Abstract
Epithelia are protected from adverse conditions by a mucous barrier. The secreted and transmembrane mucins that constitute the mucous barrier are largely unrecognized as effectors of carcinogenesis. However, both types of mucins are intimately involved in inflammation and cancer. Moreover, diverse human malignancies overexpress transmembrane mucins to exploit their role in signalling cell growth and survival. Mucins have thus been identified as markers of adverse prognosis and as attractive therapeutic targets. Notably, the findings that certain transmembrane mucins induce transformation and promote tumour progression have provided the experimental basis for demonstrating that inhibitors of their function are effective as anti-tumour agents in preclinical models.
The epithelium is a laterally connected layer of cells with apical–basal polarity that separates multicellular animals from the external environment. Most epithelia are single cell layers and as such require robust defence mechanisms to maintain the integrity of the epithelial barrier. Secreted mucins appeared early in metazoan evolution as part of that defence and further emerged as more complex transmembrane structures that participate in the protection, repair and survival of epithelia in vertebrates. The mucins function in limiting the activation of inflammatory responses at the interface with the environment. Deregulation of mucin production has therefore provided an important link between inflammation and cancer. Moreover, carcinoma cells derived from epithelia, including those of the breast, prostate, lung and pancreas, commonly overexpress transmembrane mucins to exploit their role in promoting growth and survival. In this context, certain transmembrane mucins are sufficient to induce transformation and tumours in animal models, and thereby represent highly attractive targets for anticancer treatment. Somewhat paradoxically for what evolved as a protective mechanism for epithelial cells, transmembrane mucins are also aberrantly expressed in malignant haematopoietic cells. The exploitation of mucin function therefore seems to be a strikingly common theme for promoting the survival of diverse human carcinomas and haematological malignancies. Importantly for this Review, recent work has demonstrated that certain mucins are indeed direct drug targets and that inhibitors of mucin function block survival and tumorigenicity of human tumours in experimental models.
Mucin family members
The mucin family includes proteins that contain tandem repeat structures with a high proportion of prolines, threonines and serines (which constitute the PTS domain). Mucins are further defined by extensive glycosylation of the PTS domain through GalNAc O-linkages at the threonine and serine residues. The human mucin (MUC) family consists of members — designated MUC1 to MUC21 — that have been sub-classified into secreted and transmembrane forms. The secreted mucins (for example, MUC2, MUC5AC, MUC5B and MUC6) form a physical barrier, which as a mucous gel provides protection for epithelial cells that line the respiratory and gastrointestinal tracts and form the ductal surfaces of organs such as the liver, breast, pancreas and kidney (FIG. 1a). The transmembrane mucins (for example, MUC1, MUC4, MUC13 and MUC16) have a single membrane-spanning region and contribute to the protective mucous gel through their ectodomains of O-glycosylated tandem repeats that form rod-like structures that extend over 100 nm from the cell surface and beyond the ~10 nm glycocalyx (FIG. 1b).
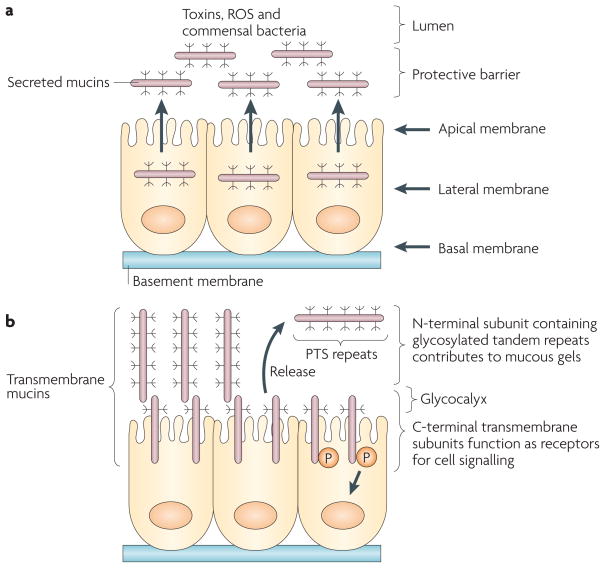
Figure 1. Secreted and transmembrane mucins form physical barriers that protect epithelia.
a| The secreted mucins are released from the apical membrane to form a protective gel that limits exposure to commensal bacteria and suppresses the inflammatory response. The gel also protects the epithelial layer from adverse conditions, for example exposure to ingested toxins, reactive oxygen species (ROS) and proteolytic enzymes in the gastrointestinal tract. Mucin 2 (MUC2) is the major secreted mucin lining the gastrointestinal mucosa. b | The transmembrane mucins are expressed in the apical cell membrane so that the region containing the glycosylated tandem proline, threonine and serine (PTS) repeats extends as a rigid structure beyond the glycocalyx and into the mucous gel. MUC1 and MUC4, and possibly MUC13, are heterodimers that are translated as single polypeptides and cleaved into amino- and carboxy-terminal subunits that in turn form a stable non-covalent complex. The N-terminal mucin subunits containing the PTS repeats are tethered to the cell surface in a complex with the C-terminal transmembrane subunit. Release of the N-terminal subunits into the mucous gel leaves the transmembrane subunits as receptors to signal the presence of inflammation and other forms of stress to the interior of the cell. Evolution of the secreted mucins to include a transmembrane component thus provided an additional level of defence to promote the growth, repair and survival of epithelial cells. MUC16 can also undergo autocleavage; however, it is not known whether MUC16 is expressed as a heterodimeric complex or whether release of the mucin region occurs after positioning of MUC16 in the apical membrane.
Secreted mucins
The secreted mucins MUC2, MUC5AC, MUC5B and MUC6 are thought to share a common ancestor with von Willebrand factor (VWF) and are encoded by a cluster of genes at the chromosomal locus 11p15 (REFS 1,2) (BOX 1). These secreted mucins contain, in addition to glycosylated PTS domains, VWF, VWC or VWD domains and cysteine-knot domains, which are responsible for dimerization in the endoplasmic reticulum. In addition, the secreted mucins have sea urchin sperm protein, enterokinase and agrin (SEA) domains, which are modules of ~120 amino acids that are widely found in O-glycosylated proteins from Caenorhabditis elegans to humans3. Among the secreted mucins, MUC2 has been linked to inflammation and cancer. MUC2 contains a large centrally located PTS domain, which is extensively O-glycosylated, and cysteine-rich domains at the amino- and carboxy- terminal regions. The MUC2 C-terminal region contributes to the formation of homodimers in the endoplasmic reticulum and, following O-glycosylation in the Golgi, MUC2 forms protease-resistant trimers through disulphide bonds at the N terminus4–7. Functionally, MUC2 suppresses inflammation in the intestinal tract and thereby inhibits the development of intestinal tumours8,9.
Box 1. Evolution of secreted and transmembrane mucins.
Evolution of the secreted mucins
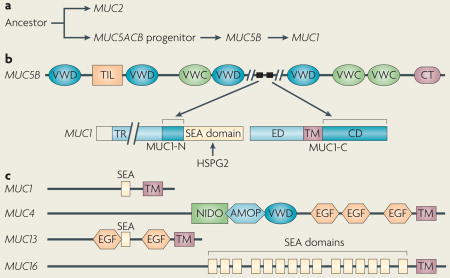
Historically, the mucin (MUC) family members have been classified according to a common biophysical structure—the glycosylated proline, threonine and serine (PTS) domain—and not according to their evolution from common ancestral genes. Nonetheless, the available evidence indicates that the secreted mucins appeared early in metazoan evolution10,21,43 and that the MUC5AC and MUC5B genes evolved from a common MUC5ACB ancestor, which arose from a progenitor of the MUC2 gene (see the figure, part a)2. As a later event, MUC1 emerged in part from MUC5B.
MUC1 sequences emerged from MUC5B
MUC1 sequences upstream of its sea urchin sperm protein, enterokinase and agrin (SEA) domain seem to have evolved from the MUC5B gene (see the figure, part b)10. In addition, the MUC1 cytoplasmic domain (MUC1-CD), which is sufficient to induce transformation, seems to have emerged from MUC5B10. MUC5B has no known role in transformation. Moreover, MUC5B does not have either the MUC1-CD phosphorylation sites for SRC, epidermal growth factor receptor, ABL, glycogen synthase kinase 3β and protein kinase Cδ or the β-catenin binding motif, which have been linked to transformation. These findings have indicated that the MUC1-CD transforming function arose by diversification after evolution from MUC5B.
Domain structures of the transmembrane mucins
In contrast to the sequences derived from MUC5B, the SEA domain structure of MUC1 emerged from heparin sulphate proteoglycan 2 (HSPG2), an inducer of tumour growth. The MUC13 SEA domain also seems to have evolved from HSPG2, whereas the MUC16 SEA domains emerged from agrin. MUC4 has no SEA domain, but has a nidogen (NIDO) domain that evolved from an ancestor common to nidogen and adhesion- associated domain in MUC4 and other proteins (AMOP) and von Willebrand factor type D (VWD) domains that originated with SUSD2 (see the figure, part c)21. In contrast to the other transmembrane mucins, MUC1 homologues are restricted to mammalian species, and except for the SEA domain, MUC1 has no sequence homology with the other transmembrane mucins10. CT, C-terminal cysteine knot domain; ED, extracellular domain; EGF, epidermal growth factor; TIL, trypsin inhibitor-like cysteine-rich domain; TM, transmembrane domain; TR, tandem repeats. Figure is modified from REF. 10 and modified, with permission, from REF. 21 © (2006) Elsevier.
Transmembrane mucins
The transmembrane mucins MUC1, MUC13 and MUC16 have SEA domains that are characteristically located between the O-glycosylated PTS repeats and the transmembrane domain. Unlike the other transmembrane mucins, MUC1 evolved from the MUC5B secreted mucin10 (BOX 1). MUC1 localizes to the apical membranes of normal secretory epithelial cells11. With transformation and loss of polarity, MUC1 is expressed at high levels over the entire surface of diverse types of carcinoma cells. MUC1 is translated as a single polypeptide that undergoes auto-proteolysis at the SEA domain to form two subunits12–14. The MUC1 N-terminal subunit (MUC1-N) contains highly conserved tandem repeats of 20 amino acids that are extensively modified by O-linked glycans15,16. Glycosylation of the MUC1-N tandem repeats is altered in human carcinomas partly as a result of changes in glycosyltransferase expression in malignant cells17. Aberrant MUC1 glycosylation has been shown to play a part in the immunosurveillance of cancer18. MUC1-N forms a stable non-covalent complex with the C-terminal transmembrane subunit (MUC1-C) and is thereby anchored to the surface of the cell. Importantly, MUC1-N, the mucin component of the heterodimer, blocks cell–cell and cell–extracellular matrix interactions19. MUC1-N is released from the cell surface, leaving MUC1-C as a putative receptor that engages diverse signalling pathways linked to transformation and tumour progression20.
Like MUC1, MUC4 is expressed as two subunits that form a complex at the cell surface. MUC4 differs from the other transmembrane mucins in that it lacks a SEA domain and has domains such as nidogen, adhesion-associated domain in MUC4 and other proteins, and VWD in the extracellular region21. MUC4 also has epidermal growth factor (EGF)-like domains (BOX 1).
The MUC13 transmembrane mucin has a domain structure similar to that of MUC4, with N-terminal tandem repeats, three EGF-like sequences and a SEA module (BOX 1). MUC3, MUC12 and MUC17 also have EGF-like domains in their extracellular regions21. By contrast, MUC16 has multiple SEA domains, but no EGF-like domains, emphasizing the diversity in the evolution and potential function of these proteins (BOX 1). On the basis of SEA domain sequences, MUC1 and MUC13 evolved from heparin sulphate proteoglycan 2 and MUC16 from agrin21. As found for MUC1, aberrant expression of MUC4, MUC13 and MUC16 is also associated with diverse human malignancies.
Mucins and epithelial cell polarity
Epithelial cells form a single cell layer and assume a unique structure in which an apical surface faces either the external environment, for example lining the respiratory and gastrointestinal tracts, or a lumen lining ducts in specialized organs. The mucins are secreted from and/or localized to the apical borders of normal epithelial cell sheets. However, in response to stress, there is a loss of polarity that occurs in association with the activation of a proliferation and survival programme22. With the loss of polarity, apical proteins, such as the transmembrane mucins, are transiently repositioned over the entire epithelial cell membrane, allowing them to interact with cell surface molecules normally sequestered at the basolateral membrane (FIG. 2). The epithelial–mesenchymal transition (EMT), which underlies such epithelial cell plasticity, is induced by diverse stimuli and can result in cancer cells with stem cell-like characteristics and aggressive traits23,24. Therefore, in cancer cells with sustained activation of the EMT, the transmembrane mucins can interact with otherwise non-apical cell surface molecules, such as the receptor tyrosine kinases.
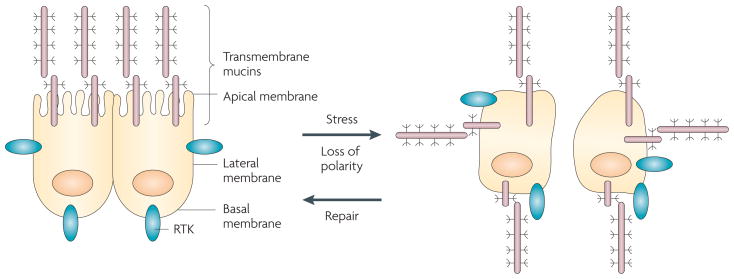
Figure 2. Epithelial stress response is associated with loss of polarity and repositioning of cell surface receptors.
Epithelial cells are polarized with the separation of the apical and basolateral membranes by specialized tight junctions between neighbouring cells. In contrast to apical positioning of the transmembrane mucins, certain other cell surface molecules, for example the receptor tyrosine kinases (RTKs), are restricted to the basolateral membranes. In the presence of inflammation and other settings that induce damage, there is loss of tight junction integrity and cell polarity. In turn, the transmembrane mucins are repositioned over the entire cell membrane and interact with RTKs. Loss of polarity allows epithelial cells to activate a programme of repair and survival22. Whereas the epithelial stress response is reversible and polarity is re-established after repair, loss of polarity is irreversible in carcinoma cells. Therefore, mucin 1 (MUC1) constitutively interacts with diverse RTKs and promotes their downstream signals, thus providing a mechanism by which carcinoma cells can exploit a physiological stress response for their own growth and survival.
The available evidence indicates that the transmembrane mucins can contribute to loss of epithelial cell polarity. Overexpression of the transmembrane mucins, as found in human cancers, may therefore promote the malignant EMT phenotype by disrupting polarity and cell–cell interactions.
Tight junctions
Tight junctions regulate cell polarity by defining the border between the apical and lateral cell membranes. Tight junctions also prevent intramembrane diffusion of cell surface structures localized to the apical and basolateral domains of the polarized epithelial cell25. The formation of tight junctions and the development of cell polarity are dependent on the partitioning defective PAR3–PAR6–atypical protein kinase C (aPKC) complex. Loss of tight junction function is of importance to the epithelial stress response and is mediated by the ERBB2 (also known as NEU and HER2) receptor tyrosine kinase22. Activation of ERBB2 disrupts polarity by associating with PAR6 and aPKC, and blocking the interaction with PAR3 (REF. 26) (FIG. 3a). Mucins participate in ERBB2 activation and can thereby contribute to the disruption of tight junctions. For example, MUC1 forms complexes with ERBB2 and functions in promoting heregulin-induced ERBB2 signalling27–29 (FIG. 3a).
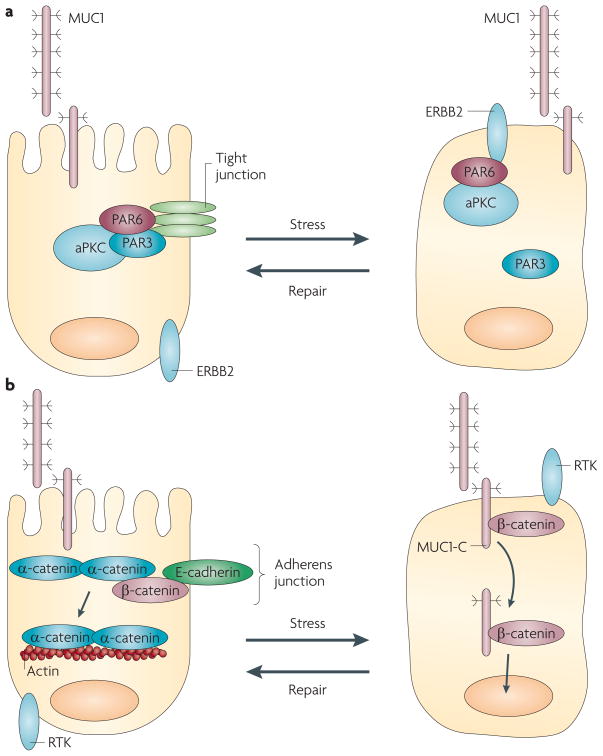
Figure 3. Transmembrane mucins, loss of polarity and disruption of cell–cell adhesion.
a| Tight junctions are dependent on a complex of the PAR6, PAR3 and atypical protein kinase C (aPKC) proteins. Activation of ERBB2, which is necessary for the epithelial stress response22, disrupts the interaction of PAR3 with PAR6 and aPKC, and thereby induces loss of polarity26. In carcinoma cells, mucin 1 (MUC1) interacts with ERBB2 and other members of the Erbb family, and promotes ERBB2 signalling. In this setting, constitutive activation of ERBB2 contributes to the irreversible disruption of tight junctions and the loss of polarity that is associated with transformation. b | In adherens junctions, β-catenin links α-catenin to E-cadherin and, in turn, α-catenin forms homodimers and interacts with the actin cytoskeleton. The MUC1 carboxy-terminal transmembrane subunit (MUC1-C) cytoplasmic domain binds to β-catenin and localizes with it in the nucleus, thus sequestering it away from adherens junctions. In addition, overexpression of MUC1 is associated with downregulation of E-cadherin expression, which disrupts adherens junctions. Moreover, β-catenin is sequestered in MUC4–ERBB2 complexes at the apical membrane, which also contributes to the disruption of adherens junction function. RTK, receptor tyrosine kinase.
Like MUC1, MUC4 activates ERBB2, but by different mechanisms. MUC4 has extracellular EGF-like domains that are of particular importance to MUC4 function in promoting tumorigenesis. MUC4 associates with ERBB2 through one of these EGF domains30,31. The MUC4–ERBB2 interaction sequesters ERBB2 in normal epithelial cells and thereby segregates it from binding to ERBB3 (REF. 32). However, with the disruption of tight junctions and loss of polarity, ERBB2 is accessible for binding to ERBB3 and other Erbb family members with resulting activation of ERBB2 signalling. The functional significance of the MUC4–ERBB2 interaction in carcinoma cells is further supported by the promotion of ERBB2 signalling by MUC4 through the induction of ERBB2 translocation to the cell surface33, stabilization of ERBB2 and the activation of downstream effectors of ERBB2 signalling34. Therefore, overexpression of MUC1 and MUC4 in carcinomas can promote and/or maintain loss of polarity by ERBB2-mediated disruption of the Par complex.
Adherens junctions
Adherens junctions maintain epithelial morphogenesis and largely consist of cadherin complexes that participate in interactions between epithelial cells35,36. The role of adherens junctions in connecting cells is in contrast to that of tight junctions in regulating epithelial cell polarity. The cell surface cadherin adhesion molecules interact with the cytoplasmic α-catenin, β-catenin and γ-catenin proteins. In epithelial cells, β-catenin links α-catenin to E-cadherin, and in turn α-catenin forms homodimers and interacts with the actin cytoskeleton37 (FIG. 3b). As a result, actin filaments form a network throughout the epithelial layer that is directed by E-cadherin–catenin complexes that form homotypic interactions between cells. The finding that the MUC1-C cytoplasmic domain binds to β-catenin, and not α-catenin38, provided the basis for subsequent work on the functional significance of the interaction. Overexpression of MUC1 in cancer cells sequesters β-catenin and thereby disrupts the function of E-cadherin in adherens junctions38 (FIG. 3b). The interaction with MUC1 stabilizes β-catenin and promotes β-catenin-mediated activation of Wnt target genes39,40. MUC1 thereby integrates the disruption of adherens junctions with the activation of the Wnt pathway, which has been linked to growth and tumorigenesis41. MUC4 has also been proposed to sequester β-catenin in MUC4–ERBB2 complexes at the apical membrane and thereby disrupt adherens junctions31.
In summary, epithelial oncogenesis is associated with the disruption of polarity and cell–cell interactions. The transmembrane mucins, particularly MUC1 and MUC4, can signal the disruption of tight junctions and adherens junctions. As a result, these mucins have emerged as being of potential importance to the EMT in tumour progression24,42.
Mucins, inflammation and cancer
MUC2, inflammatory bowel disease and tumorigenesis
The epithelial lining of the intestinal tract is exposed to luminal contents that include proteases, bile, ingested toxins and substantial numbers of commensal bacteria. For protection, the intestinal epithelium is covered by a mucous layer, which partly consists of secreted mucins. This relatively protease-resistant viscous coating provides a physical barrier that limits damage to the epithelium and attenuates activation of innate and adaptive immune responses. Evolution of the protective barrier afforded by these gel-forming mucins has prevailed from early multicellular animals to humans, emphasizing the importance of this defence for survival43.
MUC2 is the major component of the two layers of intestinal mucus44,45. The outer layer, which contains bacteria, is less dense than the inner layer owing to proteolytic cleavage of MUC2. The more densely packed inner layer is comprised of uncleaved MUC2, is attached to the epithelium and is free from bacterial colonization45. Therefore, loss of MUC2 confers a microenvironment in which bacteria can activate an inflammatory response at the epithelial surface. Ulcerative colitis is a major form of idiopathic inflammatory bowel disease (IBD) that is characterized by marked inflammation, superficial mucosal ulceration, infiltration of neutrophils and depletion of goblet cell mucins46. IBD is thought to result from deregulated responses of the immune system to bacteria in the commensal flora47. Importantly, chronic inflammation that is associated with IBD increases the risk of colon cancer, potentially by promoting a micro-environment that results in genomic instability48. The observation that the extent of disease in patients with ulcerative colitis is associated with decreases in MUC2 production has suggested that the mucosal barrier is important to the pathogenesis of this form of IBD49. In support of a crucial role for MUC2, mice deficient in MUC2 spontaneously develop inflammation of the colon and superficial erosions consistent with ulcerative colitis9,50. Mice deficient in the anti-inflammatory cytokine interleukin-10 (Il-10) are defective in colonic MUC2 synthesis and also develop chronic colitis after colonization with commensal bacteria51. Moreover, mice deficient in both Muc2 and Il-10 develop colitis that is more severe than that in Muc2−/− mice, indicating that combined defects in the mucous barrier and immunoregulation further exacerbate the colitis52. These findings have collectively supported a model in which MUC2 is essential for the protection of the intestinal epithelium against commensal bacteria and potentially other environmental factors in the lumen that promote inflammation.
Loss of MUC2 expression in Muc2−/− mice is associated with the increased proliferation and survival of intestinal epithelial cells in response to increased exposure to luminal contents and induction of inflammation8. Notably, Muc2−/− mice develop adenomas in the small and large intestine that progress to invasive adenocarcinomas8. Mutations in the adenomatous polyposis coli (APC) gene promote intestinal tumorigenesis through aberrant regulation of the Wnt–β-catenin signalling pathway53. Muc2−/− mice crossed with mouse models carrying inactivated Apc1638N/+ or ApcMin/+ alleles exhibit increases in intestinal tumour development54. Moreover, the increased tumour burden in the colon observed in the Muc2−/−;Apc-mutant mice is similar to that found in ApcMin/+ mice that are induced to mount an inflammatory response54. These studies have led to the conclusion that Muc2−/− tumours develop as a result of inflammation, and that this response complements tumorigenesis in the mice carrying Apc mutations. Interestingly, the secretory phospholipase PLA2G2A is expressed in goblet cells and confers resistance to Muc2−/−-induced intestinal tumorigenesis55. PLA2G2A modulates intestinal inflammation and so, like MUC2, protects the intestinal mucosa. Collectively, these findings have provided compelling evidence that MUC2 is important for the prevention of intestinal inflammation and so tumorigenesis.
MUC2 is clustered on chromosome 11 with MUC5AC, MUC5B and MUC6, which also encode secreted mucins. However, it is not known whether, like MUC2, these other secreted mucins protect epithelial cells from inflammation and tumorigenesis. The role of MUC2 as a tumour suppressor may seem paradoxical to reports that MUC2 is expressed at increased levels in certain malignancies, including carcinomas of the gastrointestinal tract56–59. In this regard, MUC2 expression in carcinomas might reflect the origin of these tumours from cells that normally expressed MUC2, rather than a role for this mucin in the malignant process itself. MUC2 is activated by various effectors linked to transformation, for example galectin 3, AP1 and the forkhead box transcription factors60,61. Therefore, increases in MUC2 expression in certain carcinomas might reflect the activation of these pathways. Alternatively, expression of the MUC2, MUC5AC, MUC5B and MUC6 gene cluster is also regulated by epigenetic mechanisms in carcinoma cells62 and could account for the aberrant expression of MUC2 in certain carcinomas, as well as of MUC5B in gastric and breast cancers63,64. Overexpression of MUC2 and other secreted mucins by human cancers might, through the generation of a mucous barrier, protect against recognition by anti-tumour immune effectors and thereby contribute to the malignant phenotype. In this context, MUC2 could have a tumour suppressor function in inflammation and contribute to oncogenesis in other settings. Indeed, mucus hypersecretion has also been linked to bacterial overgrowth and the induction of inflammatory responses that could promote tumour development65,66.
MUC1 and chronic inflammation
The association of MUC2 with inflammation and cancer extends to the transmembrane mucins (FIG. 4). MUC1 is also an important component of the mucosal barrier that is upregulated in response to infection with pathogenic bacteria67,68. MUC1 has been proposed to suppress inflammation that is induced by pathogenic bacteria69. In other studies, the Il-10−/− mouse model of IBD (as described above51) was crossed with human MUC1-transgenic mice70. The offspring developed MUC1-positive IBD with more pronounced inflammation at an earlier age than IL-10−/− mice, and had a marked increase in colon cancers compared with the IL-10−/− mice70.
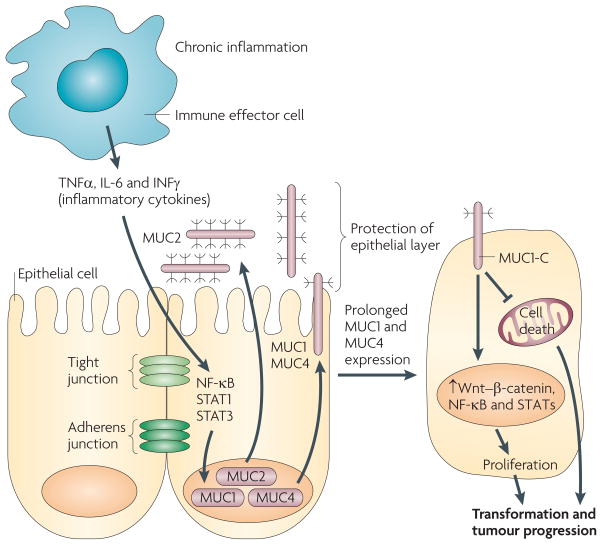
Figure 4. Mucins, chronic inflammation and cancer.
In this proposed model of the association of mucins with chronic inflammation and cancer, the production of inflammatory cytokines by immune effector cells activates transcription factors, for example nuclear factor-κB (NF-κB), signal transducer and activator of transcription 1 (STAT1) and STAT3, in epithelial cells. In turn, these transcription factors upregulate mucin expression to enhance the mucous barrier and protect the epithelial layer. Mucin 2 (MUC2) limits the inflammatory response at the apical membrane and inhibits transformation. Upregulation of the MUC1 and MUC4 transmembrane mucins similarly contributes to the protective barrier and loss of polarity in the epithelial stress response. Activation of MUC1 is associated with targeting of the MUC1 C-terminal transmembrane subunit (MUC1-C) to the nucleus, where it promotes a gene programme for proliferation and survival. Targeting of MUC1-C to the mitochondria also blocks cell death to prevent loss of the epithelial barrier. However, with chronic inflammation and prolonged stimulation of this protective response, epithelial cells may become susceptible to the accumulation of genetic mutations that induce transformation in a setting with downregulation of pathways that would otherwise protect against oncogenic events. IL-6, interleukin-6; IFNγ, interferon-γ; TNFα, tumour necrosis factor-α
The findings that MUC1 suppresses inflammation on the one hand and promotes the inflammatory response and cancer on the other hand would seem to be contradictory. Nonetheless, MUC1 expression is induced by inflammatory cytokines (tumour necrosis factor-α (TNFα), interferon-γ (IFNγ) and IL-6) and, in the absence of IL-10 and a corresponding anti-inflammatory response, deregulation of MUC1 activation could contribute to chronic inflammation and cancer (FIG. 4). In this regard, the activation of the canonical inhibitor of nuclear factor-κB kinase-β (IKKβ)–nuclear factor-κB (NF-κB) pathway is a likely mediator of inflammation-induced cancer progression71 as MUC1-C activates both IKKβ72 and the NF-κB family member RELA73. Chronic inflammation that is induced by other microorganisms has also been associated with MUC1 and the development of cancer. For example, Helicobacter pylori infection results in increased MUC1 expression and is a major contributor to gastric cancer74. MUC1 expression is also induced by the Epstein-Barr virus (EBV) latent membrane protein 1 and may contribute to tumorigenesis that is associated with chronic EBV infection75.
Therefore, MUC1 has a role in protection against inflammation. However, prolonged activation of MUC1 in chronic inflammation that is induced by a deregulated response can lead to exploitation of its growth- and survival-promoting effects, as well as the development of cancer (FIG. 4).
Effects of mucins on cellular signalling pathways
The transmembrane mucins, unlike their secreted counterparts, also protect the epithelial cell layer by functioning in cellular signalling pathways that promote growth and survival. Human cancers have exploited these protective functions by overexpressing the transmembrane mucins, particularly MUC1 and MUC4.
MUC1 and receptor tyrosine kinase signalling
MUC1 is aberrantly expressed in ~900,000 of the 1.4 million tumours (carcinomas and certain haematological malignancies) diagnosed each year in the United States, making MUC1 overexpression one of the more common alterations in human cancers. The overexpression of MUC1 can be partly attributed to amplification of the MUC1 locus76 and/or auto-inductive loops involving the activation of the MUC1 promoter through NF-κB and other transcription factors that are effectors of the inflammatory response (as discussed above)73. As has recently been reviewed77 and summarized below, much of the more recent work on MUC1 has focused on the MUC1-C subunit and how it functions as an oncoprotein.
The available evidence indicates that MUC1-C contributes to the malignant phenotype by regulating gene transcription, blocking stress-induced apoptosis and necrosis, and attenuating activation of death receptor pathways20,77,78. Other work has demonstrated that the expression of MUC1-C with point mutations in the cytoplasmic domain is sufficient to block anchorage-independent growth and tumorigenicity of carcinoma cells, indicating that these mutants function as dominant-negative mutants for transformation39,77. The involvement of MUC1 in oncogenesis is further supported by transgenic mouse models in which MUC1 overexpression induces mammary gland tumorigenesis79. Moreover, transforming growth factor-α (TGFα)- and EGF receptor (EGFR)-induced tumorigenesis is attenuated by the downregulation of MUC1 (REF. 80). More recent work (described below) has shown that an inhibitor of MUC1-C oligomerization is highly effective in killing MUC1-positive human breast and prostate cancer cells growing in vitro and as tumour xenografts in nude mice81,82. Collectively, these findings have provided compelling support for the importance of MUC1-C in oncogenesis and the dependence of certain carcinoma cells on this oncoprotein. In this regard, constitutive activation of MUC1-C in carcinoma cells represents a selective target when taken in the context of the inactivated apical form of MUC1-C in normal epithelial cells. Notably, in contrast to many other oncoproteins, there is no evidence that MUC1-C-induced transformation is associated with activating mutations.
The MUC1-C transmembrane subunit is a protein of 158 amino acids that is sufficient to induce transformation40,83. The MUC1-C 58-amino-acid extracellular domain contains an aspartic acid residue at position 36 that is modified by N-glycosylation and functions as a binding site for galectin 3, a β-galactoside-interacting protein that is upregulated in many cancers84 (FIG. 5a). N-glycosylated MUC1-C contributes to the upregulation of galectin 3 expression and, in turn, galectin 3 serves as an extracellular bridge between MUC1-C and EGFR84. The interaction between MUC1 and EGFR blocks EGFR degradation and so promotes EGFR signalling85. MUC1-C associates with other receptor tyrosine kinases, including ERBB2–4, fibroblast growth factor receptor 3 (FGFR3), platelet-derived growth factor-β (PDGFRβ) and MET, and participates in their downstream signalling pathways20,77 (FIG. 5a). Notably, constitutive interactions between MUC1 and receptor tyrosine kinases have been identified in carcinoma cells and are not expected to occur in normal epithelial cells, other than in the response to stress with a transient loss of polarity.
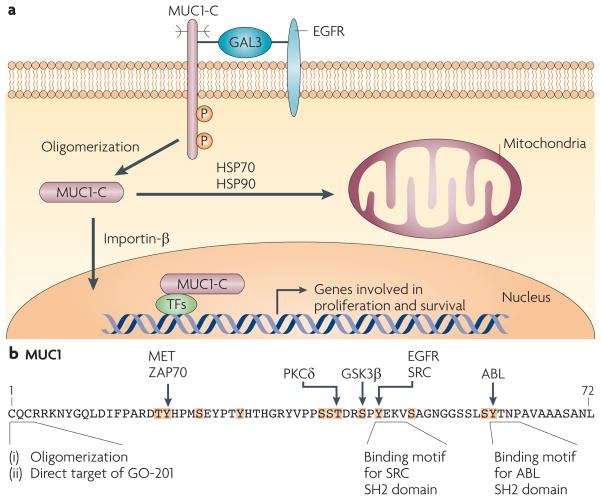
Figure 5. Activation of MUC1-C in stress and transformation.
a| The mucin 1 (MUC1) C-terminal transmembrane subunit (MUC1-C) forms complexes with the epidermal growth factor receptor (EGFR) that are mediated at least in part by galectin 3 (GAL3) lattices. MUC1-C also forms complexes with ERBB2–4 and with other receptor tyrosine kinases (not shown), although it is not known whether these interactions require galectin 3. MUC1-C–RTK complexes are transiently formed in non-malignant epithelial cells in response to inflammatory cytokines and are constitutively formed in carcinoma cells. MUC1-C oligomers are targeted to the nucleus by an importin-β-dependent mechanism, where MUC1-C associates with several transcription factors (TFs)77. MUC1-C thereby promotes the transcription of genes that encode proteins involved in proliferative and pro-survival signals by affecting the recruitment of co-activators and co-repressors93,125 and/or regulating the latency of transcription factor occupancy73. MUC1-C is also targeted to the mitochondrial outer membrane by heat shock protein 70 (HSP70) and HSP90, where it blocks stress-induced loss of the mitochondrial transmembrane potential28,29,89 and the induction of cell death in response to DNA damage, reactive oxygen species, hypoxia and glucose deprivation28,150–153. b | MUC1-C intracellular signalling is mediated in part by phosphorylation of the cytoplasmic domain, which contains five documented and seven potential phosphorylation sites (highlighted in orange with known kinases indicated). The CQC sequence is necessary for oligomerization of MUC1-C and targeting of MUC1-C to the nucleus and mitochondria, as well as binding of MUC1-C to diverse effectors, such as nuclear factor-κB73. Direct targeting of the MUC1-C CQC sequence with GO-201 induces complete regression of established human breast and prostate tumour xenografts in mice81,82. GSK3β, glycogen synthase kinase 3β; P, phosphorylation; PKCδ, protein kinase Cδ; SH2, Src homology 2.
The 72-amino-acid MUC1-C cytoplasmic domain (FIG. 5b) includes a CQC motif adjacent to the transmembrane region that is subject to palmitoylation and which regulates the recycling of MUC1 from endosomes to the cell membrane86. The CQC motif also contributes to the formation of MUC1-C oligomers87 and perhaps heterodimers, although the identity of the binding protein remains unknown88. In malignant cells, MUC1-C accumulates in the cytosol following release from the cell membrane and/or redirection from the endoplasmic reticulum. Oligomerization of cytoplasmic MUC1-C is necessary for targeting of MUC1-C to the nucleus87 and mitochondria28,29,89 (FIG. 5b); this targeting is apparently transient in the response to stress and, by contrast, constitutive in carcinoma cells77.
The MUC1-C cytoplasmic domain contains 12 documented and putative phosphorylation sites, and functions as a substrate for receptor tyrosine kinases, Src family kinases, ABL, PKCδ, glycogen synthase kinase 3β (GSK3β) and ZAP70 (REFS 20,77,90) (FIG. 5b). Phosphorylation of the MUC1-C cytoplasmic domain regulates interactions between MUC1-C and the Wnt pathway effector, β-catenin38,77,91,92. The MUC1-C cytoplasmic domain also interacts with p53 and regulates p53-mediated transcription in response to genotoxic stress93. In addition, MUC1-C activates the NF-κB pathway through direct interactions with IKKα, IKKβ and RELA72,73. Therefore, MUC1-C has been implicated in the regulation of the Wnt–β-catenin, p53 and NF-κB pathways, all of which are linked to tumour progression.
MUC4 in survival, growth and invasion pathways
In experimental models, expression of rat MUC4 is associated with the disruption of cell–cell and cell–extracellular matrix interactions94 and the development of metastases95. Cells expressing rat MUC4 exhibit reduced binding of ERBB2-specific antibodies, such as trastuzumab (Herceptin), and thus can attenuate the therapeutic effects of these antibodies96. MUC4 also confers resistance to killing by immune effectors97. In addition, the suppression of tumour cell apoptosis by MUC4 has indicated that this mucin contributes to tumour progression by promoting cell survival98–100. The effects of MUC4 on conferring resistance to apoptosis are mediated by both ERBB2-dependent and ERBB2-independent mechanisms, indicating that MUC4 can promote cell survival through multiple pathways100. MUC4 also increases growth, motility and invasiveness of carcinoma cells101,102. In concert with these findings, and like MUC1, overexpression of MUC4 in rodent fibroblasts is sufficient to induce transformation and tumorigenicity103. The 22-amino-acid MUC4 cytoplasmic domain includes 2 serines that are predicted phosphorylation sites. However, it is not known whether the MUC4 cytoplasmic domain is phosphorylated or whether it participates in cell signalling.
MUC13 and MUC16 in oncogenesis
The MUC13 transmembrane mucin has a 69-amino-acid cytoplasmic tail that includes 5 potential serine phosphorylation sites104. Overexpression of MUC13 has been found in gastrointestinal105–107 and ovarian108 carcinomas. In ovarian cancer cells, overexpression of exogenous MUC13 is associated with increases in cell motility, proliferation and tumorigenesis109. Overexpression of MUC13 has been associated with upregulation of ERBB2 levels109. Cell motility that is induced by MUC13 is also mediated, at least in part, by activation of the Jun N-terminal kinase (JNK) pathway109.
The ovarian cancer antigen CA125 (REF. 110) was found — some 20 years after its discovery — to be encoded by a gene with an amino acid sequence containing tandem repeats with homology to SEA domains, a transmembrane domain and a 31-amino-acid cytoplasmic tail111,112. This antigen was designated MUC16; it is overexpressed in around 80% of epithelial ovarian cancers110 and is detectable in the serum of patients with ovarian cancer113. MUC16 binds to mesothelin, a glycophosphatidylinositol (GPI)-anchored glycoprotein, and has thus been postulated to confer adhesion of ovarian cancer cells to the peritoneum114,115. What is not clearly known is whether overexpression of MUC16 contributes to the initiation or progression of ovarian tumorigenesis. The MUC16 cytoplasmic domain contains three potential tyrosine phosphorylation sites; however, there is presently no evidence that MUC16 is involved in cell signalling.
Mucins and haematological malignancies
The mucins evolved as a protective mechanism for the apical membranes of epithelial cells. However, and perhaps paradoxically, MUC1 is aberrantly expressed in haematological malignancies. In this context, MUC1 has been found at high levels in multiple myeloma cells116, lymphomas117 and myeloid leukaemias118,119. In human multiple myeloma cells, MUC1 expression is associated with the amplification of MUC1 (REF. 120). In addition, silencing of MUC1 expression in multiple myeloma cells has supported a role for MUC1 in promoting growth and survival120. In B cell lymphomas, MUC1 is activated by chromosomal translocation, rearrangement and amplification117. The basis for aberrant MUC1 expression in myeloid leukaemias is not presently known, but it is found in CD34+ acute myelogenous leukaemia (AML) cells119. In chronic myelogenous leukaemia (CML) cells, MUC1 expression is undetectable in CD34+ cells from patients with chronic-phase disease, but is markedly upregulated in CML blasts121. MUC1 stabilizes BCR–ABL in the CML blasts and contributes to the pathogenesis of CML by promoting self-renewal and inhibiting differentiation and death121. Therefore, as in carcinoma cells, MUC1 seems to be important for the growth and survival of malignant haematopoietic cells. To date, MUC1 is the only mucin that is functionally associated with haematological malignancies, indicating that it might be unique in this regard or that the other mucins have not been the focus of sufficient research in these disorders.
Mucins in cancer diagnosis and prognosis
MUC1
Early work demonstrating that MUC1 is aberrantly overexpressed in human breast carcinomas led to the finding that the MUC1-N subunit is detectable at increased levels in the serum of patients with breast cancer11,122. The measurement of circulating MUC1-N levels, as determined by the CA15-3 assay that has been approved by the US Food and Drug Administration, has been used to monitor the clinical course of patients with breast cancer during treatment and to detect early disease recurrence (TABLE 1). Numerous studies have examined overexpression of MUC1-N as a marker in breast tumours and indicate that aberrant localization to non-apical membranes and the cytosol confers a worse prognosis123. More recent work has identified MUC1-C-induced gene expression patterns that, when applied to breast and lung cancer databases, predict significant decreases in disease-free and overall survival124. MUC1-C associates with oestrogen receptor-α on oestrogen-responsive promoters, and antagonizes the inhibitory effects of tamoxifen125. Related to these observations, the analysis of two databases derived from patients with breast cancer who were treated with adjuvant tamoxifen demonstrated that patients with a MUC1-C-induced lipid gene signature experience significant decreases in disease-free and overall survival126. Gene expression profiling of human prostate cancers has also shown that MUC1 is upregulated in subgroups with an increased risk of recurrence127 (TABLE 1). As further evidence, comparative genome hybridization and cDNA microarray analyses of papillary thyroid cancers identified MUC1 as an independent marker of aggressive behaviour and poor survival128. In summary, MUC1 expression confers a poor prognosis in several different types of carcinomas.
Table 1.
Transmembrane mucins in cancer diagnosis, prognosis and therapy
| Mucin | Diagnosis and prognosis | Therapy |
|---|---|---|
| MUC1 |
|
|
| MUC4 | Overexpression in pancreatic cancer is a marker of poor prognosis129 | None |
| MUC16 | MUC16 (CA125) serum assay (approved by the US FDA) used to detect early-stage disease and monitor clinical course in ovarian cancer145 | Antibodies: cytotoxic drug conjugates147 and antibodies targeting the MUC16–mesothelin interaction115 |
FDA, Food and Drug Administration; L-BLP25, BLP25 liposome vaccine; MUC1-C, mucin 1 C-terminal transmembrane subunit; MUC1-N, mucin 1 N-terminal subunit; NSCLC, non-small-cell lung cancer.
MUC4
Overexpression of MUC4 and loss of restriction to the apical membrane has been identified in carcinomas of the pancreas129,130, gall bladder131, ovary132,133, breast134 and lung135,136. MUC4 expression is upregulated in precancerous pancreatic intraepithelial lesions, but to a lesser extent than in pancreatic cancers137,138. MUC4 has thus been identified as a potential marker for the diagnosis of pancreatic cancer in fine-needle aspirates139 and is associated with a poor prognosis129 (TABLE 1). Similarly, detection of MUC4 correlates with poor outcome in patients with cholangiocarcinoma and extrahepatic bile duct carcinomas140,141. By contrast, MUC4 overexpression in ovarian cancer is not a predictor of patient survival108. The overexpression of MUC4 in lung cancers has also been used to distinguish these tumours from malignant mesothelioma142. Ongoing work is anticipated to expand the usefulness of MUC4 in cancer diagnosis and prognosis, and as a potential target for cancer treatment.
MUC16
MUC16 has been especially important as a circulating marker for monitoring the clinical course of patients with ovarian cancer and for the detection of early-stage disease. The importance of MUC16 as an ovarian cancer biomarker has been summarized elsewhere143–145 (TABLE 1).
Mucins as targets for cancer treatment
The aberrant overexpression of the transmembrane mucins in diverse carcinomas and the identification of MUC1 and MUC4 as oncogenes have established them as highly attractive targets for the development of antibodies, vaccines and therapeutic inhibitors (TABLE 1). However, there are currently no approved agents directed against MUC1 or the other transmembrane mucins. Nonetheless, there are important leads suggesting that MUC1 is a promising target for vaccines and that the MUC1-C cytoplasmic domain is a direct drug target.
Antibodies
Regarding the MUC1-specific antibodies, targeting the shed MUC1-N subunit has not proved to be an effective approach, presumably because of the large pool of circulating MUC1-N that needs to be overcome for antibodies to reach the surface of carcinoma cells20. Another approach has therefore been undertaken to develop antibodies against the cell-bound MUC1-C subunit in the region that interacts with MUC1-N146. Antibodies have also been generated against MUC4 (REF. 103); however, their therapeutic potential is currently unknown. Although MUC16 has not been identified as an oncogene, the overexpression of MUC16 in ovarian cancer cells represents a potential target for therapeutic antibodies. In this regard, an antibody against the MUC16 tandem repeats has been conjugated to the cytotoxic auristatins and shown to be active against human OVCAR-3 ovarian tumour xenografts in mice147. In addition, the interaction between MUC16 and mesothelin contributes to the adhesion of ovarian cancer cells to the peritoneum, and this has been blocked with an antibody against the MUC16 binding domain on mesothelin. This is a potential therapeutic approach.
Vaccines
Two vaccines against MUC1 are in Phase III trials. The BLP25 liposome vaccine (L-BLP25, also known as stimuvax) (Oncothyreon, Merck KGaA, EMD Serono) is a liposome-based vaccine designed to induce an immune response against the MUC1 tandem repeats. In a Phase IIb trial for stage IIIb/IV non-small-cell lung cancer (NSCLC), patients who received L-BLP25 had a median survival of 30.6 months versus 13.3 months for those who did not receive the vaccine. L-BLP25 is now in Phase III trials for unresectable stage III NSCLC and for hormone-sensitive primary breast cancer. TG4010 (Transgene) is a modified vaccinia virus expressing MUC1 and IL-2 that was studied in a Phase IIb trial for patients with NSCLC who received the vaccine with chemotherapy. In patients with normal levels of activated natural killer cells at baseline (approximately 75%), median survival for the vaccine arm was 17.1 months versus 11.3 months for the control arm (patients who received chemotherapy alone). TG4010 has been approved for a Phase III trial in patients with advanced NSCLC. PANVAC (National Cancer Institute) is another promising vaccinia or fowlpox-based vaccine expressing MUC1 and co-stimulatory molecules that is now in Phase II trials for several types of carcinomas148.
Drug inhibitors
MUC1-C has no kinase or enzymatic activity and so is devoid of a catalytic site that could be targeted by small molecules. Moreover, disrupting the interactions between the MUC1-C cytoplasmic domain and its binding partners poses considerable challenges that require targeting flat protein surfaces. Nonetheless, one approach has recently been reported in which a peptide derived from the MUC1-C cytoplasmic domain (designated PMIP) has been used as a decoy for binding to β-catenin and a potential substrate for EGFR and SRC phosphorylation149. PMIP treatment is associated with partial inhibition of human breast cancer cell growth in vitro and in animal models. Another strategy involves direct targeting of the MUC1-C CQC motif with a peptide (GO-201) that inhibits MUC1-C oligomerization81. Treatment with GO-201 has been associated with the induction of breast and prostate cancer cell death in vitro and the complete regression of several breast and prostate tumour xenograft models with little toxicity81,82. These findings have provided the first evidence that the MUC1-C cytoplasmic domain is a direct target for drug development and that breast and prostate cancer cells are indeed dependent on MUC1 for survival and tumorigenicity. Therefore, targeting oligomerization of other transmembrane mucins, for example MUC4, could also represent an effective therapeutic strategy.
Conclusions and perspectives
The development of an epithelial barrier to separate metazoans from the external environment necessitated the evolution of defence mechanisms that involve the secreted and transmembrane mucins. The extensively glycosylated tandem repeats that are characteristic of the mucins afford a physical barrier that protects the epithelium from inflammation and other forms of stress. Deregulation of mucin production, for example of MUC2 in the intestinal tract, contributes to chronic inflammation and tumorigenesis. In addition to the formation of a protective barrier, the transmembrane mucins evolved with the capacity to transduce growth and survival signals to maintain the integrity of the epithelial layer. As an integral component of the epithelial stress response, transmembrane mucins contribute to the disruption of polarity and cell–cell interactions, and thereby the EMT. Overexpression of the MUC1 and MUC4 transmembrane mucins, as found in malignant epithelial cells, is sufficient to induce transformation and tumorigenicity, indicating that their function in protecting the epithelium has been exploited by human carcinomas. The observation that MUC1 is aberrantly expressed in malignant haematopoietic cells has further supported the involvement of this mucin throughout diverse human cancers. On the basis of their overexpression in malignant cells, the transmembrane mucins have been useful in cancer diagnosis and prognosis. MUC1 has also emerged as a highly attractive target for the development of vaccines. Importantly, we now have sufficient information about how MUC1 induces transformation to provide the basis for the development of anticancer agents that inhibit MUC1 function. It is expected that MUC4 and the other transmembrane mucins will also be identified as direct drug targets with therapeutic promise.
At a glance.
Epithelial cells form a layer in which an apical surface is exposed to the external environment and to other forms of stress in ducts in specialized organs. As such, epithelial cells require robust defence mechanisms to avoid sustaining damage.
Secreted mucins are highly glycosylated proteins that form a physical barrier, which protects epithelial cells from stress-induced damage. Transmembrane mucins also contribute to the physical barrier and transmit growth and survival signals to the interior of the cell.
Deregulation of secreted mucin 2 (MUC2) production has provided an important link between inflammation and cancer. Expression of the transmembrane mucin MUC1 is upregulated in response to chronic inflammation.
Aberrant overexpression of transmembrane mucins is associated with diverse human carcinomas and, somewhat paradoxically, certain haematological malignancies. Human cancers have exploited the function of these mucins in promoting growth and survival.
Overexpression of transmembrane mucins contributes to oncogenesis by promoting receptor tyrosine kinase signalling, loss of epithelial cell polarity, constitutive activation of growth and survival pathways (for example, the Wnt-β-catenin and nuclear factor-κB pathways), and downregulation of stress-induced death pathways.
Gene expression profiling and analysis of protein levels have demonstrated that overexpression of transmembrane mucins is associated with a poor prognosis in several different types of carcinomas. Circulating levels of the transmembrane mucins MUC1 and MUC16 are used to monitor the clinical course of patients with breast and ovarian cancer, respectively.
Overexpression of the transmembrane mucins in human cancers has made them highly attractive targets for the development of vaccines, antibodies and drug inhibitors. Recent work has demonstrated that the MUC1 cytoplasmic domain is a direct drug target and that inhibition of MUC1 function blocks survival and tumorigenicity of human breast and prostate cancers in preclinical models.
Acknowledgments
D.W.K. is supported by National Cancer Institute grants CA97098, CA42802 and CA100707.
- Apical–basal polarity
epithelial cells are polarized, with an apical membrane that faces the external environment or a lumen and is opposite the basolateral membrane, which functions in cell–cell interactions and contacts the basement membrane
- Glycosylation
The enzymatic process of attaching oligosaccharides, for example O-N-acetylgalactosamine (O-GalNAc), to the hydroxy oxygen of serine and threonine side chains for the generation of O-linked glycans
- Glycocalyx
A carbohydrate layer on the surface of cells comprised of oligosaccharide side chains of glycoprotein and glycolipid components in the cell membrane
- Von Willebrand factor
VWF, An adhesive glycoprotein that functions in attaching platelets to the vessel wall
- ERBB2
A receptor tyrosine kinase that functions as a heterodimerization partner with other Erbb family members. It is overexpressed in 25–30% of human breast cancers and is the target of the monoclonal antibody trastuzumab (Herceptin)
- Heregulin
The ERBB3 receptor, which lacks intrinsic kinase activity, is activated by heregulin (also known as neuregulin-1), and ERBB3 in turn forms heterodimers with other Erbb family members, such as ERBB2. Heregulin also functions as a ligand for the ERBB4 receptor
- Commensal bacteria
The human digestive tract is normally colonized by ~1013 bacteria, known as commensal bacteria, which exist in an ecosystem without the activation of innate and adaptive immune responses, partly owing to the mucin barrier
- Goblet cell
A specialized cell with apical granules in the gastrointestinal and respiratory tracts that is dedicated to the production of secreted mucins, such as MUC2
- Helicobacter pylori
A Gram-negative microorganism that can colonize the human stomach despite the low pH. H. pylori penetrates the gastric mucosal barrier and attaches to the gastric epithelium, where it induces an inflammatory response
- Epstein-Barr virus
EBV, A herpes virus that is closely associated with the development of nasopharyngeal carcinoma, Burkitt’s lymphoma and Hodgkin’s lymphoma. Latent membrane protein 1 is the major EBV oncoprotein that induces MUC1 expression
- BCR–ABL
A fusion protein that results from the reciprocal translocation between chromosome 9 and chromosome 22 (t(9;22) (q34;q11)) and is responsible for the induction of chronic myelogenous leukaemia
- Tamoxifen
A non-steroidal anti-oestrogen that targets the oestrogen receptor and increases disease-free and overall survival in breast cancer
Footnotes
Competing interests statement
The author declares competing financial interests: see web version for details.
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
APC
UniProtKB: http://www.uniprot.org
α-catenin | β-catenin | E-cadherin | EGFR | ERBB2 | galectin 3 | IKKβ | IL-10 | MUC1 | MUC2 | MUC4 | MUC5AC | MUC5B | MUC6 | MUC13 | MUC16 | PLA2G2A | RELA
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Desseyn JL, et al. Evolutionary history of the 11p15 human mucin gene family. J Mol Evol. 1998;46:102–106. doi: 10.1007/pl00006276. [DOI] [PubMed] [Google Scholar]
- 2.Desseyn JL, Aubert JP, Porchet N, Laine A. Evolution of the large secreted gel-forming mucins. Mol Biol Evol. 2000;17:1175–1184. doi: 10.1093/oxfordjournals.molbev.a026400. [DOI] [PubMed] [Google Scholar]
- 3.Bork P, Patthy L. The SEA module: a new extracellular domain associated with O-glycosylation. Protein Sci. 1995;4:1421–1425. doi: 10.1002/pro.5560040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann A, et al. Studies on the “insoluble” glycoprotein complex from human colon. Identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J Biol Chem. 1999;274:15828–15836. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 6.Godl K, et al. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 7.Lidell ME, et al. The recombinant C-terminus of the human MUC2 mucin forms dimers in Chinese-hamster ovary cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem J. 2003;372:335–345. doi: 10.1042/BJ20030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 9.van der Sluis M, et al. MUC2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;1:117–129. doi: 10.1053/j.gastro.2006.04.020. This paper demonstrated that MUC2 deficiency in the Muc2−/− mouse model leads to the spontaneous development of colitis. Previous work by Velcich et al. (reference 8) had shown that these mice develop colorectal tumours. [DOI] [PubMed] [Google Scholar]
- 10.Duraisamy S, Kufe T, Ramasamy S, Kufe D. Evolution of the human MUC1 oncoprotein. Int J Oncology. 2007;31:671–677. [PubMed] [Google Scholar]
- 11.Kufe D, et al. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 12.Ligtenberg MJ, et al. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992;267:6171–6177. [PubMed] [Google Scholar]
- 13.Levitin F, et al. The MUC1 SEA module is a self-cleaving domain. J Biol Chem. 2005;280:33374–33386. doi: 10.1074/jbc.M506047200. [DOI] [PubMed] [Google Scholar]
- 14.Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nature Struct Mol Biol. 2006;13:71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui J, et al. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA. 1988;85:2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell JA. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988;263:12820–12823. [PubMed] [Google Scholar]
- 17.Ichige K, Perey L, Vogel CA, Buchegger F, Kufe D. Expression of the DF3-P epitope in human ovarian carcinomas. Clin Cancer Res. 1995;1:565–571. [PubMed] [Google Scholar]
- 18.Finn OJ. Immunological weapons acquired early in life win battles with cancer late in life. J Immunol. 2008;181:1589–1592. doi: 10.4049/jimmunol.181.3.1589. [DOI] [PubMed] [Google Scholar]
- 19.Ligtenberg MJL, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:223–232. [PubMed] [Google Scholar]
- 20.Kufe D. Targeting the MUC1 oncoprotein: a tale of two proteins. Cancer Biol Ther. 2008;7:81–84. doi: 10.4161/cbt.7.1.5631. [DOI] [PubMed] [Google Scholar]
- 21.Duraisamy S, Ramasamy S, Kharbanda S, Kufe D. Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 and MUC16. Gene. 2006;373:28–34. doi: 10.1016/j.gene.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Vermeer PD, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 24.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 25.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 26.Aranda V, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nature Cell Biol. 2006;8:1220–1222. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, et al. Heregulin targets γ-catenin to the nucleolus by a mechanism dependent on the DF3/MUC1 protein. Mol Cancer Res. 2003;1:765–775. [PubMed] [Google Scholar]
- 28.Ren J, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anti-cancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. These findings provided the first evidence that overexpression of MUC1, as found in human malignancies, confers resistance to DNA damage-induced apoptosis and led to the demonstration that MUC1 also blocks death in response to oxidative stress, hypoxia and glucose deprivation (references 150–153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren J, et al. MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene. 2006;25:20–31. doi: 10.1038/sj.onc.1209012. [DOI] [PubMed] [Google Scholar]
- 30.Carraway KL, et al. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 31.Carraway KL, Ramsauer VP, Carraway CA. Glycoprotein contributions to mammary gland and mammary tumor structure and function: roles of adherens junctions, ErbBs and membrane MUCs. J Cell Biochem. 2005;96:914–926. doi: 10.1002/jcb.20612. [DOI] [PubMed] [Google Scholar]
- 32.Carraway CA, Carraway KL. Sequestration and segregation of receptor kinases in epithelial cells: implications for ErbB2 oncogenesis. Sci STKE. 2007;2007:re3. doi: 10.1126/stke.3812007re3. [DOI] [PubMed] [Google Scholar]
- 33.Funes M, Miller JK, Lai C, Carraway KL, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281:19310–19319. doi: 10.1074/jbc.M603225200. [DOI] [PubMed] [Google Scholar]
- 34.Chaturvedi P, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nature Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 36.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 37.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, et al. MUC1 cytoplasmic domain coactivates Wnt target gene transcription and confers transformation. Cancer Biol Ther. 2003;2:702–706. [PubMed] [Google Scholar]
- 40.Huang L, et al. MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. This work showed that the MUC1-C cytoplasmic domain is sufficient to induce anchorage-independent growth and tumorigenicity and that this effect is partly mediated by stabilization of β-catenin. [DOI] [PubMed] [Google Scholar]
- 41.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nature Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 42.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 43.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gum JR, Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to preprovon Willebrand factor. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 45.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 47.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Investigation. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nature Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 49.Van Klinken BJ, Van der Wal JW, Einerhand AW, Büller HA, Dekker J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut. 1999;44:387–393. doi: 10.1136/gut.44.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwerbrock NM, et al. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10:811–823. doi: 10.1097/00054725-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 52.van der Sluis M, et al. Combined defects in epithelial and immunoregulatory factors exacerbate the pathogenesis of inflammation: mucin 2-interleukin 10-deficient mice. Lab Invest. 2008;88:634–642. doi: 10.1038/labinvest.2008.28. [DOI] [PubMed] [Google Scholar]
- 53.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Yang K, et al. Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: potential role of chronic inflammation. Cancer Res. 2008;68:7313–7322. doi: 10.1158/0008-5472.CAN-08-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fijneman RJ, et al. Expression of Pla2g2a prevents carcinogenesis in Muc2-deficient mice. Cancer Sci. 2008;99:2113–2119. doi: 10.1111/j.1349-7006.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leteurtre E, et al. Relationships between mucinous gastric carcinoma, MUC2 expression and survival. World J Gastroenterol. 2006;12:3324–3331. doi: 10.3748/wjg.v12.i21.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takikita M, et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009;69:2950–2955. doi: 10.1158/0008-5472.CAN-08-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirabayashi K, et al. Alterations in mucin expression in ovarian mucinous tumors: immunohistochemical analysis of MUC2, MUC5AC, MUC6, and CD10 expression. Acta Histochem Cytochem. 2008;41:15–21. doi: 10.1267/ahc.08008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 2008;16:3329–3341. doi: 10.1002/pmic.200800040. [DOI] [PubMed] [Google Scholar]
- 60.Song S, et al. Galectin-3 modulates MUC2 mucin expression in human colon cancer cells at the level of transcription via AP-1 activation. Gastroenterology. 2005;129:1581–1591. doi: 10.1053/j.gastro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 61.van der Sluis M, et al. Forkhead box transcription factors Foxa1 and Foxa2 are important regulators of MUC2 mucin expression in intestinal epithelial cells. Biochem Biophys Res Commun. 2008;369:1108–1113. doi: 10.1016/j.bbrc.2008.02.158. [DOI] [PubMed] [Google Scholar]
- 62.Vincent A, et al. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene. 2007;26:6566–6576. doi: 10.1038/sj.onc.1210479. [DOI] [PubMed] [Google Scholar]
- 63.Perrais M, et al. Aberrant expression of human mucin gene MUC5B in gastric carcinoma and cancer cells. Identification and regulation of a distal promoter. J Biol Chem. 2001;276:15386–15396. doi: 10.1074/jbc.M010534200. [DOI] [PubMed] [Google Scholar]
- 64.Sonora C, et al. Immunohistochemical analysis of MUC5B apomucin expression in breast cancer and non-malignant breast tissues. J Histochem Cytochem. 2006;54:289–299. doi: 10.1369/jhc.5A6763.2005. [DOI] [PubMed] [Google Scholar]
- 65.Guilmeau S, et al. Intestinal deletion of Pofut1 in the mouse inactivates notch signaling and causes enterocolitis. Gastroenterology. 2008;135:849–860. doi: 10.1053/j.gastro.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun. 2004;72:6040–6049. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McAuley JL, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindén SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLoS One. 2008;3:e3952. doi: 10.1371/journal.pone.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ueno K, et al. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38:263–268. doi: 10.1165/rcmb.2007-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: transgenic expression of human MUC1 in IL-10−/− mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol. 2007;179:735–739. doi: 10.4049/jimmunol.179.2.735. This work and reference 67 provided evidence that MUC1 is important in the development of inflammatory bowel disease and colon cancer. [DOI] [PubMed] [Google Scholar]
- 71.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev Immunol. 2005;10:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 72.Ahmad R, et al. MUC1 oncoprotein activates the IκB kinase β complex and constitutive NF-κB signaling. Nature Cell Biol. 2007;9:1419–1427. doi: 10.1038/ncb1661. These findings demonstrated that MUC1-C activates the NF-κB pathway by interacting with IKKβ and led to the identification of a direct interaction between MUC1-C and the NF-κB family member RELA (reference 73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmad R, et al. MUC1-C oncoprotein functions as a direct activator of the NF-κB p65 transcription factor. Cancer Res. 2009;69:7013–7021. doi: 10.1158/0008-5472.CAN-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vinall LE, et al. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology. 2002;123:41–49. doi: 10.1053/gast.2002.34157. [DOI] [PubMed] [Google Scholar]
- 75.Kondo S, et al. MUC1 induced by Epstein-Barr virus latent membrane protein 1 causes dissociation of the cell-matrix interaction and cellular invasiveness via STAT signaling. J Virol. 2007;81:1554–1562. doi: 10.1128/JVI.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merlo G, et al. DF3 tumor-associated antigen gene is located in a region on chromosome 1q frequently altered in primary human breast cancer. Cancer Res. 1989;49:6966–6971. [PubMed] [Google Scholar]
- 77.Kufe D. Functional targeting of the MUC1 oncogen in human cancers. Cancer Biol Ther. 2009;8:1199–1205. doi: 10.4161/cbt.8.13.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agata N, et al. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008;68:6136–6144. doi: 10.1158/0008-5472.CAN-08-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schroeder JA, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23:5739–5747. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 80.Pochampalli MR, Bitler BG, Schroeder JA. Transforming growth factora-dependent cancer progression is modulated by MUC1. Cancer Res. 2007;67:6591–6598. doi: 10.1158/0008-5472.CAN-06-4518. [DOI] [PubMed] [Google Scholar]
- 81.Raina D, et al. Direct targeting of the MUC1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. This work represents the first demonstration that the MUC1-C cytoplasmic domain is a direct drug target and that inhibition of MUC1-C oligomerization is effective in inducing the complete regression of established human breast tumour xenografts in preclinical models. A subsequent report has demonstrated similar findings in MUC1-positive human prostate tumor xenografts (reference 82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joshi MD, et al. MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol Cancer Ther. 2009;8:3056–3065. doi: 10.1158/1535-7163.MCT-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22:6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramasamy S, et al. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pochampalli MR, el Bejjani RM, Schroeder JA. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693–1701. doi: 10.1038/sj.onc.1209976. [DOI] [PubMed] [Google Scholar]
- 86.Kinlough CL, et al. Recycling of MUC1 is dependent on its palmitoylation. J Biol Chem. 2006;281:12112–12122. doi: 10.1074/jbc.M512996200. [DOI] [PubMed] [Google Scholar]
- 87.Leng Y, et al. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 88.Zrihan-Licht S, Baruch A, Keydar I, Wreschner DH. Phosphorylation of the MUC1 breast cancer membrane proteins: cytokine receptor-like molecules. FEBS Lett. 1994;356:130–137. doi: 10.1016/0014-5793(94)01251-2. [DOI] [PubMed] [Google Scholar]
- 89.Ren J, et al. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res. 2006;4:873–883. doi: 10.1158/1541-7786.MCR-06-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carson D. The cytoplasmic tail of MUC1: a very busy place. Sci Signal. 2008;1:pe35. doi: 10.1126/scisignal.127pe35. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3β with the DF3/MUC1 carcinoma-associated antigen and β-catenin. Mol Cell Biol. 1998;18:7216–7224. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3β and β-catenin. J Biol Chem. 2001;276:6061–6064. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 93.Wei X, Xu H, Kufe D. Human MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–178. doi: 10.1016/j.ccr.2005.01.008. These findings demonstrate that MUC1-C interacts with p53, occupies promoters of p53 target genes and regulates p53-dependent transcription. Subsequent work demonstrated that MUC1-C interacts with oestrogen receptor-α (reference 125) and RELA (reference 73) on promoters of their target genes. [DOI] [PubMed] [Google Scholar]
- 94.Komatsu M, Carraway C, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–3354. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- 95.Komatsu M, Tatum L, Altman NH, Carothers Carraway CA, Carraway KL. Potentiation of metastasis by cell surface sialomucin complex (rat MUC4), a multifunctional anti-adhesive glycoprotein. Int J Cancer. 2000;87:480–486. doi: 10.1002/1097-0215(20000815)87:4<480::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 96.Price-Schiavi SA, et al. Rat MUC4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 97.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- 98.Komatsu M, Jepson S, Arango ME, Carothers Carraway CA, Carraway K. MUC4/sialomucin complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene. 2001;20:461–470. doi: 10.1038/sj.onc.1204106. This work provided evidence that the interaction between MUC4 and ERBB2 promotes tumour growth and suppresses apoptosis, and led to the findings that expression of MUC4 is sufficient to induce transformation (reference 103) and that multiple mechanisms are responsible for the anti-apoptotic effect (reference 100) [DOI] [PubMed] [Google Scholar]
- 99.Chaturvedi P, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;4:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 100.Workman HC, Sweeney C, Carraway KL. The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms. Cancer Res. 2009;69:2845–2852. doi: 10.1158/0008-5472.CAN-08-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 102.Moniaux N, et al. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97:345–357. doi: 10.1038/sj.bjc.6603868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bafna S, et al. MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 2008;68:9231–9238. doi: 10.1158/0008-5472.CAN-08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams SJ, et al. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 105.Walsh MD, et al. The MUC13 cell surface mucin is highly expressed by human colorectal carcinomas. Hum Pathol. 2007;38:883–892. doi: 10.1016/j.humpath.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 106.Shimamura T, et al. Overexpression of MUC13 is associated with intestinal-type gastric cancer. Cancer Sci. 2005;96:265–273. doi: 10.1111/j.1349-7006.2005.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Packer LM, Williams SJ, Callaghan S, Gotley DC, McGuckin MA. Expression of the cell surface mucin gene family in adenocarcinomas. Int J Oncol. 2004;25:1119–1126. [PubMed] [Google Scholar]
- 108.Chauhan SC, et al. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125) Mod Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- 109.Chauhan SC, et al. Expression and functions of transmembrane mucin MUC13 in ovarian cancer. Cancer Res. 2009;69:765–774. doi: 10.1158/0008-5472.CAN-08-0587. [DOI] [PubMed] [Google Scholar]
- 110.Bast RC, Jr, et al. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 112.O’Brien TJ, et al. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001;22:348–66. doi: 10.1159/000050638. [DOI] [PubMed] [Google Scholar]
- 113.Bast RC, Jr, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. These findings and those in reference 110 identified MUC16 as an ovarian tumor-associated antigen and led to the use of the MUC16 plasma assay for the early detection of patients with ovarian cancer. [DOI] [PubMed] [Google Scholar]
- 114.Rump A, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 115.Kaneko O, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takahashi T, et al. Expression of MUC-1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol. 1994;153:2102–2109. [PubMed] [Google Scholar]
- 117.Dyomin VG, et al. MUC1 is activated in a B-cell lymphoma by the t(1;14)(q21;q32) translocation and is rearranged and amplified in B-cell lymphoma subsets. Blood. 2000;95:2666–2671. [PubMed] [Google Scholar]
- 118.Brossart P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;29:6846–6850. [PubMed] [Google Scholar]
- 119.Fatrai S, et al. Mucin1 expression is enriched in the human stem cell fraction of cord blood and is upregulated in majority of the AML cases. Exp Hematol. 2008;36:1254–1265. doi: 10.1016/j.exphem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 120.Kawano T, Ahmad R, Nogi H, Agata N, Anderson K, Kufe D. MUC1 oncoprotein promotes growth and survival of human multiple myeloma cells. Int J Oncology. 2008;33:153–159. [PMC free article] [PubMed] [Google Scholar]
- 121.Kawano T, et al. MUC1 oncoprotein regulates Bcr-Abl stability and pathogenesis in chronic myelogenous leukemia cells. Cancer Res. 2007;67:11576–11584. doi: 10.1158/0008-5472.CAN-07-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hayes DF, et al. Use of a murine monoclonal antibody for detection of circulating DF3 antigen levels in breast cancer patients. J Clin Invest. 1985;75:1671–1678. doi: 10.1172/JCI111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001;91:1973–1982. doi: 10.1002/1097-0142(20010601)91:11<1973::aid-cncr1222>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 124.Khodarev N, et al. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69:2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor a. Mol Cell. 2006;21:295–305. doi: 10.1016/j.molcel.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 126.Pitroda S, Khodarev N, Beckett M, Kufe D, Weichselbaum R. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci USA. 2009;106:5837–5841. doi: 10.1073/pnas.0812029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wreesmann VB, et al. Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res. 2004;64:3780–3789. doi: 10.1158/0008-5472.CAN-03-1460. [DOI] [PubMed] [Google Scholar]
- 129.Saitou M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nagata K, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–254. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 131.Miyahara N, et al. MUC4 interacts with ErbB2 in human gallbladder carcinoma: potential pathobiological implications. Eur J Cancer. 2008;44:1048–1056. doi: 10.1016/j.ejca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 132.Giuntoli RL, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546–5550. [PubMed] [Google Scholar]
- 133.Davidson B, Baekelandt M, Shih I. MUC4 is upregulated in ovarian carcinoma effusions and differentiates carcinoma cells from mesothelial cells. Diagn Cytopathol. 2007;35:756–760. doi: 10.1002/dc.20771. [DOI] [PubMed] [Google Scholar]
- 134.Rakha EA, et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 135.Kwon KY, et al. MUC4 expression in non-small cell lung carcinomas: relationship to tumor histology and patient survival. Arch Pathol Lab Med. 2007;131:593–598. doi: 10.5858/2007-131-593-MEINCL. [DOI] [PubMed] [Google Scholar]
- 136.Karg A, Dinç ZA, Başok O, Uçvet A. MUC4 expression and its relation to ErbB2 expression, apoptosis, proliferation, differentiation, and tumor stage in non-small cell lung cancer (NSCLC) Pathol Res Pract. 2006;202:577–583. doi: 10.1016/j.prp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 137.Andrianifahanana M, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 138.Swartz MJ, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–796. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- 139.Jhala N, et al. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 140.Shibahara H, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–229. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 141.Tamada S, et al. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12:4257–4264. doi: 10.1158/1078-0432.CCR-05-2814. [DOI] [PubMed] [Google Scholar]
- 142.Llinares K, et al. Diagnostic value of MUC4 immunostaining in distinguishing epithelial mesothelioma and lung adenocarcinoma. Mod Pathol. 2004;2:150–157. doi: 10.1038/modpathol.3800027. [DOI] [PubMed] [Google Scholar]
- 143.Rustin GJ, et al. Use of CA-125 in clinical trial evaluation of new therapeutic drugs for ovarian cancer. Clin Cancer Res. 2004;10:3919–3926. doi: 10.1158/1078-0432.CCR-03-0787. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Z, et al. Combining multiple serum tumor markers improves detection of stage I epithelial ovarian cancer. Gynecol Oncol. 2007;107:526–531. doi: 10.1016/j.ygyno.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nature Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rubinstein DB, et al. The MUC1 oncoprotein as a functional target: immunotoxin binding to alpha/beta junction mediates cell killing. Int J Cancer. 2009;124:46–54. doi: 10.1002/ijc.23910. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y, et al. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res. 2007;67:4924–4932. doi: 10.1158/0008-5472.CAN-06-4512. [DOI] [PubMed] [Google Scholar]
- 148.Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev Immunol. 2007;27:451–462. doi: 10.1615/critrevimmunol.v27.i5.40. [DOI] [PubMed] [Google Scholar]
- 149.Bitler B, et al. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res. 2009;15:100–109. doi: 10.1158/1078-0432.CCR-08-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yin L, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem. 2003;278:35458–35464. doi: 10.1074/jbc.M301987200. [DOI] [PubMed] [Google Scholar]
- 151.Yin L, Huang L, Kufe D. MUC1 oncoprotein activates the FOXO3a transcription factor in a survival response to oxidative stress. J Biol Chem. 2004;279:45721–45727. doi: 10.1074/jbc.M408027200. [DOI] [PubMed] [Google Scholar]
- 152.Yin L, Kharbanda S, Kufe D. Mucin 1 oncoprotein blocks hypoxia-inducible factor 1α activation in a survival response to hypoxia. J Biol Chem. 2007;282:257–266. doi: 10.1074/jbc.M610156200. [DOI] [PubMed] [Google Scholar]
- 153.Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein promotes autophagy in a survival response to glucose deprivation. Int J Oncol. 2009;34:1691–1699. doi: 10.3892/ijo_00000300. [DOI] [PMC free article] [PubMed] [Google Scholar]