Abstract
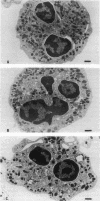
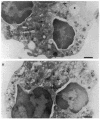
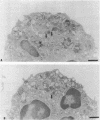
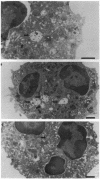
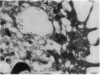
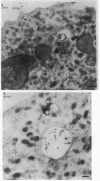
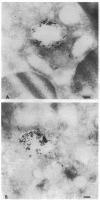
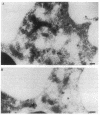
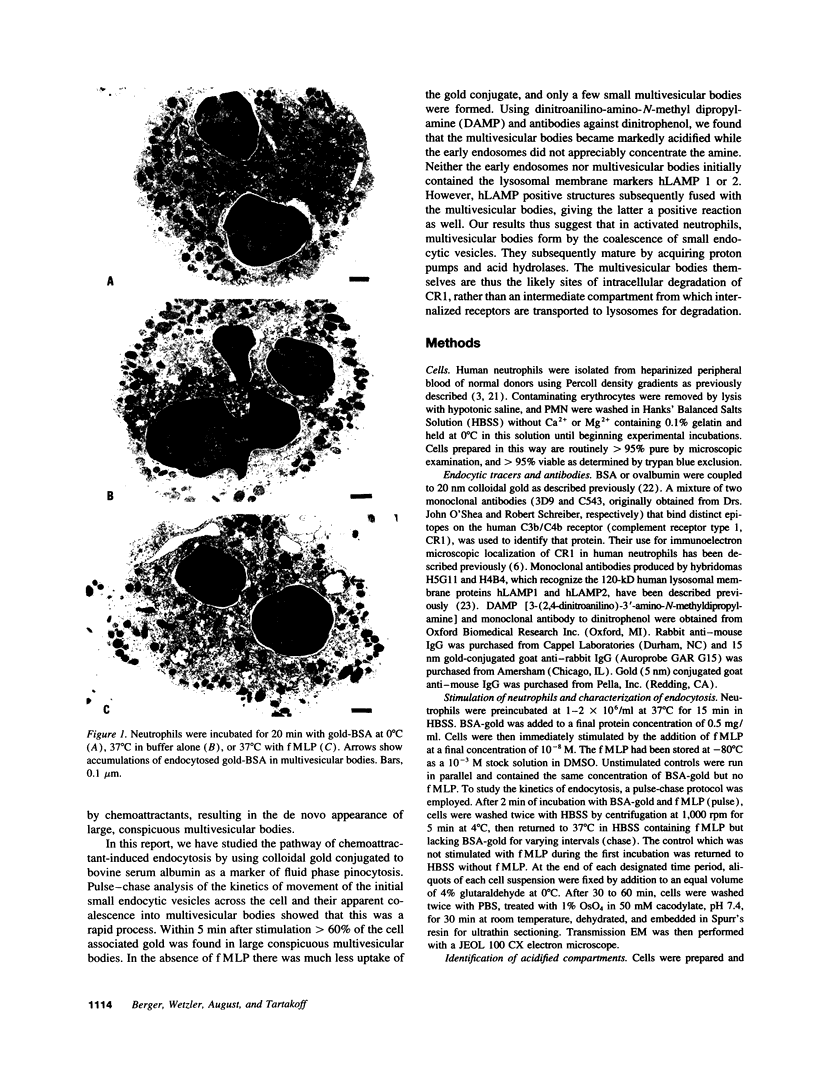
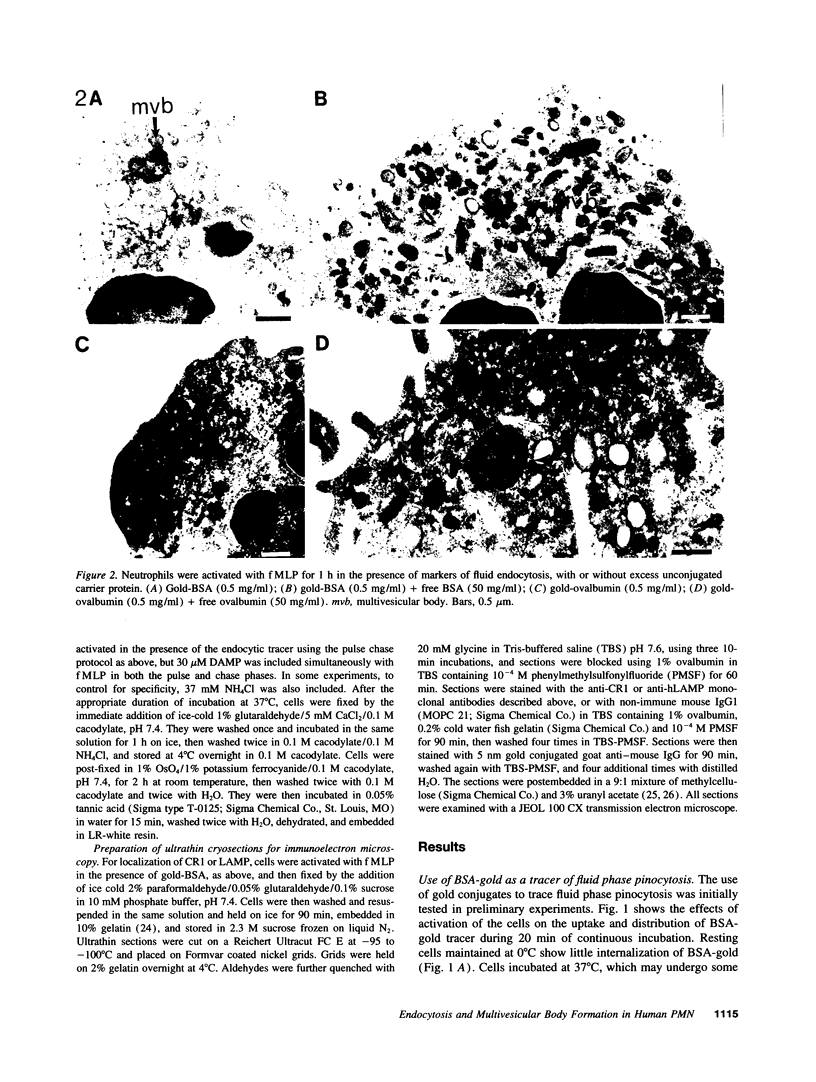
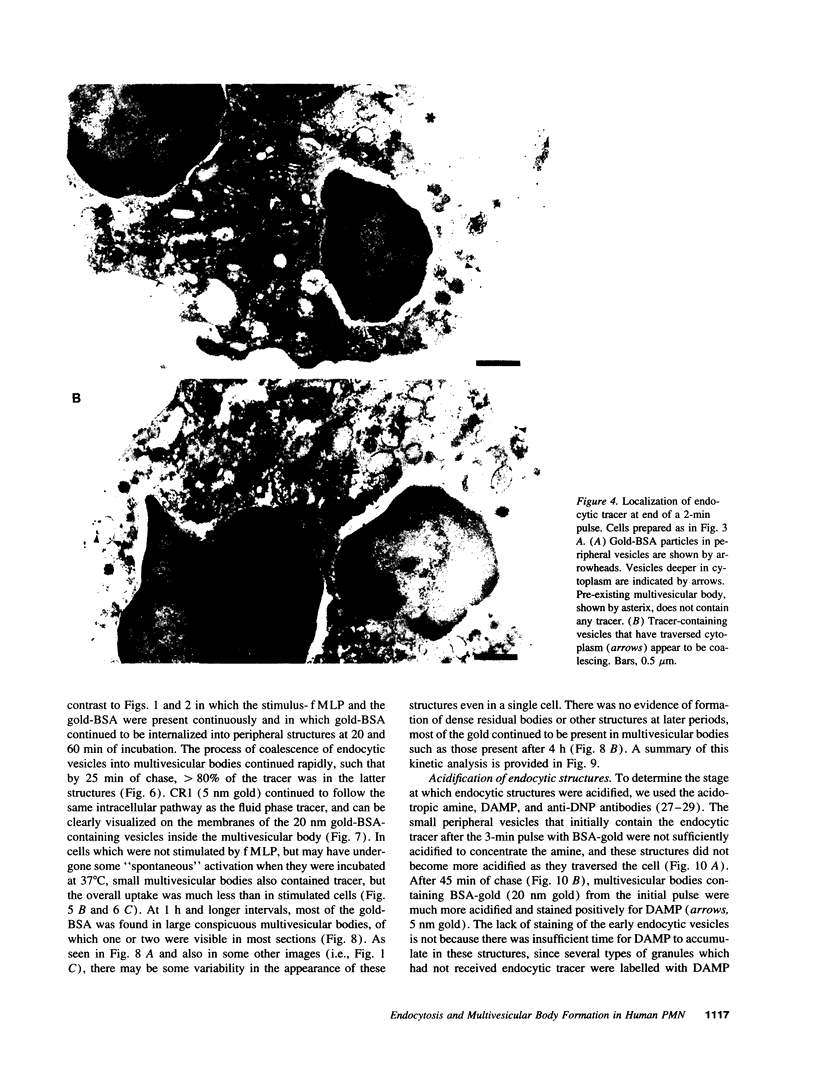
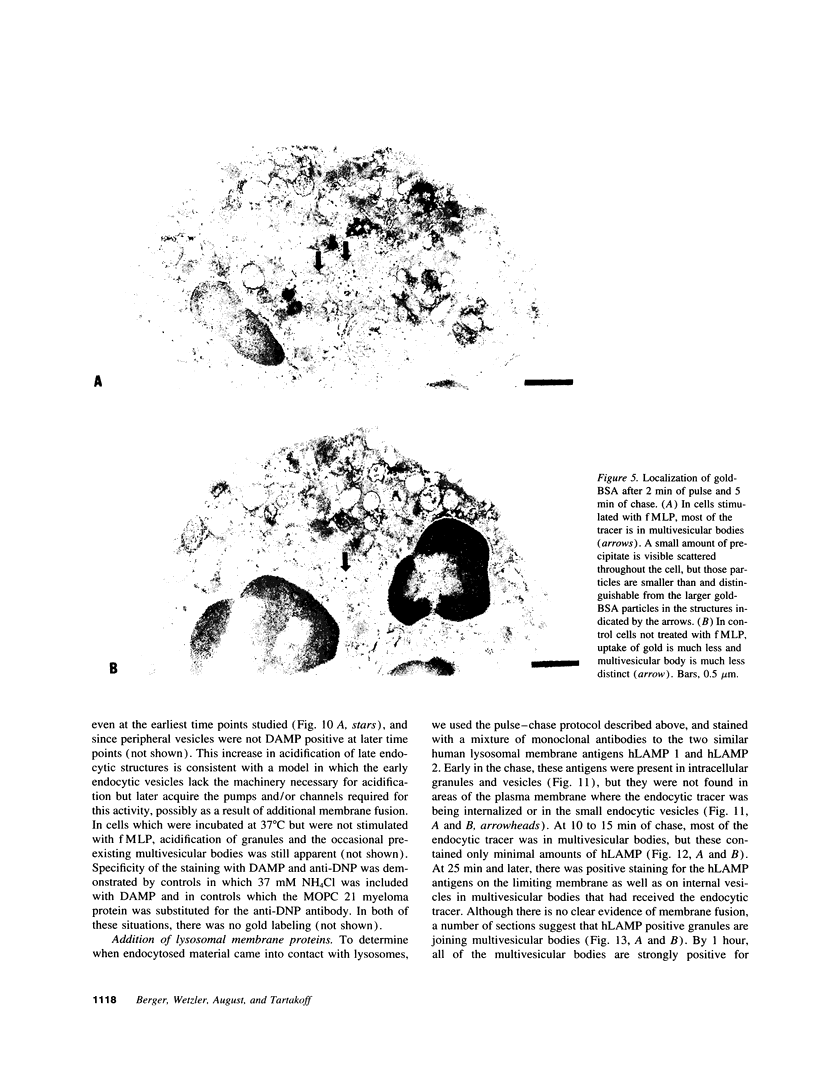
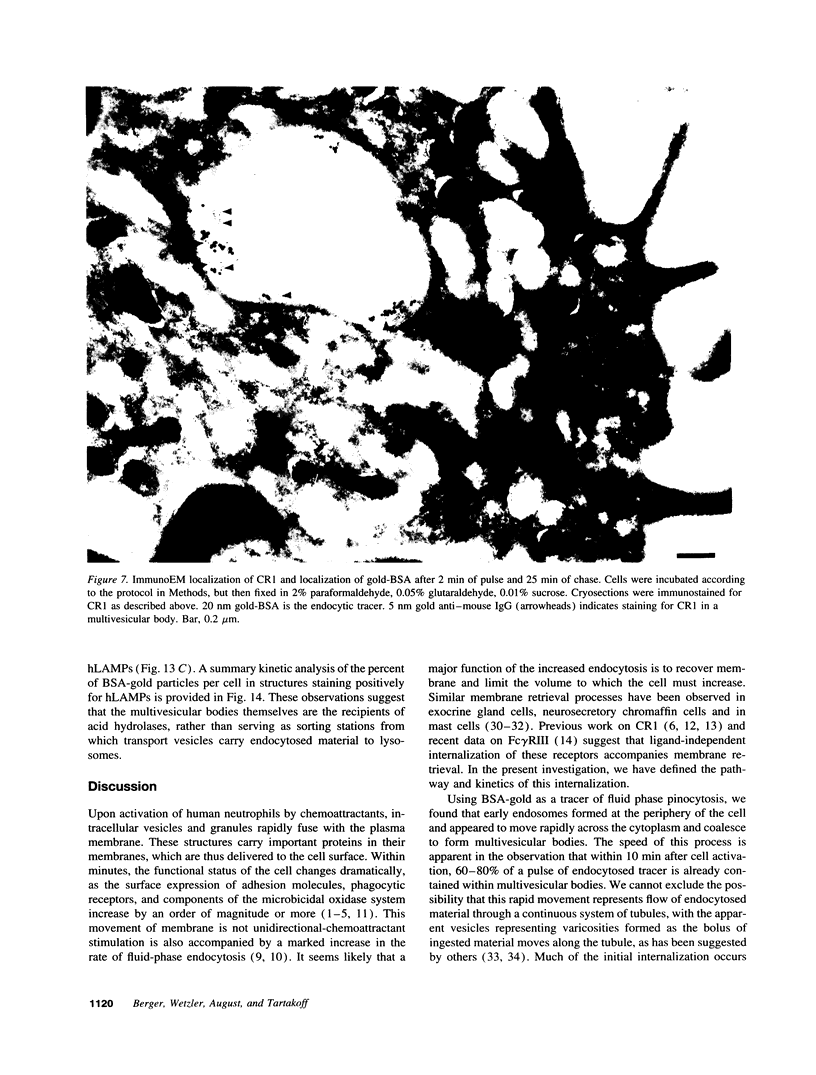
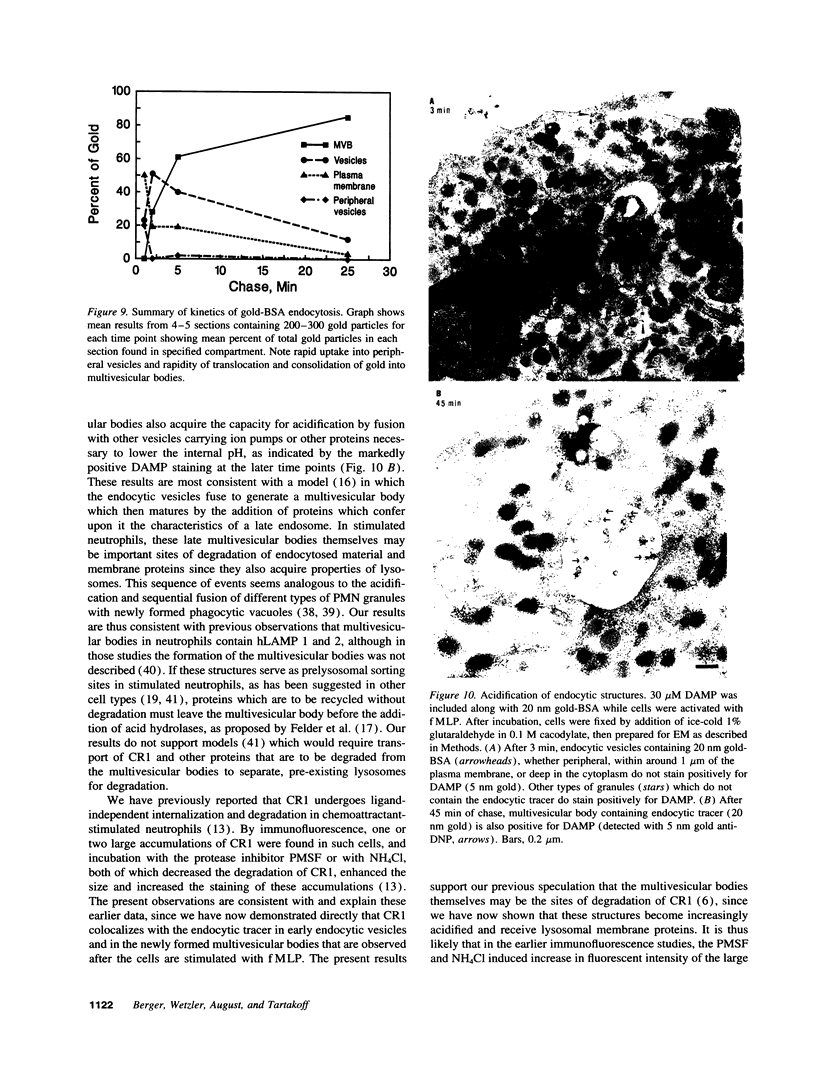
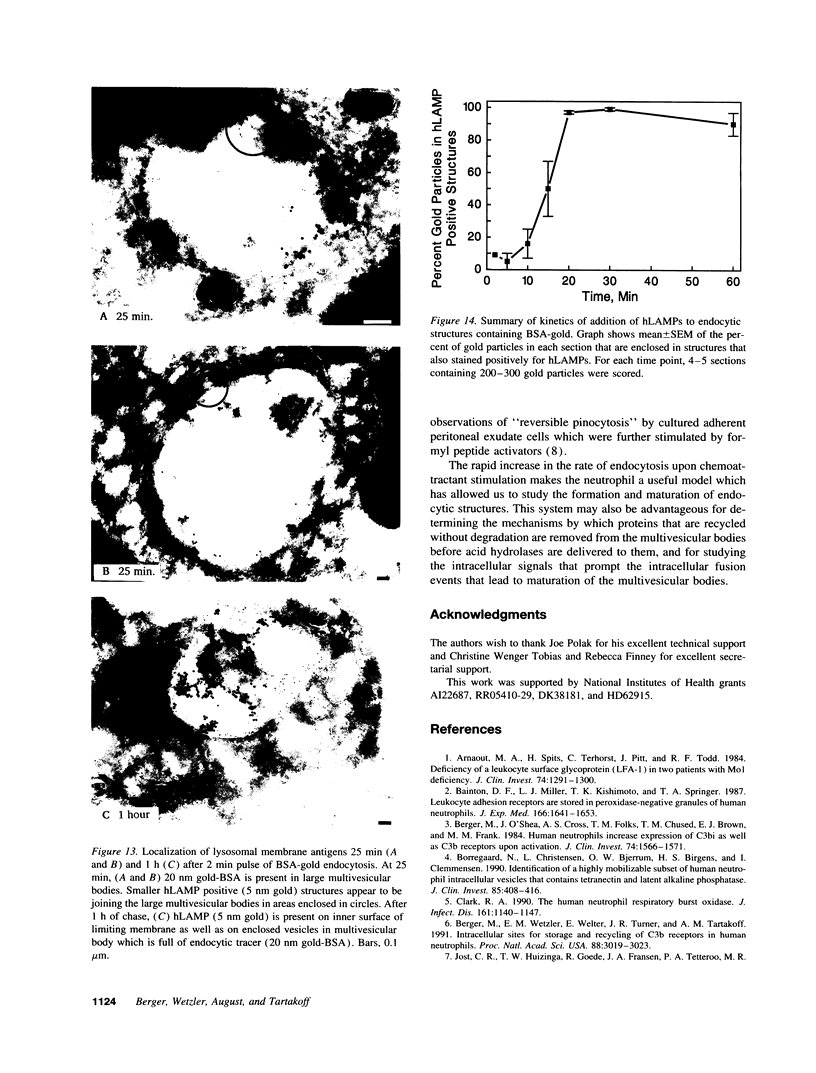
Upon activation of human neutrophils by chemoattractants, functionally important proteins are rapidly transported from intracellular granules and storage vesicles to the plasma membrane. This is accompanied by a marked increase in the rate of endocytosis and by ligand-independent internalization of type 1 complement receptors (CR1). To define the pathway of endocytosis, we used gold-conjugated BSA in a pulse-chase protocol. This tracer was initially internalized into small endocytic vesicles which rapidly traversed the cytoplasm and coalesced to form large, conspicuous multivesicular bodies. Within 5 min after addition of the chemoattractant, multivesicular bodies contained > 60% of the cell-associated BSA-gold. CR1 colocalized with the endocytic tracer in both the early endosomes and multivesicular bodies. In unstimulated cells, there was much less uptake of BSA-gold and multivesicular bodies were rarely seen. Using the acidotropic amine, DAMP, and anti-DNP antibodies, we found that the multivesicular bodies were acidified but the early endosomes did not concentrate DAMP. Neither the early endosomes nor the multivesicular bodies initially contained the lysosomal membrane antigens hLAMP 1 or 2, but hLAMP-positive structures subsequently joined the multivesicular bodies. The rapid activation of the endocytic pathway upon stimulation of neutrophils allowed us to visualize the de novo formation and maturation of multivesicular bodies. Our observations suggest that vesicles containing ion pumps and acid hydrolases fuse with multivesicular bodies, giving them characteristics of lysosomes, and that these are the probable sites of degradation of CR1. The observations do not support models which would require transport of CR1 from multivesicular bodies to defined, pre-existing lysosomes for degradation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Fearon D. T. Endocytosis of the C3b receptor of complement within coated pits in human polymorphonuclear leukocytes and monocytes. Lab Invest. 1983 Feb;48(2):162–168. [PubMed] [Google Scholar]
- Anderson R. G., Falck J. R., Goldstein J. L., Brown M. S. Visualization of acidic organelles in intact cells by electron microscopy. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4838–4842. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Spits H., Terhorst C., Pitt J., Todd R. F., 3rd Deficiency of a leukocyte surface glycoprotein (LFA-1) in two patients with Mo1 deficiency. Effects of cell activation on Mo1/LFA-1 surface expression in normal and deficient leukocytes. J Clin Invest. 1984 Oct;74(4):1291–1300. doi: 10.1172/JCI111539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., O'Shea J., Cross A. S., Folks T. M., Chused T. M., Brown E. J., Frank M. M. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Wetzler E. M., Welter E., Turner J. R., Tartakoff A. M. Intracellular sites for storage and recycling of C3b receptors in human neutrophils. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3019–3023. doi: 10.1073/pnas.88.8.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Christensen L., Bejerrum O. W., Birgens H. S., Clemmensen I. Identification of a highly mobilizable subset of human neutrophil intracellular vesicles that contains tetranectin and latent alkaline phosphatase. J Clin Invest. 1990 Feb;85(2):408–416. doi: 10.1172/JCI114453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Kjeldsen L., Rygaard K., Bastholm L., Nielsen M. H., Sengeløv H., Bjerrum O. W., Johnsen A. H. Stimulus-dependent secretion of plasma proteins from human neutrophils. J Clin Invest. 1992 Jul;90(1):86–96. doi: 10.1172/JCI115860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Lew D. P., Paccaud J. P., Gil R., Iacopetta B., Kazatchkine M., Stendahl O., Pozzan T. Internalization pathway of C3b receptors in human neutrophils and its transmodulation by chemoattractant receptors stimulation. Cell Regul. 1991 Jan;2(1):41–55. doi: 10.1091/mbc.2.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Daukas G., Lauffenburger D. A., Zigmond S. Reversible pinocytosis in polymorphonuclear leukocytes. J Cell Biol. 1983 Jun;96(6):1642–1650. doi: 10.1083/jcb.96.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. H., McCabe E., Langweiler M. Characterization of f-Met-Leu-Phe-stimulated fluid pinocytosis in human polymorphonuclear leukocytes by flow cytometry. Cytometry. 1986 May;7(3):251–262. doi: 10.1002/cyto.990070305. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Walter R. J., Pearson C. B., Becker E. L., Oliver J. M. Membrane activity and topography of F-Met-Leu-Phe-Treated polymorphonuclear leukocytes. Acute and sustained responses to chemotactic peptide. Am J Pathol. 1982 Aug;108(2):206–216. [PMC free article] [PubMed] [Google Scholar]
- Dunn K. W., Maxfield F. R. Delivery of ligands from sorting endosomes to late endosomes occurs by maturation of sorting endosomes. J Cell Biol. 1992 Apr;117(2):301–310. doi: 10.1083/jcb.117.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Friend D. S. Cytochemical staining of multivesicular body and golgi vesicles. J Cell Biol. 1969 Apr;41(1):269–279. doi: 10.1083/jcb.41.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Hasilik A., von Figura K. Possible pathways for lysosomal enzyme delivery. J Cell Biol. 1985 Dec;101(6):2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Stoorvogel W., Strous G. J., Slot J. W., Bleekemolen J. E., Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988 Dec;107(6 Pt 2):2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Brands R., Burke B., Louvard D., Warren G. Viral membrane proteins acquire galactose in trans Golgi cisternae during intracellular transport. J Cell Biol. 1982 Dec;95(3):781–792. doi: 10.1083/jcb.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Gruenberg J. The arguments for pre-existing early and late endosomes. Trends Cell Biol. 1991 Jul;1(1):5–9. doi: 10.1016/0962-8924(91)90047-d. [DOI] [PubMed] [Google Scholar]
- Harbeck R. J., Hoffman A. A., Redecker S., Biundo T., Kurnick J. The isolation and functional activity of polymorphonuclear leukocytes and lymphocytes separated from whole blood on a single percoll density gradient. Clin Immunol Immunopathol. 1982 Jun;23(3):682–690. doi: 10.1016/0090-1229(82)90331-2. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Gibson A., Shipman M., Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990 Jul 26;346(6282):335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost C. R., Huizinga T. W., de Goede R., Fransen J. A., Tetteroo P. A., Daha M. R., Ginsel L. A. Intracellular localization and de novo synthesis of FcRIII in human neutrophil granulocytes. Blood. 1990 Jan 1;75(1):144–151. [PubMed] [Google Scholar]
- Jost C. R., de Goede R., Fransen J. A., Daha M. R., Ginsel L. A. On the origin of the FcRIII (CD16)-containing vesicle population in human neutrophil granulocytes. Eur J Cell Biol. 1991 Apr;54(2):313–321. [PubMed] [Google Scholar]
- Luo Z. R., Robinson J. M. Co-localization of an endocytic marker and acid phosphatase in a tubular/reticular compartment in macrophages. J Histochem Cytochem. 1992 Jan;40(1):93–103. doi: 10.1177/40.1.1729356. [DOI] [PubMed] [Google Scholar]
- Mane S. M., Marzella L., Bainton D. F., Holt V. K., Cha Y., Hildreth J. E., August J. T. Purification and characterization of human lysosomal membrane glycoproteins. Arch Biochem Biophys. 1989 Jan;268(1):360–378. doi: 10.1016/0003-9861(89)90597-3. [DOI] [PubMed] [Google Scholar]
- Miller K., Beardmore J., Kanety H., Schlessinger J., Hopkins C. R. Localization of the epidermal growth factor (EGF) receptor within the endosome of EGF-stimulated epidermoid carcinoma (A431) cells. J Cell Biol. 1986 Feb;102(2):500–509. doi: 10.1083/jcb.102.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Artault J. C. Membrane retrieval following exocytosis in isolated neurosecretory nerve endings. Neuroscience. 1992 Jul;49(1):201–207. doi: 10.1016/0306-4522(92)90088-j. [DOI] [PubMed] [Google Scholar]
- Patzak A., Winkler H. Exocytotic exposure and recycling of membrane antigens of chromaffin granules: ultrastructural evaluation after immunolabeling. J Cell Biol. 1986 Feb;102(2):510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Strous G. J., Slot J. W., Geuze H. J. Immunoelectron microscopic localization of acidic intracellular compartments in hepatoma cells. EMBO J. 1985 Apr;4(4):899–904. doi: 10.1002/j.1460-2075.1985.tb03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Montesano R. Plasma cell endocytosis: is it related to immunoglobulin secretion? Eur J Cell Biol. 1981 Dec;26(1):188–197. [PubMed] [Google Scholar]
- Thilo L. Selective internalization of granule membrane after secretion in mast cells. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1711–1715. doi: 10.1073/pnas.82.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Tosi M. F., Zakem H. Surface expression of Fc gamma receptor III (CD16) on chemoattractant-stimulated neutrophils is determined by both surface shedding and translocation from intracellular storage compartments. J Clin Invest. 1992 Aug;90(2):462–470. doi: 10.1172/JCI115882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougard C., Louvard D., Picart R., Tixier-Vidal A. Antibodies against a lysosomal membrane antigen recognize a prelysosomal compartment involved in the endocytic pathway in cultured prolactin cells. J Cell Biol. 1985 Mar;100(3):786–793. doi: 10.1083/jcb.100.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M., Berger M. Intracellular degradation of the complement C3b/C4b receptor in the absence of ligand. J Biol Chem. 1988 Apr 5;263(10):4914–4920. [PubMed] [Google Scholar]
- Zabucchi G., Menegazzi R., Soranzo M. R., Patriarca P. Uptake of human eosinophil peroxidase by human neutrophils. Am J Pathol. 1986 Sep;124(3):510–518. [PMC free article] [PubMed] [Google Scholar]