Abstract
Background:
Recently, it has been shown that emphysematous destruction of the lung is associated with a decrease in the total number of terminal bronchioles. It is unknown whether a similar decrease is visible in the more proximal airways. We aimed to assess the relationships between proximal airway count, CT imaging measures of emphysema, and clinical prognostic factors in smokers, and to determine whether airway count predicts the BMI, airflow obstruction, dyspnea, and exercise capacity (BODE) index.
Methods:
In 50 smokers, emphysema was measured on CT scans and airway branches from the third to eighth generations of the right upper lobe apical bronchus were counted manually. The sum of airway branches from the sixth to eighth generations represented the total airway count (TAC). For each subject, the BODE index was determined. We used logistic regression to assess the ability of TAC to predict a high BODE index (≥ 7 points).
Results:
TAC was inversely associated with emphysema (r = −0.54, P < .0001). TAC correlated with the modified Medical Research Council dyspnea score (r = −0.42, P = .004), FEV1% predicted (r = 0.52, P = .0003), 6-min walk distance (r = 0.36, P = .012), and BODE index (r = −0.55, P < .0001). The C-statistics, which correspond to the area under the receiver operating characteristic curve, for the ability of TAC alone and TAC, emphysema, and age to predict a high BODE index were 0.84 and 0.92, respectively.
Conclusions:
TAC is lower in subjects with greater emphysematous destruction and is a predictor of a high BODE index. These results suggest that CT imaging-based TAC may be a unique COPD-related phenotype in smokers.
COPD is defined as incompletely reversible expiratory airflow obstruction due to emphysematous destruction of the lung parenchyma and remodeling of the small airways.1 Although these two processes are classically thought of as being pathologically distinct, with emphysema being a purely parenchymal disease,2-4 an early report5 found that emphysematous lungs tended to have fewer visible small airways than those with less parenchymal destruction. More recently, McDonough et al6 and Hogg et al7 substantiated this finding using micro-CT scanning. They found that subjects with severe disease had a significant decrease in their number of terminal bronchioles,6,7 presumably lost during the destructive parenchymal process that manifests microscopically and macroscopically as emphysema.
Although Montaudon et al8 reported no differences in airway count in smokers and nonsmokers with normal lung function, we and other groups have observed anecdotally that subjects with severe emphysema on their chest CT scan tend to have fewer proximal airways available for quantitative analysis. One possible explanation for this finding is that the loss of airways associated with emphysema is not limited to the terminal bronchioles and beyond but extends more proximally as well. Our goal was to assess objectively the relationship between central airway count and CT scan burden of emphysema in smokers. Using volumetric CT scans obtained as part of the Lung Tissue Research Consortium (LTRC) (www.ltrcpublic.com), we undertook a detailed examination of the airways originating from the apical bronchus of the right upper lobe (RB1). Our hypothesis was that subjects with the greatest burden of emphysema would have the fewest visible airways in the proximal tracheobronchial tree. We also sought to determine whether airway dropout was predictive of the BMI, airflow obstruction, dyspnea, and exercise capacity (BODE) index, which is a multidimensional score for predicting mortality in subjects with COPD.
Materials and Methods
The study and manuscript were approved according to the procedures of the LTRC detailed elsewhere.9 This study was also approved by the Institutional Review Board at Brigham and Women’s Hospital.
Subject Selection
This is a convenience sample from the LTRC. Subjects were eligible if they had a ≥ 10 pack-year smoking history and a high-resolution volumetric CT scan. Among 125 subjects meeting these initial criteria, 16 were then eliminated because they had a bullous disease of the right lung or an artifact introduced by an intravenous catheter or pacemaker that precluded visual examination of the airways (4); a large mass in the right upper lobe (4); or evidence of previous surgery on the right lung (8). A total of 109 subjects were subsequently deemed eligible for this study. Following densitometric assessment of CT scans (see “Imaging Assessment”), 50 subjects were selected and clustered into two groups: 25 with a high (≥ 25%) and 25 with a low (< 25%) emphysema percentage.10 Because a limited number of subjects had a high percentage of emphysema on CT scan, all meeting the criterion of 25% or higher were included in our study (n = 25). Then, subjects with < 25% emphysema on CT scan were selected by consecutive sampling from a list ordered by ascending emphysema percentage (ie, selected subject 1 was number 2 in the list, selected subject 2 was number 5, and so on) until to complete 25 subjects. This was done to ensure that the population with low emphysema was equally distributed across this range of interest.
Imaging Assessment
CT image acquisition was performed according to the LTRC protocol described elsewhere.9,11 Emphysema was defined as low attenuation areas below −950 Hounsfield units (HU).12,13 Quantitative measures of emphysema for both the whole lung and for the upper, middle, and lower one-thirds were performed by using open-source software (www.airwayinspector.org). Unless otherwise specified, whole-lung emphysema determined by CT scan is referred to as emphysema. The thirds were defined as equal divisions of the total lung volume. The total lung volume on CT scan was measured at full inspiration and reported as total lung capacity (TLC).14,15
The CT images were visually inspected using a window width of 1000 HU and a level of −500 HU. RB1 was identified and its segmental bronchus designated as a third airway generation (AG). RB1 was selected based on both prior investigation16 and its generally perpendicular orientation to the CT scanning plane. From that segmental bronchus, detailed slice-by-slice examination of CT regionally interpolated images17 was performed to identify and count its daughter branches based on Weibel’s model of airway anatomy18,19 (Fig 1). The expected number of daughter branches was calculated by the equation × = 2n where n is the AG of interest, starting in the third AG where n was considered to be 0. Based on the assumption that all subjects would have the same number of daughter branches in the third AG, fourth AG, and fifth AG, the airway dropout was summarized as the total airway count (TAC) of the branches from the sixth AG to the eighth AG. Twenty CT scans were assessed independently following the above method a second time 1 month later by the first reader and by a second reader to assess the intra- and interreader reproducibility of the airway counts.
Figure 1.
Illustrative case of CT images interpolated (smooth) showing visualization of distal airway branches of the right upper lobe apical bronchus (RB1). Panels show airway branches (arrows) of the sixth (A), seventh (B) and eighth (C) generations of RB1.
Clinical and Physiologic Assessments
Dyspnea was measured by using the modified Medical Research Council (mMRC) score.20 All subjects underwent standardized spirometry, single-breath diffusing capacity for carbon monoxide (Dlco), and 6-min walk distance (6MWD) testing.21,22 The postbronchodilator FEV1 and FVC, and Dlco are expressed as percentages of predicted values using standardized prediction equations.23,24 Each subject’s BODE index was also calculated.25
Statistical Analysis
The intra- and interreader reproducibility of airway counts was conducted using the intraclass correlation coefficient26 and Bland-Altman analysis.27 Comparisons between baseline characteristics of subjects with < 25% and ≥ 25% emphysema were performed using t tests and χ2 tests.
Correlation and linear regression analysis was used to assess the relationship between TAC and emphysema. Pairwise relationships of TAC with clinical and physiologic variables and with BODE index were assessed via Pearson correlation coefficients. Furthermore, we assessed the ability of TAC to predict a categorized BODE (≥ 7 vs < 7 points) through logistic regressions. BODE is a validated summary index of several clinical predictors of COPD to predict mortality. A BODE score of 7 or higher predicts the highest risk of death.20 We assessed the prognostic utility of TAC by using the C-statistic,28 which corresponds to the area under the receiver operating characteristic (ROC) curve of TAC. Additional candidate predictors were age and gender, which were selected based on a P value < .05 and/or a change in C-statistic > 0.01 units when included in the model. Analyses were performed with SAS 9.1 (SAS Institute; Cary, NC) and S-plus 8 (Insightful Corporation; Seattle, WA). P values < 0.05 were considered significant.
Results
Reliability of Airway Counts
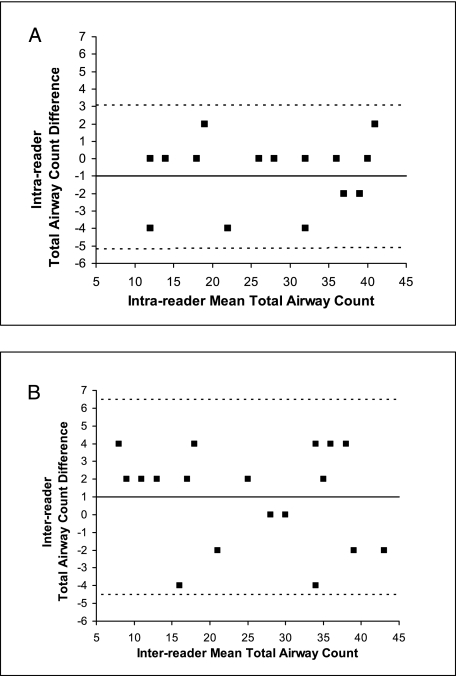
The intra- and interreader reproducibility of airway counts was high, as suggested by the intraclass correlation coefficients. The intraclass correlation coefficients for the TAC and the individual sixth AG, seventh AG, and eight AG were 0.98, 0.85, 0.95, and 0.97 for the intrareader assessments and 0.97, 0.75, 0.90, and 0.86 for the interreader assessments, respectively (Fig 2).
Figure 2.
Bland-Altman analysis of the intrareader (A) and interreader (B) reproducibility of the total airway count (TAC) for the sixth to eighth generations of the right upper lobe apical bronchus in 20 chest CT scans. The solid line is the mean difference in TAC, and the broken lines are the upper and lower limits of agreement. (Note that fewer than 20 points are visible in either figure because in both cases some of the points are superimposed upon each other.)
Population Description
Baseline characteristics of the 50 subjects by emphysema groups are shown in Table 1. Subjects with ≥ 25% emphysema were significantly younger, had a higher mMRC dyspnea score, TLC, and BODE index, lower FEV1% predicted and Dlco% predicted, and shorter 6MWD than their counterparts with < 25% emphysema. There were no differences between subjects’ characteristics (except for TLC) as a function of the brand of CT scanner (Table 2).
Table 1.
—Clinical, Physiologic, and CT Imaging Data of 50 Subjects by Emphysema Group
| Characteristic | Subjects With < 25% Emphysema (n = 25) | Subjects With ≥ 25% Emphysema (n = 25) | P Value |
| Age, y | 66.6 (8.5) | 61.2 (7.9) | .03 |
| Male gender, No. (%) | 17 (68) | 14 (56) | .38 |
| BMI, kg/m2 | 26.6 (4.3) | 25.5 (4.8) | .40 |
| Smoking history, pack-y | 57.5 (38.7) | 51.1 (26.2) | .50 |
| mMRC dyspnea score | 2 (1.3) | 3.9 (1.5) | < .0001 |
| FEV1% predicteda | 69.3 (16.8) | 31.6 (15.4) | < .0001 |
| FEV1/FVC ratio | 0.6 (0.1) | 0.3 (0.1) | < .0001 |
| Dlco % predicted | 62.4 (17.9) | 39.3 (16.4) | < .0001 |
| TLC, L | 5.5 (1.4) | 6.7 (1.4) | .004 |
| 6MWD, m | 393.9 (130.3) | 219.5 (140.9) | < .0001 |
| BODE index | 1.5 (2.0) | 6.7 (2.7) | < .0001 |
| Score 1-6, No. (%) | 24 (96) | 9 (36) | |
| Score 7-10, No. (%) | 1 (4) | 16 (64) | < .0001 |
| Emphysema percentage | |||
| Whole lung | 12.7 (7.0) | 37.8 (9.6) | < .0001 |
| Upper one-third | 16.4 (10.8) | 44.8 (12.6) | < .0001 |
| Middle one-third | 11.6 (7.0) | 38.5 (10.6) | < .0001 |
| Lower one-third | 9.9 (6.1) | 30.6 (14.4) | < .0001 |
Data are presented as mean (SD), except for male gender and BODE index categories, which are expressed as No. (%). P values were computed with the t test or χ2 test (or the Fisher exact test). 6MWD = 6-min walk distance; BODE = BMI, airflow obstruction, dyspnea, and exercise capacity; Dlco = single-breath diffusing capacity for carbon monoxide; mMRC = modified Medical Research Council; TLC = total lung capacity.
The postbronchodilator FEV1% predicted and FEV1/FVC ratio data are missed for one subject. The BODE index for this subject was computed with his prebronchodilator FEV1% predicted of 86%.
Table 2.
—Clinical, Physiologic, and CT Imaging Data of 50 Subjects by CT Scanner Brand
| Characteristic | Siemens CT (n = 14) | GE CT (n = 36) | P Value |
| Age, y | 65.5 (8.6) | 63.3 (8.6) | .42 |
| Male gender, No. (%) | 9 (64.3) | 22 (61.1) | .84 |
| BMI, kg/m2 | 25.6 (4.4) | 26.2 (4.7) | .65 |
| Smoking history, pack-y | 46.2 (24.1) | 57.4 (35.5) | .21 |
| mMRC dyspnea score | 2.3 (1.6) | 3.2 (1.7) | .15 |
| FEV1% predicteda | 51.0 (23.1) | 49.7 (25.8) | .88 |
| FEV1/FVC ratio | 0.46 (0.2) | 0.47 (0.2) | .87 |
| Dlco % predicted | 48.3 (16.6) | 52.5 (22.1) | .52 |
| TLC, L | 6.8 (1.6) | 5.8 (1.5) | .036 |
| 6MWD, m | 367.5 (150.5) | 283.0 (160.1) | .096 |
| BODE index | 2.9 (1.6) | 4.5 (3.5) | .14 |
| Score 1-6, No. (%) | 11 (79) | 22 (61) | |
| Score 7-10, No. (%) | 3 (21) | 14 (39) | .33 |
| Emphysema percentage | 23.4 (15.3) | 29.8 (14.3) | .18 |
| Total airway count of RB1 (generations 6 to 8) | 24.7 (9.4) | 23.9 (13.1) | .84 |
| Sixth generation | 7.6 (1.2) | 6.7 (2.0) | .07 |
| Seventh generation | 9.9 (4.3) | 9.1 (5.0) | .63 |
| Eighth generation | 7.3 (4.9) | 8.1 (7.1) | .0.64 |
Data are presented as mean (SD), except for male gender and BODE index categories, which are expressed as n (%). P values were computed with the t test or χ2 test (or the Fisher exact test). CT scan slice thicknesses of the Siemens and GE scanners were 1.25 mm and 1 mm, respectively, with 50% overlap. More details about the CT imaging protocol are in Han et al.9 GE = General Electric; RB1 = apical bronchus of right upper lobe. See Table 1 legend for expansion of other abbreviations.
The postbronchodilator FEV1% predicted and FEV1/FVC ratio data are missed for one subject. The BODE index for this subject was computed with his prebronchodilator FEV1% predicted of 86%.
Total and Generational Airway Counts
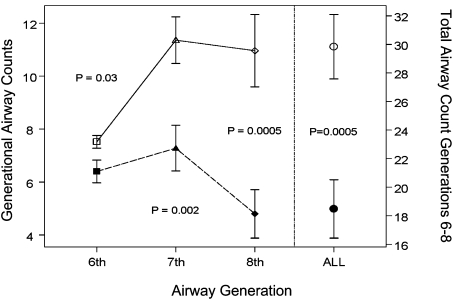
Those subjects with ≥ 25% emphysema had a lower TAC (18.5 ± 10.2) than those with < 25% emphysema (29.8 ± 11.3) and the differences were statistically significant (P = .0005) (Fig 3). After adjustment for age, gender (female = 1), BMI, and TLC, the mean TACs for the high and low emphysema groups were 19.3 and 29.0, respectively (P model < .0001). This difference in airway count between the two emphysema groups was also noted when analyzing the sixth AG (6.4 ± 2.2 vs 7.5 ± 1.2, P = .03), seventh AG (7.3 ± 4.3 vs 11.4 ± 4.4, P = .002), and eighth AG (4.8 ± 4.6 vs 11.0 ± 6.8, P = .0005) (Fig 3). For the whole population, the ratios of real airway count to Weibel’s theoretic airway count from the sixth AG through the eighth AG were 0.87, 0.58, and 0.24, respectively. There were no significant differences in the airway counts by scanner brand (Table 2).
Figure 3.
Airway count from generations 6 to 8 and the total airway count of the apical bronchus of the right upper lobe in subjects with emphysema. In the left y-axis, comparison of the individual airway count obtained in generations 6 to 8 between subjects with ≥ 25% (solid line with empty symbols) and < 25% (dashed line with solid symbols) emphysema is shown. In the right y-axis, comparison of the total airway count between subjects with high (○) and low (●) emphysema is also shown. Data are presented as mean ± SEM. P values were computed via t tests.
Relationships Between Airway Counts and Emphysema
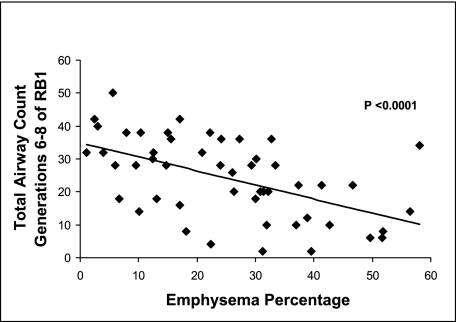
There was an inverse and statistically significant association (r = −0.54, P < .0001) (Fig 4) between TAC and whole-lung emphysema. Results were comparable when analyzing emphysema and airway counts of the sixth AG (r = −0.40, P = .004), seventh AG (r = −0.49, P = .0003), or eighth AG (r = −0.52, P = .0001) of RB1. Correlations of airways counts and upper-zone emphysema were comparable. The correlation coefficient for TAC and the upper-zone emphysema was −0.61 (P < .0001) and for the sixth to eighth generational airway counts and upper-zone emphysema were −0.48 (P = .0004), −0.59 (P < .0001), and −0.57 (P < .0001), respectively.
Figure 4.
Plot of total airway count (the sum of the branches from the sixth to eighth generations of the apical bronchus of the right upper lobe [RB1]) to emphysema, defined as low attenuation areas below −950 Hounsfield units in 50 smokers. See Figure 1 legend for expansion of abbreviation.
Relationships Among Airway Counts, Clinical and Physiologic Variables, and BODE Index
Airway counts were correlated with dyspnea, FEV1% predicted, Dlco% predicted, exercise capacity, and BODE index, which are known to have prognostic value in subjects with COPD. Correlations of TAC and generational airway counts were stronger with BODE index than with mMRC dyspnea score, FEV1% predicted, Dlco% predicted, and 6MWD (Table 3). Those subjects with lower TAC had a higher mMRC dyspnea score and BODE index, and a lower FEV1% predicted, Dlco% predicted, and 6MWD.
Table 3.
—Pearson Correlation Coefficients (r) Between Airway Counts of RB1, Dyspnea Score, Lung Function, 6MWD, and BODE Index in 50 Subjects
| Airway Counts of RB1 | mMRC Dyspnea Score | FEV1% Predicted | Dlco % Predicted | TLC, L | 6MWD, m | BODE Index |
| Total airway count (generations 6-8) | −0.42 | 0.52 | 0.45 | −0.01 | 0.36 | −0.55 |
| Sixth generation | −0.30 | 0.34 | 0.36 | 0.13 | 0.39 | −0.45 |
| Seventh generation | −0.41 | 0.45 | 0.43 | −0.04 | 0.38 | −0.53 |
| Eighth generation | −0.40 | 0.53 | 0.43 | −0.03 | 0.28 | −0.50 |
Predictive Ability of TAC for BODE
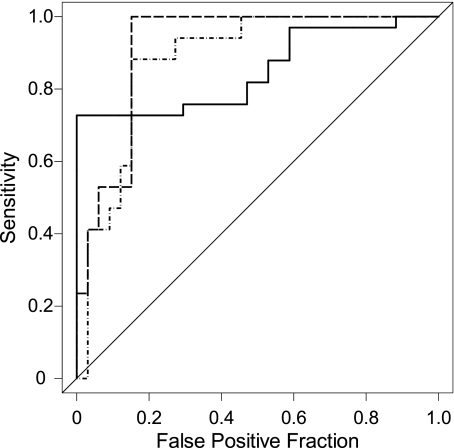
TAC was a good predictor of a high (worse) BODE index, as suggested by the area under the ROC curve of TAC: 0.84 (95% CI, 0.73-0.95) (Fig 5). TAC (mean ± SD, 14.9 ± 8.5 airways) for subjects with BODE ≥ 7 points was statistically significantly lower than for subjects with BODE < 7 (28.9 ± 10.9 airways, P < .0001).
Figure 5.
Receiver operating characteristic (ROC) curves of prognostic rule based on total airway count alone (solid line; area under the ROC curve [AUC-ROC] = 0.84); emphysema alone (dotted line; AUC-ROC = 0.88); and total airway count, emphysema, and age (dashed line; AUC-ROC = 0.92). This last ROC curve was obtained using the risk-score of the logistic regression model.
To improve the prognostic accuracy of CT image assessment for smoking-related lung disease, we examined the ability of TAC, emphysema, age, and gender jointly in predicting a BODE ≥ 7 points with a logistic regression model. Although age became not statistically significant when included in a model with TAC and emphysema, it increased the predictive ability of the model by 0.02 units (area under the ROC curve = 0.92 [95% CI, 0.84-0.99]) (Table 4). Gender neither was a statistically significant predictor nor added predictive power to the model. Both CT imaging indices were statistically significant predictors of a high BODE index (for TAC, P = .02, for emphysema, P = .03).
Table 4.
—Logistic Regression Models for a BODE Index ≥ 7 Points
| Predictor | Odds Ratio (95% CI) | P Value | Model AUC-ROC (C-statistic) |
| Single predictor, crude analysis | |||
| Total airway count generations 6-8 of RB1 | 0.88 (0.82, 0.95) | < .0001 | 0.84 (0.73, 0.95) |
| Multiple predictors, adjusted analysis | |||
| Total airway count generations 6-8 of RB1 | 0.91 (0.84, 0.99) | .02 | 0.92 (0.84, 0.99) |
| Emphysema | 1.08 (1.01, 1.16) | .03 | |
| Age | 0.93 (0.82, 1.05) | .23 | |
Discussion
In this study of a subset of subjects enrolled in the LTRC, we performed a detailed visual assessment of the third through eighth AG arising from the RB1. There was an inverse association among the TAC, the airway count of the sixth AG to the eighth AG of RB1, and emphysema. Although TAC and airway counts of the sixth AG to the eighth AG were directly related to both spirometric measures of lung function and the distance walked by a subject in 6 min, they were inversely related to both mMRC dyspnea score and BODE index. A subsequent regression analysis showed that TAC was a predictor of a high (worse) BODE index.
TAC-Emphysema Association
To our knowledge, there are data only on airway branch counts on clinical CT scans of smokers with normal lung function but not on smokers with emphysema.8 In the cohort reported here, we found an inverse association between TAC and emphysema and its physiologic surrogate, Dlco. Those subjects with ≥ 25% emphysema had significantly lower TAC and generational airway counts than those subjects with < 25% emphysema. This finding is in agreement with an early study by Scott and Steiner,29 which reported a decrease in macroscopic airway count by bronchography with tantalum in subjects with a high emphysema percentage measured by histologic means compared with those with a low emphysema percentage. Later, Montaudon et al8 reported that there were no differences in the total and generational airway counts visible on CT scans between smokers and nonsmokers with normal lung function. Although emphysema was not reported in that study, we speculate that they may have not observed differences in the airway count because their smoking cohort did not have significant emphysema.
TAC-Association With Clinical Variables and Ability to Predict BODE Index
There is little information regarding the clinical relevance of proximal airway count. In this study, pairwise correlation analysis showed that the total count of visible airways was associated directly with FEV1% predicted, Dlco% predicted, and 6MWD, and was associated inversely with mMRC dyspnea score and BODE index. The observed association between lung function and airway count is in agreement with a similar finding reported by Leader et al.30 They used the airway count section (not the generational airway count) and found an association between this CT imaging metric and the magnitude of the FEV1 decrement. Although the association between emphysema, dyspnea, exercise capacity, and BODE index has been reported,9,31 to our knowledge there are no previous reports on the association between airway count performed on CT scan and these parameters. A relevant finding in this study was the high ability of TAC and of TAC jointly with emphysema to predict a high (worse) BODE index, a multidimensional disease severity score that predicts mortality in subjects with COPD. The ability of TAC and emphysema to predict a worse BODE index in smokers is consistent with a recent study that suggested that subjects with emphysema may have accelerated death and they might be identified by BODE index.9 A larger study, however, is needed to validate these CT imaging indices as predictors of mortality risk in subjects with emphysema and COPD. We believe that despite the modest size of our study cohort, the findings described here suggest that TAC may be a clinically meaningful phenotype of disease independent of standard CT imaging measures of emphysema and airway remodeling.
Study Limitations
This study has some limitations. First, this was a convenience sample from the LTRC initiative selected to represent the breadth of emphysema seen in clinical practice. Furthermore, there is inherent selection bias because subjects were enrolled before undergoing either surgical treatment or diagnosis for their condition (COPD, interstitial lung diseases, and others). Hence, they may not be fully representative of all smokers. However, the differences found between the two emphysema groups are similar to those reported by others.31
Second, the study lacks histopathologic corroboration. The apparent pruning of the proximal airway tree we observed may have been due to the distortion and collapse of the airways related to the change in tissue properties associated with emphysema, rather than the outright absence of airways as observed by Hogg et al.7 Although this is certainly possible, the airways surveyed in our study were the more central cartilaginous bronchi and sufficient distortion to render them invisible seems unlikely. Additionally, the increase in contrast between low attenuating areas of emphysema and an adjacent airway may, to a degree, mitigate this phenomenon by enhancing structure visibility.
Third, the airway count was performed manually on a slice-by-slice basis and not in a volumetric reconstruction of the airway tree. Although this two-dimensional approach may lead to confusion regarding the ascertainment of local AG, such manual methods have been employed previously as a standard reference to validate more automated software-based analysis of the airways.32 To further support our method, the intra- and interreader agreement in the TAC was high.
Fourth, the presence of visually apparent emphysema on the CT scan may bias the reader to underreport TAC. We did not find, however, that the differences in airway count between the two readings were associated with emphysema percentage (data not shown).
Finally, we assumed that the airway count of RB1 was representative of the distribution of airway count through the whole lung. It may be that such an assumption is not appropriate because it has been shown that there are regional variations in airway morphometry on CT scan.32,33 Further investigation is needed to clarify airway count distribution by lung lobes by means of CT scanning.
Conclusions
In summary, the total CT imaging-based count of airways present in the sixth through eighth AG (TAC) of a subset of smokers enrolled in the LTRC was inversely related to densitometric measures of emphysema and was also a predictor of a high BODE index. This latter observation suggests that CT imaging airway count may be a unique COPD-related clinically relevant phenotype. Additional investigation is needed in a larger sample to further explore the clinical significance of emphysema-associated pruning of the airway tree and to validate its use as a CT imaging phenotype of lung disease in smokers. Software development to reconstruct the bronchial tree and count airway branches by generation with high accuracy and reproducibility is desirable.
Acknowledgments
Author contributions: Dr Diaz: contributed to the writing and final approval of the manuscript.
Dr Valim: contributed to the statistical analysis and the writing and final approval of the manuscript.
Dr Yamashiro: contributed to the reading of CT scans for the reproducibility analysis of visual airway count, and to the writing and final approval of the manuscript.
Dr San José Estépar: contributed to software development, which allowed the CT imaging analysis of emphysema, and to the writing and final approval of the manuscript.
Mr Ross: contributed to software development, which allowed the CT imaging analysis of emphysema, and to the writing and final approval of the manuscript.
Dr Matsuoka: contributed to the writing and final approval of the manuscript.
Dr Bartholmai: contributed to the collection of CT scan data, and to the writing and final approval of the manuscript.
Dr Hatabu: contributed to the writing and final approval of the manuscript.
Dr Silverman: contributed to the writing and final approval of the manuscript.
Dr Washko: contributed to the writing and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Silverman received an honorarium for a talk on COPD genetics in 2006, and grant support and consulting fees from GlaxoSmithKline for two studies of COPD genetics; an honorarium from Bayer for a symposium at the European Respiratory Society Meeting in 2005; and honoraria in 2007 and 2008 and consulting fees from AstraZeneca. Drs Diaz, Valim, Yamashiro, San José Estépar, Matsuoka, Bartholmai, Hatabu, and Washko, and Mr Ross have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- 6MWD
6-min walk distance
- AG
airway generation
- BODE
BMI, airflow obstruction, dyspnea, and exercise capacity
- Dlco
single-breath diffusing capacity for carbon monoxide
- HU
Hounsfield unit
- LTRC
Lung Tissue Research Consortium
- mMRC
modified Medical Research Council
- RB1
apical bronchus of the right upper lobe
- ROC
receiver operating characteristic
- TAC
total airway count
- TLC
total lung capacity
Funding/Support: This work was supported by the National Institutes of Health [Grants K23HL089353-01A1 and U01089856] and by a grant from the Parker B. Francis Foundation.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 2.McLean KH. The histology of generalized pulmonary emphysema. II. Diffuse emphysema. Australas Ann Med. 1957;6(3):203–217. doi: 10.1111/imj.1957.6.3.203. [DOI] [PubMed] [Google Scholar]
- 3.Leopold JG, Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957;12(3):219–235. doi: 10.1136/thx.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean KH. The pathogenesis of pulmonary emphysema. Am J Med. 1958;25(1):62–74. doi: 10.1016/0002-9343(58)90199-2. [DOI] [PubMed] [Google Scholar]
- 5.Matsuba K, Thurlbeck WM. The number and dimensions of small airways in emphysematous lungs. Am J Pathol. 1972;67(2):265–275. [PMC free article] [PubMed] [Google Scholar]
- 6.McDonough JE, Sanchez PG, Elliott WM, et al. Small airway obstruction in COPD [abstract] Am J Respir Crit Care Med. 2009;179:A2970. [Google Scholar]
- 7.Hogg JC, McDonough JE, Sanchez PG, et al. Micro-computed tomography measurements of peripheral lung pathology in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(6):546–549. doi: 10.1513/pats.200905-029DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montaudon M, Berger P, Lederlin M, Marthan R, Tunon-de-Lara JM, Laurent F. Bronchial morphometry in smokers: comparison with healthy subjects by using 3D CT. Eur Radiol. 2009;19(6):1328–1334. doi: 10.1007/s00330-008-1284-3. [DOI] [PubMed] [Google Scholar]
- 9.Han MK, Bartholmai B, Liu LX, et al. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6(6):459–467. doi: 10.3109/15412550903341513. [DOI] [PubMed] [Google Scholar]
- 10.Mishima M, Hirai T, Itoh H, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 1999;96(16):8829–8834. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Bruesewitz MR, Bartholmai BJ, McCollough CH. Selection of appropriate computed tomographic image reconstruction algorithms for a quantitative multicenter trial of diffuse lung disease. J Comput Assist Tomogr. 2008;32(2):233–237. doi: 10.1097/RCT.0b013e3180690d89. [DOI] [PubMed] [Google Scholar]
- 12.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94(4):782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 13.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 14.Brown MS, McNitt-Gray MF, Goldin JG, et al. Automated measurement of single and total lung volume from CT. J Comput Assist Tomogr. 1999;23(4):632–640. doi: 10.1097/00004728-199907000-00027. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of Plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137(5):1108–1115. doi: 10.1378/chest.09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 17.Washko GR, Dransfield MT, Estépar RS, et al. Airway wall attenuation: a biomarker of airway disease in subjects with COPD. J Appl Physiol. 2009;107(1):185–191. doi: 10.1152/japplphysiol.00216.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weibel ER, Gomez DM. Architecture of the human lung. Use of quantitative methods establishes fundamental relations between size and number of lung structures. Science. 1962;137:577–585. doi: 10.1126/science.137.3530.577. [DOI] [PubMed] [Google Scholar]
- 19.Weibel E. Morphometry of the Human Lung. Berlin, Gottinngen. Heidelberg, Germany: Springer-Verlag; 1963. [Google Scholar]
- 20.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 22.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 23.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 24.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127(3):270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- 25.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Measurement error and correlation coefficients. BMJ. 1996;313(7048):41–42. doi: 10.1136/bmj.313.7048.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 28.Nam B, D’ Agostino R. Discrimination index, the area under the ROC curve. In: Huber-Carol C, Balakrishnam N, Nikulin M, et al., editors. Goodness-of-Fit Tests and Model Validity. Boston, MA: Birkhauser; 2002. pp. 267–280. [Google Scholar]
- 29.Scott KW, Steiner GM. Postmortem assessment of chronic airways obstruction by tantalum bronchography. Thorax. 1975;30(4):405–414. doi: 10.1136/thx.30.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leader J, Coxson H, Zheng B, et al. The relation between lung function decline and airway count [abstract] Am J Respir Crit Care Med. 2009;179:A5576. [Google Scholar]
- 31.Boschetto P, Quintavalle S, Zeni E, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61(12):1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montaudon M, Berger P, de Dietrich G, et al. Assessment of airways with three-dimensional quantitative thin-section CT: in vitro and in vivo validation. Radiology. 2007;242(2):563–572. doi: 10.1148/radiol.2422060029. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka S, Kurihara Y, Nakajima Y, Niimi H, Ashida H, Kaneoya K. Serial change in airway lumen and wall thickness at thin-section CT in asymptomatic subjects. Radiology. 2005;234(2):595–603. doi: 10.1148/radiol.2342031466. [DOI] [PubMed] [Google Scholar]