Abstract
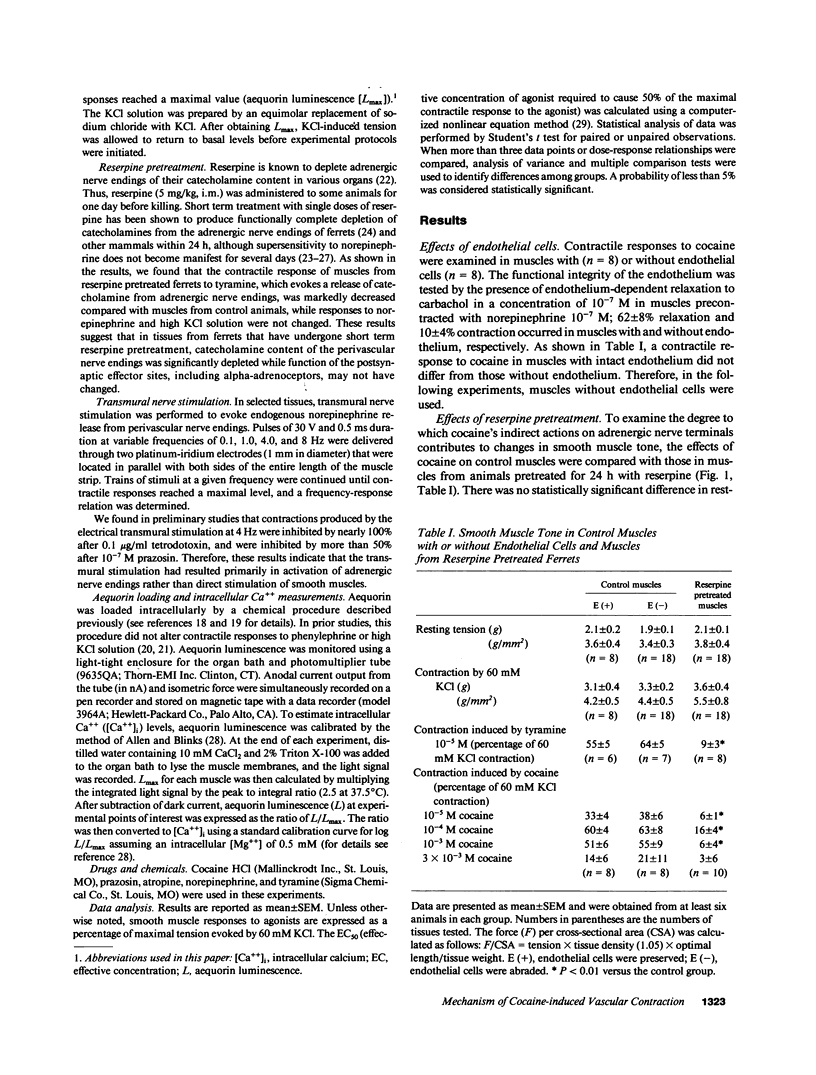
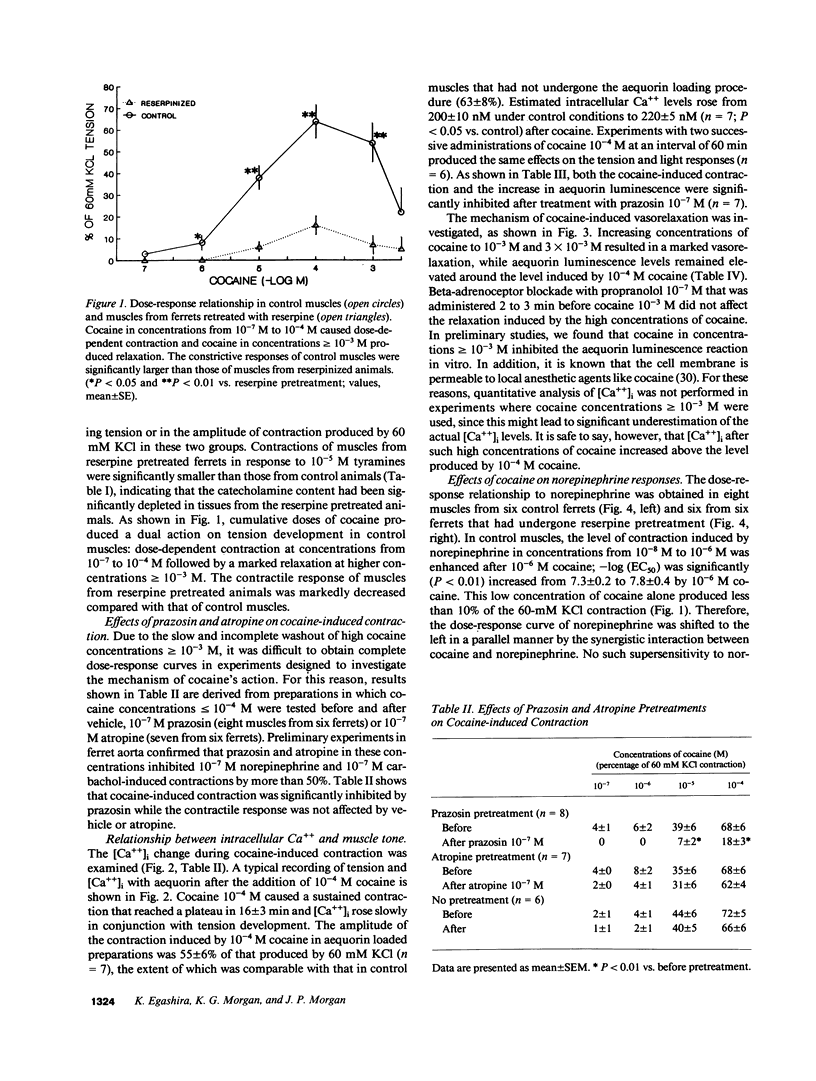
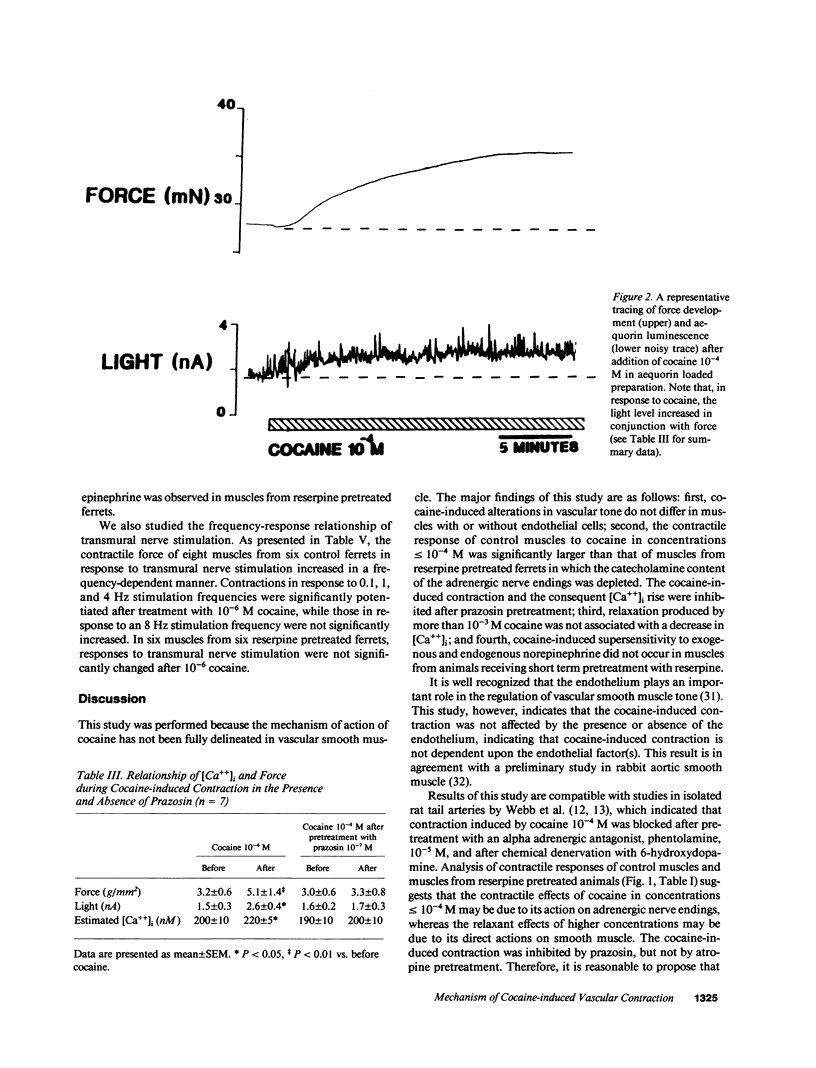
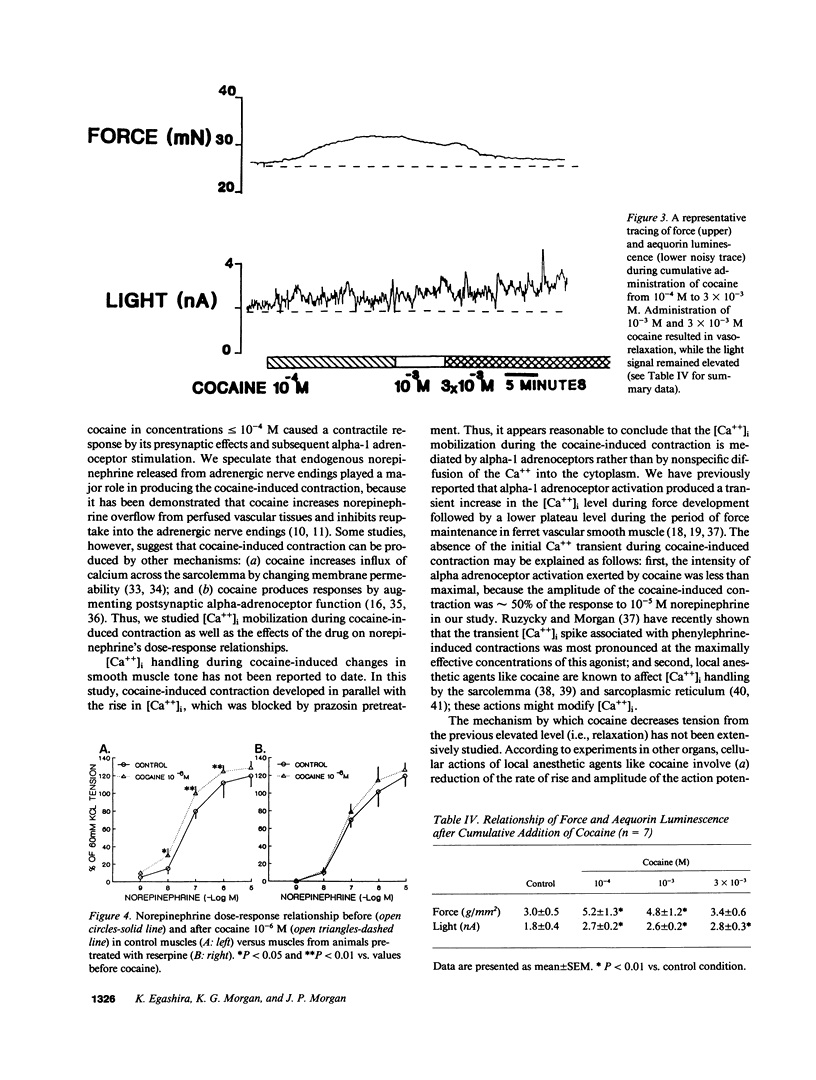
The mechanism by which cocaine alters vascular tone is not fully understood. We determined the effects of cocaine on excitation-contraction coupling of isolated ferret aorta. Cocaine in concentrations less than or equal to 10(-4) M caused a contractile response in a dose-dependent manner. The response of control muscle was significantly larger than that in muscle from ferrets pretreated with reserpine. Cocaine-induced contraction was not affected by endothelial factors, but was significantly inhibited by prazosin 10(-7) M pretreatment. The intracellular calcium [( Ca++]i), as measured with aequorin, rose in conjunction with cocaine-induced contraction. The degree of contraction generated by 10(-4) M cocaine decreased after higher concentrations of cocaine greater than or equal to 10(-3) M, while aequorin luminescence remained elevated above the levels before 10(-6) M cocaine. The dose-response relationships of norepinephrine and sympathetic nerve stimulation were enhanced by 10(-6) M cocaine in control muscles; this did not occur in muscles from reserpine pretreated ferrets. In conclusion, (a) cocaine in concentrations less than or equal to 10(-4) M caused vascular contraction presumably by its presynaptic action with consequent alpha-1 adrenoceptor activation and consequent [Ca++]i rise; (b) high concentrations of cocaine greater than or equal to 10(-3) M reduced muscle tone by decreasing the Ca++ sensitivity of the contractile proteins; and (c) supersensitivity to norepinephrine was mediated by cocaine's action on adrenergic nerve endings.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes J. M., Barnes N. M., Costall B., Naylor R. J., Tattersall F. D. Reserpine, para-chlorophenylalanine and fenfluramine antagonise cisplatin-induced emesis in the ferret. Neuropharmacology. 1988 Aug;27(8):783–790. doi: 10.1016/0028-3908(88)90092-5. [DOI] [PubMed] [Google Scholar]
- Bigger J. T., Jr, Mandel W. J. Effect of lidocaine on the electrophysiological properties of ventricular muscle and purkinje fibers. J Clin Invest. 1970 Jan;49(1):63–77. doi: 10.1172/JCI106224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Bradley A. B., Morgan K. G. Alterations in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. J Physiol. 1987 Apr;385:437–448. doi: 10.1113/jphysiol.1987.sp016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. G., Lee A. B., Bolson E. L., Dodge H. T. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation. 1984 Jul;70(1):18–24. doi: 10.1161/01.cir.70.1.18. [DOI] [PubMed] [Google Scholar]
- Catchlove R. F. The influence of CO 2 and pH on local anesthetic action. J Pharmacol Exp Ther. 1972 May;181(2):298–309. [PubMed] [Google Scholar]
- Cregler L. L., Mark H. Medical complications of cocaine abuse. N Engl J Med. 1986 Dec 4;315(23):1495–1500. doi: 10.1056/NEJM198612043152327. [DOI] [PubMed] [Google Scholar]
- Davis L. D., Temte J. V. Electrophysiological actions of lidocaine on canine ventricular muscle and Purkinje fibers. Circ Res. 1969 May;24(5):639–655. doi: 10.1161/01.res.24.5.639. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Gawin F. H., Ellinwood E. H., Jr Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med. 1988 May 5;318(18):1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Grassby P. F., Broadley K. J. Responses mediated via beta-1 adrenoceptors but not beta-2 adrenoceptors exhibit supersensitivity after chronic reserpine pretreatment. J Pharmacol Exp Ther. 1986 Jun;237(3):950–958. [PubMed] [Google Scholar]
- Greenberg R., Innes I. R. The role of bound calcium in supersensitivity induced by cocaine. Br J Pharmacol. 1976 Jul;57(3):329–334. doi: 10.1111/j.1476-5381.1976.tb07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes I. R., Mailhot R. Effect of cocaine on the affinity of -adrenoceptors for noradrenaline. Br J Pharmacol. 1973 May;48(1):139–143. doi: 10.1111/j.1476-5381.1973.tb08231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isner J. M., Estes N. A., 3rd, Thompson P. D., Costanzo-Nordin M. R., Subramanian R., Miller G., Katsas G., Sweeney K., Sturner W. Q. Acute cardiac events temporally related to cocaine abuse. N Engl J Med. 1986 Dec 4;315(23):1438–1443. doi: 10.1056/NEJM198612043152302. [DOI] [PubMed] [Google Scholar]
- Johnson P. N., Inesi G. The effect of methylxanthines and local anesthetics on fragmented sarcoplasmic reticulum. J Pharmacol Exp Ther. 1969 Oct;169(2):308–314. [PubMed] [Google Scholar]
- Kuriyama H., Suyama A. Multiple actions of cocaine on neuromuscular transmission and smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1983 Apr;337:631–654. doi: 10.1113/jphysiol.1983.sp014646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z., Draskóczy P. R., Trendelenburg U. Time course of the development of supersensitivity to various amines in the nictitating membrane of the pithed cat after denervation or decentralization. J Pharmacol Exp Ther. 1967 Aug;157(2):255–273. [PubMed] [Google Scholar]
- Lee T. J., Westfall D. P., Fleming W. W. The correlation between spontaneous contractions and postjunctional supersensitivity of the smooth muscle of the rat vas deferens. J Pharmacol Exp Ther. 1975 Jan;192(1):136–148. [PubMed] [Google Scholar]
- Maxwell R. A., Wastila W. B., Eckhardt S. B. Some factors determining the response of rabbit aortic strips to dl-norepinephrine-7-H3 hydrochloride and the influence of cocaine, guanethidine and methylphenidate on these factors. J Pharmacol Exp Ther. 1966 Feb;151(2):253–261. [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Mudge G. H., Jr, Grossman W., Mills R. M., Jr, Lesch M., Braunwald E. Reflex increase in coronary vascular resistance in patients with ischemic heart disease. N Engl J Med. 1976 Dec 9;295(24):1333–1337. doi: 10.1056/NEJM197612092952401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis A., Mackell M. A., Graham M. Disposition of cocaine in fatal poisoning in man. J Anal Toxicol. 1985 Sep-Oct;9(5):227–229. doi: 10.1093/jat/9.5.227. [DOI] [PubMed] [Google Scholar]
- Przywara D. A., Dambach G. E. Direct actions of cocaine on cardiac cellular electrical activity. Circ Res. 1989 Jul;65(1):185–192. doi: 10.1161/01.res.65.1.185. [DOI] [PubMed] [Google Scholar]
- Ruzycky A. L., Morgan K. G. Involvement of the protein kinase C system in calcium-force relationships in ferret aorta. Br J Pharmacol. 1989 Jun;97(2):391–400. doi: 10.1111/j.1476-5381.1989.tb11966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART D. M., ROGERS W. P., MAHAFFEY J. E., WITHERSPOON S., WOODS E. F. EFFECT OF LOCAL ANESTHETICS ON THE CARDIOVASCULAR SYSTEM OF THE DOG. Anesthesiology. 1963 Sep-Oct;24:620–624. doi: 10.1097/00000542-196309000-00005. [DOI] [PubMed] [Google Scholar]
- Saida K., Suzuki A. Mode of action of prilocaine on sarcoplasmic reticulum in skinned skeletal muscle fibers. J Pharmacol Exp Ther. 1981 Dec;219(3):815–820. [PubMed] [Google Scholar]
- Schachne J. S., Roberts B. H., Thompson P. D. Coronary-artery spasm and myocardial infarction associated with cocaine use. N Engl J Med. 1984 Jun 21;310(25):1665–1666. doi: 10.1056/NEJM198406213102511. [DOI] [PubMed] [Google Scholar]
- Shibata S., Hattori K., Sakurai I., Mori J., Fujiwara M. Adrenergic innervation and cocaine-induced potentiation of adrenergic responses of aortic strips from young and old rabbits. J Pharmacol Exp Ther. 1971 Jun;177(3):621–632. [PubMed] [Google Scholar]
- Simpson R. W., Edwards W. D. Pathogenesis of cocaine-induced ischemic heart disease. Autopsy findings in a 21-year-old man. Arch Pathol Lab Med. 1986 Jun;110(6):479–484. [PubMed] [Google Scholar]
- Smith H. W., 3rd, Liberman H. A., Brody S. L., Battey L. L., Donohue B. C., Morris D. C. Acute myocardial infarction temporally related to cocaine use. Clinical, angiographic, and pathophysiologic observations. Ann Intern Med. 1987 Jul;107(1):13–18. doi: 10.7326/0003-4819-107-1-13. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. The supersensitivity caused by cocaine. J Pharmacol Exp Ther. 1959 Jan;125(1):55–65. [PubMed] [Google Scholar]
- Van Dyke C., Barash P. G., Jatlow P., Byck R. Cocaine: plasma concentrations after intranasal application in man. Science. 1976 Feb 27;191(4229):859–861. doi: 10.1126/science.56036. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- WHITBY L. G., HERTTING G., AXELROD J. Effect of cocaine on the disposition of noradrenaline labelled with tritium. Nature. 1960 Aug 13;187:604–605. doi: 10.1038/187604a0. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Vanhoutte P. M., Bohr D. F. Inactivation of released norepinephrine in rat tail artery by neuronal uptake. J Cardiovasc Pharmacol. 1980;2(2):121–132. doi: 10.1097/00005344-198003000-00004. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Vanhoutte P. M. Cocaine-induced release of noradrenaline in rat tail artery. J Pharm Pharmacol. 1982 Feb;34(2):134–136. doi: 10.1111/j.2042-7158.1982.tb04207.x. [DOI] [PubMed] [Google Scholar]
- Wilkerson R. D. Cardiovascular effects of cocaine: enhancement by yohimbine and atropine. J Pharmacol Exp Ther. 1989 Jan;248(1):57–61. [PubMed] [Google Scholar]
- Winek C. L., Wahba W. W., Rozin L., Janssen J. K. An unusually high blood cocaine concentration in a fatal case. J Anal Toxicol. 1987 Jan-Feb;11(1):43–46. doi: 10.1093/jat/11.1.43. [DOI] [PubMed] [Google Scholar]
- Zimmerman F. H., Gustafson G. M., Kemp H. G., Jr Recurrent myocardial infarction associated with cocaine abuse in a young man with normal coronary arteries: evidence for coronary artery spasm culminating in thrombosis. J Am Coll Cardiol. 1987 Apr;9(4):964–968. doi: 10.1016/s0735-1097(87)80256-5. [DOI] [PubMed] [Google Scholar]



