Abstract
Background
Our previous studies showed that high levels of soluble CD25 (sCD25) in the serum of patients with hepatocellular carcinoma (HCC) correlated with blunted effector T cells (Teff) responses, tumor burden and poor survival. Understanding the interactions between Teff, CD4+CD25+ regulatory T cells (Treg) and soluble factors can identify novel therapeutic targets.
Aims
In this study, we characterize the mechanisms by which HCC serum and sCD25 mediate suppression of Teff. We also evaluate the effect of sCD25 on the suppression assays with normal healthy control cells (NHC) at a 1:1 Treg to Teff cell ratio to determine if sCD25 has any impact on Treg suppression.
Results
HCC serum and sCD25 suppressed Teff proliferation and down regulated CD25 expression on HCC Teffs in a dose dependent fashion with sCD25 doses above 3,000 pg/ml. Tregs from HCC and cirrhosis patients suppressed proliferation of target CD4+CD25− Teff in serum free medium (SFM). HCC Tregs showed a higher degree of suppression than cirrhosis derived Tregs. In contrast, Tregs from NHC did not suppress target Teff in SFM. However, isolated Tregs from all three study subjects (HCC, cirrhosis, NHC) suppressed CD4+CD25− Teff in serum conditions or in the presence of sCD25 in the range 6,000–12,000 pg/mL.
Conclusion
Down regulation of CD25 cell surface expression on Teffs is part of the overall suppressive mechanism of sCD25 and HCC serum on Teff responses. The observed sCD25 and HCC serum mediated suppression is further influenced via novel immune-inhibitory interaction between CD4+CD25+ Tregs and sCD25.
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer related death in the world and in developed countries it is expected to continue to increase due to the epidemic of chronic hepatitis C virus (HCV) infection [1]. Most patients present with advanced disease with limited treatment options that are palliative. As such, novel therapies are urgently needed in HCC. During the development of HCC the tumor microenvironment has been shown to play a major role in promoting progression via a variety of immunological mechanisms. Our present understanding is limited but shows that HCC is associated with blunted immunity and that it involves a complex interaction between effectors T cells (Teff), CD4+CD25+ regulatory cells (Tregs), suppressive soluble factors such as soluble CD25 (sCD25) and tolerogenic dendritic cells in the tumor microenvironment [2–4]. In the initial stage of HCC development tumor related antigens engage naïve CD4+ T cells during the tumor interaction/elimination phase of the adaptive immune response. Naïve CD4+ T cell activation is a critical step in the development of an adaptive immunological response and is also essential to effectively activate and optimize CD8+ T cell function [5, 6]. The importance of naïve CD4+ T cell activation is reflected in studies showing that a higher CD4+:CD8 T-cell ratio is associated with improved clinical outcome in HCC [7]. Previous studies also showed that Tregs infiltrating HCC tumors were an indicator of poor prognosis [6]. Tregs mediate suppression by a plethora of mechanisms [8–14] including cell – cell contact with CD4+CD25− Teff cell population, but not apoptosis induction [15].
Central to an effective immune response is the activation of naïve CD4+ T cells which requires IL-2 binding to its high affinity IL-2 receptor (IL-2R) for optimal signaling. The high affinity form of the IL-2R consists of three chains that include the alpha (CD25), beta (CD122) and gamma (CD132) chains [16]. Both beta and gamma chains are constitutively expressed on lymphocytes and have long cytoplasmic domains that activate the cytoplasmic proteins of the JAK-STAT pathway following the binding of IL-2 to the trimeric receptor. The alpha chain is inducible and high levels of CD25 expression on CD4 T cells are seen after IL-2 activation through the T cell receptor. The alpha chain lacks signaling function due to its short cytoplasmic domain. The main function of CD25 is to bind IL2 and promote optimal IL-2 signaling through the high affinity IL-2R upon its association with the beta and gamma chains. Intracellular signaling begins with recruitment of JAK which then leads to activation of transcription factors such as STAT-5 resulting in T cell proliferation. A set threshold of IL-2R must be activated in order for the T cell to commit to cytokinesis and subsequent clonal expansion [2].
We have previously shown that serum from patients with HCC impairs Teff responses and that high serum levels of sCD25 is a major player in diminishing Teff responses [17]. In this study, we hypothesize that HCC serum mediates suppression of CD4+CD25− Teffs by decreasing the level of CD25 expression in response to mitogenic stimulation thereby preventing formation of the high affinity IL-2R, IL-2 signaling and Teff activation. We present a series of in vitro experiments showing the phenotype and proliferative responses of target CD4+CD25− Teff in response to HCC serum and sCD25. We also characterize the impact of sCD25 on suppression assays to determine its effect on Treg function. Here we demonstrate that soluble factors in HCC serum such as sCD25 promote CD4+CD25− T cell suppression by decreasing CD25 expression which blunts the IL2 pathway. We also show that Treg mediated suppression is enhanced with sCD25 which represents a novel immuno-inhibitory mechanism.
Materials and Methods
Isolation of human CD4+CD25− Teff and Tregs
Peripheral blood mononuclear cells (PBMC), CD4+CD25− Teff and CD4+CD25+ T cells were isolated from patients with HCC and age/sex matched disease controls with HCV related HCC (DC) and normal healthy controls (NHC) as described previously [18]. Briefly, CD4+T cells were isolated by negative selection using magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer protocol. The total CD4 T cell fraction was incubated with anti CD25 beads (Miltenyi Biotec) and the CD4+CD25− fraction was isolated by negative selection and the CD4+CD25+ cells were recovered by positive selection from the column. The purity of the CD4+CD25+ T cells were >85% by flow cytometry. To further evaluate the purity of the isolated CD4+CD25− and CD4+CD25+ cells used in our assays, we also performed staining for the APC marker CD80. We found that in both cell populations isolated with magnetic beads the percentage of CD80+ cells was <2%.
Cell Cultures and Reagents
CD4+CD25− Teff and Tregs were cultured under the following conditions: with serum free medium (SFM; CTL media, Shaker Heights, Ohio), autologous serum, or allogenic serum with no mitogen (negative control), with 5 ug/ml of lectin from Phaseolus vulgaris PHA B (Sigma, St Louis, MO, USA) (positive control) or supplemented with various doses of recombinant sCD25 (Bender Systems, Burlingame, CA). Cells were incubated in 5% CO2 at 37 C for 72 hours. The doses of recombinant sCD25 used in this study included: 3000 pg/ml which represents the borderline value for healthy controls; 6000 pg/ml which represent the higher point in our previous ROC range; and 12000pg/ml which represent the average sCD25 content in the serum from HCC patients with multifocal (intermediate stage) HCC [17].
Suppression Assay
7.5* 10 4 CD4+CD25− Teff cells and same number of Tregs cells were seeded into 96-well plates in triplicate, total volume 200 μL and cultured for 72 h with or without PHA in 5% CO2 and 37 C. After 72 hrs, [3H]-thymidine (Amersham Biosciences) was added (1□Ci/well) for 18 hrs, cells were maintained at 5% CO2 and 37 C. Next day, [3H]-thymidine incorporation was measured on a □-scintillation counter. Results were expressed as the mean counts per min with standard error (mean cpm + SE).
Flow cytometry
CD4+CD25− Teffs were cultured in SFM (CTL media, Shaker Heights, OH, USA) with or without PHA and increasing concentrations of recombinant sCD25(3000,6000 and 12000pg/ml), autologous/allogenic serum and supplemented with IL2(500 units/ml). After a 72 hrs of culture, the cells were stained using the antibodies: CD4 anti human PE, CD25 anti human PE-Cy5-A (BD Biosciences Pharmingen, SanJose, California, USA). Antihuman LAP TGFB I –PerCP were purchased from R&D (Mineapolis,MN,USA). Cells were detected using flow cytometryperformed on a flow cytometer LSR-II (Becton Dickinson BD, Franklin Lakes, NJ USA). Ten thousand-gated events were acquired for each condition, and data was analyzed using FACSDiva software (BD).
Statistical Analysis
Data are expressed as mean + SE. Statistical analyses were performed using the Mann-Whitney test using the SPSS 17.0 program (Chicago, IL). All p values are 2 tailed and statistically significant when p<0.05 (*p<0.05 and **p<0.01).
Results
Tregs from HCC Patients Retain Suppression in SFM and Serum
Our previous studies showed a dose dependent effect of HCC serum on suppression of Teffs. In order to minimize these suppressive effects during our assays a dose response curve between serum concentration and Teff proliferation was performed for each group (HCC, NHC and DC). This curve confirmed 0.5% serum concentration as the optimal value for cell culture with minimal suppression and maximal support (data not show).
Treg mediated suppression of target CD4+CD25− Teff in a suppression assay [19, 20] is presently the best way to confirm that the CD4+CD25+ T cell subset represents the suppressive Treg population. Both CD4+CD25+ T cells isolated from NHC and HCC subjects were able to suppress target CD4+CD25− Teff proliferation in standard suppression assays with serum containing medium, serving as proof that the isolated CD4+CD25+ cells represents the Treg population.
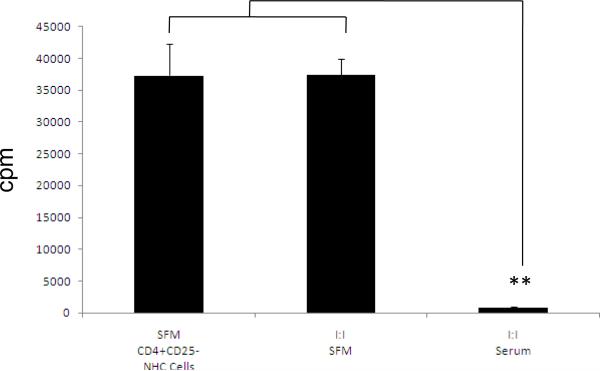
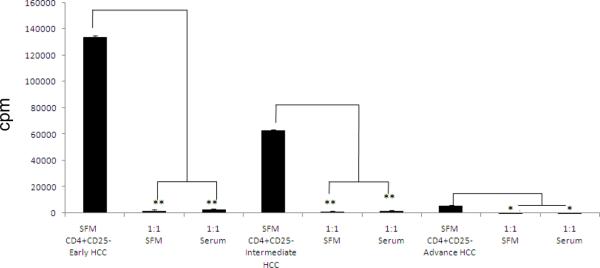
In order to determine if the presence or absence of serum in the culture medium influences Tregs, a series of suppression assays were performed using Tregs and CD4+CD25− Teffs isolated from NHC (fig 1). CD4+CD25− Teffs from NHC yielded a baseline, mean proliferative response of 37,330 + 4,882 cpm (mean + SE). Tregs from NHC failed to suppress Teff proliferation in 1:1 co-cultures in SFM (37,236 + 2466 cpm) but were able to suppress target Teffs in the presence of autologous serum (486 + 254). In contrast, Tregs isolated from HCC patients suppressed Teff proliferation in 1: 1 cultures irrespective of tumor stage (early, intermediate, and advanced stage) in SFM (early 2026 + 47 cpm; intermediate 1366 + 27 cpm; advanced 126 + 148 cpm) and in serum (early 2940.5 + 216.5 cpm; intermedate 2023.5 + 405.5 cpm, advanced 140 + 111 cpm) (fig 2).
Figure 1. In SFM conditions NHC CD4+CD25+ can not suppress NHC CD4+CD25− cells proliferation.
Results are shown as mean cell proliferation ± SE (cpm). NHC CD4+CD25− cells alone (37329.5 ± 4881.5; n = 3). *p<0.05, **p< 0.01, next, CD4+CD25− cells at a cell ratio I:I with NHC CD4+CD25+ cells in SFM conditions, 37235.6 ± 2465.5 no suppression, and finally, CD4+CD25− cells at a cell ratio I:I with NHC CD4+CD25+ cells in autologous serum containing conditions 486 ±254,suppression is observed.
Figure 2. HCC CD4+CD25+ cells can suppress proliferation of HCC CD4+CD25− cells stimulated with PHA in SFM conditions.
HCC CD4+CD25− cells from an early stage HCC donor cultured alone and in SFM conditions (134372 ± 911 cpm) and with HCC CD4+CD25− cells 1:1 in SFM (2026 ± 478) and autologous HCC serum conditions (2940.5 ± 216.5). HCC CD4+CD25− cells from an Intermediate stage HCC donor, cultured in SFM conditions (62927.5 ± 405.5 cpm) and with HCC CD4+CD25+ cells 1:1 in SFM (1366 ± 27) and autologous HCC serum (2023.5 ± 405.5). HCC CD4+CD25− cells from an advance stage HCC donor, SFM conditions (5713.5 ± 365.5 cpm) and with HCC CD4+CD25+ cells 1:1 in SFM (126 ± 148 and autologous serum (140 ± 111). *p<0.05, **p< 0.01.
Tregs from HCC Patients are More Suppressive than Tregs from Controls
To further confirm that HCC derived Tregs are more suppressive than controls, cell titration experiments at different Treg:Teff ratios were performed. We broadened our cell ratios in the NHC suppression assays to include 1:1, 1:10, 1:20 and 1:30 (fig 3). The introduction of Tregs in the NHC co-cultures failed to suppress the baseline Teff proliferation (18330.3 + 732.2 cpm) at all the various Treg:Teff ratios: 1:1 Treg/Teff ratio 17929.48 + 1663 (P= 0.63); 1:10 Treg/Teff ratio 17651.4 + 7 1972.9 cpm (p= 0.08); 1:20 Treg/Teff ratio 18373.3 + 1481.3 (P= 0.98); and 1:30 Treg/Teff ratio 19590.9+ 1597.4 cpm (P= 0.51). In contrast, the Tregs in the suppression co-cultures from HCC patients suppressed Teff proliferation (baseline 3020.5 + 115.4 cpm) in a graded fashion during all the co-cultures: 1:1 Treg/Teff ratio 191.5 + 25.5 (P= 0.00016); 1:10 Treg/Teff ratio 501.5 + 22.5 cpm (P= 0.0002), 1:20 Treg/Teff ratio 1147.5 + 67.5 (P= 0.0002); and the 1:30 Treg/Teff ratio 1990.5 + 194.5 cpm (P= 0.045).
Figure 3. HCC CD4+CD25+ cells are more suppressive that NHC CD4+CD25+ cells in serum free media conditions at various Treg/Teff ratios (1:1, 1:10, 1:20; 1:30).
NHC derived CD4+CD25+ Tregs fail to suppress the baseline proliferative response of target CD4+CD25− Teffs (18330.3 ± 732.2 cpm) at any of the Treg/Teff ratios in the co-cultures: 1:1 ratio 17929.48 ± 1663 (P= 0.63), 1:10 ratio 17651.4 ± 7 1972.9 cpm (P= 0.08), 1:20 ratio 18373.3 ± 1481.3 (P= 0.98) and 1:30 ratio 19590.9± 1597.4 cpm (P= 0.51). In contrast, HCC derived CD4+CD25+ Tregs suppress CD4+CD25− Teff at all the four Treg/Teff ratios examined in a graded fashion: baseline 3020.5 ± 115.4 cpm, 1:1 ratio 191.5 ± 25.5 (P= 0.00016); 1:10 ratio 501.5 ± 22.5 cpm (P= 0.0002); 1:20 ratio 1147.5 ± 67.5 (P= 0.0002); and 1:30 ratio 1990.5 ± 194.5 cpm (P= 0.045)
To account for the influence of cirrhosis on Tregs which co-exists in the patients with HCC, a series of co-cultures with Tregs and CD4+CD25− Teffs at a 1:1 ratio using cells from patients with HCV related cirrhosis (DC) were performed (fig 4). In general, Tregs from DC suppressed the baseline proliferative response of target Teff proliferation from 63841.5 + 11309.9 cpm to 11514.1 + 4665.6 cpm at a 1:1 ratio in SFM conditions and 14603.3 + 7110.1 cpm at a 1:1 ratio in autologous serum containing conditions. Comparison with the HCC 1:1 co-cultures (SFM: 1176.333 + 371.5 cpm, p= 0.075; serum containing medium: 1696.5 + 537.5 cpm, p=0.083) showed a statistical trend suggesting that HCC derived Tregs were more suppressive than cirrhosis derived Tregs (figure 4).
Figure 4. HCC and DC CD4+CD25+ cells can suppress proliferation of CD4+CD25− cells stimulated with PHA in SFM.
Results are shown as mean cell proliferation ± SE (cpm). DC (black bar) and HCC (grey bar) CD4+CD25− cells alone (63841.5 ± 11309.96 and 67457.83 ± 23651.3; n = 3). p<=0.225, next, CD4+CD25− cells at a cell ratio I:I with CD4+CD25+ cells in SFM conditions, DC coculture (black bar) 11514.14 ± 4665.6, HCC coculture 1176.333 ± 371.5; n = 3 both cocultures show suppression and are not statistically different, p= 0.075 and finally, CD4+CD25− cells at a cell ratio I:I with CD4+CD25+ cells in autologous serum containing conditions DC coculture 14603.3 ± 7110.1; n = 3 and HCC coculture 1696.5 ± 537.5, both cocultures show suppression and are not statistically different, p= 0.083 although a statistical trend is observed.
Recombinant sCD25 Enhances Treg Mediated Suppression of Teff in SFM
Previously we investigated the effects of increasing concentrations of recombinant sCD25 on CD4+CD25− Teff proliferative responses when stimulated with PHA [17]. We observed that NHC Teffs were suppressed by higher doses of sCD25 (above 60000 pg/ml) when added to SFM cultures. Here, we explored the effect of increasing concentrations of sCD25 on NHC CD4+CD25− Teff proliferation in the presence of Tregs during in vitro suppression assays in SFM conditions. Three concentrations of sCD25 (3000, 6000 and 12000 pg/ml) were added to 1:1 Teff to Treg cocultures stimulated with PHA to determine its effect on Treg mediated suppression. These three concentrations were chosen because they represented levels typically observed in normal healthy controls (3000 pg/ml), the cut-off value between controls and patients with HCC (6000pg/ml) and levels in the serum of patients with intermediate HCC from our previous studies [17]. The baseline mean proliferative response by [3H]-thymidine incorporation of CD4+CD25− Teffs from NHC was 37329.5 + 4881.5 cpm (fig 5A). Physiologic (low) doses of sCD25 (3,000 pg/ml) to 1:1 Teff to Treg co-cultures did not reduce the mean proliferative response of the Teffs (32407.5 + 1689.5) (fig 4). However, introduction of the intermediate and higher dose of sCD25 to the 1:1 co-cultures led to significant reductions in the baseline mean CD4+CD25− Teff proliferative response (37329.5 + 4881.5 cpm) with 6,000 pg/ml of sCD25 (15555 + 1900 cpm; p<0.01) and with 12,000 pg/ml of sCD25 (967.5 + 255.5 cpm; p<0.01).
Figure 5. CD4+CD25− cells from NHC are suppressed in SFM conditions when recombinant sCD25 is added to the medium.
(A)Suppression is dose dependent and starts to be significant at 6000 pg/ml. NHC CD4+CD25− cells alone (37329.5 ± 4881.5 cpm; n = 3 **p< 0.01), CD4+CD25− cells at a cell ratio I:I with NHC CD4+CD25+ cells in SFM conditions (35383.5 ± 199.5), CD4+CD25− cells at a cell ratio I:I with NHC CD4+CD25+ cells in SFM conditions plus 3000 pg/ml of sCD25 (32407.5 ± 1689.5), CD4+CD25− cells at a cell ratio I:I with NHC CD4+CD25+ cells in SFM conditions plus 6000 pg/ml of sCD25 (15555 ± 1900) and CD4+CD25− cells at a cell ratio I:I with NHC CD4+CD25+ cells in SFM conditions plus 12000 pg/ml of sCD25 (967.5 ± 255.5). (B) Enhanced Suppression of CD4+CD25+ Tregs from NHC in SFM is Specific for sCD25. To evaluate if the effect of sCD25 (alpha chain) in the NHC Tregs/Teffs co-cultures at a 1:1 ratio in serum free conditions is specific for sCD25, 6000 pg/ml of sCD25, beta receptor of CD25 and alpha receptor of IL-6 were added to 1:1 suppression assays. In the absence of soluble receptors, Tregs fail to suppress Teffs under SFM conditions: 18330.3 + 732.2 cpm for CD4+CD25− Teff cells alone; and 17929.5 + 1663 cpm when Treg/Teff cocultured at 1:1 ration, n=4, P= 0.61. While the Tregs in the 1:1 co-cultures with sCD25 showed suppression (393.8 + 169.1 cpm, P<0.001) of the baseline Teff proliferative response (18330.3 + 732.2 cpm), the Tregs in the 1:1 co-cultures containing the beta CD25 receptor (17527.5 + 962.3 cpm, P= 0.53) and the soluble IL-6 alpha receptor (20622.9 + 710.8, P= 0.03) at a similar dose of 6000 pg/ml did not show suppression.
Enhanced Suppression of Tregs from NHC in SFM is Specific for sCD25
In order to confirm that the observed sCD25 enhancement of Treg suppression is specific for sCD25, a series of Treg/Teff co-cultures were performed with other soluble receptors (fig 5B). While the Tregs in the 1:1 co-cultures with sCD25 showed suppression (393.8 + 169.1 cpm, P<0.001) of the baseline Teff proliferative response (18330.3 + 732.2 cpm), the Tregs in the 1:1 co-cultures containing the beta CD25 receptor (17527.5 + 962.3 cpm, P= 0.53) and the soluble IL-6 alpha receptor (20622.9 + 710.8, P= 0.03) at a similar dose of 6000 pg/ml did not show suppression. Interestingly, the addition of sIL-6 did lead to enhanced Teff proliferation. These results support our finding that the sCD25 enhancement of Treg suppression is specific to the presence of sCD25 in the Treg/Teff co-cultures.
Recombinant sCD25 and HCC Serum Decreases Teff CD25 Surface Expression
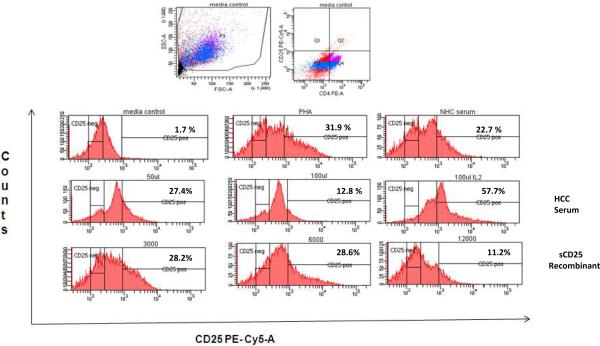
In our prior work, HCC serum and recombinant sCD25 suppressed Teff IFN-γ production, ATP production and cell proliferation which appeared to be related to impairment in the IL2 signaling pathway [17]. In order to determine if HCC serum and sCD25 negatively influenced formation of the high affinity IL-2R and thus lead to impairment in IL-2 signaling, we measured the levels of CD25 surface expression on CD4+CD25− Teff from HCC in the presence of HCC serum or increasing doses of recombinant sCD25. In general, HCC serum and increasing sCD25 concentrations (3000, 6000 and 12000 pg/ml) decreases induced CD25 surface expression on CD4+CD25− Teff from HCC subjects in a dose dependent manner (figure 6). In the sCD25 supplemented cultures, the decrease in CD25 cell surface expression began with the 6000 pg/ml concentration which was the same dose at which we observed changes in cellproliferation during the suppression assays. The addition of IL2 (500 U/ml) to the cultures reversed the observed suppression of CD25 surface expression despite the presence of suppressive doses of HCC serum and sCD25.
Figure 6. sCD25 and HCC serum decreases induced CD25 expression on HCC CD4+CD25− cells.
HCC CD4+CD25− cells were cultured for 3 days after stimulation with PHA and supplementation with recombinant sCD25 and HCC serum, later, cells were stained for CD25 and CD4 and analyzed by flow cytometry. The percentage of CD25 positive cells from the total analyzed is shown. Figures are representative of three separate experiments (three separate donors). First row left, media (non PHA), next PHA only, and NHC serum. Second row left, HCC serum 5%, HCC serum 10% and, HCC serum plus IL2 (500 units per ml). Third row left: sCD25 dose 1 (3000 pg/ml), dose 2 (6000 pg/ml) and dose 3 (12000 pg/ml).
Tregs from HCC Patients Express more TGFβ1 than Tregs from NHC and DC controls
The baseline cell surface expression of TGFβ1 on CD4+CD25+ cells were evaluated by flow cytometry using PBMCs from controls and patients with advanced HCC. The gating strategy and percentage of TGFβ1 positive CD4+CD25+ cells for controls and HCC patients are presented in figure 7. Cell surface staining of Tregs for TGFβ1 showed a 2 to 4 fold higher level of staining in patients with advanced HCC (22.3%, 21.2%, and 24.4%) than controls (6.4% in NHC and 10.3% in DC).
Figure 7. Tregs from HCC Patients Express more TGFβ1 than Tregs from NHC and DC controls.
The baseline cell surface expression of TGFβ1 on CD4+CD25+ cells were evaluated by flow cytometry using PBMCs from controls and patients with advanced HCC. The gating strategy and percentage of TGF β1 positive CD4+CD25+ cells for controls and HCC patients are shown. Cell surface staining of Tregs for TGFβ1 showed a 2 to 4 fold higher level of staining in patients with advanced HCC (22.3%, 21.2%, and 24.4%) than controls (6.4% in NHC and 10.3% in DC).
Discussion
A number of studies have demonstrated a critical role for Tregs and Teff anergy in the development of HCC [7, 18, 21, 22]. In this study, we demonstrate that the function of Tregs derived from NHC and HCC depends on the medium conditions. HCC derived Tregs retain their suppressive function regardless of presence or absence of serum in culture while NHC Tregs require serum for suppression. We also demonstrate that the soluble factor sCD25 restores the suppressive function of Tregs derived from NHC in the absence of serum. Our evaluation to determine why T cell activation is blunted by soluble factors in HCC serum shows that sCD25 decreases CD25 up regulation on naïve CD4+CD25− Teff thereby impairing formation of the high affinity IL-2R and optimal IL-2 signaling essential for CD4 T cell activation. While other mechanisms are likely at play, the enhancement of Treg suppressor function and induction of Teff anergy by sCD25 decreasing CD25 surface expression in a dose related fashion are novel findings. There is a paucity of data regarding mechanisms that enhance the suppressor function of Tregs and blunt Teffs due to our limited knowledge of regulatory pathways. Targeting of sCD25 with the aim of manipulating sCD25 mediated immuno-inhibitory properties may hold therapeutic promise by enhancing anti-HCC immunity.
The finding that HCC derived Tregs suppress target Teffs in the absence of serum, in contrast to NHC Tregs suggest that HCC derived Tregs are more inherently suppressive than NHC Tregs. Prior studies have shown that Tregs display a preactivated state before culture that makes them functionally serum independent. As such, HCC derived Tregs compared to NHC derived Tregs may have a higher state of preactivation and this may partially explain our finding of differential suppressor function but additional studies are needed [23]. The requirement of NHC derived Tregs for serum to exert suppression on target Teffs may reflect their lower level of preactivation and the need for co-stimulatory signals. While some studies have shown that Tregs are able to suppress in serum free conditions [28], others also show a reduced capacity of Tregs to suppress proliferation under serum free conditions and full recovery of Treg suppression with the addition of serum [24]. All these variations in studies highlight how results of suppression assays may have different outcomes with modifications in the in vitro environment such as different serum free media, presence/absence of serum, and type of polyclonal stimulants used in culures. Another important concern is if the inability of Tregsto suppress is related to poor cellular survival. In a recent study, the lack of NHC Tregsuppression in serum free conditions was not the result of low Treg cellular survivalsince they retained their ability to suppress, suggesting that the requirement of co-stimulatory signals is at play [24]. The suppressive function of Treg has also been reported to be influenced by the presence of TGFβ1 that can exist on their cell surface and allow suppression of target Teffs [24]. The higher expression of TGFβ1 in HCC derived Tregs compared to control Tregs may provide another explanation to our findings that Tregs from HCC are more suppressive. Further, it will be valuable to determine if the ability to suppress target cells in vitro in the absence of serum is unique to HCC derived Tregs or if it is a common feature in Tregs derived from patients with other solid malignancies as this may represent a potential target for future therapy.
While NHC derived Tregs failed to suppress Teffs in the absence of serum, the introduction of non-physiologic doses of recombinant sCD25 (6,000 pg/ml and 12,000 pg/ml) to the 1:1 suppression assays led to suppression of Teff responses. In our current study, the 3,000 pg/ml concentration represents the typical physiologic level of sCD25 in the serum of NHC that we have previously reported [17]. In the presence of low or physiologic doses of sCD25 Teff viability and responses increase and have no significant effects on Treg mediated suppression [24, 25]. However, higher or non-homeostatic doses of sCD25 typical of levels seen in patients with HCC promote Treg suppression and/or decrease Teff responses which are unique findings not previously described. Our data suggest a synergistic effect between Tregs and the soluble factor sCD25. Previously, we reported that supra-physiologic doses of sCD25 similar the levels observed in patients with advanced HCC are needed for sCD25 to impair CD4 Teff responses directly. The ability of NHC derived Tregs to suppress Teff responses in the absence of serum with lower concentrations of sCD25 than previously published further support the existence of a synergistic effect between Treg and sCD25 [17, 26].
A mechanism that partially explains the immuno-inhibitory properties of sCD25 at higher doses is its down regulation of CD25 surface expression in naïve CD4+CD25− Teffs. Up regulation of CD25 and subsequent expression on the cell surface serves as an early marker of T cell activation. Cell surface expression of CD25 on Teff cells allow optimal IL-2 binding and signaling via the constitutively expressed transducer gamma and delta chains (CD122 and CD132). The high levels of sCD25 and the presence of Tregs which do constitutively express the high affinity IL-2 receptor avidly bind IL-2 in the suppression cultures starving naïve CD4+CD25− T cells of IL-2 signaling. This may represent an important level of regulating responsiveness of Teff activation to IL-2 both in the tumor microenvironments in HCC patients explaining the observed blunted immunity. The reversal of the blunted HCC derived naïve CD4+CD25− Teff responses with IL 2 supplementation suggest a potential role for this cytokine in improving immune responsiveness to tumor [27].
In conclusion, in these series of suppression co-cultures we show that serum and sCD25 influences Treg suppressive function. The Tregs isolated from normal subjects appear to be dependent on serum to suppress target Teff but suppression can be elicited with the introduction of moderate levels of sCD25 to the cultures. In contrast, Tregs from HCC patients suppress target Teffs independent of presence or absence of serum and when compared on a cell per cell basis to controls with HCV related cirrhosis suppress to a higher degree. These findings suggest that the tumor likely accounts for the more robust activated Treg suppressive function and that the inhibition of surface expression of CD25 on naïve Teff by supra-physiologic doses of sCD25 is an important regulatory pathway. Targeting IL-2 signaling in the tumor microenvironment may be an effective strategy to induce immunity to the tumor and overcome this Treg-sCD25 synergistic immune-inhibitory pathway. Current studies are underway to examine the effectiveness of IL-2 therapy on immunological parameters in patients with hepatocellular carcinoma.
Acknowledgments
We want to thank Steve McClellan from the UF ICBR flow lab for expert technical assistance. This work was supported by the KL2 NIH scholar award, Bankhead-Coley new investigator cancer research award and the NIH LRP (R.C.).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001 Oct 15;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Smith KA, Popmihajlov Z. The quantal theory of immunity and the interleukin-2-dependent negative feedback regulation of the immune response. Immunol Rev. 2008 Aug;224:124–40. doi: 10.1111/j.1600-065X.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 3.Cao M, Cabrera R, Xu Y, et al. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab Invest. 2007 Jun;87:582–90. doi: 10.1038/labinvest.3700540. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann H, Cobbold S. Regulatory T cells: context matters. Immunity. 2009 May;30:613–5. doi: 10.1016/j.immuni.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Foss FM. Immunologic mechanisms of antitumor activity. Semin Oncol. 2002 Jun;29:5–11. doi: 10.1053/sonc.2002.33076. [DOI] [PubMed] [Google Scholar]
- 6.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 7.Unitt E, Marshall A, Gelson W, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006 Aug;45:246–53. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem. 2002 Aug 16;277:29355–8. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009 Oct;21:1105–11. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 10.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004 Apr;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009 Oct;70:326–36. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009 May;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008 Jul;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cellmediated suppression. J Exp Med. 2006 Mar 20;203:489–92. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vercoulen Y, Wehrens EJ, van Teijlingen NH, de Jager W, Beekman JM, Prakken BJ. Human regulatory T cell suppressive function is independent of apoptosis induction in activated effector T cells. PLoS One. 2009;4:e7183. doi: 10.1371/journal.pone.0007183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera R, Ararat M, Cao M, et al. Hepatocellular Carcinoma Immunopathogenesis: Clinical Evidence for Global T Cell Defects and an Immunomodulatory Role for Soluble CD25 (sCD25) Dig Dis Sci. 2009 Aug 28; doi: 10.1007/s10620-009-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera R, Tu Z, Xu Y, et al. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004 Nov;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 19.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998 Jul 20;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintainedby CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998 Dec;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 21.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005 Mar 15;65:2457–64. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 22.Yang XH, Yamagiwa S, Ichida T, et al. Increase of CD4+ CD25+ regulatory Tcells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006 Aug;45:254–62. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Szymczak-Workman AL, Workman CJ, Vignali DA. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. J Immunol. 2009 May 1;182:5188–92. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brusko TM, Wasserfall CH, Hulme MA, Cabrera R, Schatz D, Atkinson MA. Influence of membrane CD25 stability on T lymphocyte activity: implications fo immunoregulation. PLoS One. 2009;4:e7980. doi: 10.1371/journal.pone.0007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen AE, Lauritsen JP. CD25 shedding by human natural occurring CD4+CD25+ regulatory T cells does not inhibit the action of IL-2. Scand J Immunol. 2009 Jul;70:40–3. doi: 10.1111/j.1365-3083.2009.02268.x. [DOI] [PubMed] [Google Scholar]
- 26.Maier LM, Anderson DE, Severson CA, et al. Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol. 2009 Feb 1;182:1541–7. doi: 10.4049/jimmunol.182.3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sereti I, Gea-Banacloche J, Kan MY, Hallahan CW, Lane HC. Interleukin 2 leads to dose-dependent expression of the alpha chain of the IL-2 receptor on CD25-negative T lymphocytes in the absence of exogenous antigenic stimulation. Clin Immunol. 2000 Dec;97:266–76. doi: 10.1006/clim.2000.4929. [DOI] [PubMed] [Google Scholar]
- 28.Oberg HH, Wesch D, Lenke J, Kabelitz D. An optimized method for the functional analysis of human regulatory T cells. Scand J Immunol. 2006 Sep;64(3):353–60. doi: 10.1111/j.1365-3083.2006.01825.x. [DOI] [PubMed] [Google Scholar]