Abstract
Fatigue is a major complaint among cancer patients, yet it is unknown whether cancer-related fatigue experienced during the day relates to sleep/wake cycles or to the quality and quantity of sleep obtained at night. Although it is not well defined or well understood at present, cancer-related fatigue is generally regarded as a form of tiredness that does not improve following rest or sleep. Objectively recorded sleep and biological rhythms have not been well investigated in these patients, but it appears that most cancer patients may in fact not be getting a good night’s sleep. Evidence is accumulating that sleep is often disturbed in cancer patients, probably owing to a variety of causes. We posit that some degree of cancer-related fatigue experienced during the day may relate to sleep/wake cycles or to the quality and quantity of sleep obtained at night. Different components or dimensions of fatigue (physical, attentional/cognitive, emotional/affective, etc.) are probably associated in some way with disrupted sleep and desynchronized sleep/wake rhythms. These associations may change in measurable ways prior to treatment, during treatment and after treatment completion. In cancer patients, as in other medically ill patients, sleep that is inadequate or unrefreshing may be important not only to the expression of fatigue, but to the patients’ quality of life and their tolerance to treatment, and may influence the development of mood disorders and clinical depression. This review summarizes the state of the literature on fatigue, sleep and circadian rhythms.
Keywords: sleep, fatigue, cancer, circadian rhythms, chronotherapy
INTRODUCTION
Fatigue is one of the most frequent and disturbing complaints of cancer patients (Richardson 1995; Stein et al. 1998), with over 75% of patients undergoing chemotherapy or radiation therapy reporting feeling tired and weak (Winningham et al. 1994; Smets et al. 1996). As recently as the 1999 meeting of the American Society of Clinical Oncology, the Fatigue Coalition (Curt et al. 1999) reported that 76% of patients who had received chemotherapy reported fatigue at least once a week and 18% identified it as the most significant problem during therapy. Fatigue interferes with daily life, reduces quality of life (Frank Stromborg & Wright 1984; Richardson 1995; Visser & Smets 1998) and is often one of the key reasons for patients discontinuing treatment (Winningham et al. 1994). The Fatigue coalition recommended that the assessment and evaluation of fatigue as well as treatment options should be routinely considered.
Sleep disruption is also a very common complaint in patients with cancer (Derogatis et al. 1979; Hu & Silberfarb 1991; Silberfarb et al. 1993). It is unknown, however, whether fatigue has any relationship to the quality or quantity of sleep or to the sleep/wake circadian rhythm cycle. In fact, few studies have examined the circadian rhythms of cancer patients (Mormont & Levi 1997; Berger 1998; Miaskowski & Lee 1999; Morrow et al. 2000). This review attempts to bridge the gap between the growing awareness of fatigue that is secondary to or related to the cancer (i.e. cancer-related fatigue) and the possible contribution to this by sleep and biological rhythms.
BACKGROUND
There is no universally accepted definition of fatigue (Glaus 1998). Fatigue has been defined by patients as a decrease in strength and performance, tiredness, weakness, lack of energy, lethargy, depression, difficulty with concentration, lack of motivation and sleepiness (Winningham et al. 1994; Glaus 1998). Fatigue is believed to be caused by physical factors and psychological factors, as well as social factors (Stone et al. 1998) (Fig. 1). Physical factors of fatigue include cachexia, weight loss and biochemical, haematological and endocrine abnormalities (Stone et al. 1998). Studies examining nutritional status and fatigue, however, have not found overwhelming evidence to indicate a causal relationship in cancer patients (Bruera et al. 1989; Morant et al. 1993). Biochemical abnormalities, such as anaemia, are known to both cause fatigue and be present in patients with cancer. One recent study examined the incremental effect of increasing haemoglobin on quality of life (QOL) and found that improving the anaemia only improved QOL up to a point, beyond which there was no further improvement (Cleeland et al. 1999). Few studies, however, have examined the relationship between biochemical abnormalities and fatigue (Bruera et al. 1989), or between endocrine abnormalities and fatigue (Stone et al. 1998), in cancer patients.
Figure 1.
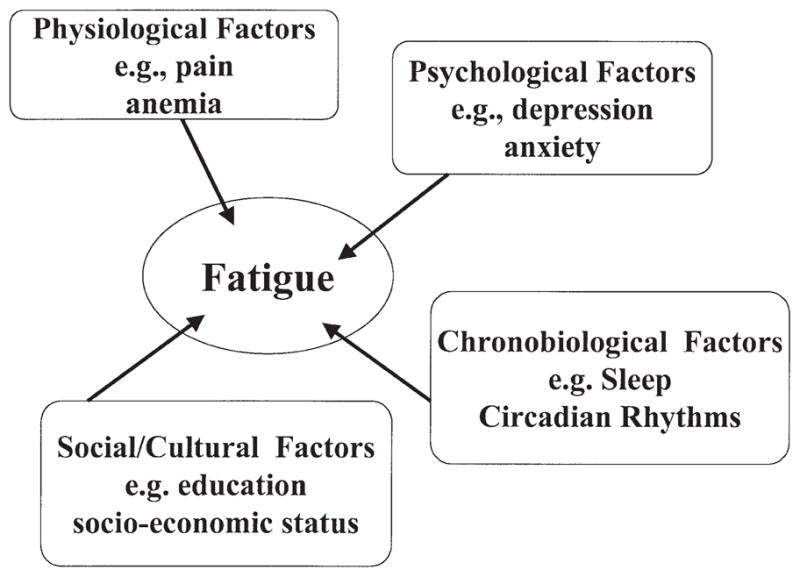
Diagrammatic representation of possible factors affecting fatigue as described in multiple studies (see text).
In addition to the physical factors of fatigue, there are psychological components of fatigue. Depression is common both in cancer patients and in patients reporting fatigue (e.g. Bruera et al. 1989; Morant et al. 1993; Smets et al. 1996; Broeckel et al. 1998). Broeckel et al. (1998) showed a relationship between self-reported fatigue and self-reports of depression, self-reports of menopausal symptoms and self-reports of poor sleep quality and sleeping during the day. However, cause and effect have not been determined.
Few studies have followed patients over time, but there is some evidence that, in breast cancer patients, the amount of fatigue may vary before and after treatment (Haes et al. 1987; Greenberg et al. 1992, 1993; Visser & Smets 1998). Other clinical studies have indicated that breast cancer patients experience fatigue for months after therapy has been completed (Bruera et al. 1989; Dow et al. 1996; Ferrell et al. 1996; Ganz et al. 1996; Goldstein et al. 2000). Jacobsen and colleagues examined fatigue in women for 1 year following completion of chemotherapy and found that, compared with women with no history of breast cancer, patients reported more severe fatigue, a poorer quality of life owing to fatigue and more menopausal symptoms (Broeckel et al. 1998). It is unclear whether these findings would hold true across different cancer groups or whether these effects are treatment and/or stage specific.
The few studies that have examined fatigue have primarily used questionnaires to measure it. However, in her review of the literature, Richardson (1995) noted that most questionnaires have not been validated and often fatigue was just one question in a more general instrument. Without a clear definition of fatigue and without a standardized way of measuring it, the study of fatigue was very difficult. More recently, new fatigue scales, such as the Fatigue Symptom Inventory (Hann et al. 1998) and the Multidimensional Fatigue Symptom Inventory (Stein et al. 1998), have been developed to be used specifically with cancer patients. The use of these validated scales might help to clarify some of the causes of and possible treatments for fatigue.
SLEEP DISRUPTION IN CANCER PATIENTS
While the results of some fatigue instruments have been compared with scales of sleepiness, the relationship between fatigue and the objectively measured quality and quantity of sleep has not been fully determined (Richardson 1998), as few studies to date have examined objectively measured sleep. In an older review of over 1500 cancer patients, Derogatis et al. (1979) found that the most frequent psychotropic prescription was for hypnotics, accounting for 48% of total prescriptions. In addition, out of 814 total prescriptions for hypnotics, ‘sleep’ was the physician’s stated reason for the prescription in 85% (n = 692) of cases compared with 14% (n = 114) for ‘medical procedure’, 1% (n = 8) for ‘nausea/vomiting’, 1% (n = 8) for ‘psychological distress’, none for ‘pain’ and none for ‘other’. Ten years later the same results were replicated (Stiefel et al. 1990), with 44% of 400 prescriptions for 200 consecutive cancer clinic patients being for hypnotic medications. These data suggest that sleep difficulty is a major problem in cancer patients. The patient sample in this particular study encompassed a broad range of cancer diagnoses and disease severities, and the time since diagnosis varied from 1 to 204 months (mean 23 months). The reported data, unfortunately, are not broken down by any of these categories, so it is unknown whether any of the findings are more applicable to certain groups of cancer patients than others.
There are multiple causes of sleep disruption in cancer patients. As outlined by Hu & Silberfarb (1991), patients may suffer from insomnia or from excessive daytime sleepiness (hypersomnia) or both. Possible causes include pain and affective or other psychiatric disorders (e.g. depression and anxiety). In addition, chemotherapy and radiation therapy are both known to produce some sleep disturbance. However, at this time, it is unknown whether the disruptions in sleep are partially secondary to pain, for example, and/or to the medications used to counteract the pain, or to what extent anxiety, depression, and chemotherapy and/or radiation therapy have an impact on sleep. Additional, well-controlled research studies are needed to tease out the answers to these questions.
Sleep, independent of its relationship to fatigue, may play an important role in the life of a cancer patient (Hu & Silberfarb 1991). Although circadian rhythms (24-h cycles of biological processes) and sleep have been widely studied in health and other illnesses, little is known about the links between these domains in cancer (see section on Circadian rhythms and treatment of cancer: chronotherapy). There is growing evidence to suggest, however, that disruptions in biological rhythmicity are relevant to cancer, the mitotic properties of cancerous cells themselves, the treatments of cancer and the time of day of their administration, and possibly the quality of life in cancer patients (Mormont & Levi 1997).
While some previous research has suggested that women with breast cancer do experience sleep problems (Knobf 1986; Berglund et al. 1991; Silberfarb et al. 1993), most have suggested that the sleep disturbance is secondary to the pain and psychological distress experienced by these patients (Hu & Silberfarb 1991). However, the true cause of the sleep problems has not been determined. The remainder of this section will focus on studies that have examined patients’ reports about their sleep (see Table 1 for a summary of the major studies).
Table 1.
Key findings of sleep and rhythm studies in cancer patients
| Number and type of patient | Fatigue measurement | Sleep/Rhythm measurements | Results | |
|---|---|---|---|---|
| Berger (1998) | 17 breast CA | Piper Fatigue Scale | Actigraphy | Fatigue and night-time restlessness ↑during chemotherapy |
| Berger & Farr (1999) | 72 breast CA | Piper Fatigue Scale | Actigraphy | Those less active during the day (i.e. had lower peak activity scores) had ↑awakenings, ↑levels of fatigue |
| Beszterczey & Lipowski (1977) | 47 radiotherapy | None | Self-report questionnaire | Insomnia was positively correlated with depression and anxiety but not with pain. |
| Cimprich (1999) | 74 breast CA | Symptom Distress Scale; POMS | None | High levels of distress related to insomnia, fatigue (tiredness) and loss of concentration. Insomnia most common complaint (88%) with >50% indicating high levels of distress owing to insomnia |
| Engstrom et al. (1999) | 150 CA – Phase I 42 CA – Phase II |
None | Telephone interviews | Phase I: 44% reported sleep difficulty; Phase II: 45% reported sleep difficulty, with half as severe; most frequent problems were mid-night awakenings, sleeping fewer hours, trouble getting back to sleep |
| Kaye et al. (1983) | 30 CA 28 cardiac 24 controls |
None | Sleep habits questionnaire | CA patients had more difficulty staying asleep compared with controls |
| Miaskowski & Lee (1999) | 24 bone metastases patients | Lee Fatigue Scale | Actigraphy | Fatigue ratings ↑ in evening and ↓in morning; SE ↓; fatigue associated with periods of greater inactivity; sleep fragmented |
| Mormont et al. (1996) | 30 colorectal CA | None | Actigraphy | Patients had less differentiation in rest/activity between night and day |
| Mormont et al. (2000) | 200 colorectal CA | None | Actigraphy | Survival at 2 years was fivefold higher in those with marked activity rhythms |
| Morrow et al. (1999) | 78 breast CA | Multidimensional Assessment of Fatigue; Fatigue Symptom Checklist; POMS | Actigraphy | More robust and consistent circadian rhythm patterns associated with ↓fatigue scores, even after controlling for depression |
| Owen et al. (1999) | 15 CA | None | Self-report; Pittsburgh Sleep Quality Inventory | Cancer patients reported significantly ↓sleep quality, ↓SE, ↑SOL |
| Silberfarb et al. (1993) | 15 breast CA 17 lung CA 32 insomniacs 32 Controls |
None | PSG measures of SE, SOL, WASO | Lung CA patients had ↓SE, ↑SOL and WASO compared with breast CA patients and controls |
CA, cancer; PSG, polysomnogram; SE, sleep efficiency; SOL, sleep onset latency; WASO, wake after sleep onset or the total time spent awake during the night.
Two early studies examined self-report about sleep in cancer patients, but sleep was not measured and the severity of the sleep disturbance was not examined (Beszterczey & Lipowski 1977; Kaye et al. 1983). The complaints ranged from difficulty falling asleep to difficulty staying asleep with frequent and prolonged nighttime awakenings. Patients reported these complaints both prior to treatment (Cimprich 1999) and during treatment (Engstrom et al. 1999; Owen et al. 1999). Information regarding sleep quality after treatment completion is scant.
Owen et al. (1999) compared reports of sleep quality over the previous month in patients with different types of cancer who were undergoing different types of treatment. Cancer patients reported significantly poorer overall sleep quality and more daytime dysfunction than previously published normative data using the same survey. Cimprich (1999) administered self-report items relating to sleep quality, fatigue and distress to breast cancer patients who had not yet undergone treatment of any kind. Insomnia was correlated with high levels of distress and was the most frequent symptom reported, with 88% (n = 65) of the sample reporting difficulty sleeping. It was found that subjective reports of distress and anxiety were correlated with the perception of insomnia and that, even before treatment had begun, self-ratings of fatigue and sleep difficulty were high. In patients whose self-ratings of anxiety (as well as anger) were low, insomnia and fatigue were still at high levels. This contrasts with the general notion that disturbed sleep prior to treatment is attributable to the increased anxiety and stress accompanying the recent diagnosis of a life-threatening illness. However, as treatment continues, so does the sleep disruption and, as Engstrom et al. (1999) posit, pain may be the cause of nocturnal awakenings, but the usual return to sleep is prevented by psychological distress.
Others have also attributed much of the insomnia reported by cancer patients to pain (Strang & Qvarner 1990). Lewin & Dahl (1999) point out that, in a variety of medical conditions, the management of pain interrelates with sleep quality in many ways. There are surprisingly few studies, however, supporting the notion that pain leads to disrupted sleep. Lewin and Dahl theorize that, as sleep leads to recovery and repair of tissue and may offer a temporary cessation of the psychological awareness of pain, poor sleep can lead to difficulty managing pain. In this way, a cycle of pain and poor sleep may become self-perpetuating.
In a study by Silberfarb et al. (1993), 32 cancer patients (15 breast cancer, 17 lung cancer), 32 age- and sex-matched normal volunteers and 32 patients with insomnia were compared. When pain was examined, only breast cancer patients complained of pain prior to bedtime; however, their sleep quality was not significantly affected. On the other hand, the poor sleep quality of the insomnia and lung cancer patients was not associated with reports of pain.
Other sleep survey studies have also been done in cancer patients. Engstrom et al. (1999) administered an extensive and sleep-specific telephone survey to 150 patients with lung and with breast cancer in various stages of treatment undergoing a variety of treatments. Of those interviewed, 44% (n = 66) reported a sleep problem in the previous month, but only about 17% (n = 25) communicated the problem to their doctors. In the second phase of the survey, another group of patients was interviewed, 45% (n = 20) of whom reported a sleep problem in the prior month, half of whom rated the sleep problem as moderate, severe or intolerable. The most frequent type of sleep problem was waking during the night, reported by more than 90% of patients (n = 18). About 85% (n = 17) complained of sleeping fewer hours than normal, 75% (n = 15) complained of difficulty in getting back to sleep and 39% (n = 8) reported napping at unusual times, such as mid-morning and mid-afternoon. These results help to identify the type of sleep complaints that cancer patients experience.
In another survey, the amount of insomnia in cancer patients was as high as the amount of insomnia found in depressed patients (Holland & Plumb 1977). As insomnia is so common in cancer patients, the possibility that it may indicate some depression should not be overlooked by clinicians (McDaniel et al. 1995). In all, the small number of studies that have looked at reports of sleep problems in cancer patients suggest that disrupted sleep may play an important role in the discomfort experienced by these patients; nevertheless, additional, well-controlled studies are needed.
LABORATORY STUDIES IN BIOLOGICAL RHYTHMS AND SLEEP IN CANCER
Objective laboratory recordings of sleep in cancer patients are few (see Table 2 for a glossary of terms). As mentioned above, Silberfarb et al. (1993) compared polysomnographically recorded sleep of 32 cancer patients (15 breast cancer, 17 lung cancer) with 32 age- and sex-matched normal volunteers and 32 patients with insomnia. As expected, the patients with insomnia had the shortest total sleep time of all the groups. Although the lung cancer patients spent more time in bed, they did not sleep more than the breast cancer patients or the normal controls. Therefore, lung cancer patients had lower sleep efficiency (the percentage of time in bed actually spent asleep) as well as longer sleep onset latency (the amount of time it takes to fall asleep initially) and spent more time awake during the night than those with breast cancer or the normal sleepers. There were no differences between the cancer patients and normal controls in self-ratings of stress, in clinician-evaluated emotional state, or in reported total time spent sleeping. Upon being questioned in the morning about the previous night’s sleep, cancer patients reported falling asleep easier and sleeping longer than the insomnia patients, but unlike the insomnia patients, did not under-report their total sleep time or exaggerate their sleep loss during the night. In fact, the lung cancer patients over-reported total amount of sleep, which contrasts with the tendency by insomniacs to under-report their sleep.
Table 2.
Glossary of sleep and rhythm related terms
| Term | Definition |
|---|---|
| Actigraphy | A measure of wrist movement which can be related to rest/activity or sleep/wake. Simply, one moves one’s wrist when awake and tends to be still when asleep Algorithms that have been correlated to the gold standard EEG are used to distinguish wake from sleep |
| Circadian | 24h or approximately 1 day; coming from the Greek circa (about) and dia (day) |
| Circadian rhythms | Biological rhythms that take about 24h to complete one cycle. Examples of circadian rhythms would include core body temperature, certain hormones (e.g. cortisol, prolactin), sleep/wake |
| Chronotherapy | The administration of treatment based on times of the circadian rhythm to optimize treatment effects and outcome |
| Polysomnogram | A recording of sleep that generally includes measures of EEG (brain waves), EOG (eye movement), submental EMG (muscle tension), respiration, ECG (heart rate), tibialis EMG |
| Time in bed (TIB) | Total time person spends in bed from lights out to final time out of bed |
| Total sleep time (TST) | Total time spent sleeping during time in bed |
| Sleep efficiency (SE) | The ratio of the amount of total sleep time (TST) to the amount of time spent in bed (TIB). SE = TST:TIB. The higher the SE, the better the sleep |
| Sleep onset latency (SOL) | Time from lights out to first appearance of sleep |
| Wake time after sleep onset (WASO) | Total time spent awake from sleep onset to final arising time |
In the same study, the frequency of body movements were also similar across groups and none of the cancer patients were found to have sleep apnoea. However, there was a higher preponderance of periodic limb movements during sleep (PLMS, a disorder of leg jerks or kicks during the night) in the cancer patients than the controls and insomnia patients. As PLMS is treatable (Earley & Allen 1996), it might be important to rule it out as a cause of sleep disturbance in patients with cancer.
There is growing interest in examining the biological rhythms of cancer patients. In order to accomplish this, several studies have begun to use actigraphy to study rest/activity patterns in cancer patients (Mormont et al. 1996; Berger 1998). Actigraphy allows for non-invasive, continuous ambulatory measurements of circadian rhythms and sleep/wake activity via motion-sensitive accelerometers placed on the non-dominant wrist (Ancoli-Israel 2000). This is an approximate measure of sleep, with about 90% accuracy compared with polysomnography (Cole et al. 1992). Morrow et al. (2000) used actigraphy to measure the relationship between fatigue, sleep and rhythms in cancer patients and suggested that circadian rhythm disruption plays a role in the psychological experience of fatigue. Berger (1998) used actigraphy to study fatigue and rhythms during adjuvant chemotherapy in 17 women with breast cancer. She recorded wrist actigraphy for 96h beginning on the morning of treatment and for 72h at the midpoint for each of three cycles of chemotherapy. Subjective measurements of fatigue were collected. Patients reported more fatigue during treatment and less fatigue at cycle midpoints, in a ‘roller coaster’ pattern. Activity levels were negatively correlated with reports of fatigue, i.e. those with more fatigue showed less activity. Activity levels were reduced during the three treatment sessions compared with the mid-cycle points. Activity levels therefore showed the reverse ‘roller coaster’ pattern and changed simultaneously with fatigue scores, albeit in the opposite direction. Patients tended to have more night-time restlessness at treatment times compared with cycle midpoints when higher activity during the day prevailed and there were fewer night-time awakenings.
Unexpectedly, night-time awakenings were not associated with higher fatigue scores. Limitations of this study include the lack of baseline data, the possibility of bias toward patients who were less fatigued (as the extremely fatigued may not have been able to complete the study) and the small sample size. Another study reiterated the basic finding that daytime inactivity and night-time restlessness are associated with higher subjective ratings of cancer-related fatigue (Berger & Farr 1999).
Miaskowski & Lee (1999) recorded wrist actigraphy over a 48-h period in 24 patients at various points during radiation therapy for bone metastases. Ratings of fatigue were collected in the morning and evening. Five of the patients were also on antidepressant drugs, some of which may have had different alerting or sedating profiles. There were no apparent diurnal influences in pain ratings with pain intensity scores remaining fairly constant over the 48h. In contrast, fatigue ratings were higher in the evening and lower in the morning.
One possible interpretation is that sleep decreased some component of fatigue in these patients, even if their sleep was disturbed. Physical fatigue, for example, might be expected to respond to rest in this way. Affective states of distress in depression or other signs of emotional fatigue tend to show a pronounced circadian effect in the opposite direction.
In the same Miaskowski and Lee study, as radiation therapy progressed, the subjective sleep complaints increased. Sleep efficiency also declined, and frequent urination, rather than pain intensity, was reported to be the main cause of awakening in the night (Miaskowski & Lee 1999). As in Berger (1998) and Berger & Farr (1999), the periods in which the greatest fatigue was experienced were periods of diminished dichotomization of the sleep/wake schedule, i.e. periods less activity during the day and more awakenings during the night. More specifically, during these times, instead of activity occurring during the day and rest taking place only at night, a polyphasic rhythm emerged suggesting daytime naps and less daytime activity. Thus, rather than increased fatigue resulting from increased activity, fatigue was associated with periods of greater inactivity. Perhaps not coincidentally, sleep during these times was broken up with periods of intermittent wakefulness, and was fragmented and non-consolidated.
Subjective ratings of fatigue and alertness may not be the only important consequence of consolidated circadian rhythms in cancer patients. Mormont et al. (1996) found an association between specific patterns of rest/activity in patients with metastatic colon and rectal cancer. All subjects wore an actigraph for 2–5 days. As in the studies above, the contrast between daytime activity and nighttime inactivity was smaller in the cancer patients than in healthy individuals. The cancer patients had altered rest/activity cycles with more interpatient variability and less differentiation between night and day. In particular, patterns characterized by a clear dichotomy between activity and rest (as defined by Minors et al. 1996) were associated with better outcome from treatment, i.e. longer survival. However, there was a high degree of variability between patients. Whether or not cancer patients have altered rest-activity circadian patterns owing to their diagnosis or as a result of a vicious cycle of fatigue and inactivity remains to be established.
Winningham et al. (1994) remarked that such a cycle of decreasing activity and increasing fatigue, leading to increasing deterioration and deconditioning, may occur in the chronically ill. As the patient becomes less active, the patient fatigues more quickly upon exertion, leading to an ever-worsening cycle of avoidance and continued deterioration. Thus, lengthening rest by staying in bed may be detrimental not only for fatigue, but may result in decreased night-time sleep quality. This cycle, leading to decreased activity during the day, may also lead to decreased exposure to bright sunlight which could lead to an attenuation of the intensity and regularity of body rhythms, to a decrease in rhythm amplitude and possibly to an alteration in circadian phase placement of the rhythms, all which would additionally decrement sleep quality.
In fact, in a study by Mock et al. (1997) activity was used as a treatment rather than as an outcome. Daytime activity was specifically promoted in a group of breast cancer patients, and its effects on physical functioning, fatigue, pain, and self-rated difficulty in sleeping and emotional stress, were examined. A second group received usual care. Once again, fatigue was a common complaint, reported by all subjects. However, going for a self-paced walk three or four times per week for each of 6 weeks of radiation therapy was associated with lower ratings of fatigue, as well as lower ratings of anxiety, depression and difficulty sleeping.
Walking subjects in this study may have benefited from this mild exercise programme for a variety of reasons, one of which is that the mild exercise served to synchronize their rest/activity rhythms. There is evidence from experimental animals to suggest that this is a strong possibility (e.g. Edgar & Dement 1991). Unlike the walkers, for patients in the usual care group, the delineations between rest and activity began to blur over time, with the normally active daytime period being interrupted with increasing periods of rest and bursts of activity invading the night-time period. This resulted in a flattened or weakened rhythm of rest/activity. By encouraging a specific amount of exercise and activity during the day, the flattening effect may have been countered.
A second unaccounted for benefit may have been increased exposure to bright light. As some of the walkers in this study exercised outdoors, their exposure to bright sunlight during the day may have further promoted daytime alertness. The usual care group may have had less exposure to bright sunlight compared with the walkers. It has been argued that the presence of sunlight serves to enforce vigilance and alertness at appropriate times of the day (Edgar et al. 1993), through input from the retinal hypothalamic pathway. When not stimulated sufficiently, vigilance no longer remains anchored to the daytime hours and diffuses into the nocturnal hours. Activity during the daytime alone, however, may have been sufficient to consolidate the activity/rest behaviour rhythm. Future studies might clarify this finding by including additional control groups, such as a group of patients who do not exercise but are exposed to bright light during the day.
Smolensky and colleagues studied rhythms with actigraphy in cancer patients and found that some go to bed early (8:30–9:00 pm) and awaken early (4:40–5:30 am), suggesting an advanced sleep phase, while others showed more typical sleep patterns (Brown et al. 1990). Although abnormal rhythms can be synchronized, more information is needed about individual rhythms before treatment trials can be designed to address these issues. Future studies should assess pretreatment levels of activity and rest as well as treatment levels to better understand any changes that might occur in ratings of fatigue, in sleep and in rhythms.
Circadian rhythms and treatment of cancer: chronotherapy
Many disorders have rhythmicity. It has long been known that most heart attacks occur in the morning hours. Osteoarthritis causes more discomfort in the afternoon and evening. Thrombotic stroke is more likely to occur in the morning while haemorrhagic stroke is likely to occur in the evening. Ulcer pain is usually manifested during the early hours of sleep. It is therefore probable that administering medications at different times of the circadian cycles might have different outcomes. This method of therapy, called chronotherapy, is a relatively new area of study.
Chronotherapy involves choosing the timing of administration of a drug in order to minimize its side-effects and maximize its therapeutic effects. The effects of chronotherapy on toxicity have been studied in many fields of medicine and have documented that both toxicity and antitumour activity of cancer drugs are time dependent. Clinical trials have confirmed these results for several cancer drugs (Hrushesky et al. 1992; Levi 1996). In fact, within the field of oncology, studies have examined the effect of chronotherapy in ovarian, lung (non-small cell), renal cell, pancreas, breast and upper digestive tract carcinomas. Studies in mice and rats have shown that the tolerability of 30 different anticancer drugs varies by as much as 50% or more depending on the time of administration in relation to the circadian rhythms (Levi 1996). Not only is toxicity reduced, but dose intensity can be increased without enhancing the side-effects.
Circadian rhythms can influence tissues and cells as well as sleep/wake activity and this rhythmicity can be used to help guide therapy. Mormont & Levi (1997) reviewed circadian influences on a variety of measures in tumour cells and in healthy tissues in cancer by administering drugs at different times of the day and night. The same dose of an anticancer drug became lethally toxic only when administered at certain times of day, whereas at other times of day, a 10-fold increase in dose was tolerated (Mormont et al. 1989).
Other aspects of rhythmicity may be relevant, in that certain tumour types appear to undergo mitosis (cell division) at certain times of day. This may also have implications for treatment. Additional data indicate that, in certain cancers, with advancing illness, the patient’s rest/activity rhythms deteriorate and become less organized with regard to time. It is unknown whether patients in terminal stages of illness are in fact less responsive to the time cues of the external environment or whether the environment is no longer providing meaningful time cues owing to round-the-clock-care, such as continuous low-level light in place of a light/dark cycle. In some cases, tumour cell mitosis also appears to fall out of phase with the patient’s rhythms. In other words, there are indications that the tumour as well as the host (i.e. the patient) show signs of abnormal circadian rhythmicity as the disease progresses.
Entrained circadian rhythmicity serves to coordinate physiological function on multiple levels (Wood & Hrushesky 1996), from the molecular and cellular to the behavioural level. Whether or not different cell types may be responsive to different types of circadian cues is a mystery currently being unravelled at the molecular level (Balsalobre et al. 1998).
More recently, Mormont et al. (2000) studied the circadian rhythm of the rest/activity cycle (with actigraphy) and serum cortisol, leucocyte counts and neutrophil counts, in 200 patients with metastatic colorectal cancer for three consecutive days prior to beginning chronomodulated chemotherapy. Maximum tumour response to therapy was then measured every 2 months for the first 6 months of therapy. Complete response was defined as a disappearance of all signs of cancer for 4 weeks, while a partial response was defined as a reduction of at least 50%. Patients with marked activity rhythms (i.e. greater activity when out of bed than when in bed), had a fivefold higher survival at 2-year follow-up than those with less synchronized rhythms. Patients with marked activity rhythms also had better quality of life and reported significantly less fatigue. Circadian rhythms in activity and in white blood cells were jointly prognostic of response. The authors concluded that the rest/activity cycle can be used to determine prognosis for cancer patients’ survival and tumour response.
The circadian rhythm of the immune system has also been examined in patients with cancer to determine if the timing of therapeutic agents might either positively or negatively effect outcome (Levi et al. 1994; Zuchowska-Vogelgesang et al. 1996; Aveta et al. 1997). Aveta et al. (1997) reported that 50 patients were treated with chronobiological infusion of floxuridine resulting in 12% showing a long period of stable disease and low toxicity. Zuchowska-Vogelgesang et al. (1996) studied 40 patients with testicular cancer and 40 patients with ovarian cancer. Half the patients were administered cytostatics in the evening and half in the morning. The response rates and the 3-year survival were the same in both groups; however, there were less haematological and renal sides effects, milder nausea and less vomiting in the group receiving chemotherapy in the evening. Levi et al. (1997) adjusted the administration of chemotherapy (oxaliplatin, fluorouracil and folinic acid) to coincide with relevant circadian rhythms and compared it against a constant rate infusion method in patients with colorectal cancer. Results indicated that the chronotherapy was significantly less toxic and more effective than the constant-rate infusion. An objective response was obtained in 51% (n = 47) of those receiving chronotherapy versus 29% (n = 27) in the constant rate infusion group. In addition, chronotherapy reduced the rate of mucosal toxicity fivefold and halved the functional impairment from peripheral sensory neuropathy. In addition to reduced toxicity, other benefits have been improved quality of life and fewer days of hospitalization (Wood & Hrushesky 1996).
The effect of chronotherapy on quality of life has been examined more directly. Bertolini et al. (1995) compared traditional treatment with chronotherapy in patients with metastatic cancer. Quality-of-life measures as well as measures of anxiety and depression were recorded at seven time points from pretreatment to the sixth cycle of treatment. Results showed that there was better psychosocial adaptation (including better social relations, less feeling of loss of independence, less anxiety, less depression and less somatic discomfort) in patients receiving chronotherapy. Nordlinger et al. (1994) concluded that, although 5-fluorouracil is the drug of reference in patients with advanced colorectal cancers, various routes and schedules of administration of folinic acid and methotrexate (e.g. continuous infusion, hepatic artery infusions and chronotherapy) led to significant improvement in response rates as well as in quality of life.
Figure 2 is a schematic representation of the possible effects of chronotherapy in cancer patients.
Figure 2.
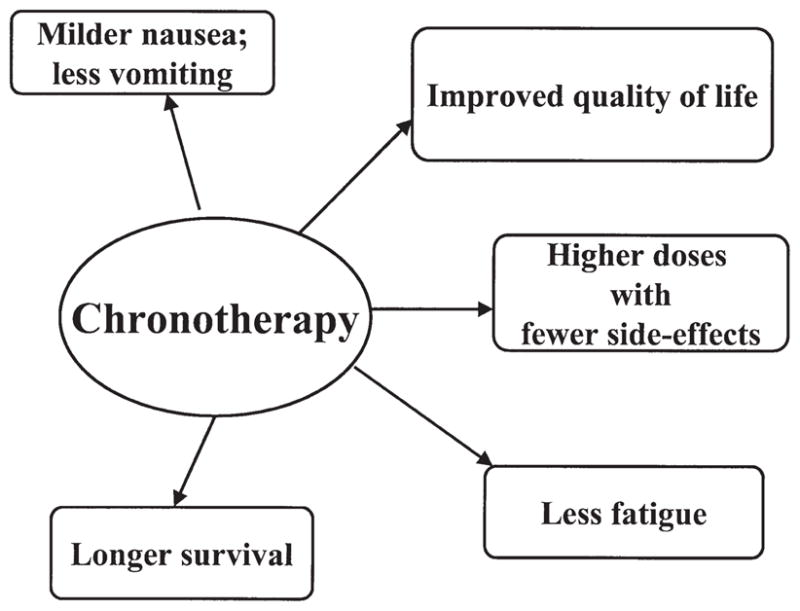
Diagrammatic representation of the effects of chronotherapy as described in multiple studies (see text).
CONCLUSION
The long-term goal of this line of research is to illuminate approaches that might improve the quality of life during treatment and/or the course of treatment itself. However, before treatment trials can be started, descriptive studies are needed to determine the baseline levels of sleep and circadian rhythms. More objective sleep studies are needed. Sleep disruption in cancer has been attributed to many possible causes such as anxiety and stress, depression and pain. Interestingly, even when pain has abated or is well controlled, sleep disruption may persist.
This degree of sleep disruption is not trivial. It is known that disruptions in circadian rhythms can affect sleep quality in and of the rhythms themselves and thus disrupt a variety of physiological mechanisms pertaining to fatigue. Other disruptions in circadian rhythms, such as a lack of entrainment to day–night cycle and sleeping-in, can lead to feelings of grogginess akin to those of jet lag. It is unclear whether naps are truly helpful in reducing fatigue in cancer patients as has been believed; there is some evidence that the opposite may be true, that naps may result in increased fatigue levels (Miaskowski & Lee 1999). In fact, in non-cancer patients, it is well established that inconsistent naps reduce sleep quality in the subsequent night and may produce daytime fatigue the next day (Feinberg et al. 1985; 1992). Entraining the sleep/wake rhythm to the day/night cycle will help to entrain sleep/wake to other circadian rhythms, such as core body temperature and other functions. The core body temperature rhythm has a well-established connection to mental performance tests and thus relates closely to the cognitive dimension of fatigue.
Although fatigue is one of the most frequent and most debilitating symptoms in cancer patients, little is known about the relationship between fatigue, sleep and circadian rhythms. Jacobsen (1991), in a review of an article by Hu & Silberfarb (1991), wrote that much remains to be learned about sleep problems in patients with cancer. Mormont & Levi (1997), in a review of circadian system changes in cancer patients, concluded that cancer patients’ individual circadian rhythms need to be explored in order to estimate the best approach of treatment, particularly in patients with circadian rhythm disturbances. Our laboratory is now beginning to address these very issues.
Acknowledgments
This study was supported by the following: NCI CA85264, NIA AG02711, NIA AG08415, NHLBI HL44915, UCSD Cancer Center, the Department of Veterans Affairs VISN-22 Mental Illness Research, Education and Clinical Center (MIRECC) and the Research Service of the Veterans Affairs San Diego Healthcare System.
Contributor Information
S. ANCOLI-ISRAEL, Department of Psychiatry, University of California San Diego and Veterans Affairs San Diego Healthcare System, and University of California San Diego Cancer Center, San Diego.
P.J. MOORE, Department of Psychiatry, University of California San Diego and Veterans Affairs San Diego Healthcare System, and University of California San Diego Cancer Center, San Diego.
V. JONES, University of California San Diego Cancer Center, San Diego, and Department of Medicine, University of California San Diego, San Diego, USA.
References
- Ancoli-Israel S. Actigraphy. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3. W.B. Saunders; Philadelphia: 2000. pp. 1295–1301. [Google Scholar]
- Aveta P, Terrone C, Neira D, et al. Chemotherapy with FUDR in the management of metastatic renal cell carcinoma. Annales D Urologie. 1997;31:159–163. [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells [see comments] Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncology Nursing Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- Berger AM, Farr L. The influence of daytime inactivity and night-time restlessness on cancer-related fatigue. Oncology Nursing Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- Berglund G, Bolund C, Fornander T, et al. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality of life among breast cancer patients. Cancer. 1991;27:1075–1081. doi: 10.1016/0277-5379(91)90295-o. [DOI] [PubMed] [Google Scholar]
- Bertolini R, Focan C, Bartholome F, et al. Comparative psychological aspects of two different types of chemotherapeutic administration (chronotherapy vs. traditional chemotherapy) on quality of life of cancer patients at advanced stage. In Vivo. 1995;9:583–587. [PubMed] [Google Scholar]
- Beszterczey A, Lipowski ZJ. Insomnia in cancer patients. Canadian Medicine Association Journal. 1977;116:355. [PMC free article] [PubMed] [Google Scholar]
- Broeckel J, Jacobsen PB, Horton J, et al. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. Journal of Cinical Oncology. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- Brown AC, Smolensky MH, D’Alonzo GE, et al. Actigraphy: a means of assessing circadian patterns in human activity. Chronobiology International. 1990;7 (2):125–133. doi: 10.3109/07420529009056964. [DOI] [PubMed] [Google Scholar]
- Bruera E, Brenneis C, Michaud M, et al. Association between asthenia and nutritional status, lean body mass, anaemia, psychological status, and tumour mass in patients with advanced breast cancer. Journal of Pain Symptom Management. 1989;4:59–63. doi: 10.1016/0885-3924(89)90023-7. [DOI] [PubMed] [Google Scholar]
- Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nursing. 1999;22:185–194. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- Cleeland C, Demetri G, Glaspy J, et al. Identifying hemoglobin level for optimal quality of life: Results of an incremental analysis. Proceedings of the American Society of Clinical Oncology. 1999;18:574a. [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, et al. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Curt G, Breitbart W, Cella DF, et al. Impact of cancer-related fatigue on the lives of patients. Proceedings of the American Society of Clinical Oncology. 1999;18:573a. [Google Scholar]
- Derogatis LR, Feldstein M, Morrow G, et al. A survey of psychotropic drug prescriptions in an oncology population. Cancer. 1979;44:1919–1929. doi: 10.1002/1097-0142(197911)44:5<1919::aid-cncr2820440555>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Dow KH, Ferrell BR, Leigh S, et al. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Research Treatment. 1996;39:261–273. doi: 10.1007/BF01806154. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Allen RP. Pergolide and carbidopa/levodopa treatment of the restless legs syndrome and periodic leg movements in sleep in a consecutive series of patients. Sleep. 1996;19:801–810. doi: 10.1093/sleep/19.10.801. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. American Journal of Physiology. 1991;261:R928–R933. doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in Sleep-Wake regulation. Journal of Neuroscience. 1993;13 (3):1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom CA, Strohl RA, Rose L, et al. Sleep alterations in cancer patients. Cancer Nursing. 1999;22:143–148. doi: 10.1097/00002820-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Maloney T, March JD. Precise conservation of NREM period 1 delta across naps and nocturnal sleep: Implications for REM latency and NREM/REM alteration. Sleep. 1992;15:400–403. doi: 10.1093/sleep/15.5.400. [DOI] [PubMed] [Google Scholar]
- Feinberg I, March JD, Floyd TE, et al. Homeostatic changes during post-nap sleep maintain baseline levels of delta EEG. Electroencephalography and Clinical Neurophysiology. 1985;61:134–137. doi: 10.1016/0013-4694(85)91051-x. [DOI] [PubMed] [Google Scholar]
- Ferrell BR, Grant M, Funk B, et al. Quality of life in breast cancer. Cancer Practice. 1996;4:331–340. [PubMed] [Google Scholar]
- Ganz P, Coscarelli A, Fred C, et al. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Research Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- Glaus A. Fatigue in patients with cancer: analysis and assessment. Springer; Berlin: 1998. pp. 1–172. [PubMed] [Google Scholar]
- Goldstein D, Scott E, Barbara B, et al. Post malignancy fatigue syndrome – results of long term fellow-up in a cross-sectional study in women following adjuvant treatment for breast cancer. ASCO. 2000;19:623a. [Google Scholar]
- Greenberg DB, Gray JL, Mannix CM, et al. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. Journal of Pain and Symptom Management. 1993;8:196–200. doi: 10.1016/0885-3924(93)90127-h. [DOI] [PubMed] [Google Scholar]
- Greenberg DB, Sawicka J, Eisenthal S, et al. Fatigue syndrome due to localized radiation. Journal of Pain and Symptom Management. 1992;7:38–45. doi: 10.1016/0885-3924(92)90106-r. [DOI] [PubMed] [Google Scholar]
- Haes JD, Raategever J, Van der Berg M, et al. Evaluation of the quality of life of patients with advanced ovarian cancer treated with combination chemotherapy. In: Aaronson N, Beckmann J, editors. Quality of Life of Cancer Patients. Raven Press; New York: 1987. pp. 215–226. [Google Scholar]
- Hann DM, Jacobsen PB, Afzzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Quality of Life Research. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Holland JC, Plumb M. A comparative study of depressive symptoms in patients with advanced cancer. Proceedings of the American Association Cancer Research. 1977;18:201. [Google Scholar]
- Hrushesky WJM, Martynowicz M, Markiewicz M, et al. Chronotherapy of cancer – a major drug-delivery challenge. Advanced Drug Delivery Review. 1992;9:1–83. [Google Scholar]
- Hu D, Silberfarb PM. Management of sleep problems in cancer patients. Oncology. 1991;5:23–27. [PubMed] [Google Scholar]
- Jacobsen PB. Management of sleep problems in cancer patients: article review. Oncology. 1991;5:28. [PubMed] [Google Scholar]
- Kaye J, Kaye K, Madow L. Sleep patterns in patients with cancer and patients with cardiac disease. Journal of Psychology. 1983;114:107–113. doi: 10.1080/00223980.1983.9915403. [DOI] [PubMed] [Google Scholar]
- Knobf MT. Physical and psychological distress associated with adjuvant chemotherapy in women with breast cancer. Journal of Clinical Oncology. 1986;4:678–684. doi: 10.1200/JCO.1986.4.5.678. [DOI] [PubMed] [Google Scholar]
- Levi F. Chronopharmacology and chronotherapy of cancers. Pathologie Biologie. 1996;44:631–644. [PubMed] [Google Scholar]
- Levi F, Bourin P, Depres-Brummer P, Adam R. Chronobiology of the immune system: Implication for the delivery of therapeutic agents. Clinical Immunotherapy. 1994;2:53–64. [Google Scholar]
- Levi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet. 1997;350:681–686. doi: 10.1016/s0140-6736(97)03358-8. [DOI] [PubMed] [Google Scholar]
- Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. Journal of Developmental and Behavioural Pediatrics. 1999;20:244–252. doi: 10.1097/00004703-199908000-00007. [DOI] [PubMed] [Google Scholar]
- McDaniel JS, Musselman DL, Porter MR, et al. Depression in patients with cancer: diagnosis, biology and treatment. Archives of General Psychiatry. 1995;52:89–99. doi: 10.1001/archpsyc.1995.03950140007002. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Lee KA. Pain, fatigue and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. Journal of Pain Symptom Management. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Minors D, Akerstedt T, Atkinson G, et al. The difference between activity when in bed and out of bed. I. Healthy subjects and selected patients. Chronobiology Internation. 1996;13:27–34. doi: 10.3109/07420529609040839. [DOI] [PubMed] [Google Scholar]
- Mock V, Hassey D, Meares CJ, et al. Effects of exercise on fatigue, physical functioning and emotional distress during radiation therapy for breast cancer. Oncology Nursing Forum. 1997;24:991–1000. [PubMed] [Google Scholar]
- Morant R, Stiefel F, Berchtold W, et al. Preliminary results of a study assessing asthenia and related psychological and biological phenomena in patients with advanced cancer. Support Care Cancer. 1993;1:101–107. doi: 10.1007/BF00366904. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Boughattas NA, Levi F. Mechanisms of circadian rhythms in the toxicity and the efficacy of anti-cancer drugs: Relevance for the development of new analogs. In: Lemmer B, editor. Chronopharmacology: Cellular and Biochemical Interactions. Marcel Dekker; New York: 1989. pp. 395–437. [Google Scholar]
- Mormont MC, De Prins J, Levi F. Assessment of activity rhythms by wrist actigraphy: preliminary results in 30 patients with colorectal cancer. Pathology Biology. 1996;44:165–171. [PubMed] [Google Scholar]
- Mormont MC, Levi F. Circadian system alterations during cancer processes: a review. International Journal of Cancer. 1997;70:241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Waterhouse J, Bleuzen P, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6:3038–3045. [PubMed] [Google Scholar]
- Morrow G, Tian L, Roscoe J, et al. The relationship between circadian rhythm and fatigue in breast cancer patients. Annals of Behavioural Medicine. 2000;22:S188. [Google Scholar]
- Nordlinger B, Levy E, Vaillant JC, et al. Treatment of metastases of colorectal cancers. Revue Du Practicien. 1994;44:2733–2738. [PubMed] [Google Scholar]
- Owen DC, Parker KP, McGuire DB. Comparison of subjective sleep quality in patients with cancer and healthy subjects. Oncology Nursing Forum. 1999;26:1649–1651. [PubMed] [Google Scholar]
- Richardson A. Fatigue in cancer patients: a review of the literature. European Journal of Cancer Care. 1995;4:20–32. doi: 10.1111/j.1365-2354.1995.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Richardson A. Measuring fatigue in patients with cancer. Support Care Cancer. 1998;6:94–100. doi: 10.1007/s005200050141. [DOI] [PubMed] [Google Scholar]
- Silberfarb PM, Hauri PJ, Oxman TE, et al. Assessment of sleep in patients with lung cancer and breast cancer. Journal of Clinical Oncology. 1993;11:997–1004. doi: 10.1200/JCO.1993.11.5.997. [DOI] [PubMed] [Google Scholar]
- Smets EMA, Garssen B, Cull A, et al. Application of the multidimensional fatigue inventory in cancer patients receiving radiotherapy. British Journal of Cancer. 1996;73:241–245. doi: 10.1038/bjc.1996.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DM, et al. A multidimensional measure of fatigue for use with cancer patients. Cancer Practice. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- Stiefel FC, Kornblith AB, Holland JC. Changes in the prescription patterns of psychotropic drugs over a 10-year period. Cancer. 1990;65:1048–1053. doi: 10.1002/1097-0142(19900215)65:4<1048::aid-cncr2820650434>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Stone P, Richards M, Hardy J. Fatigue in patients with cancer. European Journal of Cancer Care. 1998;34:1670–1676. doi: 10.1016/s0959-8049(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Strang P, Qvarner H. Cancer-related pain and its influence on quality of life. Anticancer Research. 1990;10:109–112. [PubMed] [Google Scholar]
- Stromborg MF, Wright P. Ambulatory cancer patients’ perception of the physical and psychosocial changes in their lives since the diagnosis of cancer. Cancer Nursing. 1984;7:117–130. [PubMed] [Google Scholar]
- Visser MRM, Smets EMA. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience; the state of the knowledge. Oncology Nursing Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- Wood PA, Hrushesky WJM. Circadian rhythms and cancer chemotherapy. Critical Reviews in Eukaryotic Gene Expression. 1996;6:299–343. doi: 10.1615/critreveukargeneexpr.v6.i4.10. [DOI] [PubMed] [Google Scholar]
- Zuchowska-Vogelgesang B, Pernal J, Zemelka T. Toxicity and efficacy of chemotherapy dependent on circadian time of cytostatic drug administration. Przeglad Lekarski. 1996;53:870–873. [PubMed] [Google Scholar]


