Abstract
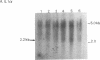
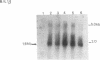
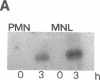
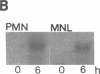
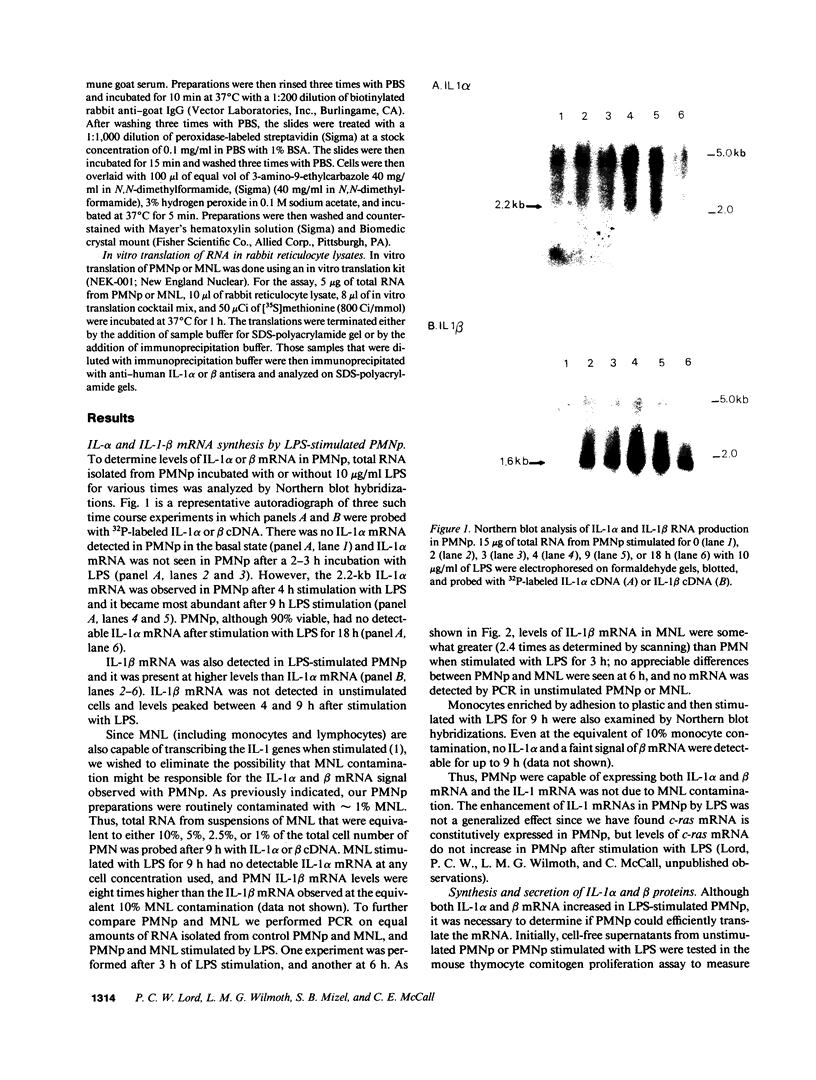
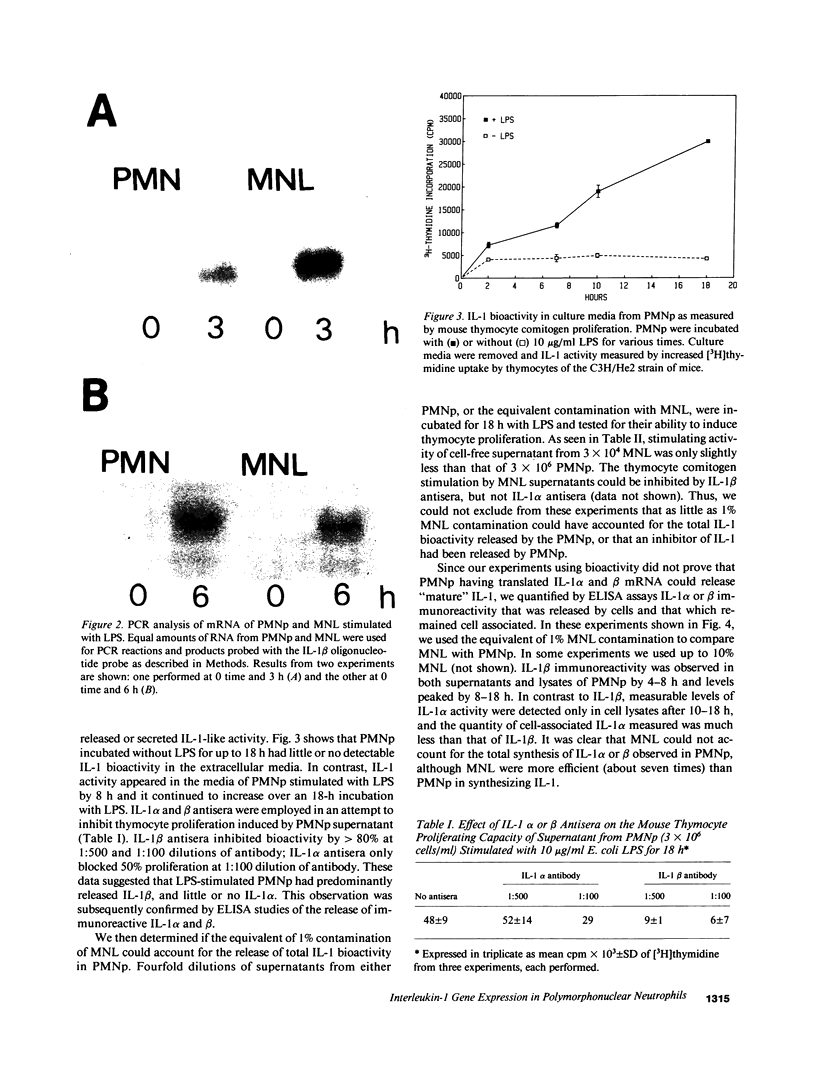
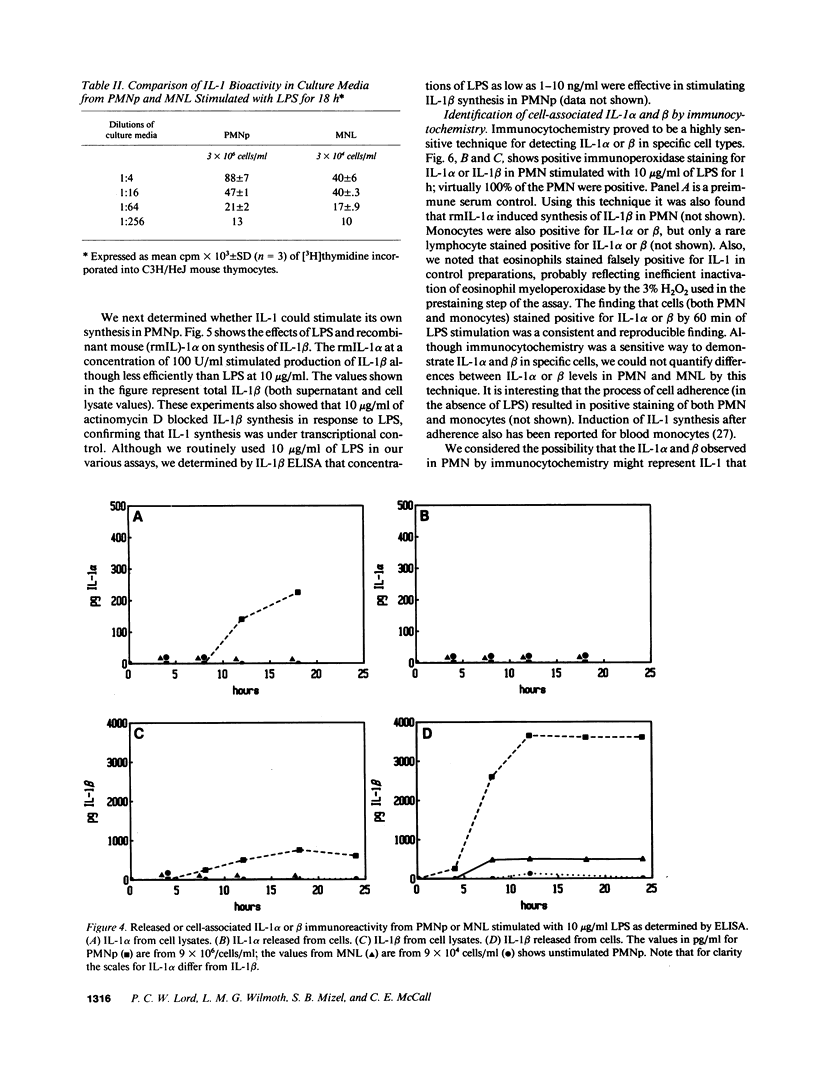
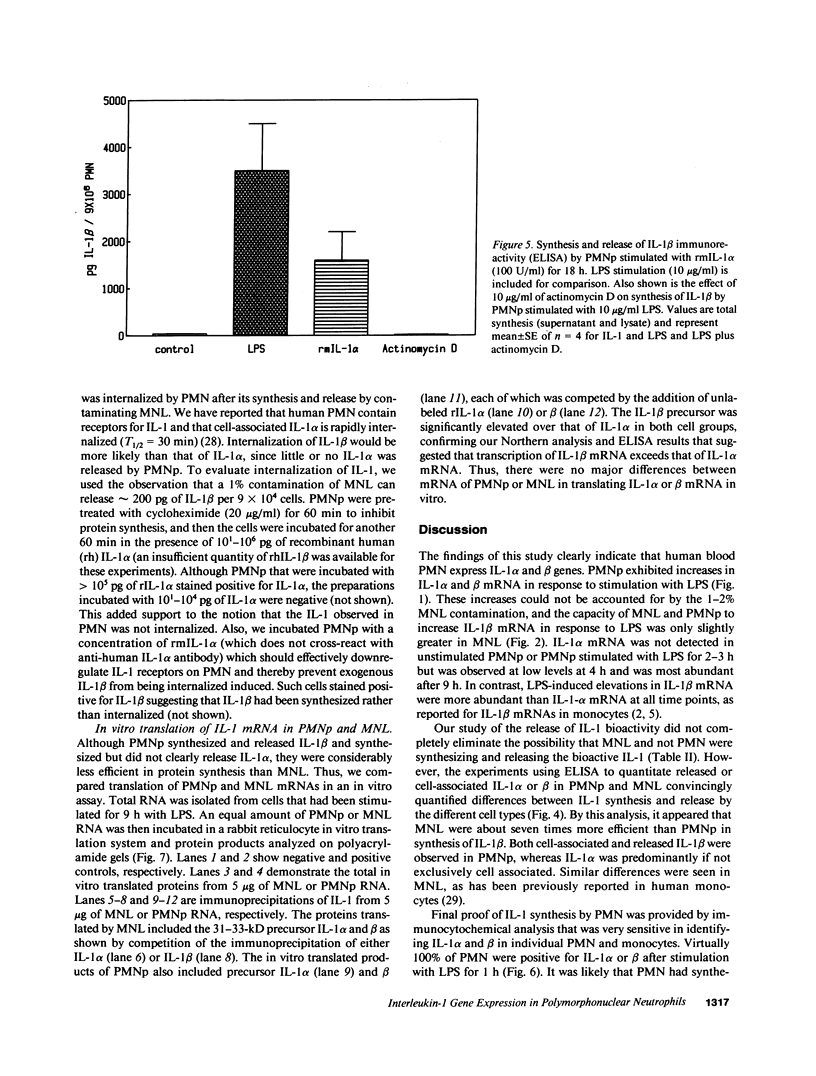
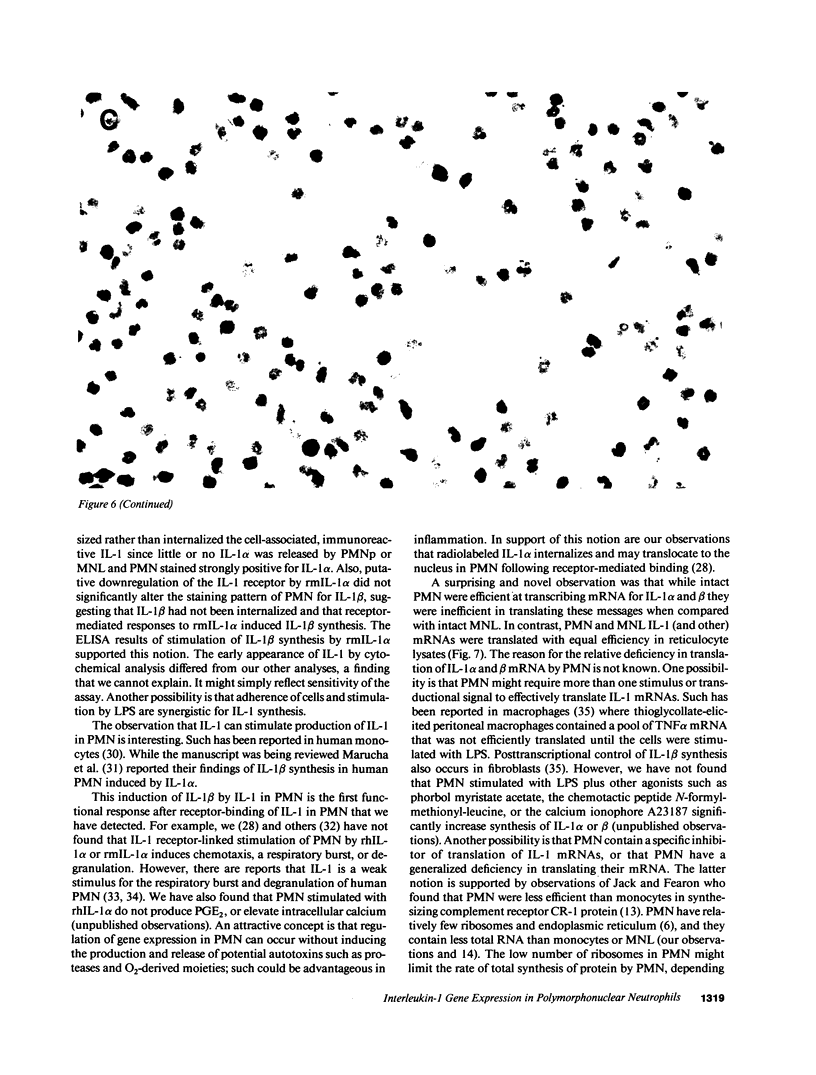
Expression of IL-1 alpha and beta genes was studied in human blood PMN with close monitoring of the effects of contaminating mononuclear leukocytes (MNL). We provide evidence that PMN both transcribe and translate IL-1 alpha and beta genes after stimulation with LPS or IL-1 alpha. A combination of mouse thymocyte comitogen proliferation assay, ELISA, and immunocytochemistry was required to establish that IL-1 alpha and beta synthesis observed in preparations of PMN could not be accounted for by the low level of contaminating MNL. Synthesis of IL-1 beta in PMN exceeded that of IL-1 alpha, but little or no IL-1 alpha was released by PMN. Although increases in IL-1 mRNA after stimulation of PMN and MNL with LPS were similar, PMN were less efficient than MNL in translating IL-1 mRNA. In contrast, PMN and MNL IL-1 alpha and beta mRNAs were translated with equal efficiency in rabbit reticulocyte lysates, suggesting that synthesis of IL-1 in PMN is subject to some form of translational control. We conclude that PMN stimulated with LPS efficiently transcribe but inefficiently translate IL-1 genes relative to MNL. IL-1 beta transcription and translation predominates over that of IL-1 alpha, and IL-1 beta is the predominant IL-1 protein released by PMN. IL-1 can induce its own synthesis in PMN.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Warner S. J., Webb A. C., Cannon J. G., Bernheim H. A., McAdam K. J., Rosenwasser L. J., LoPreste G., Mucci S. F., Dinarello C. A. Studies on the molecular nature of human interleukin 1. J Immunol. 1987 Mar 1;138(5):1447–1456. [PubMed] [Google Scholar]
- BENNETT I. L., Jr, BEESON P. B. Studies on the pathogenesis of fever. II. Characterization of fever-producing substances from polymorphonuclear leukocytes and from the fluid of sterile exudates. J Exp Med. 1953 Nov;98(5):493–508. doi: 10.1084/jem.98.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu A. D., Lang F., Belles-Isles M., Poubelle P. Protein biosynthetic activity of polymorphonuclear leukocytes in inflammatory arthropathies. Increased synthesis and release of fibronectin. J Rheumatol. 1987 Aug;14(4):656–661. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Remick D. G., Shmyr-Forsch C., Beals T. F., Kunkel S. L. Immunohistochemical demonstration of cytoplasmic and membrane-associated tumor necrosis factor in murine macrophages. Am J Pathol. 1988 Dec;133(3):564–572. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Eid N. S., Kravath R. E., Lanks K. W. Heat-shock protein synthesis by human polymorphonuclear cells. J Exp Med. 1987 May 1;165(5):1448–1452. doi: 10.1084/jem.165.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Reynolds M. M., Kotloff R. M., Kern J. A. Fibroblast interleukin 1 beta: synergistic stimulation by recombinant interleukin 1 and tumor necrosis factor and posttranscriptional regulation. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6171–6175. doi: 10.1073/pnas.86.16.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Chaplin D. D., Kiely J. M., Unanue E. R. Regulation of interleukin 1 gene expression by adherence and lipopolysaccharide. J Immunol. 1987 Jun 1;138(11):3799–3802. [PubMed] [Google Scholar]
- Georgilis K., Schaefer C., Dinarello C. A., Klempner M. S. Human recombinant interleukin 1 beta has no effect on intracellular calcium or on functional responses of human neutrophils. J Immunol. 1987 May 15;138(10):3403–3407. [PubMed] [Google Scholar]
- Goto F., Nakamura S., Goto K., Yoshinaga M. Production of a lymphocyte proliferation potentiating factor by purified polymorphonuclear leucocytes from mice and rabbits. Immunology. 1984 Dec;53(4):683–692. [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Vassalli J. D., Reich E. Secretion of plasminogen activator by human polymorphonuclear leukocytes. Modulation by glucocorticoids and other effectors. J Exp Med. 1977 Dec 1;146(6):1693–1706. doi: 10.1084/jem.146.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. H. Polymorphonuclear leukocyte elastase activity is increased by bacterial lipopolysaccharide: a response inhibited by glucocorticoids. Blood. 1984 Feb;63(2):421–426. [PubMed] [Google Scholar]
- Iribe H., Koga T., Kotani S., Kusumoto S., Shiba T. Stimulating effect of MDP and its adjuvant-active analogues on guinea pig fibroblasts for the production of thymocyte-activating factor. J Exp Med. 1983 Jun 1;157(6):2190–2195. doi: 10.1084/jem.157.6.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack R. M., Fearon D. T. Selective synthesis of mRNA and proteins by human peripheral blood neutrophils. J Immunol. 1988 Jun 15;140(12):4286–4293. [PubMed] [Google Scholar]
- La Fleur M., Beaulieu A. D., Kreis C., Poubelle P. Fibronectin gene expression in polymorphonuclear leukocytes. Accumulation of mRNA in inflammatory cells. J Biol Chem. 1987 Feb 15;262(5):2111–2115. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Meuer S. C., Blohm D., Mertelsmann R. H., Herrmann F. Granulocyte/macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988 Feb 1;140(3):837–839. [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Ziegler-Heitbrock H. W., Mertelsmann R., Herrmann F. Granulocyte-macrophage colony-stimulating factor induces cytokine secretion by human polymorphonuclear leukocytes. J Clin Invest. 1989 Apr;83(4):1308–1312. doi: 10.1172/JCI114016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Marucha P. T., Zeff R. A., Kreutzer D. L. Cytokine regulation of IL-1 beta gene expression in the human polymorphonuclear leukocyte. J Immunol. 1990 Nov 1;145(9):2932–2937. [PubMed] [Google Scholar]
- Matsushima K., Procopio A., Abe H., Scala G., Ortaldo J. R., Oppenheim J. J. Production of interleukin 1 activity by normal human peripheral blood B lymphocytes. J Immunol. 1985 Aug;135(2):1132–1136. [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Kume S. Potentiation of neutrophil function by recombinant DNA-produced interleukin 1a. J Leukoc Biol. 1987 Dec;42(6):621–627. doi: 10.1002/jlb.42.6.621. [DOI] [PubMed] [Google Scholar]
- Rhyne J. A., Mizel S. B., Taylor R. G., Chedid M., McCall C. E. Characterization of the human interleukin 1 receptor on human polymorphonuclear leukocytes. Clin Immunol Immunopathol. 1988 Sep;48(3):354–361. doi: 10.1016/0090-1229(88)90029-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Epps D. E., Justen J. M., Sam L. M., Wynalda M. A., Fitzpatrick F. A., Yein F. S. Human neutrophil activation with interleukin-1. A role for intracellular calcium and arachidonic acid lipoxygenation. Biochem Pharmacol. 1987 Nov 15;36(22):3851–3858. doi: 10.1016/0006-2952(87)90449-7. [DOI] [PubMed] [Google Scholar]
- Tiku K., Tiku M. L., Liu S., Skosey J. L. Normal human neutrophils are a source of a specific interleukin 1 inhibitor. J Immunol. 1986 May 15;136(10):3686–3692. [PubMed] [Google Scholar]
- Tiku K., Tiku M. L., Skosey J. L. Interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1986 May 15;136(10):3677–3685. [PubMed] [Google Scholar]