Abstract
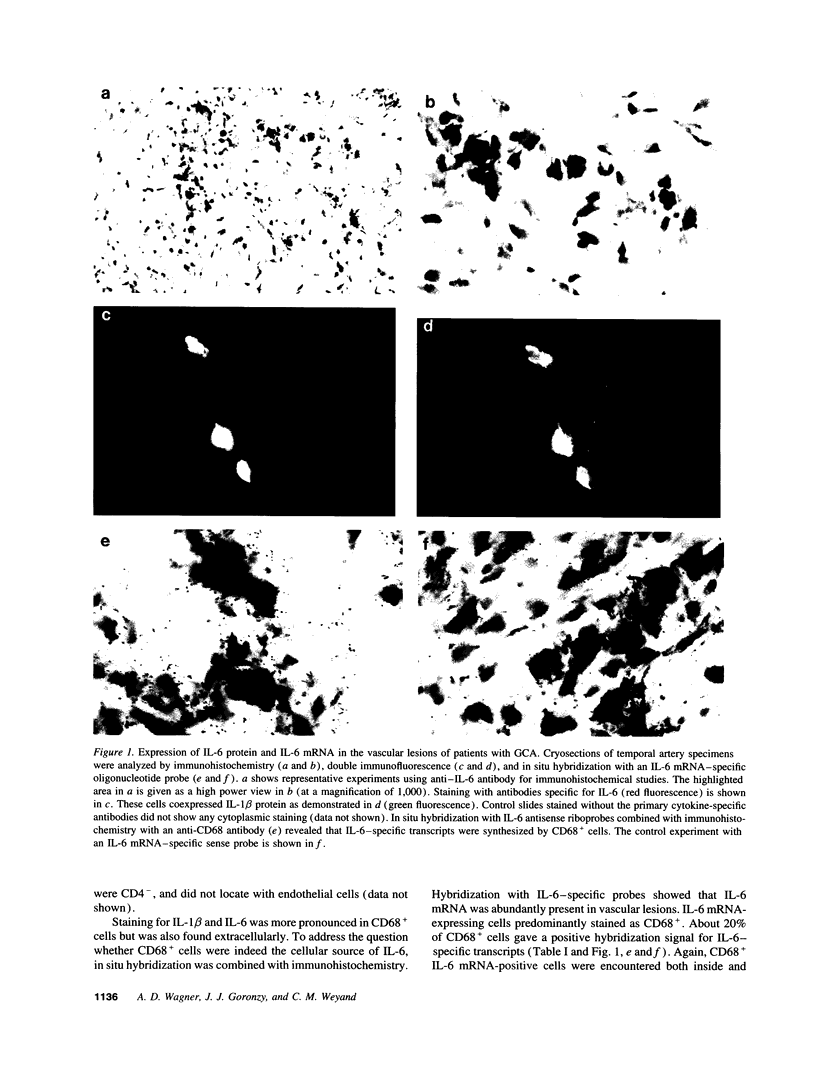
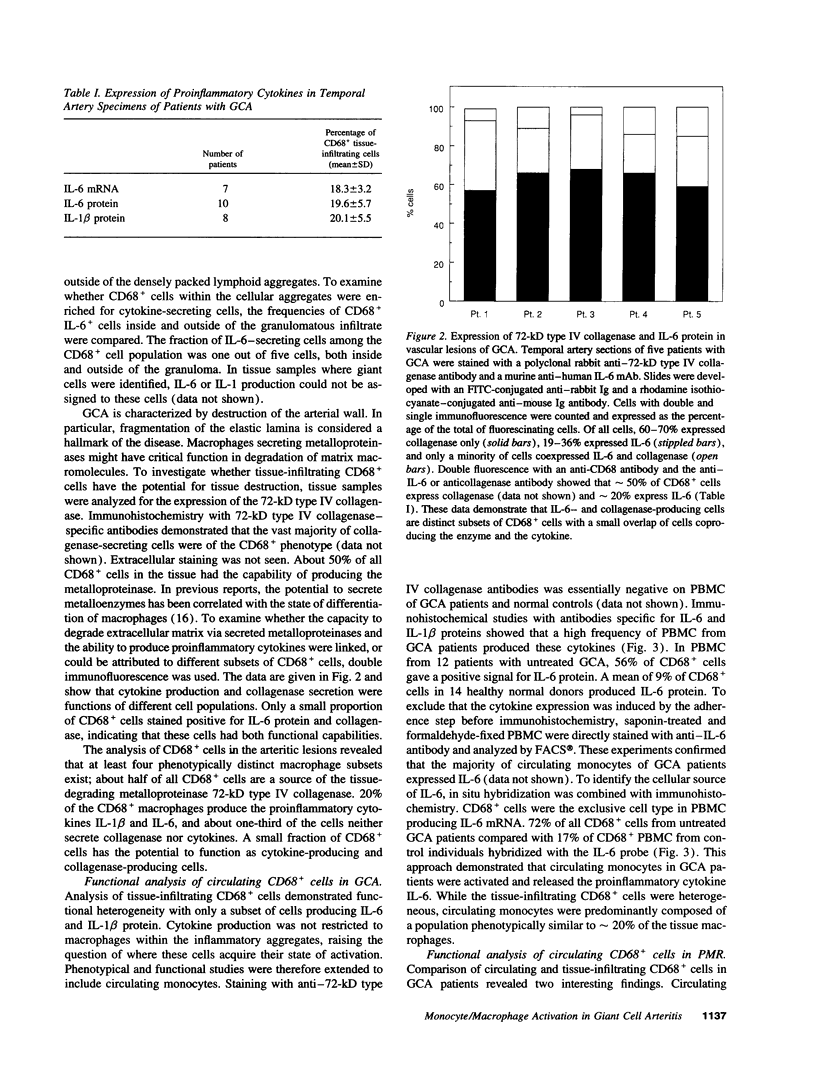
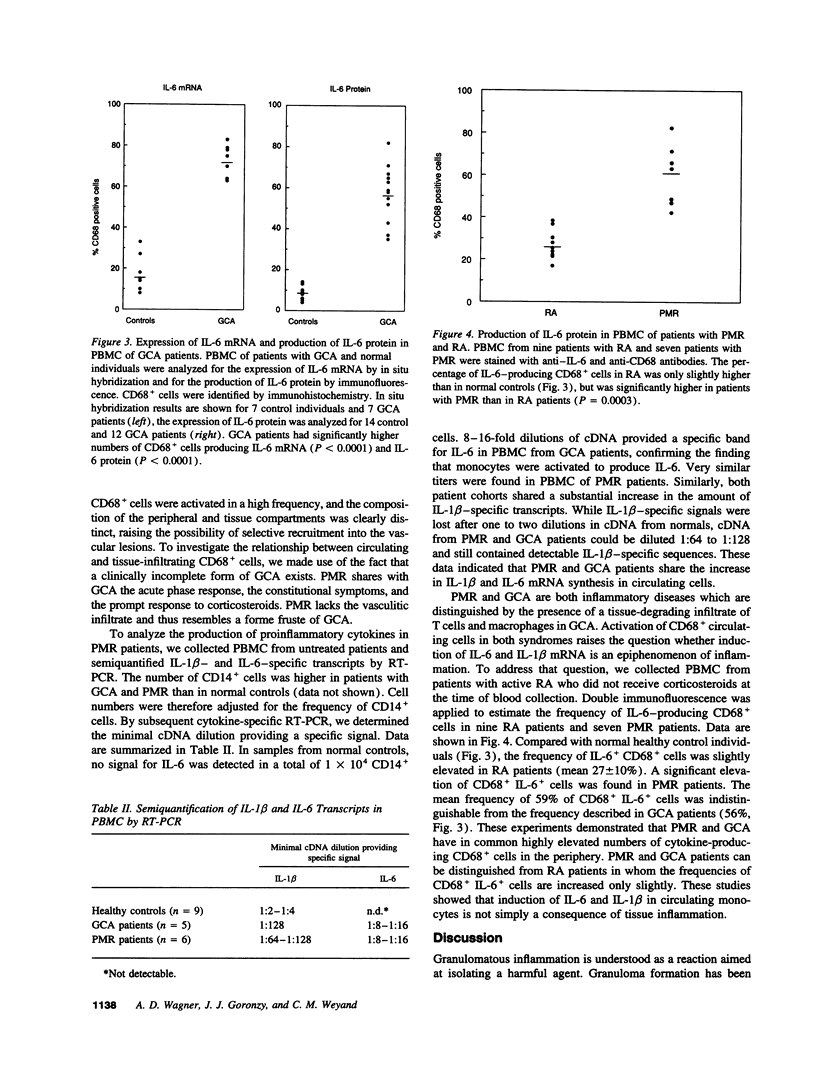
Macrophages represent a critical component in the inflammatory lesions of giant cell arteritis. By combining immunohistochemistry and in situ hybridization, we have analyzed the functional heterogeneity of tissue-infiltrating macrophages in patients with untreated vasculitis. 20% of macrophages in temporal artery tissue synthesized IL-6-specific mRNA and produced IL-6 and IL-1 beta proteins. IL-6 and IL-1 beta production was not limited to CD68+ cells in the lymphoid aggregates but was a feature of CD68+ cells dispersed throughout the tissue. 50% of tissue-infiltrating CD68+ cells synthesized 72-kD type IV collagenase. Only a small subset of CD68+ cells produced cytokines as well as collagenase, indicating functional specialization or distinct differentiation stages of CD68+ cells in the inflamed tissue. Activation of CD68+ cells was not restricted to tissue-infiltrating cells. Expression of IL-6 and IL-1 beta was found in 60-80% of circulating monocytes of patients with untreated giant cell arteritis, whereas collagenase production was restricted to tissue macrophages. IL-6 and IL-1 beta production by the majority of circulating monocytes was a shared feature of patients with giant cell arteritis and polymyalgia rheumatica but was not found in rheumatoid arthritis. These data suggest that giant cell arteritis has two components of disease, an inflammatory reaction in vessel walls and a systemic activation of monocytes. Systemic monocyte activation can manifest independently without vasculitis as exemplified in patients with polymyalgia rheumatica.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bernaudin J. F., Yamauchi K., Wewers M. D., Tocci M. J., Ferrans V. J., Crystal R. G. Demonstration by in situ hybridization of dissimilar IL-1 beta gene expression in human alveolar macrophages and blood monocytes in response to lipopolysaccharide. J Immunol. 1988 Jun 1;140(11):3822–3829. [PubMed] [Google Scholar]
- Bignon J. D., Ferec C., Barrier J., Pennec Y., Verlingue C., Cheneau M. L., Lucas V., Muller J. Y., Saleun J. P. HLA class II genes polymorphism in DR4 giant cell arteritis patients. Tissue Antigens. 1988 Nov;32(5):254–258. doi: 10.1111/j.1399-0039.1988.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Calamia K. T., Moore S. B., Elveback L. R., Hunder G. G. HLA-DR locus antigens in polymyalgia rheumatica and giant cell arteritis. J Rheumatol. 1981 Nov-Dec;8(6):993–996. [PubMed] [Google Scholar]
- Chuang T. Y., Hunder G. G., Ilstrup D. M., Kurland L. T. Polymyalgia rheumatica: a 10-year epidemiologic and clinical study. Ann Intern Med. 1982 Nov;97(5):672–680. doi: 10.7326/0003-4819-97-5-672. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Devergne O., Peuchmaur M., Humbert M., Navratil E., Leger-Ravet M. B., Crevon M. C., Petit M. A., Galanaud P., Emilie D. In vivo expression of IL-1 beta and IL-6 genes during viral infections in human. Eur Cytokine Netw. 1991 May-Jun;2(3):183–194. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and tumor necrosis factor: effector cytokines in autoimmune diseases. Semin Immunol. 1992 Jun;4(3):133–145. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold K. N., Weyand C. M., Goronzy J. J. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994 Jun;37(6):925–933. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Zuraw B. L., Vaughan J. H., Carson D. A., Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989 Feb;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunder G. G., Bloch D. A., Michel B. A., Stevens M. B., Arend W. P., Calabrese L. H., Edworthy S. M., Fauci A. S., Leavitt R. Y., Lie J. T. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990 Aug;33(8):1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- Hunder G. G., Lie J. T., Goronzy J. J., Weyand C. M. Pathogenesis of giant cell arteritis. Arthritis Rheum. 1993 Jun;36(6):757–761. doi: 10.1002/art.1780360604. [DOI] [PubMed] [Google Scholar]
- Huston K. A., Hunder G. G., Lie J. T., Kennedy R. H., Elveback L. R. Temporal arteritis: a 25-year epidemiologic, clinical, and pathologic study. Ann Intern Med. 1978 Feb;88(2):162–167. doi: 10.7326/0003-4819-88-2-162. [DOI] [PubMed] [Google Scholar]
- Lie J. T. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum. 1990 Aug;33(8):1074–1087. doi: 10.1002/art.1780330804. [DOI] [PubMed] [Google Scholar]
- Lowenstein M. B., Bridgeford P. H., Vasey F. B., Germain B. F., Espinoza L. R. Increased frequency of HLA-DR3 and DR4 in polymyalgia rheumatica-giant cell arteritis. Arthritis Rheum. 1983 Jul;26(7):925–927. doi: 10.1002/art.1780260717. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K. A., Rigney E. M., Micklem K. J., Jones M., Stross W. P., Gatter K. C., Mason D. Y. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche N. E., Fulbright J. W., Wagner A. D., Hunder G. G., Goronzy J. J., Weyand C. M. Correlation of interleukin-6 production and disease activity in polymyalgia rheumatica and giant cell arteritis. Arthritis Rheum. 1993 Sep;36(9):1286–1294. doi: 10.1002/art.1780360913. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Functional domains on HLA-DR molecules: implications for the linkage of HLA-DR genes to different autoimmune diseases. Clin Immunol Immunopathol. 1994 Feb;70(2):91–98. doi: 10.1006/clin.1994.1015. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Hunder G. G., Goronzy J. J. The HLA-DRB1 locus as a genetic component in giant cell arteritis. Mapping of a disease-linked sequence motif to the antigen binding site of the HLA-DR molecule. J Clin Invest. 1992 Dec;90(6):2355–2361. doi: 10.1172/JCI116125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Hunder N. N., Hicok K. C., Hunder G. G., Goronzy J. J. HLA-DRB1 alleles in polymyalgia rheumatica, giant cell arteritis, and rheumatoid arthritis. Arthritis Rheum. 1994 Apr;37(4):514–520. doi: 10.1002/art.1780370411. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Schönberger J., Oppitz U., Hunder N. N., Hicok K. C., Goronzy J. J. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994 Mar 1;179(3):951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. D., Firestein G. S., Taetle R., Kaushansky K., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989 Mar;83(3):876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]