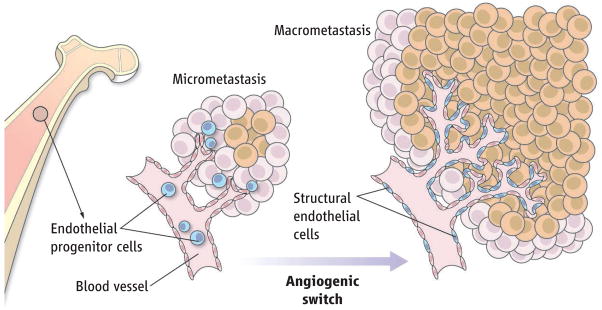
The rapid formation of blood vessels, a process known as the angiogenic switch, is required for progression of dormant or micrometastatic tumors to macrometastatic invasive tumors. New blood vessels may either sprout from preexisting mature ones or form de novo by recruiting circulating endothelial progenitor cells derived from the bone marrow (1–3). Although these progenitors can incorporate into human tumors and transplanted tissue (4, 5), they do so in small numbers, raising doubt about their physiological contribution to neo-angiogenic processes. On page 195 of this issue, Gao et al. cast any doubt aside by showing that notwithstanding their low numbers, recruitment of these endothelial progenitor cells is pivotal for the progression of avascular micrometastatic tumors to lethal macrometastatic ones (6).
Why has there been so much confusion? It may be that only certain types of tumors (7), producing distinct proangiogenic chemokines (1, 8–11), demand bone marrow-derived endothelial progenitor cells to initiate (12) and possibly maintain nascent vessels within specific primary and metastatic lesions (13). Indeed, the extent of progenitor cell incorporation is maximal in relapsing tumors (9) and in certain aggressive carcinomas (14). But the main reason for inconsistent results is probably the phenotypic similarities between hematopoietic cells and true endothelial progenitor cells (those with bone marrow repopulating potential) (2, 3), and the technical hurdles involved in distinguishing and localizing them in the lumen of functional blood vessels. In some mouse studies, the majority of the bone marrow–derived cells in tumors was not endothelial, but of hematopoietic lineage, and were positioned perivascularly rather than incorporated into the vessel lumen (15, 16). However, in these reports, it was unclear whether true endothelial progenitors were transplanted into recipient bone marrow.
To circumvent these problems, Gao et al. tracked endothelial progenitor cells by labeling with green fluorescent protein and by assessing the expression of vascular-specific molecular markers, including the cell adhesion molecules VE-cadherin and CD31, VEGFR2 (a receptor for vascular endothelial growth factor), and Id1, a transcription factor that promotes angiogenesis. Using mouse models of lung (metastatic mouse Lewis Lung carcinoma) and breast (spontaneous MMTV-PyMT) cancer models, the authors demonstrate that about 12% of the endothelial cells within macrometastases were derived from bone marrow. Remarkably, when the expression of Id1 was reduced in these small number of progenitors, their mobilization from bone marrow decreased by 96%, angiogenesis was blocked, tumor formation decreased, and the animal’s survival improved.
The inhibition of Id1 did not affect tumor cell dissemination or the initial colonization of organs by malignant cells, but rather, shut off the mobilization and recruitment of particular endothelial progenitor cells (those expressing Id1, VE-cadherin, and low amounts of CD31). These specific progenitor cells infiltrated micrometastatic lesions and produced proangiogenic growth factors before the initiation of macrometastases. Ultimately, subsets of these progenitor cells differentiated (to express VE-cadherinand increased amounts of CD31) and integrated into the lumen of tumor neovessels. These data suggest that bone marrow–derived endothelial progenitors are unique in providing both instructive (paracrine) and structural (vessel incorporation) roles to promote tumor macrometastasis (see the figure). They also extend previous studies demonstrating that hematopoietic progenitor cells initiate metastatic colonization (10), whereas endothelial progenitor cells promote progression of the metastatic lesion.
Figure. Few but mighty.
Bone marrow–derived endothelial progenitor cells contribute instructively to micrometastasis and structurally to the emergence of macrometastatic tumor nodules.
How can bone marrow–derived endothelial progenitor cells be distinguished from preexisting tumor endothelium or hematopoietic cells (17)? A phenotypic definition by specific molecules (including the presence of Id1, VE-cadherin, VEGFR2, CD31, CD13, and the growth factor receptor c-Kit, but absence of the cell adhesion molecule CD11b and the phosphatase CD45) have been used previously to mark endothelial progenitor cells incorporated in the lumen of tumor vasculature (12). However, some of these markers are expressed by subsets of hematopoietic lineages, and therefore phenotyping must be carefully performed (2, 3). Another complicating factor is the potential contribution of recently discovered cells similar to endothelial progenitor cells resident within organs other than the bone marrow (18). Lack of functional standardized bioassays to quantify the scarce populations of true endothelial progenitor cells is a major hurdle in assessing whether bone marrow transplantations performed in different laboratories (2, 3, 15, 16) results in engraftment of sufficient numbers of progenitor cells to interrogate their contribution to tumor neo-angiogenesis. Therefore, establishing standardized in vivo functional assays to detect and quantify repopulating progenitors is urgently needed.
Major issues still need to be resolved. It is unclear why endothelial progenitor cells are recruited only by certain tumors. And the role of proangiogenic factors elaborated by (or specific to) endothelial progenitor cells needs further investigation. Whether their continuous recruitment contributes to maintaining stabilized tumor vessels also has yet to be determined.
Contributor Information
Shahin Rafii, Email: srafii@med.cornell.edu.
David Lyden, Email: dcl2001@med.cornell.edu.
References
- 1.Lyden D, et al. Nat Med. 2001;7:1194. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 2.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Nat Rev Cancer. 2002;2:826. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 3.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. Nat Rev Cancer. 2006;6:835. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 4.Peters BA, et al. Nat Med. 2005;11:261. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 5.Minami E, Laflamme MA, Saffitz JE, Murry CE. Circulation. 2005;112:2951. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 6.Gao D, et al. Science. 2008;319:195. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 7.Ruzinova MB, et al. Cancer Cell. 2003;4:277. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 8.Shaked Y, et al. Cancer Cell. 2005;7:101. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Shaked Y, et al. Science. 2006;313:1785. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan RN, et al. Nature. 2005;438:820. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petit I, Jin D, Rafii S. Trends Immunol. 2007;28:299. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan DJ, et al. Genes Dev. 2007;21:1546. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duda DG, et al. Blood. 2005;107:2774. [Google Scholar]
- 14.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Proc Natl Acad Sci USA. 2005;102:18111. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Palma M, et al. Cancer Cell. 2005;8:211. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Gothert JR, et al. Blood. 2004;104:1769. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 17.Yoder MC, et al. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aicher A, et al. Circ Res. 2007;100:581. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]