Abstract
Objectives:
Cachexia is common in pancreatic cancer and may have an influence on longterm survival but few studies have investigated this in patients with operable tumours. Therefore, this study was carried out to document body composition status in patients with pancreatic adenocarcinoma (PCa) presenting for a Whipple's procedure (WP) and to relate the findings to histopathology and longterm survival.
Methods:
Body composition was measured 1 day before a WP for ductal PCa in 36 patients (15 men, 21 women) aged 41–81 years. Results for total body nitrogen (TBN), nitrogen index (NI), total body water (TBW), fat mass (FM) and total body potassium (TBK) were compared with results in 73 age- and sex-matched controls. Patients' survival and details from histopathology synoptic reports were documented.
Results:
Patients undergoing WPs had low TBK values (P < 0.001) and females had lower body fat (P= 0.007) compared with controls. Five of 36 presented with significant protein deficiency, but this was not associated with a prolonged length of stay or reduced survival. The 12 patients who had involved surgical margins had larger tumours and reduced weight (P= 0.015), FM (P= 0.001), TBN (P= 0.045), TBK (P= 0.014) and survival (P= 0.036). However, multivariate Cox's regression analysis only included FM along with vascular invasion and margin status as independent predictors of survival.
Conclusions:
PCa patients undergoing a WP have reduced body fat and TBK compared with community controls while those with stage III tumours had greater deficits of fat, TBK and protein stores. However, preoperative body composition was a poor predictor of postoperative survival after pathological data were considered.
Keywords: pancreatic neoplasms, Whipple's procedure, resection, survival, body composition, body protein, body fat, body water, surgery, bioelectrical impedance
Introduction
Pancreatic cancer is associated with weight loss and nutritional impairment which influence inpatient morbidity and mortality.1 Despite this, the perioperative mortality rate of patients undergoing Whipple's procedure (WP) has declined in recent years, with reports in the literature indicating significantly lower mortality rates in more experienced centres (3.8%) compared with those in less experienced centres (7.5–17.6%).2 Cameron et al.3 reported an exceptional series of 1000 cases operated over a 34-year period of practice in which mortality was as low as 1%, indicating that a safe operation can be offered to these patients when the procedure is undertaken by experienced surgeons in well-equipped hospitals. This has now become the standard of care that patients expect.
Now that low rates of operative mortality have been consistently achieved, longterm survival has become a more important endpoint to study. Ahmad et al.4 have demonstrated 5-year survival of 19% and 7-year survival of 11% after WP, confirming the fact that resection in patients with pancreatic adenocarcinoma (PCa) can result in relatively longterm survival, especially when combined with adjuvant therapies. Others have repeated these findings.2,5 Furthermore, in a study of longterm outcome results, a significant 5-year survival advantage was reported in high-volume centres operating on more than 25 cases per year.6 In such centres, 5-year survival after surgical resection ranges from approximately 10% to almost 30%,7–9 probably as a result of patient and cultural variability. In a surgical study, cachexia, defined as a loss of >10% of pre-illness weight, was associated with more advanced disease.10 Cachexia in pancreatic cancer patients was also associated with greater reductions in body fat as measured by computerized tomography (CT) scanning and this resulted in reduced survival. Resection rate was also reduced in patients with as little as 5% weight loss, but was more substantial in those with >10% weight loss.10
Recognized negative prognostic factors of PCa include a poorly differentiated tumour, involved surgical margins, lymph node involvement and tumour diameter >2 cm.7–9 In the large series reported by Cameron et al., involved surgical margins and lymph node metastases had negative influences on survival.3 Preoperative weight loss and malnutrition, as assessed by body mass index (BMI), have also been shown to influence longterm survival.11
This paper reports the body composition of patients presenting for WP with the diagnosis of PCa and tests the hypothesis that these measures are the most sensitive at detecting early cachexia which may have an impact on longterm survival. This study uses body composition analysis techniques which utilize the nuclear physics technology of in vivo neutron capture analysis (IVNCA),12 total body potassium (TBK)13,14 and anthropometric techniques15 to measure the metabolically active compartments of the body and to relate these to the stage of the tumour at diagnosis and to survival. As histopathological factors are known to be important predictors of survival, their role in evaluating the effect of body composition on survival is included in this study.
Materials and methods
Patients
Patients presenting with masses in the pancreatic head in which surgical resection by WP was indicated were invited to undergo body composition studies prior to surgery. All WPs were performed by RCS, whose operative policy was to take the renal fascia behind the head of the pancreas and the anterior fold of the pancreatic fascia up to the superior mesenteric artery, where neural tissue was cleared from the right-hand side, to give the best chance of clear margins at tumour resection. Nutritional support was provided in the postoperative period with total parenteral nutrition until the introduction of oral intake. During this period 17 WP patients who also underwent preoperative body composition measurements were excluded from the analysis because their pathology was not pancreatic ductal adenocarcinoma. They had a wide variety of pathologies, including islet cell tumours, duodenal carcinoma, cystadenoma, chronic pancreatitis and cystadenocarcinoma.
Blood values were recorded from the hospital pathology record with the mean and standard deviation (SD) calculated for each. Assessment of nutritional risk of complication (ANSB) was computed from values for haemoglobin, lymphocyte count and albumin.16
For the purposes of survival analysis, the patients were followed up for 72 months postoperatively and death was chosen as the endpoint.
The study was approved by the Royal North Shore Hospital's Medical (Human) Research Ethics Committee and the Radiation Safety Committee. All subjects gave informed, written consent to their participation in the study.
Body composition analysis
Body composition measurements were carried out 1 day before surgery and included total body nitrogen (TBN), total body water (TBW), total body potassium (TBK) and percentage body fat (%BFat). Anthropometric measurements included skin-fold thicknesses, circumferences (chest, waist, mid-thigh, mid-calf and mid-upper arm), height and weight. The equations of Durnin and Womersley15 were then used to estimate the %BFat from skin-fold thickness measurements. Lean body mass (LBM) and total body fat (TBF)/fat mass (FM) were then calculated from weight and %BFat.
Total body nitrogen was measured non-invasively using the IVNCA technique.12 In this technique, a small dose of radiation of <0.2 mSv of fast neutrons was delivered to the patient. The overall radiation received by the patient was approximately 10% of annual background radiation. Nitrogen index (NI) was also calculated by expressing TBN as a percentage of that in age-, sex- and height-matched controls.17 These measures allowed the assessment of TBN with a precision and accuracy of 97.0% and 95.5%, respectively. An NI value of 0.95–1.05 (or 95–105%) is considered to be within the normal range and generally patients with an NI value of <0.95 are considered to be malnourished.
Total body water measurements were carried out directly after TBN measurements while the patient was still on the IVNCA bed. Values for TBW were obtained using the bioelectrical impedance analysis (BIA) technique. These measurements were carried out with the patient in the supine position using two leads attached to the non-dominant hand and two leads attached to the ipsilateral foot (tetrapolar arrangement). The body's resistance was then measured in triplicate using a swept frequency bioelectrical impedance meter (SEAC® SFB23; UniQuest Pty Ltd, Brisbane, Qld, Australia). The 50-kHz measurements used the equations of Kushner and Schoeller18 to calculate the patient's TBW. The phase angle (PhA) was determined by the equations of Cohn et al.19 The PhA is derived from the resistance and reactance measurements obtained from BIA and is considered indicative of cellular health and membrane integrity.
Total body potassium was measured using supine geometric sodium iodide whole body counting.13,14 This test relies on the natural K radiation from the body to estimate TBK. The TBK was then used to estimate the patients' fat-free mass (FFM) on the assumption that the potassium content of FFM is 2.26 g/kg and 2.52 g/kg in women and men, respectively.19 The precision and accuracy of this technique are 98.5% and 95.5%, respectively.14
Data for two age- and sex-matched control subjects for each patient, accumulated over 15 years, were employed as community controls. Controls were selected by excluding normal individuals aged <41 years. Control subjects' ages ranged from 41 to 85 years.
Descriptive pathological assessments were undertaken prospectively by three pathologists. In addition, the archived slides from all cases were subsequently reviewed by one specialist pathologist (AJG) and placed into a synoptic format.20 Tumours were graded using the TNM (tumour, node, metastasis) grading system21 into four categories ranging from grade 1 (well differentiated) to grade 4 (undifferentiated). Tumours were also assigned a pathological stage using the American Joint Committee on Cancer staging scheme (6th edition, 2002).21 The resection margins were placed into three categories: definitively involved (malignant cells present at the margins); close and likely to be involved (malignant cells at <1 mm of the margin), and clear (malignant cells >1 mm from all margins).
Statistical analyses
All acquired data and results were stored electronically in Microsoft FoxPro® and Microsoft Access® 2003 databases. Data manipulation and statistical analyses were carried out in Microsoft Excel® 2003 and spss® Version 14 for Windows® (SPSS, Inc., Chicago, IL, USA).
Preoperative values for each of the sex-matched groups were compared using Student's t-test. A general linear model (GLM) was undertaken to compare body composition results between controls and PCa patients using sex as a fixed factor and to compare baseline values between patients with involved and clear surgical margins using sex as a fixed factor. A P-value of ≤0.05 was taken to be significant. Kaplan–Meier survival analysis using the log-rank and Breslow (general Wilcoxon) tests for significance was used to compare survival data between groups. Cox's regression was carried out to investigate the proportional hazards of different histological factors and body composition parameters when a log-rank or univariate analysis indicated a potential influence on survival (P < 0.25).
Results
A total of 36 patients with pancreatic ductal adenocarcinoma scheduled for WP underwent preoperative body composition studies. They included 15 men and 21 women with a median age of 71.4 years (range 41.0–81.6 years), whose median hospital stay was 16 days (range 10–24 days). Preoperative values (mean ± SD) for serum albumin (37 ± 6 g/l), white cell count ([7.2 ± 2.5]× 106/l), lymphocyte count ([1.61 ± 0.61]× 109/l) and haemoglobin (122 ± 31 g/l) were within the normal range, whereas that for serum bilirubin was elevated to 108 ± 114 µmol/l. The automated nutritional score (ANSB) indicated increased risk for morbidity in only five patients, in whom two or more of the above markers showed depressed values. Postoperative hospital stay was not prolonged in these patients. Preoperative body composition studies indicated that values for weight, LBM and TBN were similar to those for the sex- and age-matched controls, but mean TBK and TBK/Ht values were significantly lower in PCa patients (P < 0.001). Mean %BFat values were lower in female patients (P= 0.007) compared with their controls, but not in the cohort of male patients (Table 1). Patient BMI values ranged from 15.9 kg/m2 to 39.7 kg/m2 (median 23.7 kg/m2) and did not differ from control values.
Table 1.
Body composition values in women and men with pancreatic cancer compared with those in sex- and age-matched community controls
|
Women |
Men |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Controls, n= 40 |
PCa patients, n= 21 |
P-value |
Controls, n= 33 |
PCa patients, n= 15 |
P-value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age, years | 66.2 | 9.7 | 70.7 | 10.6 | 0.12 | 62.6 | 10.8 | 63.4 | 13.2 | 0.82 |
| %BFat, % | 37.3 | 4.7 | 32.5 | 7.6 | 0.007 | 26.4 | 5 | 26.2 | 5.9 | 0.85 |
| BMI, w/h2 | 26 | 4.4 | 24.2 | 3.2 | 0.12 | 25.9 | 2.7 | 25.4 | 5.9 | 0.72 |
| LBM, kg | 40.5 | 5.9 | 38.1 | 5.2 | 0.13 | 57.6 | 7.4 | 56.3 | 9.3 | 0.60 |
| TBN, g | 1411.6 | 237.6 | 1350.4 | 236.3 | 0.36 | 2245.3 | 397.3 | 2274.6 | 620.1 | 0.84 |
| NI | 1.01 | 0.14 | 0.99 | 0.14 | 0.58 | 1.06 | 0.15 | 1.06 | 0.26 | 0.97 |
| TBK, g | 93.7 | 15.6 | 75.7 | 13.1 | 0.001 | 147.6 | 26 | 130.4 | 24.2 | 0.001 |
| TBK/Ht, g/m | 0.578 | 0.086 | 0.480 | 0.071 | 0.001 | 0.847 | 0.135 | 0.748 | 0.131 | 0.001 |
PCa, pancreatic adenocarcinoma; SD, standard deviation; %BFat, percentage body fat; BMI, body mass index; LBM, lean body mass; TBN, total body nitrogen; NI, nitrogen index; TBK, total body potassium; TBK/Ht, total body potassium/height
Histopathology details
Three of 36 patients had tumours in the periampullary region of the pancreas. Twelve of 36 patients had definitive histological evidence of incomplete excision with malignant cells at the margins of resection (i.e. involved). In an additional three patients the retroperitoneal margin was close (carcinoma <1 mm of the margin). The tumours' median maximal diameter was 30 mm and poor prognostic features included: nodal spread (n= 25); perineural invasion (n= 23), and vascular invasion (n= 18). In half the patients the tumours were at least stage IIB and were grade 3 or 4 (poorly differentiated or undifferentiated). Spearman's correlation coefficients indicated significant correlations between margin involvement and tumour size (P= 0.018). Vascular invasion was associated with tumour size (P= 0.033), tumour grade (P= 0.004) and nodal involvement (P= 0.044).
There was a significant but weak influence of margin involvement on survival, whereas the influence of vascular invasion was more definite when Kaplan–Meier survival curves were examined (Figs 1 and 2). The tumours were 27.7 ± 11.3 mm in diameter when the margins were uninvolved and 35.0 ± 9.7 mm when the margins were involved (t= 2.22, P= 0.03). Cox's stepwise regression analysis indicated independent influences of vascular invasion and margin involvement on survival for which the hazard ratio (HR) and confidence interval (CI) were 0.21 and 0.06–0.52 (P= 0.001), and 2.85 and 1.24–6.54 (P= 0.013), respectively.
Figure 1.
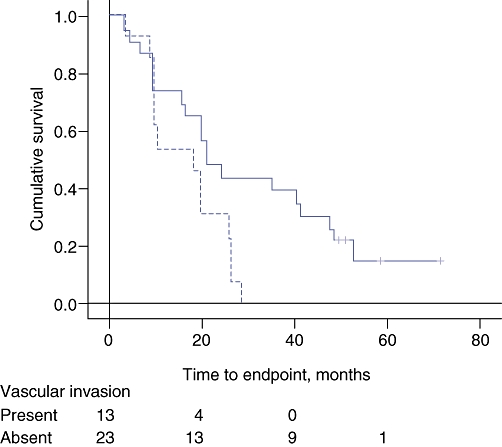
Kaplan–Meier survival curves for the effect of vascular invasion on survival. The broken line indicates cases with vascular invasion by tumour. Log-rank significance: P= 0.004
Figure 2.
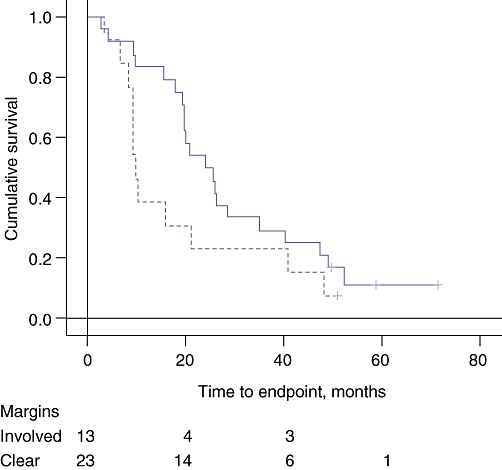
Kaplan–Meier survival curves for the effect of involved surgical margins on survival. The broken line indicates cases with involved surgical margins. Log-rank significance: P= 0.048
Nutritional indices and margin status
Patients with clear and involved surgical margins had similar ages (Mann–Whitney test, P= 0.6) and preoperative values for albumin, haemoglobin, lymphocyte count and bilirubin. There was no difference in hospital stay between the clear margin group (median 17.0 days, range 14–24 days) and those with involved surgical margins (median 17.8 days, range 10–30 days) (Mann–Whitney test, P= 0.9).
Body weight was found to be reduced in subjects with involved surgical margins (P= 0.015) when sex was included as a factor in the GLM program. Both the LBM and FM measurements were also reduced in patients with involved surgical margins: TBK (P= 0.014); TBK/Ht (P= 0.02); TBN (P= 0.045); FM (P= 0.005); %BFat (P= 0.004); TBW (P= 0.019), and LBM (P= 0.009) (Table 2).
Table 2.
The significance of involved surgical margins and preoperative body composition measures for women and men with pancreatic cancer
| Body composition measures | Margin status |
Women |
Men |
P for multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SEM | n | Mean | SEM | |||
| Weight, kg | Involved | 6 | 53.8 | 3.5 | 7 | 69.2 | 6.1 | 0.015 |
| Clear | 15 | 62.2 | 2.6 | 8 | 86 | 6.9 | ||
| TBN, g | Involved | 6 | 1264 | 92 | 7 | 1993 | 189 | 0.045 |
| Clear | 15 | 1385 | 62 | 8 | 2531 | 238 | ||
| NI units | Involved | 6 | 1.01 | 0.07 | 7 | 0.96 | 0.08 | NS |
| Clear | 15 | 0.99 | 0.03 | 8 | 1.16 | 0.1 | ||
| %BFat, % | Involved | 6 | 26.3a | 2.9 | 7 | 22.8a | 1.8 | 0.004 |
| Clear | 15 | 35 | 1.7 | 8 | 29.2 | 2.1 | ||
| FM, kg | Involved | 6 | 14.5a | 2.3 | 7 | 16.4 | 2.8 | 0.005 |
| Clear | 15 | 22.1 | 1.8 | 8 | 25.9 | 4 | ||
| TBW, kg | Involved | 6 | 25.8a | 1.2 | 7 | 35.9 | 2.4 | 0.019 |
| Clear | 15 | 29.1 | 0.7 | 8 | 39.1 | 1.3 | ||
| BMI, g/h2 | Involved | 6 | 23.2 | 1.4 | 7 | 23.1 | 2.1 | NS |
| Clear | 15 | 24.6 | 0.8 | 8 | 27.7 | 2.1 | ||
| TBK, g | Involved | 6 | 65.3b | 4.1 | 7 | 120.7 | 10.5 | 0.014 |
| Clear | 15 | 79.8 | 3.1 | 8 | 138.5 | 7.3 | ||
| TBK/Ht, g/m | Involved | 6 | 0.43a | 0.02 | 7 | 0.7 | 0.06 | 0.02 |
| Clear | 15 | 0.5 | 0.02 | 8 | 0.79 | 0.04 | ||
| LBM, kg | Involved | 6 | 34.4a | 2 | 7 | 52 | 3.2 | 0.009 |
| Clear | 15 | 39.6 | 1.2 | 8 | 60.1 | 3.3 | ||
P for multivariate analysis used the GLM program with sex as a fixed factor
Comparison of means within sex subgroup using t-test:
P < 0.05;
P < 0.001
SEM, standard error of the mean; NS, not significant; TBN, total body nitrogen; NI, nitrogen index, %BFat, percentage body fat; FM, fat mass; TBW, total body water; BMI, body mass index; TBK, total body potassium; TBK/Ht, total body potassium/height; LBM, lean body mass
Influence of preoperative nutritional status on longterm survival
None of the measures of body composition had an influence on survival when tested by univariate analysis: weight (P= 0.22); LBM (P= 0.16); FM (P= 0.17), and TBN (P= 0.11). Kaplan–Meier curves were examined to compare survival of patients with NI values <0.95 with survival of those with NI values >0.95 (Fig. 3). There was no influence of a low NI on longterm survival. Cox's backward stepwise regression analysis also failed to demonstrate influence of body composition indices, serum bilirubin (P= 0.12) and white blood cell count (P= 0.12).
Figure 3.
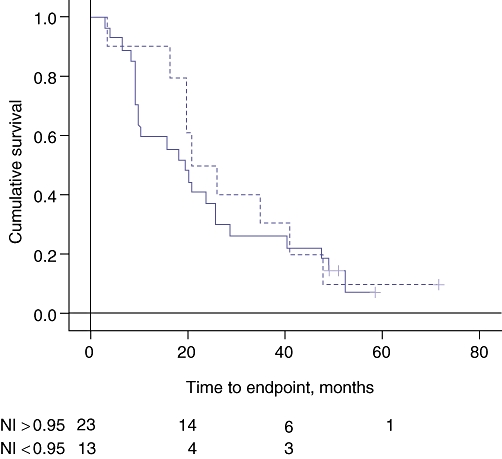
Kaplan–Meier survival curves for the effect of nitrogen index (NI) on survival. The broken line indicates NI < 0.95. Log-rank significance: P= 0.44
Interaction of nutritional status and histopathology on survival
Pathological indices which had a significant influence on survival in multivariate Cox's regression (vascular invasion, margin involvement and posterior margin) were tested with the nutritional measures which demonstrated potential influence on survival. There was an independent predictive effect of vascular invasion (P= 0.010), margin involvement (P= 0.001) and FM (P= 0.039) (Table 3). Although interaction effects demonstrated trends, these did not reach significance at the P= 0.05 level. There was no improvement in the equation when age and sex were included.
Table 3.
Prediction of longterm survival by histological measures and body composition in patients with pancreatic cancer
| Value |
Cox's regression analysis |
|||
|---|---|---|---|---|
| Univariate probability | Multivariate probability | HR and 95% CI for Cox's multivariate analysis | ||
| Stage, n | 0.740 | |||
| IA | 3 | |||
| IB | 1 | |||
| IIA | 14 | |||
| IIB | 16 | |||
| III | 2 | |||
| Grade, n | 0.074 | |||
| 1 | 1 | |||
| 2 | 20 | |||
| 3 | 13 | |||
| 4 | 2 | |||
| Margin involved, n | 12 | 0.048 | 0.001 | 0.22 (0.09–0.51) |
| Posterior margin, n | 15 | 0.081 | ||
| Neck margin, n | 3 | N/A | ||
| Neural spread, n | 23 | 0.275 | ||
| Intravascular spread, n | 18 | 0.004 | 0.010 | 2.96 (1.29–6.80) |
| Lymph node metastases, n | 25 | 0.380 | ||
| Number of involved nodes, median (range) | 1 (0–9) | 0.047 | ||
| Tumour size, mm, median (range) | 30 (5–50) | 0.013 | ||
| Fat mass, kg, mean (SD) | 20.8 (8.5) | 0.130 | 0.039 | 1.06 (0.09–0.51) |
| TBN, g, mean (SD) | 1.80 (0.20) | 0.041 | ||
HR, hazard ratio; 95% CI, 95% confidence interval; N/A, not applicable; SD, standard deviation; TBN, total body nitrogen
Discussion
Although the WP was undertaken with the intent to improve survival, only four of 36 patients remained alive at the end of 6 years. This reflects the aggressive nature of pancreatic cancer, but allows for the study of influencing factors as most patients reached the study endpoint. This study focuses on detailed body composition measurements taken at the time of presentation for surgery. Such measures are difficult to obtain in patients with severe illness when they are confronted with the need for major surgery. However, the body composition findings of this study demonstrated that total body fat and potassium, but not protein, were reduced in patients presenting for resection of PCa compared with community control subjects. These findings reflect the early nutritional effects of PCa as a consequence of the complex altered metabolism of cachexia and reduced pancreatic enzyme production for digestion of food. These patients had not developed severe cachexia. Only five of the 36 patients had evidence of protein deficiency, with low NI values indicating malnutrition, and five patients were considered to be at risk of malnutrition-related complications according to their ANSB scores. However, these five apparently malnourished patients did not have an increased length of hospital stay.
Univariate analysis and histopathology results confirmed the influence of margin status, intravascular spread, number of lymph nodes involved and tumour size on survival. Surgical margin involvement and vascular invasion demonstrated the strongest independent significance on multivariate Cox's regression. It was interesting that patients with involved surgical margins had significant reductions in body fat and LBM as shown by reduced TBK, indicating that they had significantly greater reductions in nutritional status. When Cox's regression was undertaken on the significant histological and body composition factors, survival was predicted by the independent influences of margin status, vascular invasion and fat mass, indicating that tissue reserves, complete removal of the tumour and the aggressive nature of the cancer play independent roles which determine survival.
A recent study which analysed CT scans taken at the time of work-up for surgery also demonstrated that patients with pancreatic cancer have reduced body fat. Furthermore, patients who lose >10% of their body weight were more likely to have metastatic disease and those who had resection had reduced survival.10 Muscle size did not differ between groups with greater or lesser amounts of weight loss. This measurement may be difficult to obtain because water retention associated with weight loss masks changes in protein loss.10 Nonetheless, the findings of this recent study10 are consistent with those of the present study in that PCa patients were more likely to have reduced body fat. Furthermore, those with greater amounts of fat loss had reduced longterm survival.
The interpretation of nutritional status at the time of presentation of PCa is complicated because of aetiological associations with both under- and over-nutrition, as demonstrated by the wide range of BMI values in our PCa patients. This is an important issue because obesity is more common in our community and has been shown to influence the onset of pancreatic cancer.22 The Japanese Collaborative Cohort Study for Evaluation of Cancer Risk studied 110 792 Japanese men and women enrolled between 1988 and 1990, of whom 402 developed pancreatic cancer.22 The authors analysed data on height and weight at baseline and at 20 years of age, as well as information relating to physical activity. The relative risks of pancreatic cancer mortality were 3.5-fold greater for men and 1.6-fold greater for women when their BMI was ≥30 kg/cm2. It is also interesting that in men a weight loss of ≥5 kg was associated with increased risk for pancreatic cancer, whereas in women this implied a decreased risk for pancreatic cancer. The authors concluded that the risk of pancreatic cancer in relation to BMI seems to differ according to sex and the period over which BMI is measured.22
Obesity, as well as malnutrition, may also have an influence on the outcome of pancreatic cancer. House et al.23 demonstrated that obesity, as determined by the thickness of the retro-renal fat, was associated with increased risk for complications following a WP. Alternatively, this paper finds that fat stores have a small but significant influence by which increased fat stores improve survival.
There is little information in the literature describing the effects of body composition on survival following a WP. One of the few available reports is by Gupta and Ihmaidat,24 who, as part of a report on the nutritional effects of upper gastrointestinal cancer, describe the results of short-term (7-day) body composition changes following a WP. The authors suggest that body composition may be a useful tool for assessing surgical outcomes. An example of the power of this concept is indicated by the finding that the longterm consequences of a standard WP resulted in more loss of body fat than a pylorus preserving pancreatectomy.25 Unfortunately these authors did not follow their patients from the time of surgery and the two cohorts were studied over different time periods, making comparisons difficult. However, the authors of the later study concluded that pylorus preservation seems to have advantages in terms of enduring functional and nutritional status years after surgery for pancreatic cancer.25
Whereas some studies fail to demonstrate the advantage of good margin clearance,26 others have demonstrated that a clear resection margin is the most important predictor of outcome.8,9,27–31 There is now definitive evidence that the traditional histopathological approach to reporting pancreatic cancer margins considerably underestimates the true high rate of margin positivity.5,6,8,27–35 For example, when resected cancers are carefully assessed by an experienced pancreatic pathologist paying particular attention to margin status, the rate of histologically reported incomplete excisions in WP may rise from as little as 14–59% to as much as 53–85%.34,35 In these studies, in which the margins are carefully assessed, excision status is a highly significant predictor of survival. Our results demonstrating a relationship between margin status and survival confirm these findings.29 Our additional finding, that vascular invasion was a stronger independent predictor of survival, supports the concept that the biology of individual tumours, as reflected by their ability to show vascular space invasion or their degree of independent growth, is a key factor in survival. Indeed, it is clear that the prognosis for patients who do undergo pancreatic resection is determined by multiple factors, including both the pathological stage and the molecular characteristics of the resected tumour specimen.8 Longterm survival occurs only in patients in whom the tumour is completely resected and is also influenced by tumour classification, tumour differentiation, blood loss during surgery, nutritional status and systemic adjuvant/neoadjuvant therapy.26,30,31,36,37
Nutritional consequences of surgery resulting in weight loss are also important for longterm outcome.38 Given that our patients presented with good nutritional status and that few demonstrated severe protein deficiency, it will be difficult to identify patients at risk of short prognosis from body composition status at the time of presentation. Bioelectrical impedance analysis, which is a measure of TBW and TBF, provides a bedside measure of nutritional status. The phase angle, which is derived from BIA measures, has been found to be associated with nutritional status39 and to be an independent predictor of postoperative complications.40 It has also been shown to predict survival in patients with advanced pancreatic cancer,39 breast cancer41 and colorectal cancer.42 However, we were not able to confirm this finding in our cohort of patients.
Surgery, when possible, is considered to give a survival benefit,38 but more advanced tumours with higher TNM stage and those in the pancreatic body are associated with poor survival. Because the majority of these patients will also receive chemotherapy, it is useful to examine the effect of weight loss on the outcome of chemotherapy because it usually leads to a reduced dose and an increased incidence of complications.38 A study of 1555 upper gastrointestinal cases43 demonstrated that patients with weight loss received lower chemotherapy doses initially, but this did not prevent them from developing more frequent and more severe dose-limiting toxicity, specifically plantar-palmar syndrome and stomatitis, than patients without weight loss. Consequently, patients with weight loss on average received 1 month less treatment. Weight loss correlated with shorter failure-free and overall survival, and decreased response, resulting in poorer quality of life and performance status. Weight loss at presentation was an independent prognostic variable with an HR of 1.43.44 The poorer outcome from treatment in patients with weight loss appears to occur because they receive significantly less chemotherapy and develop more toxicity, rather than because there is any specific reduction in tumour responsiveness to treatment.44 However, there are multiple other factors to consider. Previous studies in patients undergoing neoadjuvant therapy for breast cancer demonstrated that NI was the best predictor of complications from chemotherapy, resulting in a delay and reduction in treatment.17 Marechal et al.38 devised a prognostic index which includes aspartate transaminase >53 IU/l, weight loss ≥10% of body weight and Karnofsky performance status <90 as significant independent negative prognostic factors for first-line gemcitabine chemotherapy.38 Fearon et al.44 examined 170 patients and demonstrated that a three-factor profile incorporating weight loss (≥10%), low food intake (≤1500 kcal/day) and systemic inflammation (C-reactive protein ≥10 mg/l) was a better predictor of adverse events and of the patient's overall prognosis than weight loss alone.
Conclusions
In conclusion, this paper documents the detailed body composition status of patients presenting with resectable PCa and indicates that those with larger tumours and involved margins present with greater losses of body fat and lean tissue. Fat mass at presentation had a weak, but significant, predictive effect on survival. However, only a small percentage of patients presented with severe cachexia. In these patients its influence on hospital stay and survival was not evident. Furthermore, the pathological criteria of margin status and vascular invasion appear to have stronger influences on survival. Therefore, when a patient presents with significant weight loss, the doctor should be alerted to the association with a more advanced cancer and the patient should not be denied surgical treatment if the radiological characteristics indicate that clear margins can be achieved.
Acknowledgments
The authors would like to acknowledge the assistance of Sarah F. Smith PhD with the manuscript and the support of Dr Bruce A. Cooper, Body Composition Unit, Royal North Shore Hospital, St Leonards, Australia.
Conflict of interest
None declared.
References
- 1.Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26:698–709. doi: 10.1016/j.clnu.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 3.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad NA, Lewis JD, Ginsberg GG, Haller DG, Morris JB, Williams NN, et al. Longterm survival after pancreatic resection for pancreatic adenocarcinoma. Am J Gastroenterol. 2001;96:2609–2615. doi: 10.1111/j.1572-0241.2001.04123.x. [DOI] [PubMed] [Google Scholar]
- 5.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Longterm survival is superior after resection for cancer in high-volume centres. Ann Surg. 2005;242:540–544. doi: 10.1097/01.sla.0000184190.20289.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmann J, Ketterer K, Marsch C, Fechtner K, Krakowski-Roosen H, Buchler MW, et al. Pancreatic cancer-related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer. 2009;9:255. doi: 10.1186/1471-2407-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Buchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg. 2008;12:1193–1201. doi: 10.1007/s11605-008-0505-z. [DOI] [PubMed] [Google Scholar]
- 12.Allen BJ, Blagojevic N, Delaney I, Pollock CA, Ibels LS, Allman MA, et al. The role of body protein studies in clinical trials. Basic Life Sci. 1990;55:155–169. doi: 10.1007/978-1-4613-1473-8_23. [DOI] [PubMed] [Google Scholar]
- 13.Hansen RD, Allen BJ. Calibration of a total body potassium monitor with an anthropomorphic phantom. Phys Med Biol. 1996;41:2447–2462. doi: 10.1088/0031-9155/41/11/015. [DOI] [PubMed] [Google Scholar]
- 14.Hansen RD, Raja C, Aslani A, Smith RC, Allen BJ. Determination of skeletal muscle and fat-free mass by nuclear and dual-energy x-ray absorptiometry methods in men and women aged 51–84 y. Am J Clin Nutr. 1999;70:228–233. doi: 10.1093/ajcn.70.2.228. [DOI] [PubMed] [Google Scholar]
- 15.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 16.Smith RC, Ledgard JP, Doig G, Chesher D, Smith SF. An effective automated nutrition screen for hospitalized patients. Nutrition. 2009;25:309–315. doi: 10.1016/j.nut.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Aslani A, Smith RC, Allen BJ, Pavlakis N, Levi JA. The predictive value of body protein for chemotherapy-induced toxicity. Cancer. 2000;88:796–803. doi: 10.1002/(sici)1097-0142(20000215)88:4<796::aid-cncr10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Kushner RF, Schoeller DA. Estimation of total body water by bioelectrical impedance analysis. Am J Clin Nutr. 1986;44:417–424. doi: 10.1093/ajcn/44.3.417. [DOI] [PubMed] [Google Scholar]
- 19.Cohn SH, Vartsky D, Yasumura S, Sawitsky A, Zanzi I, Vaswani A, et al. Compartmental body composition based on total-body nitrogen, potassium, and calcium. Am J Physiol. 1980;239:524–530. doi: 10.1152/ajpendo.1980.239.6.E524. [DOI] [PubMed] [Google Scholar]
- 20.Gill AJ, Johns AL, Eckstein R, Samra JS, Kaufman A, Chang DK, et al. Synoptic reporting improves histopathological assessment of pancreatic resection specimens. Pathology. 2009;41:161–167. doi: 10.1080/00313020802337329. [DOI] [PubMed] [Google Scholar]
- 21.American Joint Committee on Cancer. Cancer Staging Manual. 6th. New York, NY: Springer; 2002. [Google Scholar]
- 22.Lin Y, Kikuchi S, Tamakoshi A, Yagyu K, Obata Y, Inaba Y, et al. Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. Int J Cancer. 2007;120:2665–2671. doi: 10.1002/ijc.22614. [DOI] [PubMed] [Google Scholar]
- 23.House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–278. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 24.Gupta R, Ihmaidat H. Nutritional effects of oesophageal, gastric and pancreatic carcinoma. Eur J Surg Oncol. 2003;29:634–643. doi: 10.1016/s0748-7983(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 25.Niedergethmann M, Shang E, Farag SM, Saar J, Berisha S, Willeke F, et al. Early and enduring nutritional and functional results of pylorus preservation vs. classic Whipple procedure for pancreatic cancer. Langenbecks Arch Surg. 2006;391:195–202. doi: 10.1007/s00423-005-0015-3. [DOI] [PubMed] [Google Scholar]
- 26.Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143:75–83. doi: 10.1001/archsurg.2007.17. [DOI] [PubMed] [Google Scholar]
- 27.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73:212–218. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Millikan KW, Deziel DJ, Silverstein JC, Kanjo TM, Christein JD, Doolas A, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65:618–623. [PubMed] [Google Scholar]
- 29.Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37–43. doi: 10.1097/01.mpa.0000194609.24606.4b. [DOI] [PubMed] [Google Scholar]
- 30.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996;83:625–631. doi: 10.1002/bjs.1800830512. [DOI] [PubMed] [Google Scholar]
- 31.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 32.Esposito I, Seiler C, Bergmann F, Kleeff J, Friess H, Schirmacher P. Hypothetical progression model of pancreatic cancer with origin in the centroacinar-acinar compartment. Pancreas. 2007;35:212–217. doi: 10.1097/mpa.0b013e31805d0190. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Reber HA, Dry SM, Elashoff D, Chen SL, Umetani N, et al. Unfavourable prognosis associated with K-ras gene mutation in pancreatic cancer surgical margins. Gut. 2006;55:1598–1605. doi: 10.1136/gut.2005.083063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 35.Verbeke CS, Menon KV. Variability in reporting resection margin status in pancreatic cancer. Ann Surg. 2008;247:716–717. doi: 10.1097/SLA.0b013e31816a7077. [DOI] [PubMed] [Google Scholar]
- 36.Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to longterm survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–1345. doi: 10.1016/j.gassur.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Xue A, Scarlett CJ, Jackson CJ, Allen BJ, Smith RC. Prognostic significance of growth factors and the urokinase-type plasminogen activator system in pancreatic ductal adenocarcinoma. Pancreas. 2008;36:160–167. doi: 10.1097/MPA.0b013e31815750f0. [DOI] [PubMed] [Google Scholar]
- 38.Marechal R, Demols A, Gay F, De Maertelaere V, Arvanitaki M, Hendlisz A, et al. Prognostic factors and prognostic index for chemonaive and gemcitabine-refractory patients with advanced pancreatic cancer. Oncology. 2007;73:41–51. doi: 10.1159/000120627. [DOI] [PubMed] [Google Scholar]
- 39.Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr. 2004;92:957–962. doi: 10.1079/bjn20041292. [DOI] [PubMed] [Google Scholar]
- 40.Barbosa-Silva MC, Barros AJ. Bioelectric impedance and individual characteristics as prognostic factors for postoperative complications. Clin Nutr. 2005;24:830–838. doi: 10.1016/j.clnu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Gupta D, Lammersfeld CA, Vashi PG, King J, Dahlk SL, Grutsch JF, et al. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer. 2008;8:249. doi: 10.1186/1471-2407-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta D, Lis CG, Dahlk SL, King J, Vashi PG, Grutsch JF, et al. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr J. 2008;7:19. doi: 10.1186/1475-2891-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–509. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 44.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]


