Abstract
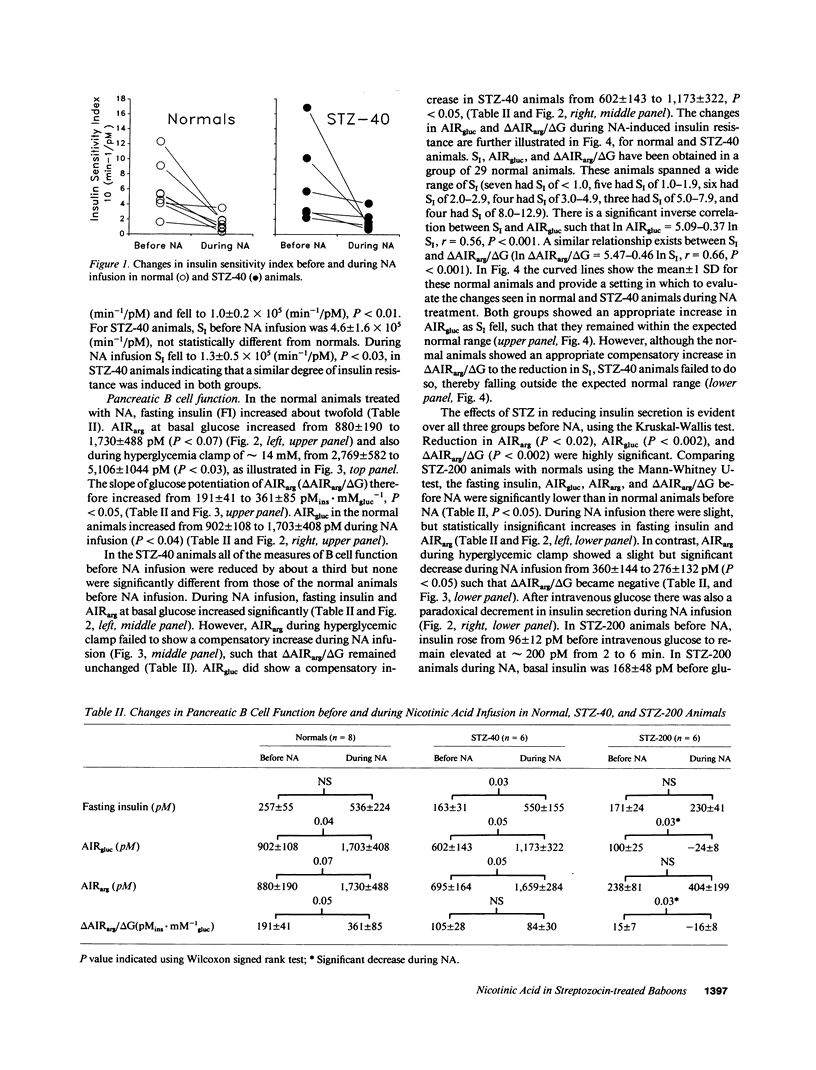
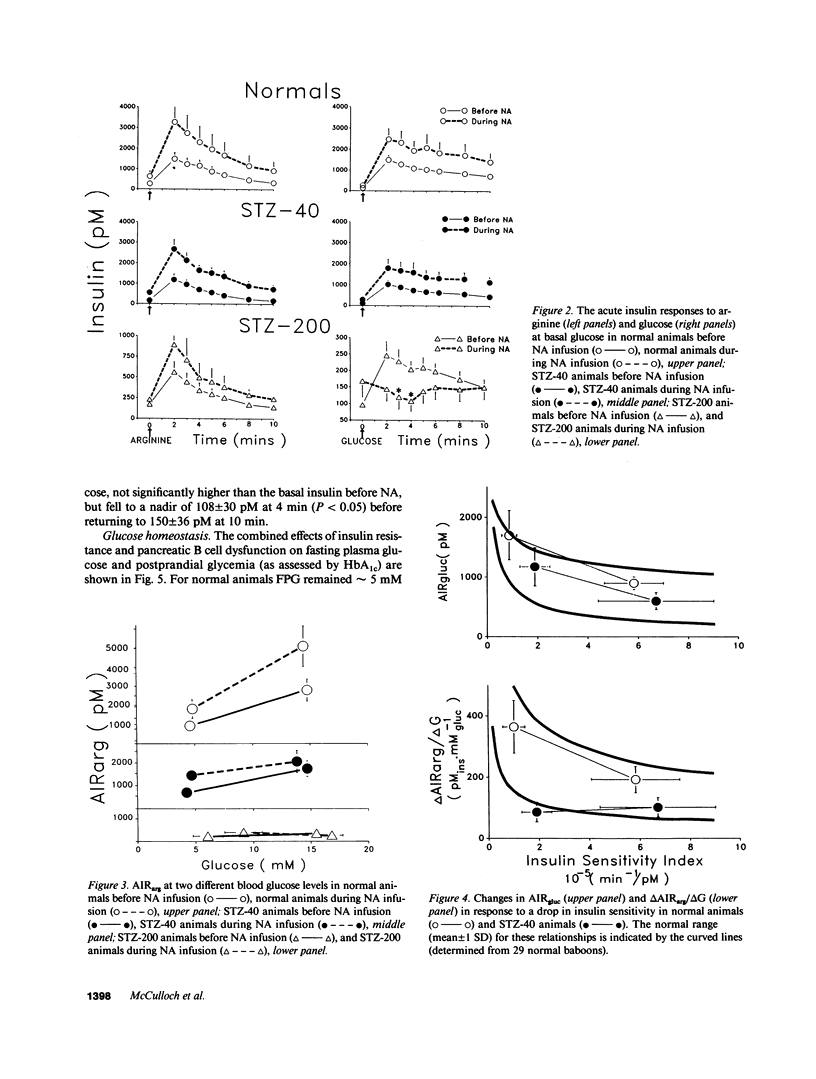
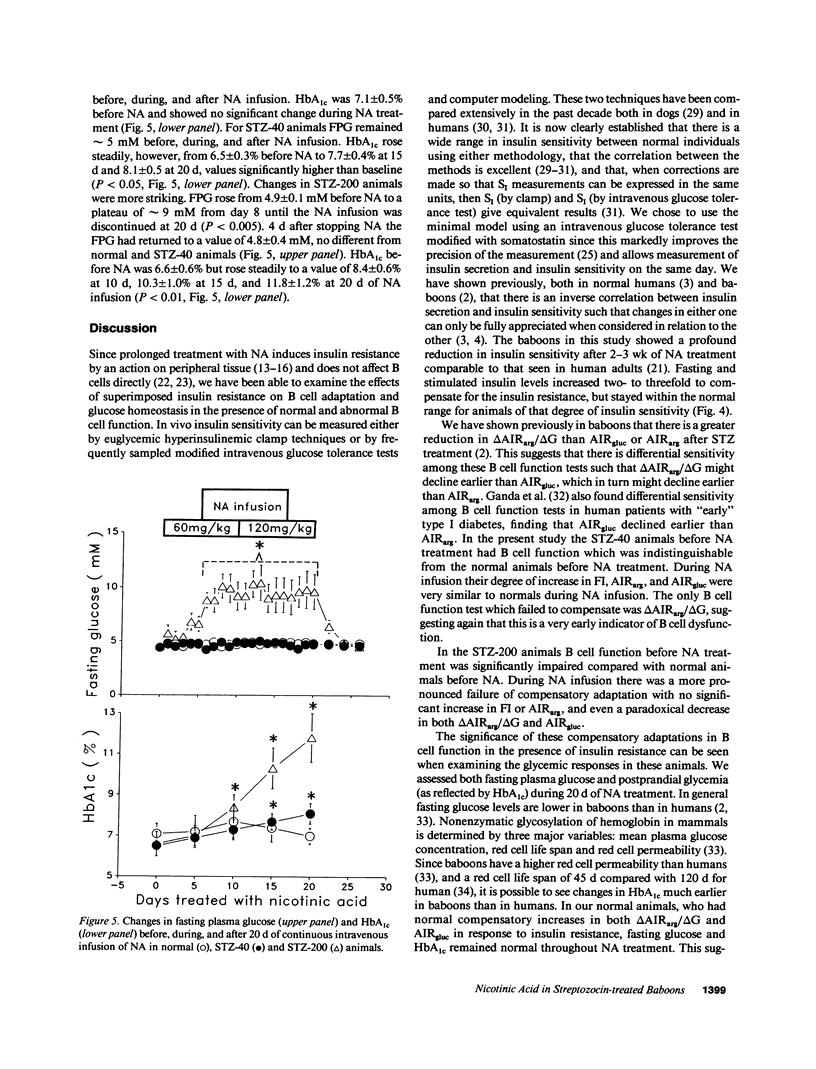
To study the interaction between insulin secretion and insulin action in maintaining glucose homeostasis, we induced experimental insulin resistance in eight normal baboons, in six baboons treated with 40 mg/kg streptozocin (STZ-40), and in six baboons treated with 200 mg/kg streptozocin (STZ-200). Insulin resistance was induced by a 20-d continuous intravenous infusion of nicotinic acid (NA). Normal animals showed compensatory increases in several measures of insulin secretion (fasting insulin [FI], acute insulin response to arginine [AIRarg], acute insulin response to glucose [AIRgluc], and glucose potentiation slope [delta AIRarg/delta G]), with no net change in fasting plasma glucose (FPG) or glycosylated hemoglobin (HbAtc). STZ-40 animals showed compensatory increases in FI, AIRarg, and AIRgluc, but delta AIRarg/delta G failed to compensate. Although FPG remained normal in this group during NA infusion, HbA1c rose significantly. STZ-200 animals failed to show compensatory changes in both AIRgluc and delta AIRarg/delta G, with both HbA1c and FPG rising. These animals showed a paradoxical inhibition of insulin secretion in response to intravenous glucose during NA infusion, at a time when they were hyperglycemic. These data indicate that a significant degree of insulin resistance does not cause hyperglycemia in the presence of normal B cell function but, in animals with reduced B cell mass and superimposed insulin resistance, the degree of hyperglycemia is proportional to the degree of pancreatic B cell dysfunction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews W. J., Vasquez B., Nagulesparan M., Klimes I., Foley J., Unger R., Reaven G. M. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984 Jul;33(7):634–642. doi: 10.2337/diab.33.7.634. [DOI] [PubMed] [Google Scholar]
- Barseghian G., Levine R. Effect of corticosterone on insulin and glucagon secretion by the isolated perfused rat pancreas. Endocrinology. 1980 Feb;106(2):547–552. doi: 10.1210/endo-106-2-547. [DOI] [PubMed] [Google Scholar]
- Beard J. C., Bergman R. N., Ward W. K., Porte D., Jr The insulin sensitivity index in nondiabetic man. Correlation between clamp-derived and IVGTT-derived values. Diabetes. 1986 Mar;35(3):362–369. doi: 10.2337/diab.35.3.362. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Ider Y. Z., Bowden C. R., Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979 Jun;236(6):E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Prager R., Volund A., Olefsky J. M. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987 Mar;79(3):790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSON L. A., ORO L. The effect of nicotinic acid on the plasma free fatty acid; demonstration of a metabolic type of sympathicolysis. Acta Med Scand. 1962 Dec;172:641–645. doi: 10.1111/j.0954-6820.1962.tb07203.x. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Bernstein J. M. The effect of nicotinic acid on growth hormone-induced lipolysis and glucose intolerance. J Lab Clin Med. 1973 Apr;81(4):568–576. [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Pacini G., Bergman R. N. The insulin sensitivity index. Correlation in dogs between values determined from the intravenous glucose tolerance test and the euglycemic glucose clamp. Diabetes. 1984 Apr;33(4):362–368. doi: 10.2337/diab.33.4.362. [DOI] [PubMed] [Google Scholar]
- Ganda O. P., Srikanta S., Brink S. J., Morris M. A., Gleason R. E., Soeldner J. S., Eisenbarth G. S. Differential sensitivity to beta-cell secretagogues in "early," type I diabetes mellitus. Diabetes. 1984 Jun;33(6):516–521. doi: 10.2337/diab.33.6.516. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Griffin J., Hamman R. F., Kolterman O. G. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985 Mar;34(3):222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- Gaut Z. N., Pocelinko R., Solomon H. M., Thomas G. B. Oral glucose tolerance, plasma insulin, and uric acid excretion in man during chronic administration of nicotinic acid. Metabolism. 1971 Nov;20(11):1031–1035. doi: 10.1016/0026-0495(71)90026-6. [DOI] [PubMed] [Google Scholar]
- Gross R. C., Carlson L. A. Metabolic effects of nicotinic acid in acute insulin deficiency in the rat. Diabetes. 1968 Jun;17(6):353–361. doi: 10.2337/diab.17.6.353. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Effects of low-carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose-tolerance tests. Lancet. 1963 Apr 13;1(7285):790–794. doi: 10.1016/s0140-6736(63)91501-0. [DOI] [PubMed] [Google Scholar]
- Halter J. B., Graf R. J., Porte D., Jr Potentiation of insulin secretory responses by plasma glucose levels in man: evidence that hyperglycemia in diabetes compensates for imparied glucose potentiation. J Clin Endocrinol Metab. 1979 Jun;48(6):946–954. doi: 10.1210/jcem-48-6-946. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Garlick R. L., Bunn H. F. Glycosylated hemoglobin in human and animal red cells. Role of glucose permeability. Diabetes. 1982 Sep;31(9):743–748. doi: 10.2337/diab.31.9.743. [DOI] [PubMed] [Google Scholar]
- Hollobaugh S. L., Tzagournis M., Folk R. L., Kruger F. A., Hamwi G. J. The diabetogenic action of huuan growth hormone: glucose--fatty acid interrelationships. Metabolism. 1968 Jun;17(6):485–491. doi: 10.1016/0026-0495(68)90039-5. [DOI] [PubMed] [Google Scholar]
- Johnston C., Raghu P., McCulloch D. K., Beard J. C., Ward W. K., Klaff L. J., McKnight B., Bergman R. N., Palmer J. P. Beta-cell function and insulin sensitivity in nondiabetic HLA-identical siblings of insulin-dependent diabetics. Diabetes. 1987 Jul;36(7):829–837. doi: 10.2337/diab.36.7.829. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Beard J. C., Schwartz M. W., Ward W. K., Ding H. L., Bergman R. N., Taborsky G. J., Jr, Porte D., Jr Increased beta-cell secretory capacity as mechanism for islet adaptation to nicotinic acid-induced insulin resistance. Diabetes. 1989 May;38(5):562–568. doi: 10.2337/diab.38.5.562. [DOI] [PubMed] [Google Scholar]
- Kalhan S. C., Adam P. A. Inhibitory effect of prednisone on insulin secretion in man: model for duplication of blood glucose concentration. J Clin Endocrinol Metab. 1975 Sep;41(3):600–610. doi: 10.1210/jcem-41-3-600. [DOI] [PubMed] [Google Scholar]
- Lager I., Lönnroth P., von Schenck H., Smith U. Reversal of insulin resistance in type I diabetes after treatment with continuous subcutaneous insulin infusion. Br Med J (Clin Res Ed) 1983 Dec 3;287(6406):1661–1664. doi: 10.1136/bmj.287.6406.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. L., Porte D., Jr Acute and steady-state insulin responses to glucose in nonobese diabetic subjects. J Clin Invest. 1972 Jul;51(7):1624–1631. doi: 10.1172/JCI106963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell H., Vessby B., Hellsing K. Changes in glucose tolerance and plasma insulin during lipid-lowering treatment with diet, clofibrate and niceritrol. Atherosclerosis. 1982 Jun;43(2-3):177–184. doi: 10.1016/0021-9150(82)90020-x. [DOI] [PubMed] [Google Scholar]
- Maclaren N. K., Riley W. J., Winter W. E., Silverstein J. H. Is insulin-dependent diabetes mellitus a preventable disease? Clin Invest Med. 1987 Sep;10(5):444–449. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco J., Calle C., Román D., Díaz-Fierros M., Villanueva M. L., Valverde I. Hyperglucagonism induced by glucocorticoid treatment in man. N Engl J Med. 1973 Jan 18;288(3):128–131. doi: 10.1056/NEJM197301182880305. [DOI] [PubMed] [Google Scholar]
- McCulloch D. K., Klaff L. J., Kahn S. E., Schoenfeld S. L., Greenbaum C. J., Mauseth R. S., Benson E. A., Nepom G. T., Shewey L., Palmer J. P. Nonprogression of subclinical beta-cell dysfunction among first-degree relatives of IDDM patients. 5-yr follow-up of the Seattle Family Study. Diabetes. 1990 May;39(5):549–556. doi: 10.2337/diab.39.5.549. [DOI] [PubMed] [Google Scholar]
- McCulloch D. K., Raghu P. K., Johnston C., Klaff L. J., Kahn S. E., Beard J. C., Ward W. K., Benson E. A., Koerker D. J., Bergman R. N. Defects in beta-cell function and insulin sensitivity in normoglycemic streptozocin-treated baboons: a model of preclinical insulin-dependent diabetes. J Clin Endocrinol Metab. 1988 Oct;67(4):785–792. doi: 10.1210/jcem-67-4-785. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Halter J. B., Robertson R. P. Paradoxical inhibition of insulin secretion by glucose in human diabetes mellitus. J Clin Endocrinol Metab. 1979 May;48(5):827–835. doi: 10.1210/jcem-48-5-827. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A., Taskinen M. R., Pelkonen R., Nikkilä E. A. Glucose tolerance and plasma insulin in man during acute and chronic administration of nicotinic acid. Acta Med Scand. 1969 Oct;186(4):247–253. doi: 10.1111/j.0954-6820.1969.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Nye E. R., Buchanan H. Short-term effect of nicotinic acid on plasma level and turnover of free fatty acids in sheep and man. J Lipid Res. 1969 Mar;10(2):193–196. [PubMed] [Google Scholar]
- Pascoe W. S., Storlien L. H. Inducement by fat feeding of basal hyperglycemia in rats with abnormal beta-cell function. Model for study of etiology and pathogenesis of NIDDM. Diabetes. 1990 Feb;39(2):226–233. doi: 10.2337/diab.39.2.226. [DOI] [PubMed] [Google Scholar]
- Pecoraro R. E., Graf R. J., Halter J. B., Beiter H., Porte D., Jr Comparison of a colorimetric assay for glycosylated hemoglobin with ion-exchange chromatography. Diabetes. 1979 Dec;28(12):1120–1125. doi: 10.2337/diab.28.12.1120. [DOI] [PubMed] [Google Scholar]
- Pereira J. N. The plasma free fatty acid rebound induced by nicotinic acid. J Lipid Res. 1967 May;8(3):239–244. [PubMed] [Google Scholar]
- Pinter E. J., Pattee C. J. Biphasic nature of blood glucose and free fatty acid changes following intravenous nicotinic acid in man. J Clin Endocrinol Metab. 1967 Mar;27(3):440–443. doi: 10.1210/jcem-27-3-440. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Chang H., Hoffman B. B. Additive hypoglycemic effects of drugs that modify free-fatty acid metabolism by different mechanisms in rats with streptozocin-induced diabetes. Diabetes. 1988 Jan;37(1):28–32. doi: 10.2337/diab.37.1.28. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Kolterman O. G., Scarlett J. A., Gray R. S., Olefsky J. M. Lack of in vivo insulin resistance in controlled insulin-dependent, type I, diabetic patients. J Clin Endocrinol Metab. 1984 Feb;58(2):353–358. doi: 10.1210/jcem-58-2-353. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Franklin G., Nelson L. Glucose homeostasis and insulin secretion during chronic treatment with cyclosporin in nondiabetic humans. Diabetes. 1989 Jan;38 (Suppl 1):99–100. doi: 10.2337/diab.38.1.s99. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Gray R. S., Griffin J., Olefsky J. M., Kolterman O. G. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982 Jul-Aug;5(4):353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- Seltzer H. S., Allen E. W., Herron A. L., Jr, Brennan M. T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967 Mar;46(3):323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. C., Malone J. I., Simpson N. E. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989 Mar 2;320(9):550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985 Mar;28(3):119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- Vague P., Moulin J. P. The defective glucose sensitivity of the B cell in non insulin dependent diabetes. Improvement after twenty hours of normoglycaemia. Metabolism. 1982 Feb;31(2):139–142. doi: 10.1016/0026-0495(82)90125-1. [DOI] [PubMed] [Google Scholar]
- Yang Y. J., Youn J. H., Bergman R. N. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol. 1987 Dec;253(6 Pt 1):E595–E602. doi: 10.1152/ajpendo.1987.253.6.E595. [DOI] [PubMed] [Google Scholar]


