlations evident at age 15. Use of more developmentally sensitive measures (e.g., initiation of smoking, less severe depressed mood) may provide more variability, reliability of estimates, and the ability to examine genetic and environmental effects at younger ages. However, our study had the advantage of assessing the same clinically relevant measures of DSM criteria across all three assessments, and the low rates of symptom endorsement at age 15 are descriptive of individuals at this age in this general population sample.
Second, while an important strength of the clinical interviews was their ability to assess symptoms during the entire period between time points, the three-year intervals may have been too long to detect the predictive effects of one of these disorders on the other. For instance, MDD may predict NicD when NicD is measured immediately following a depressive episode, with this effect weakening over longer durations of time. Future research that studies the longitudinal relationship between these constructs across the same high-risk developmental period but at shorter intervals may pinpoint a more precise timeline (especially between ages 15 and 18) when the developmental shifts in genetic and environmental effects occur.
Third, biometric modeling partitions variance into additive genetic, shared environmental, and nonshared environmental effects but does not provide information about specific genes, environmental risks, or targets for interventions and does not inform about causal processes (Rutter & Sroufe, 2000). The Cholesky decomposition models used in this study do not separate or identify gene-environment or epistatic genetic effects. Research on the interplay of specific genes and environmental risk factors across this developmental period will be important for specifying the nature of changing etiological factors.
Finally, the participants were primarily Caucasian, which reflects the community from which the twins were selected but limits generalizability of the findings to other groups. In addition, since the participants in the study were twins, the findings may not be applicable to singletons. However, various lines of research support the applicability of findings from samples of twins, such as studies finding few differences in rates of psychopathology between twins and singletons (e.g., Kendler, Martin, Heath, and Eaves, 1995).
This study also has several methodological strengths. First, the age range of participants was broad (approximately 15 years to 21 years), but participants were assessed at discrete ages (i.e., variability in age at each assessment was low), which provided the opportunity to study change across the high-risk period while also examining effects at important, distinct points during development. Second, the sample had low attrition across assessments and was a representative community sample, which makes it possible to generalize findings to non-clinical populations. Third, clinical interviews were used to assess symptoms throughout the entire period from age 15 to 21, which provides clinically relevant measures and fills gaps in behavior genetics research on depression and nicotine use in youth samples, which have almost exclusively relied on self-report measures of current problems. These strengths, especially the longitudinal design, clinical measures, discrete ages at assessments, and models that account for limitations in the previous research (e.g., stability of disorders), allowed us to address unanswered questions about age-related changes in the genetic and environmental influences on depression and nicotine dependence and longitudinal associations between depression and nicotine dependence during the critical years of adolescence and early adulthood.
Conclusion
In summary, genetic and environmental factors were involved in the developmental cascade of risks explaining the rise in symptoms of NicD and MDD between age 15, when levels of symptoms were low and individual variability in symptoms were small, and late adolescence/early adulthood, when symptoms were at peak levels and individual variability was more substantial. This high risk period, especially between ages 15 and 18 years, is a critical time for changes in etiological factors as genetic influences become more important, new genetic influences emerge, and nonshared environmental influences contribute to greater variability in susceptibility to depression and nicotine dependence. Individuals high in symptoms of one disorder were likely to be high in symptoms of the same disorder later in development. This developmental continuity was largely explained by genetic factors. Environmental factors uniquely experienced by one twin and not the other were influential in the development of both disorders, but especially depression. These environmental factors were developmentally-specific and tended to explain variability at only one age. MDD and NicD symptoms co-occurred at the same time, had small associations across ages, and had correlated age-specific risk factors at age 18. However, after accounting for the stability of the two disorders, there were no cumulative cascade effects from one disorder to the other over the assessed time intervals, calling into question the causal role of one disorder in the developmental cascade of the other disorder.
Understanding developmental changes in genetic and environmental factors has implications for intervening and treating NicD and MDD. Given the large overlap in genetic influences across the three discrete ages, early interventions that target children with high genetic-risk (e.g., a parent with the disorder) may yield “high returns” (Heckman, 2006) and thus may help reduce the economic and health burdens associated with these disorders. Furthermore, identifying intergenerational cascades of effects that link risk in one generation with risk in the next generation has practical implications for pinpointing and targeting specific predictors of risk and “buffers” that promote resilience (Serbin & Karp, 2004). For example, interventions for children of depressed parents may target building resilience to genetic risks, such as developing skills to minimize the effects of their own genetically-influenced proneness to negative affectivity, and protecting against environmental mechanisms of intergenerational transmission of risk, such as learning to cope with the fluctuating moods and parenting practices of their depressed parent. The development of effective resilience skills may ultimately reduce negative chain reactions (Rutter, 1999) and help prevent problems across development. At the same time, given evidence of age-specific genetic and environmental risk factors, preventative interventions must be ongoing, take into account individual family members' unique experiences of family risk factors, adjust to individuals' developmental levels, and consider age-specific risk factors.
Acknowledgements
This study was supported in part by National Institutes of Health Grants DA-05147, AA-09367, and 017069. We thank the participating families who generously gave their time to the study and the many staff members and research assistants who contributed to the overall conduct of the study.
Footnotes
Longitudinal relationships between categorically diagnosed MDD and NicD were also examined using a similar cross-lagged model, again accounting for the twin data by nesting twins within twin pairs. Findings from this diagnosis-based model were similar to those reported for the symptom count model. Again, no cross paths between MDD and NicD diagnoses across any ages were significant, and there was a nonsignificant decrease in the fit of the model when all cross paths between the two were constrained to zero [Δχ2(3)=3.40, p =.33].
References
- Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Author; Burlington, VT: 1991. [Google Scholar]
- Baker LA, Daniels D. Nonshared environmental influences and personality differences in adult twins. Journal of Personality and Social Psychology. 1990;58:103–110. doi: 10.1037//0022-3514.58.1.103. doi: 10.1037/0022-3514.58.1.103. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics. 2007;10(3):423–433. doi: 10.1375/twin.10.3.423. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Biglan A, Duncanm TE, Ary DV, Smolkowski K. Peer and parental influences on adolescent tobacco use. Journal of Behavioral Medicine. 1995;18(4):315–330. doi: 10.1007/BF01857657. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Koopmans JR, van Doornen LJP, Orlebeke JF. Genetic and social influences on starting to smoke: a study of Dutch adolescent twins and their parents. Addiction. 1994;89:219–226. doi: 10.1111/j.1360-0443.1994.tb00881.x. doi: 10.1111/j.1360-0443.1994.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Robinette D, Fabsitz RR. Heritability of substance use in the NAS-NRC Twin Registry. Acta Geneticae Medicae et Gemellologial. 1990;39:91–98. doi: 10.1017/s0001566000005602. [DOI] [PubMed] [Google Scholar]
- Cassano P, Fava M. Depression and public health: An overview. Journal of Psychosomatic Research. 2002;53(4):849–857. doi: 10.1016/s0022-3999(02)00304-5. [DOI] [PubMed] [Google Scholar]
- Chaiton MO, Cohen JE, O'Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BCM Public Health. 2009;9:1–11. doi: 10.1186/1471-2458-9-356. doi: 10.1186/1471-2458-9-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood: demographic predictors of continuity and change. Health Psychology. 1996;15(6):478–484. doi: 10.1037//0278-6133.15.6.478. doi: 10.1037/0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. The emergence of developmental psychopathology. Child Development. 1984;55:1–7. [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Moss HB. Early adolescent gateway drug use in sons of fathers with substance use disorders. Addictive Behaviors. 1998;23:561–566. doi: 10.1016/s0306-4603(98)00038-0. doi: 10.1016/S0306-4603(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Costello E, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Maes HH, Simonoff E, Pickles A, Rutter M, et al. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry. 1997;38(8):965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Eberhart NK, Hammen CL. Interpersonal predictors of onset of depression during the transition to adulthood. Personal Relationships. 2006;13(2):195–206. doi: 10.1111/j.1475-6811.2006.00113.x. [Google Scholar]
- Ehringer MA, Rhee SH, Young S, Corley R, Hewitt JK. Genetic and Environmental Contributions to Common Psychopathologies of Childhood and Adolescence: A Study of Twins and Their Siblings. Journal of Abnormal Child Psychology. 2006;34(1):1–17. doi: 10.1007/s10802-005-9000-0. doi: 10.1007/s10802-005-9000-0. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug and Alcohol Dependence. 2000;59(Suppl. 1):41–60. doi: 10.1016/s0376-8716(99)00164-7. doi: 10.1016/S0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Eley TC, Plomin R. Genetic analyses of emotionality. Current Opinion in Neurobiology. 1997;7(2):279–284. doi: 10.1016/s0959-4388(97)80017-7. doi: doi:10.1016/S0959-4388(97)80017-7. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stevenson J. Exploring the covariation between anxiety and depression symptoms: A genetic analysis of the effects of age and sex. Journal of Child Psychology and Psychiatry. 1999;40(8):1273–1282. doi: doi:10.1111/1469-7610.00543. [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: Evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–363. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Fendrich M, Warner V, Weissman MM. Family risk factors, parental depression, and psychopathology in offspring. Developmental Psychology. 1990;26(1):40–50. doi: 10.1037/0012-1649.26.1.40. [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Maternal depressive symptoms and depressive symptoms in adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1995;36:1161–1178. doi: 10.1111/j.1469-7610.1995.tb01363.x. doi: 10.1111/j.1469-7610.1995.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Lynskey MT, Horwood LJ. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16-year-olds. Archives of General Psychiatry. 1996;53(11):1043–1047. doi: 10.1001/archpsyc.1996.01830110081010. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Wostear G, Cooper V, Harrington R, Rutter M. The Maudsley long-term follow-up of child and adolescent depression: I Psychiatric outcomes in adulthood. British Journal of Psychiatry. 2001;179:210–217. doi: 10.1192/bjp.179.3.210. doi: 10.1192/bjp.179.3.210. [DOI] [PubMed] [Google Scholar]
- Gjone H, Stevenson J, Sundet JM, Eilertsen DE. Changes in heritability across increasing levels of behavior problems in young twins. Behavior Genetics. 1996;26(4):419–426. doi: 10.1007/BF02359486. doi: 10.1007/BF02359486. [DOI] [PubMed] [Google Scholar]
- Glowinski AL, Madden PA, Bucholz KK, Lynskey MT, Heath AC. Genetic epidemiology of self-reported lifetime DSM-IV major depressive disorder in a population-based twin sample of female adolescents. Journal of Child Psychology and Psychiatry. 2003;44(7):988–996. doi: 10.1111/1469-7610.00183. doi: 10.1111/1469-7610.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Greden JF. The burden of recurrent depression: causes, consequences, and future prospects. Journal of Clinical Psychiatry. 2001;62(Suppl 22):5–9. [PubMed] [Google Scholar]
- Griesler PC, Hu MC, Schaffran C, Kandel DB, Griesler PC, Hu M-C, et al. Comorbidity of psychiatric disorders and nicotine dependence among adolescents: findings from a prospective, longitudinal study. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(11):1340–1350. doi: 10.1097/CHI.0b013e318185d2ad. doi: 10.1097/CHI.0b013e318185d2ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Madden P, Lynskey M. The genetics of tobacco use: methods, findings and policy implications. Tobacco Control. 2002;11:119–124. doi: 10.1136/tc.11.2.119. doi: 10.1136/tc.11.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington R, Fudge H, Rutter M, Pickles A, Hill J. Adult outcomes of childhood and adolescent depression: I. Psychiatric status. Archives of General Psychiatry. 1990;47(5):465–473. doi: 10.1001/archpsyc.1990.01810170065010. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Martin NG. Statistical methods in genetic research on smoking. Statistical Methods in Medical Research. 1998;7:165–186. doi: 10.1177/096228029800700205. doi: 10.1177/096228029800700205. [DOI] [PubMed] [Google Scholar]
- Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Hewitt JK, Silberg J, Neale M, Eaves L, Erickson M. The analysis of parental ratings of children's behavior using LISREL. Behavior Genetics. 1992;22(3):293–317. doi: 10.1007/BF01066663. doi: 10.1007/BF01066663. [DOI] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB, Hu M-C, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. American Journal of Public Health. 2006;96(2):299–308. doi: 10.2105/AJPH.2004.057232. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor JT, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M, Krueger RF. Minnesota Center for Twin and Family Research. Twin Research and Human Genetics. 2006;9:978–984. doi: 10.1375/183242706779462642. [DOI] [PubMed] [Google Scholar]
- Kandela DB, Hua M-C, Grieslerb PC, Schaffranc C. On the development of nicotine dependence in adolescence. Drug and Alcohol Dependence. 2007;91(1):26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J, Hammar N, Koskenvuo M, Floderus-Myrhed B, Langinvainio H, Sarna S. Cigarette smoking and alcohol use in Finland and Sweden: a cross-national twin study. International Journal of Epidemiology. 1982;11:378–386. doi: 10.1093/ije/11.4.378. [DOI] [PubMed] [Google Scholar]
- Kardia SL, Pomerleau CS, Rozek LS, Marks JL. Association of parental smoking history with nicotine dependence, smoking rate, and psychological cofactors in adult smokers. 2003. doi:10.1016/S0306-4603%2802%2900245-9. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. Archives of General Psychiatry. 1993;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine. 1999;29:299–308. doi: 10.1017/s0033291798008022. doi: 10.1017/S0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in Swedish twins reared apart and reared together. Archives of General Psychiatry. 2000;57:886–892. doi: 10.1001/archpsyc.57.9.886. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- Keyes M, Legrand LN, Iacono WG, McGue M. Parental smoking and adolescent problem behavior: An adoption study of general and specific effects. American Journal of Psychiatry. 2008;165(10):1338–1344. doi: 10.1176/appi.ajp.2008.08010125. doi: 10.1176/appi.ajp.2008.08010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behavioral Genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. doi: 10.1023/A:1021618719735. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: Prevalence, risk factors, and clinical implications. Clinical Psychology Review. 1998;18(7):765–794. doi: 10.1016/s0272-7358(98)00010-5. doi: 10.1016/S0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Lieb R, Schreier A, Pfister H, Wittchen H. Maternal smoking and smoking in adolescents: A prospective community study of adolescents and their mothers. European Addiction Research. 2003;9:120–130. doi: 10.1159/000070980. [DOI] [PubMed] [Google Scholar]
- Lopez AD. Disease Control Priorities Project: Global burden of disease and risk factors. Oxford University Press; Washington, D.C.: 2006. [Google Scholar]
- Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, et al. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine & Tobacco Research. 2008;10(1):97–108. doi: 10.1080/14622200701705332. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. Journal of Studies on Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. Retrieved from: http://www.jsad.com. [DOI] [PubMed] [Google Scholar]
- Manke B, McGuire S, Reiss D, Hetherington EM, Plomin R. Genetic contributions to adolescents' extrafamilial social interactions: Teachers, best friends, and peers. Social Development. 1995;4:238–256. doi: 10.1111/j.1467-9507.1995.tb00064.x. [Google Scholar]
- Mathers CD, Stein C, Fat DM, Rao C, Inoue M, Tomijima N, et al. Global Burden of Disease 2000: Version 2 Methods and Results. Geneva, Switzerland: 2000. [Google Scholar]
- McCaffery JM, Niaura R, Swan GE, Carmelli D. A study of depressive symptoms and smoking behavior in adult male twins from the NHLBI twin study. Nicotine and Tobacco Research. 2003;5(1):77–83. doi: 10.1080/14622200307259. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. doi: 10.1002/1096-8628(20001009)96:5<671::AID-AJMG14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Walden B, Iacono WG. Perceptions of the parent-adolescent relationship: A longitudinal investigation. Developmental Psychology. 2005;41(6):971–984. doi: 10.1037/0012-1649.41.6.971. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- McGue M, lacono WG, Krueger R. The association of early adolescent problem behavior and adult psychopathology: a multivariate behavioral genetic perspective. Behavior Genetics. 2006;36(4):591–602. doi: 10.1007/s10519-006-9061-z. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Fifth Edition Muthén & Muthén; Los Angeles: 1998–2007. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 5th ed. Department of Psychiatry; Richmond, VA: 1999. [Google Scholar]
- O'Connor TG, McGuire S, Reiss D, Hetherington E, Plomin R. Co-occurrence of depressive symptoms and antisocial behavior in adolescence: A common genetic liability. Journal of Abnormal Psychology. 1998;107(1):27–37. doi: 10.1037//0021-843x.107.1.27. doi: 10.1037/0021-843X.107.1.27. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Neiderhiser JM, Reiss D, Hetherington E, Plomin R. Genetic contributions to continuity, change, and co-occurrence of antisocial and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry. 1998;39(3):323–336. doi: 10.1017/S0021963097002126. [PubMed] [Google Scholar]
- Patterson GR. A social learning approach to family intervention:Vol. 3. Coercive family process. Castalia; Eugene, OR: 1982. [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Wakefield M. Teen Smokers Reach Their Mid Twenties. Journal of Adolescent Health. 2006;39(2):214–220. doi: 10.1016/j.jadohealth.2005.11.027. doi:10.1016/j.jadohealth.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Pedersen W, von Soest T. Smoking, nicotine dependence and mental health among young adults: A 13-year population-based longitudinal study. 2009. doi: 10.1111/j.1360-0443.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- Pike A, McGuire S, Hetherington EM, Reiss D, Plomin R. Family environment and adolescent depressive symptoms and antisocial behavior: A multivariate genetic analysis. Developmental Psychology. 1996;32(4):590–604. doi: 10.1037/0012-1649.32.4.590. [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. doi: 10.1037/0033-2909.84.2.309. [PubMed] [Google Scholar]
- Plomin R, Reiss D, Hetherington EM, Howe GW. Nature and nurture: Genetic contributions to measures of the family environment. Developmental Psychology. 1994;30(1):32–43. doi: 10.1037/0012-1649.30.1.32. [Google Scholar]
- Prinstein MJ, Cheah CS, Guyer AE. Peer victimization, cue interpretation, and internalizing symptoms: preliminary concurrent and longitudinal findings for children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34(1):11–24. doi: 10.1207/s15374424jccp3401_2. doi: 10.1207/s15374424jccp3401_2. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [Google Scholar]
- Rao U, Hammen C, Daley SE. Continuity of depression during the transition to adulthood: A 5-year longitudinal study of young women. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:908–915. doi: 10.1097/00004583-199907000-00022. doi: 10.1097/00004583-199907000-00022. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z. Diagnostic Interview for Children and Adolescents-Revised: DSMIIIR version (DICA-R) Washington University; St. Louis, MO: 1988. [Google Scholar]
- Rende RD, Plomin R, Reiss D, Hetherington E. Genetic and environmental influences on depressive symptomatology in adolescence: Individual differences and extreme scores. Journal of Child Psychology and Psychiatry. 1993;34(8):1387–1398. doi: 10.1111/j.1469-7610.1993.tb02097.x. doi: 10.1111/j.1469-7610.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thaper A. Assessing the effects of age, sex and shared environment on the genetic aetiology of depression in childhood and adolescence. Journal of Child Psychology and Psychiatry. 2002a;43(8):1039–1051. doi: 10.1111/1469-7610.00231. doi: doi:10.1111/1469-7610.00543. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thaper A. The genetic aetiology of childhood depression: A review. Journal of Child Psychology and Psychiatry. 2002b;43(1):65–79. doi: 10.1111/1469-7610.00004. [DOI] [PubMed] [Google Scholar]
- Riley WT, Kaugers GE, Grisius TM, Page DG, Burns JC, Svirsky JA. Adult smokeless tobacco use and age of onset. Addictive Behaviors. 1996;21:135–138. doi: 10.1016/0306-4603(95)00030-5. doi: 10.1016/0306-4603(95)00030-5. [DOI] [PubMed] [Google Scholar]
- Robins LN, Baber T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Masten AS, Coatsworth JD, Tellegen A. Salient and emerging developmental tasks in the transition to adulthood. Child Development. 2004;75:123–133. doi: 10.1111/j.1467-8624.2004.00658.x. doi: 10.1111/j.1467-8624.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Russell MAH. The nicotine addiction trap: a 40-year sentence for four cigarettes. British Journal of Addiction. 1990;85:293–300. doi: 10.1111/j.1360-0443.1990.tb03085.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Resilience concepts and findings: implications for family therapy. Journal of Family Therapy. 1999;21:119–144. doi: 10.1111/1467-6427.00108. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Sroufe A. Developmental psychopathology: Concepts and challenges. Development and Psychopathology. 2000;12:265–296. doi: 10.1017/s0954579400003023. doi: doi:10.1017/S0954579400003023. [DOI] [PubMed] [Google Scholar]
- Satorra A. Scaled and adjusted restricted tests in multisample analysis of moment structures. In: Heijmails DDH, Pollock DSG, Satorra A, editors. Innovations in multivariate statistical analysis: A Festschrift for Heinz Neudecker. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 233–247. [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype greater than environmental effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Rao U. Epidemiology and etiology of adolescent smoking. Current Opinion in Pediatrics. 2005;17(5):607–612. doi: 10.1097/01.mop.0000176442.49743.31. [DOI] [PubMed] [Google Scholar]
- Schmitz S, Fulker DW, Mrazek DA. Problem behavior in early and middle childhood: An initial behavior genetic analysis. Journal of Child Psychology and Psychiatry. 1995;36(8):1443–1458. doi: 10.1111/j.1469-7610.1995.tb01674.x. doi: 10.1111/j.1469-7610.1995.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Scourfield J, Rice F, Thapar A, Harold GT, Martin N, McGuffin P. Depressive symptoms in children and adolescents: Changing aetiological influences with development. Journal of Child Psychology and Psychiatry. 2003;44(7):968–976. doi: 10.1111/1469-7610.00181. doi: 10.1111/1469-7610.00181. [DOI] [PubMed] [Google Scholar]
- Serbin LA, Karp J. The intergenerational transfer of psychosocial risk:Mediators of vulnerability and resilience. Annual Review of Psychology. 2004;55:333–363. doi: 10.1146/annurev.psych.54.101601.145228. doi: 10.1146/annurev.psych.54.101601.145228. [DOI] [PubMed] [Google Scholar]
- Shiner RL, Marmorstein NR. Family environments of adolescents with lifetime depression: Associations with maternal depression history. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37(11):1152–1160. doi: 10.1097/00004583-199811000-00014. doi: 10.1097/00004583-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Slomkowski C, Rende R, Lloyd-Richardson E, Niaura R. Sibling effects on smoking in adolescence: Evidence for social influence from a genetically informative design. Addiction. 2005;100:430–438. doi: 10.1111/j.1360-0443.2004.00965.x. doi: 10.1111/j.1360-0443.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IIIR (SCID) New York State Psychiatric Institute, Biometrics Research; New York: 1987. [Google Scholar]
- Sroufe LA, Rutter M. The domain of developmental psychopathology. Child Development. 1984;55:17–29. [PubMed] [Google Scholar]
- Stallings MC, Hewitt JK, Beresford T, Heath AC, Eaves LJ. A twin study of drinking and smoking onset and latencies from first use to regular use. Behavior Genetics. 1999;29(6):409–421. doi: 10.1023/a:1021622820644. doi: 10.1023/A:1021622820644. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine & Tobacco Research. 1999;1(1):51–57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. Retrieved from: http://ajp.psychiatryonline.org/index.dtl. [DOI] [PubMed] [Google Scholar]
- Thapar A, McGuffin P. A twin study of depressive symptoms in childhood. British Journal of Psychiatry. 1994;165(2):259–265. doi: 10.1192/bjp.165.2.259. doi: 10.1192/bjp.165.2.259. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Government Printing Office; Washington, D. C.: Health Consequences of Smoking Cessation: A Report of the Surgeon General. 1994
- Ustun T, Kessler RC. Global burden of depressive disorders: The issue of duration. British Journal of Psychiatry. 2002;181(3):181–183. doi: 10.1192/bjp.181.3.181. [DOI] [PubMed] [Google Scholar]
- Vogel JS, Hurford DP, Smith JV, Cole A. The relationship between depression and smoking in adolescents. Adolescence. 2003;38(149):57–74. [PubMed] [Google Scholar]
- Walden B, McGue M, Iacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. Journal of Abnormal Psychology. 2004;113(3):440–450. doi: 10.1037/0021-843X.113.3.440. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- Wang MQ, Fitzhugh EC, Turner L, Fu Q, Westerfield RC. Association of depressive symptoms and school adolescents' smoking: A cross-lagged analysis. Psychological Reports. 1996;79(1):127–130. doi: 10.2466/pr0.1996.79.1.127. [DOI] [PubMed] [Google Scholar]
- White VM, Hopper JL, Wearing AJ, Hill DJ. The role of genes in tobacco smoking during adolescence and young adulthood: a multivariate behaviour genetic investigation. Addiction. 2003;98:1087–1100. doi: 10.1046/j.1360-0443.2003.00427.x. doi: 10.1046/j.1360-0443.2003.00427.x. [DOI] [PubMed] [Google Scholar]