Abstract
In this issue of Immunity, Ahern et al. (2010) show that IL-23R signaling in CD4+ T cells induces effector and suppresses regulatory CD4+ T cells, which is critical for the development of intestinal but not systemic inflammation.
Different lineages of effector CD4+ T cells, which include T helper 1 (Th1), Th2 and Th17 cells, are important for the protection against different classes of pathogens. However, these effector CD4+ T cells may also respond to auto- or environmental antigens resulting in chronic inflammatory or autoimmune diseases. Therefore, the immune system has developed several mechanisms to control these effector T cells. The two most studied subsets of CD4+ regulatory T cells, which can control effector T cells, are the Foxp3+CD4+CD25+ regulatory T (Treg) cells and the T regulatory type 1 (Tr1) cells. The balance between effector and Treg cells is crucial for the maintenance of immune homeostasis, especially in the intestine, where the immune system is continuously confronted with commensal microflora (Littman and Rudensky, 2010). In contrast, imbalance between effector and Treg cells is linked to many human autoimmune and chronic inflammatory diseases; one of which is inflammatory bowel disease (IBD). The cytokine IL-23 was shown to be important for the sustenance of Th17 cell response (McGeachy et al., 2009). IL-23 also plays an important role in the development of IBD in humans and in mouse colitis models (Duerr et al., 2006; Elson et al., 2007). However, precisely how IL-23 controls the development of intestinal pathology and which cell type it acts on is unclear. In this issue of Immunity, Ahern et al. (2010) show that IL-23R signaling in CD4+ T cells induces effector and suppresses regulatory CD4+ T cells. IL-23R signaling in T cells was therefore critical for the development of intestinal pathology.
The T cell transfer colitis model is frequently used to study the balance between effector and Treg cells in the intestine. In this animal model, CD4+CD25-CD45RBhi cells (mainly naïve T cells) are transferred into an immune deficient host (Rag1-/-). Upon transfer of the T cells to this lymphopenic environment, they proliferate, differentiate into effector T cells and cause pathology especially in the colon (Read et al., 2000). T cell transfer colitis seems to be mediated by both Th1 and Th17 cells (Leppkes et al., 2009; Neurath et al., 2002). In contrast, Treg and Tr1 cells, as well as their cytokine products IL-10 and TGF-β1, have been shown to have protective functions in this model (Groux et al., 1997; Li and Flavell, 2008; Read et al., 2000).
In this issue of Immunity, Ahern et al. (2010) aimed to study directly the role of IL-23R signaling in T cells in colitis. To that end they transferred CD4+CD45RBhi T cells isolated from wild-type or Il23r-/- mice into Rag1-/- hosts. Interestingly, IL-23R signaling in T cells was critical for the development of intestinal pathology, but not of systemic inflammation. In line with this observation, the authors found that recipients of Il23r-/- T cell had reduced T cell numbers in the colon, but not in the spleen. This effect was not due to an altered migration of Il23r-/- T cell in the Rag1-/- host, but rather, was due to an impaired proliferation of effector T cells in the colon. These data indicate a tissue specific role of IL-23R signaling.
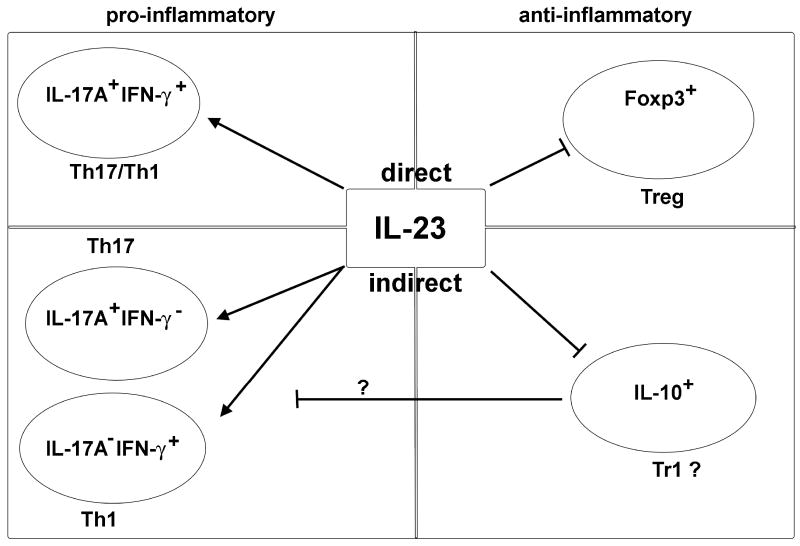
CD4+ effector T cell lineages are defined by the production of signature cytokines and also in some cases by the expression of a specific transcription factor. Based on intracellular cytokine staining for IL-17A and IFN- γ, there are three major effector T cell populations that can be identified in the colon in the T cell transfer colitis model. Those are the IL-17A+IFN-γ-, IL-17A+IFN- γ+, and IL-17A-IFN- γ+ cells. However, it is not clear whether the IL-17A and IFN- γ double producing cells are ‘Th17’ or ‘Th1’ cells. Therefore, these cells are referred as ‘Th17+Th1’ cells. Additionally, there is increasing evidence that Th17 cells are plastic and can differentiate into other T cell lineages (e.g. Th1 cells). In order to better understand the stability and function of these ‘Th17’ and ‘Th17+Th1’ cell subsets, further studies using reporter mice to specifically isolate and analyze these cells ex vivo will be essential. Nevertheless, it is interesting to elucidate which of these effector CD4+ T cell subsets is dependent on IL-23R signaling in colitis. Ahern et al. (2010) have found that Rag1-/- recipients of Il23r-/- CD4+CD45RBhi T cells showed selective reduction in the frequency of IL-17A+IFN- γ+ cells, but not in IL-17A+IFN- γ- or IL-17A-IFN- γ+ cells in the colon. In addition, the frequency of Foxp3+ Treg cells in the colon was also increased compared to Rag1-/- mice that received wild-type CD4+CD45RBhi T cells. Thus, IL-23 seems to inhibit Foxp3+ Treg cells, while promoting the emergence of IL-17A+IFN- γ+ effector T cells, which are together crucial for the development of intestinal disease.
Because recipients of Il23r-/- T cell had strongly reduced total CD4+ T cell numbers in the colon (including IL-17A+IFN- γ-, IL-17A+IFN- γ+, IL-17A-IFN- γ+ producing T cells), Ahern et al. employed an elegant co-transfer systems aimed to determine which of the T cell subsets was reduced due to a direct cell intrinsic effect of IL-23 on T cells, and whether there was a T cell extrinsic effect due to the absence of the inflammatory milieu in the mice receiving Il23r-/- T cells. To this aim, they co-transferred naïve T cells isolated from CD45.2- wild type or CD45.2+ Il23r-/- mice into Rag1-/- recipients. Interestingly, the presence of IL-23R sufficient T cells and their subsequent downstream inflammatory signals were able to overcome the requirement of IL-23R signaling for the T cells to accumulate in the intestine. These data contrast with a previous study reporting a cell intrinsic role of IL-23R expression in Th17 cell accumulation in the brain during EAE (experimental autoimmune encephalomyelitis), which could not be overcome by the presence of wild-type T cells (McGeachy et al., 2009). Together, these data again indicate a tissue specific role of IL-23R signaling, which requires further investigation of the inflammatory milieu responsible for this difference. Although the total number of CD45.2- wild-type and CD45.2+ Il23r-/- T cells in the Rag1-/- recipient mice was only slightly different, Ahern et al. (2010) still found a reduced frequency of CD45.2+IL-17A+IFN- γ+ T cells and an increased frequency of CD45.2+Foxp3+ T cells compared to the CD45.1 wild-type population in the colon. These data indicated that these two important effector and regulatory T cell subsets are directly controlled by cell intrinsic IL-23R signaling in T cells (Figure 1). Despite the difference in these subsets, the incidence and severity of colitis were similar between Rag1-/- mice receiving mixture of CD45.2-wild-type and CD45.2+ Il23r-/- T cells or wild-type naïve T cells only. This suggests that IL-23 either induces a potent pro-inflammatory response or inhibits an important anti-inflammatory factor in the IL-23 responsive wild type T cells, which then acts on the co-transferred Il23r-/- T cells.
Figure 1. Direct and indirect effects of IL-23 on T cell subsets in the colon.
IL-23R signaling in CD4+ T cells is crucial for the accumulation of effector CD4+ T cells in the intestine. It directly promotes IL-17A+IFN-γ+ CD4+ T cells, while it inhibits Foxp3+ Treg cells. In contrast, IL-23 has an indirect inhibitory effect on IL-10 producing cells (which might be Tr1 cells) via an unidentified mediator. Additionally, IL-23 is indirectly promoting Th17 and Th1 cells, which might be mediated by suppression of IL-10 production by CD4+ T cells. Therefore IL-23 shifts the balance from an anti-inflammatory to a pro-inflammatory milieu in the intestine via direct and indirect effects on CD4+ T cell subsets.
Ahern et al. found that IL-10 production by Il23r-/- T cells was increased compared to wild type T cells, when these cells were transferred alone into Rag1-/- recipients. By contrast, Il23r-/- T cells isolated from the colon of mice co-transferred with wild type cells did not demonstrate increased IL-10 production. These data suggest that IL-23 responsive T cells somehow suppress the increased IL-10 production by Il23r-/- T cells (Figure 1). This is a very interesting finding, although it warrants further exploration: Several T cell subsets including Treg, Tr1, and also Th17 cells are able to produce IL-10 (Li and Flavell, 2008). Additionally, a variety of immune cells can respond to IL-10. It is not clear, whether IL-10 acts on antigen presenting cells or directly on different T cell subsets, which include IL-17A+IFN- γ-, IL-17A+IFN- γ+, IL-17A-IFN- γ+, and also Treg cells, in the inflamed colon. Further studies using mouse models with cell-specific blockade of IL-10 signaling in combination with reporter mice seem to be essential to address this important question.
The balance between effector and regulatory CD4 T cell is of great importance for the maintenance of immune homeostasis, especially in the colon. Ahern et al. demonstrated that IL-23R signaling in T cells themselves has an important impact on this balance in the colon. They found that IL-23R signaling in T cells played a crucial role for their proliferation and accumulation in the colon. This was mediated via an indirect cell extrinsic effect on IL-17A+IFN- γ-, IL-17A-IFN- γ+ and IL-10-producing T cells. But more importantly, IL-23R signaling promoted the emergence of IL-17A+IFN- γ+ T cells, while inhibiting Foxp3+ Treg in a direct manner (Figure 1). Thus IL-23 has a dual effect acting on regulatory and effector T cells. Considering the human genetic studies, which link IL-23R signaling to the pathogenesis of IBD, the data by Ahern et al. implicate that blockade of IL-23 might restore the balance between effector and regulatory T cells in IBD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. IL-23 Drives Intestinal Inflammation Through Direct Activity on T cells. Immunity. 2010 doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cellmediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]