Abstract
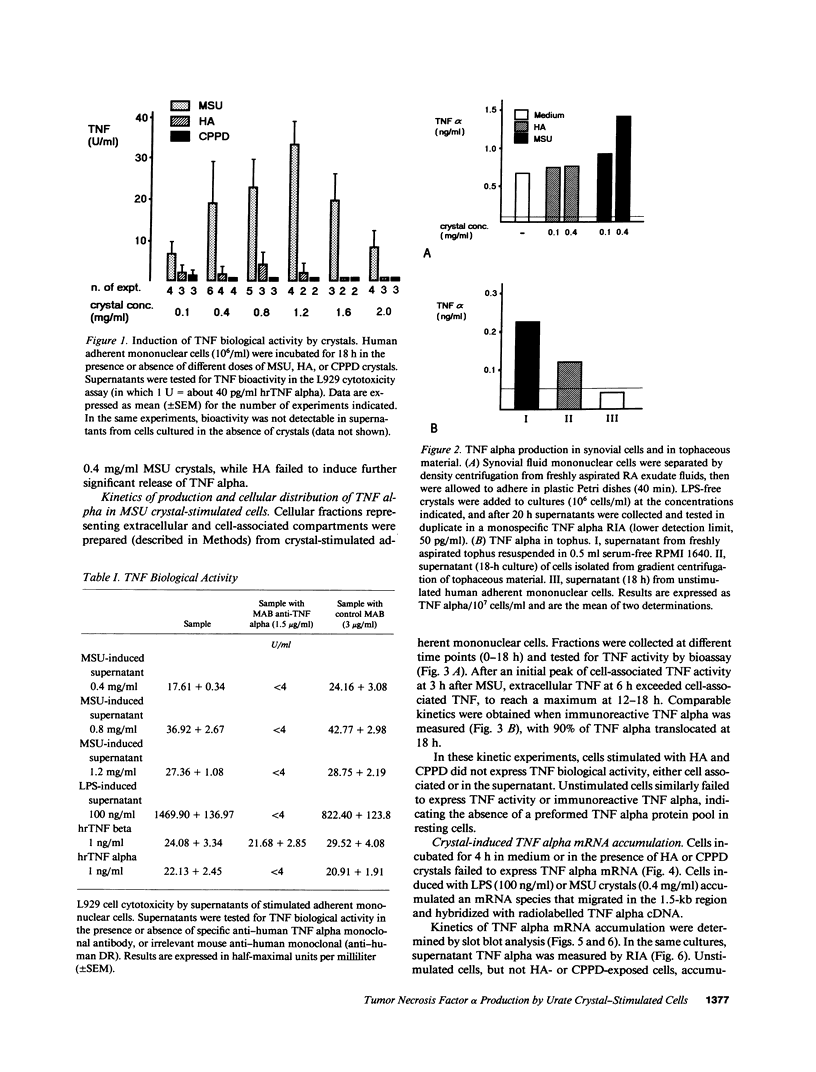
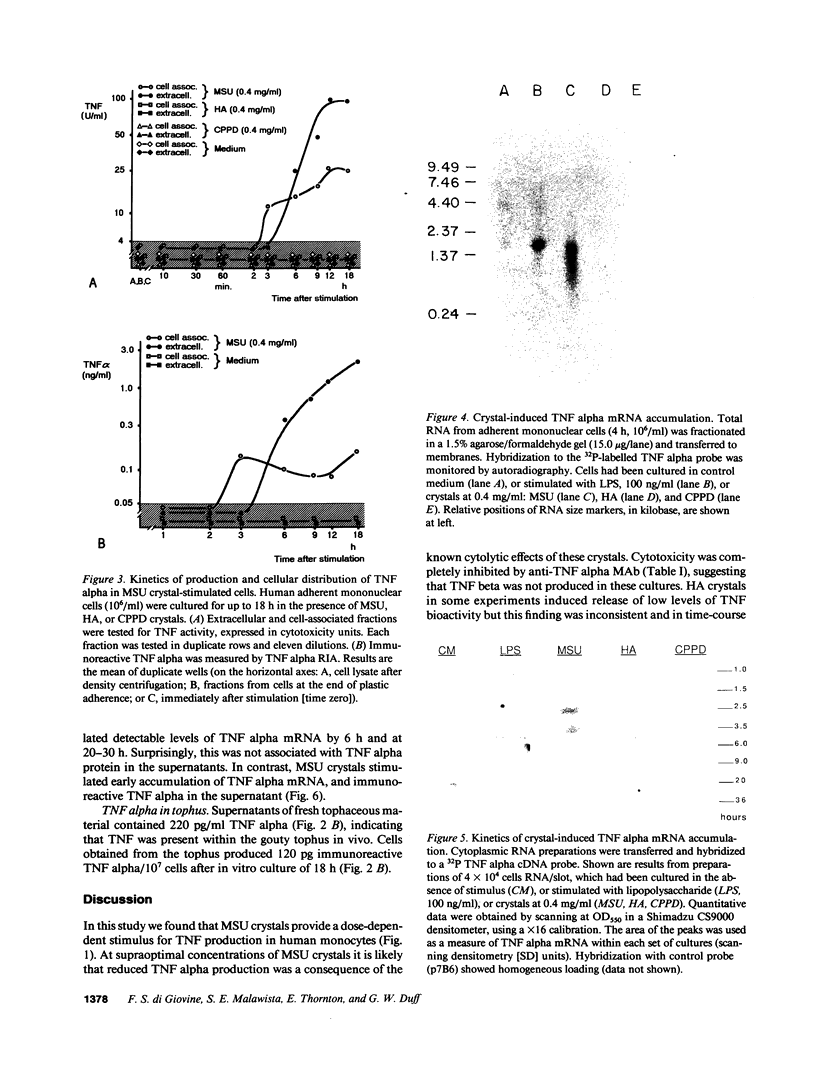
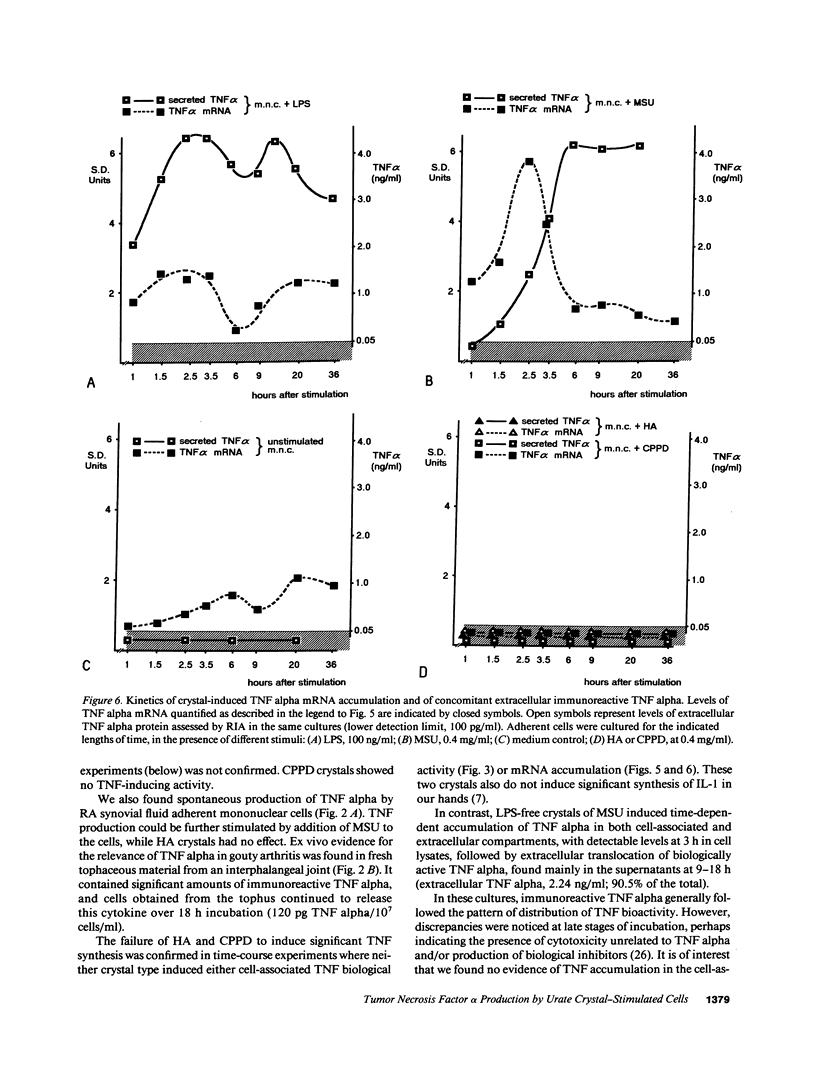
Crystals of monosodium urate (MSU) provide a dose-dependent stimulus for the production by human blood monocytes of tumor necrosis factor (TNF), a cytokine with proinflammatory properties; TNF activity was inhibited selectively by monoclonal antibody to TNF alpha. Biologically active cell-associated TNF activity peaked at 3 h and was exceeded at 6 h by extracellular activity, which peaked at 12-18 h. Comparable kinetics were observed with immunoreactive TNF alpha. TNF alpha mRNA accumulation in monocytes stimulated with MSU crystals appeared as a single peak at 2-4 h, kinetics compatible with rapid production of a short half-life transcript. In contrast, crystals of calcium pyrophosphate or of hydroxyapatite did not stimulate significant production of TNF or of message. Fresh tophaceous material from a patient with gout contained significant levels of TNF alpha and cells cultured from the tophus produced TNF alpha in vitro. In rheumatoid synovial cells, spontaneous release of TNF alpha was increased by in vitro exposure to MSU crystals. Taken together with earlier work, these results support an expanded view of gouty inflammation in which the crystal-stimulated production of cytokines provides a crucial link between crystal deposition and many of the clinical and pathological facts of both acute and chronic gouty arthritis.
Full text
PDF



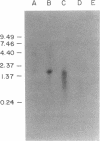
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Defilippi P., Poupart P., Tavernier J., Fiers W., Content J. Induction and regulation of mRNA encoding 26-kDa protein in human cell lines treated with recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4557–4561. doi: 10.1073/pnas.84.13.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovine F. S., Malawista S. E., Nuki G., Duff G. W. Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal-induced IL 1. J Immunol. 1987 May 15;138(10):3213–3218. [PubMed] [Google Scholar]
- Di Giovine F. S., Nuki G., Duff G. W. Tumour necrosis factor in synovial exudates. Ann Rheum Dis. 1988 Sep;47(9):768–772. doi: 10.1136/ard.47.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. The biology of interleukin 1 and comparison to tumor necrosis factor. Immunol Lett. 1987 Dec;16(3-4):227–231. doi: 10.1016/0165-2478(87)90151-9. [DOI] [PubMed] [Google Scholar]
- Duff G. W., Atkins E., Malawista S. E. The fever of gout: urate crystals activate endogenous pyrogen production from human and rabbit mononuclear phagocytes. Trans Assoc Am Physicians. 1983;96:234–245. [PubMed] [Google Scholar]
- Duff G. W., Atkins E. The detection of endotoxin by in vitro production of endogenous pyrogen: comparison with limulus amebocyte lysate gelation. J Immunol Methods. 1982 Aug 13;52(3):323–331. doi: 10.1016/0022-1759(82)90004-7. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Terkeltaub R., Zuraw B., Lotz M. Inflammatory microcrystals stimulate interleukin-6 production and secretion by human monocytes and synoviocytes. Arthritis Rheum. 1989 Nov;32(11):1443–1452. doi: 10.1002/anr.1780321114. [DOI] [PubMed] [Google Scholar]
- Hanson D. F., Murphy P. A., Windle B. E. Failure of rabbit neutrophils to secrete endogenous pyrogen when stimulated with staphylococci. J Exp Med. 1980 Jun 1;151(6):1360–1371. doi: 10.1084/jem.151.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L., Calabretta B., Baserga R. Expression of cell-cycle-dependent genes in phytohemagglutinin-stimulated human lymphocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5375–5379. doi: 10.1073/pnas.82.16.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Duff G. W., Atkins E., Cheung H. S., McCarty D. J. Crystal-induced endogenous pyrogen production. A further look at gouty inflammation. Arthritis Rheum. 1985 Sep;28(9):1039–1046. doi: 10.1002/art.1780280911. [DOI] [PubMed] [Google Scholar]
- Marmenout A., Fransen L., Tavernier J., Van der Heyden J., Tizard R., Kawashima E., Shaw A., Johnson M. J., Semon D., Müller R. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Lapierre L. A., Stolpen A. H., Brock T. A., Springer T. A., Fiers W., Bevilacqua M. P., Mendrick D. L., Gimbrone M. A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987 May 15;138(10):3319–3324. [PubMed] [Google Scholar]
- Ruggiero V., Baglioni C. Synergistic anti-proliferative activity of interleukin 1 and tumor necrosis factor. J Immunol. 1987 Feb 1;138(3):661–663. [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Imamura K., Luebbers R., Kufe D. Transcriptional and posttranscriptional regulation of tumor necrosis factor gene expression in human monocytes. J Clin Invest. 1988 May;81(5):1506–1510. doi: 10.1172/JCI113482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]
- Terkeltaub R. A., Ginsberg M. H. The inflammatory reaction to crystals. Rheum Dis Clin North Am. 1988 Aug;14(2):353–364. [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]