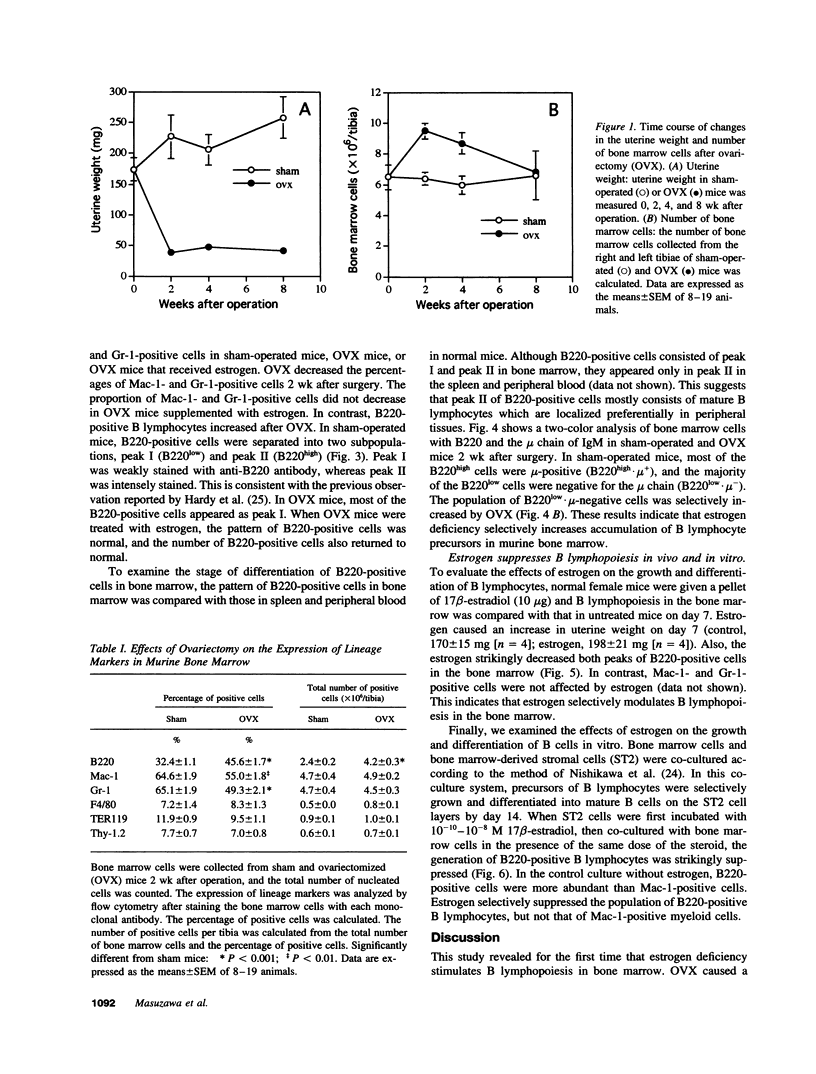
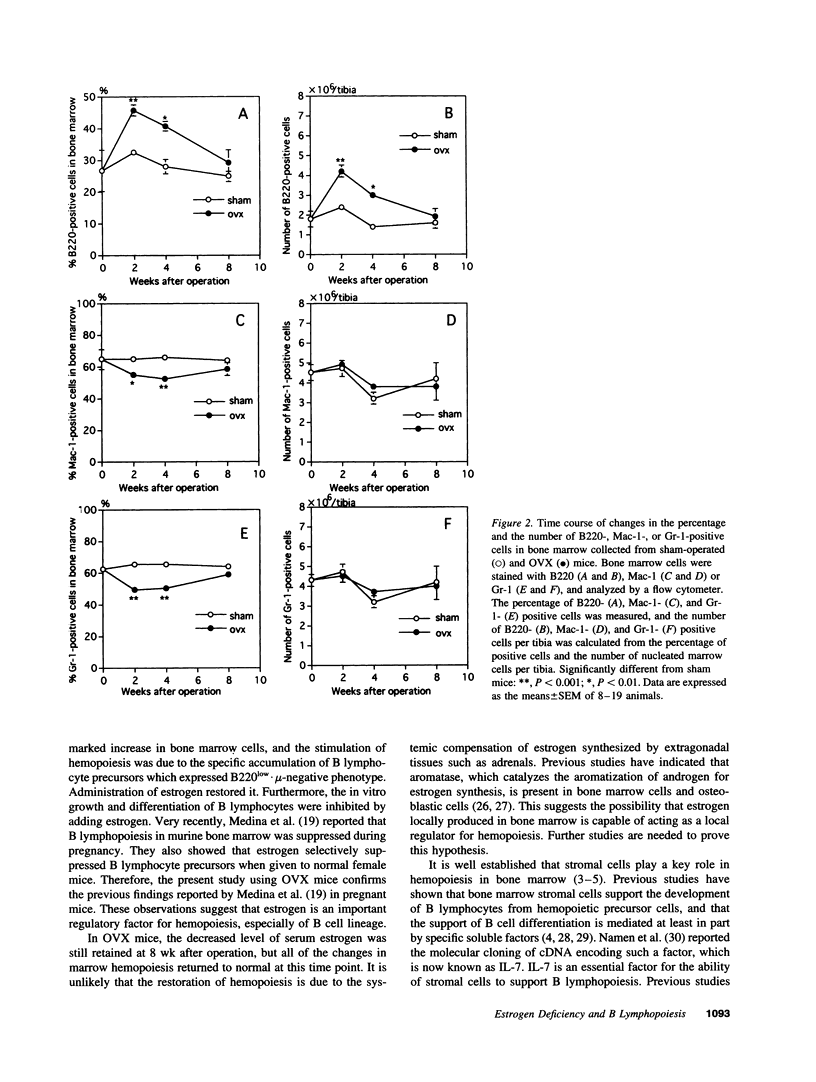
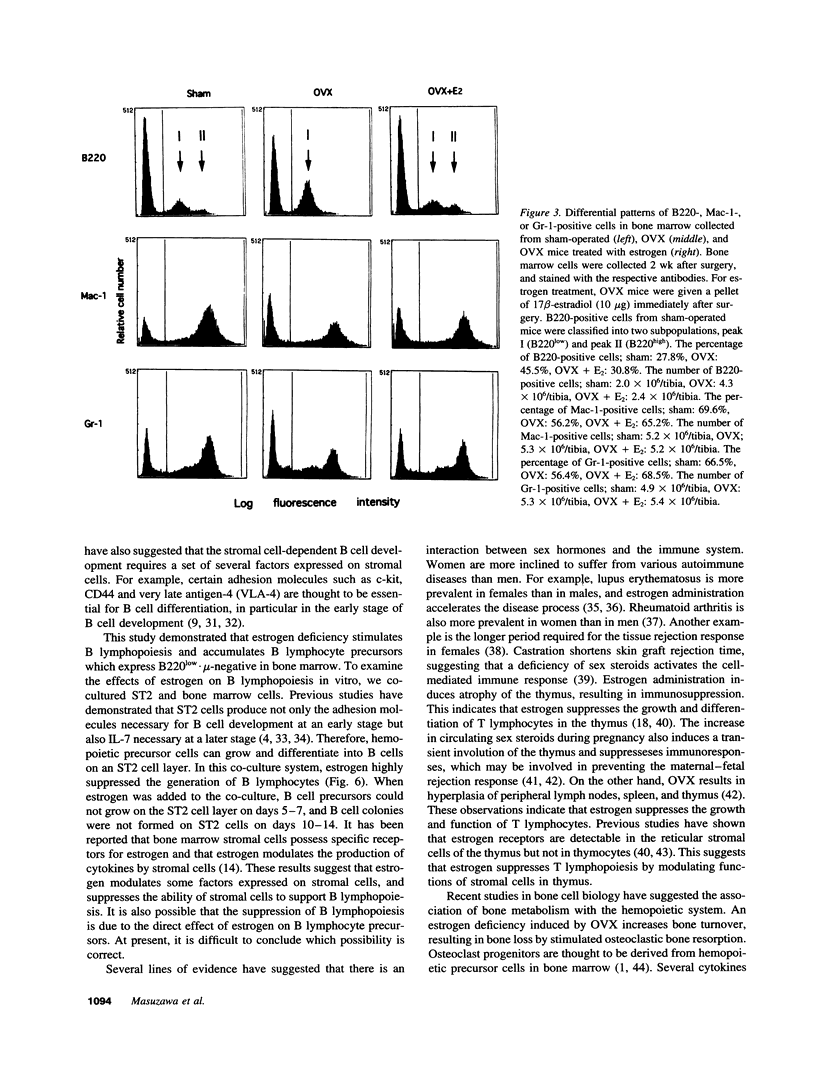
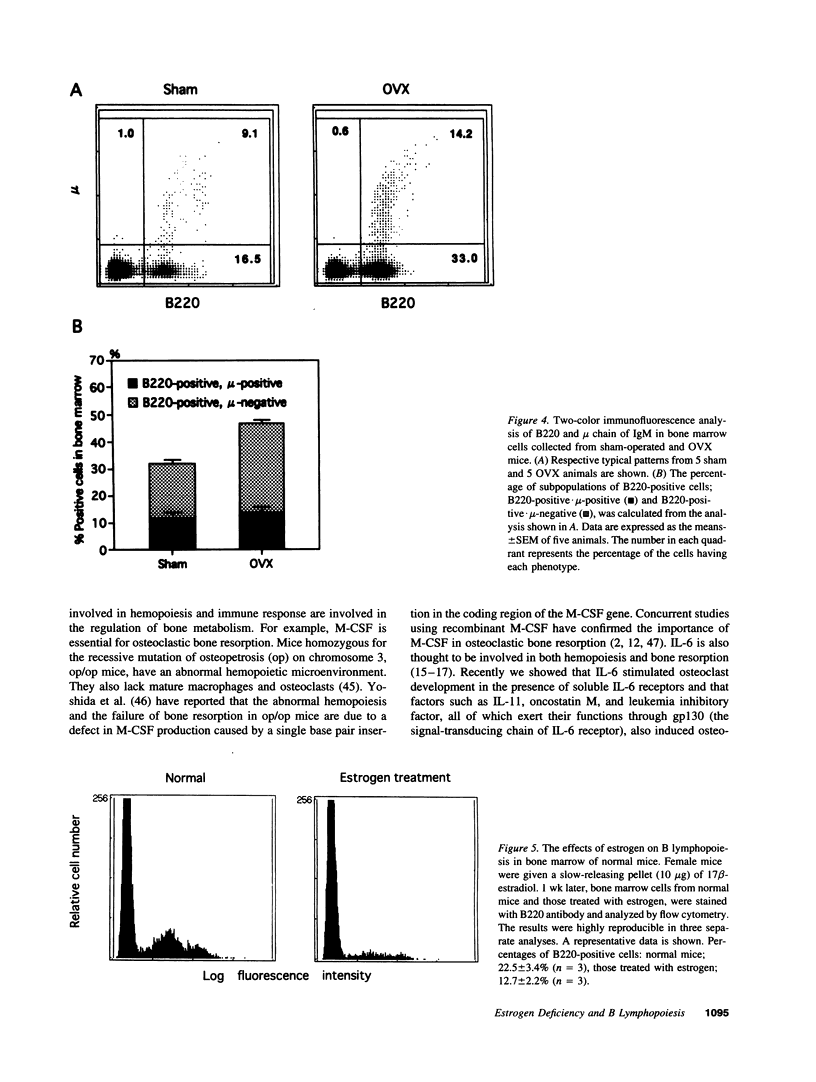
Abstract
We have found that an estrogen deficiency causes a marked increase in bone marrow cells. To examine the effect of estrogen on hemopoiesis, we characterized the increased population of bone marrow cells after ovariectomy (OVX). In OVX mice, the percentage of myeloid cells and granulocytes was decreased, whereas that of B220-positive B lymphocytes was selectively increased 2-4 wk after surgery. The total number of myeloid cells and granulocytes did not change appreciably, but that of B220-positive cells was greatly increased by OVX. When OVX mice were treated with estrogen, the increased B lymphopoiesis returned to normal. B220-positive cells were classified into two subpopulations, B220low and B220high. The majority of the B220low cells were negative for the IgM mu chain, whereas most of the B220high cells were mu-positive. OVX selectively increased the precursors of B lymphocytes identified by B220low. mu-negative phenotype, suggesting that an estrogen deficiency stimulates accumulation of B lymphocyte precursors. When bone marrow-derived stromal cells (ST2) were pretreated with estrogen then co-cultured with bone marrow cells in the presence of estrogen, the stromal cell-dependent B lymphopoiesis was greatly inhibited. The present study suggests that estrogen plays an important role in the regulation of B lymphocyte development in mouse bone marrow.
Full text
PDF

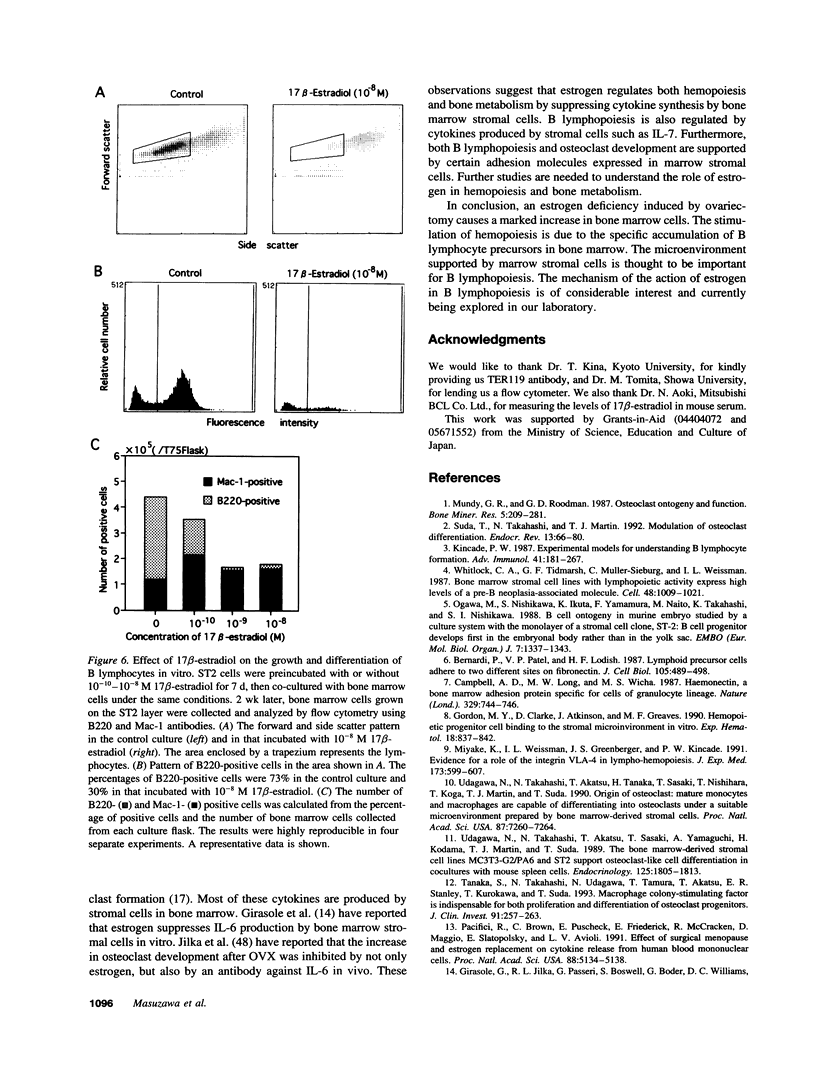

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablin R. J., Bhatti R. A., Guinan P. D., Khin W. Modulatory effects of oestrogen on immunological responsiveness. II. Suppression of tumour-associated immunity in patients with prostatic cancer. Clin Exp Immunol. 1979 Oct;38(1):83–91. [PMC free article] [PubMed] [Google Scholar]
- BARDAWIL W. A., MITCHELL G. W., Jr, McKEOGH R. P., MARCHANT D. J. Behavior of skin homografts in human pregnancy. I. Habitual abortion. Am J Obstet Gynecol. 1962 Nov 15;84:1283–1299. doi: 10.1016/s0002-9378(16)35735-0. [DOI] [PubMed] [Google Scholar]
- Barr I. G., Khalid B. A., Pearce P., Toh B. H., Bartlett P. F., Scollay R. G., Funder J. W. Dihydrotestosterone and estradiol deplete corticosensitive thymocytes lacking in receptors for these hormones. J Immunol. 1982 Jun;128(6):2825–2828. [PubMed] [Google Scholar]
- Bernardi P., Patel V. P., Lodish H. F. Lymphoid precursor cells adhere to two different sites on fibronectin. J Cell Biol. 1987 Jul;105(1):489–498. doi: 10.1083/jcb.105.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. D., Long M. W., Wicha M. S. Haemonectin, a bone marrow adhesion protein specific for cells of granulocyte lineage. Nature. 1987 Oct 22;329(6141):744–746. doi: 10.1038/329744a0. [DOI] [PubMed] [Google Scholar]
- Frisch R. E., Canick J. A., Tulchinsky D. Human fatty marrow aromatizes androgen to estrogen. J Clin Endocrinol Metab. 1980 Aug;51(2):394–396. doi: 10.1210/jcem-51-2-394. [DOI] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. Y., Clarke D., Atkinson J., Greaves M. F. Hemopoietic progenitor cell binding to the stromal microenvironment in vitro. Exp Hematol. 1990 Aug;18(7):837–842. [PubMed] [Google Scholar]
- Graff R. J., Lappé M. A., Snell G. D. The influence of the gonads and adrenal glands on the immune response to skin grafts. Transplantation. 1969 Feb;7(2):105–111. doi: 10.1097/00007890-196902000-00003. [DOI] [PubMed] [Google Scholar]
- Grossman C. J. Interactions between the gonadal steroids and the immune system. Science. 1985 Jan 18;227(4684):257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Kunisada T., Ogawa M., Sudo T., Kodama H., Suda T., Nishikawa S., Nishikawa S. Stepwise progression of B lineage differentiation supported by interleukin 7 and other stromal cell molecules. J Exp Med. 1990 May 1;171(5):1683–1695. doi: 10.1084/jem.171.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y. H., Weissman I. L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990 Sep 7;62(5):863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ishimi Y., Miyaura C., Jin C. H., Akatsu T., Abe E., Nakamura Y., Yamaguchi A., Yoshiki S., Matsuda T., Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990 Nov 15;145(10):3297–3303. [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Kincade P. W. Experimental models for understanding B lymphocyte formation. Adv Immunol. 1987;41:181–267. doi: 10.1016/s0065-2776(08)60032-2. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991 Jan 1;173(1):269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N., Bertolini D., Suda T., Akiyama Y., Roodman G. D. IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. J Immunol. 1990 Jun 1;144(11):4226–4230. [PubMed] [Google Scholar]
- Landreth K. S., Dorshkind K. Pre-B cell generation potentiated by soluble factors from a bone marrow stromal cell line. J Immunol. 1988 Feb 1;140(3):845–852. [PubMed] [Google Scholar]
- Medina K. L., Smithson G., Kincade P. W. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993 Nov 1;178(5):1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijweide P. J., Burger E. H., Feyen J. H. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986 Oct;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Ogawa M., Nishikawa S., Kunisada T., Kodama H. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur J Immunol. 1988 Nov;18(11):1767–1771. doi: 10.1002/eji.1830181117. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Nishikawa S., Ikuta K., Yamamura F., Naito M., Takahashi K., Nishikawa S. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988 May;7(5):1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Nakauchi H., Nagayoshi K., Nishikawa S., Nishikawa S., Miura Y., Suda T. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood. 1991 Oct 1;78(7):1706–1712. [PubMed] [Google Scholar]
- Pacifici R., Brown C., Puscheck E., Friedrich E., Slatopolsky E., Maggio D., McCracken R., Avioli L. V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit A., Flanagan A. M., Reed M. J. Estrogen synthesis by osteoblast cell lines. Endocrinology. 1992 Oct;131(4):2027–2029. doi: 10.1210/endo.131.4.1396346. [DOI] [PubMed] [Google Scholar]
- Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. Delayed androgen treatment prolongs survival in murine lupus. J Clin Invest. 1979 May;63(5):902–911. doi: 10.1172/JCI109390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Screpanti I., Morrone S., Meco D., Santoni A., Gulino A., Paolini R., Crisanti A., Mathieson B. J., Frati L. Steroid sensitivity of thymocyte subpopulations during intrathymic differentiation. Effects of 17 beta-estradiol and dexamethasone on subsets expressing T cell antigen receptor or IL-2 receptor. J Immunol. 1989 May 15;142(10):3378–3383. [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Sudo T., Ito M., Ogawa Y., Iizuka M., Kodama H., Kunisada T., Hayashi S., Ogawa M., Sakai K., Nishikawa S. Interleukin 7 production and function in stromal cell-dependent B cell development. J Exp Med. 1989 Jul 1;170(1):333–338. doi: 10.1084/jem.170.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Udagawa N., Takahashi N., Miyaura C., Tanaka S., Yamada Y., Koishihara Y., Ohsugi Y., Kumaki K., Taga T. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Takahashi N., Udagawa N., Tamura T., Akatsu T., Stanley E. R., Kurokawa T., Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993 Jan;91(1):257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. A., Jr The effects of estradiol upon the thymus of the sexually immature female mouse. J Steroid Biochem. 1981 Feb;14(2):167–174. doi: 10.1016/0022-4731(81)90170-9. [DOI] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Sasaki T., Yamaguchi A., Kodama H., Martin T. J., Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989 Oct;125(4):1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T. J., Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke J. P., Valkenburg H. A., Boersma J. W., Cats A., Festen J. J., Huber-Bruning O., Rasker J. J. Oral contraceptives and rheumatoid arthritis: further evidence for a preventive effect. Lancet. 1982 Oct 16;2(8303):839–842. doi: 10.1016/s0140-6736(82)90809-1. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Tidmarsh G. F., Muller-Sieburg C., Weissman I. L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]



