Abstract
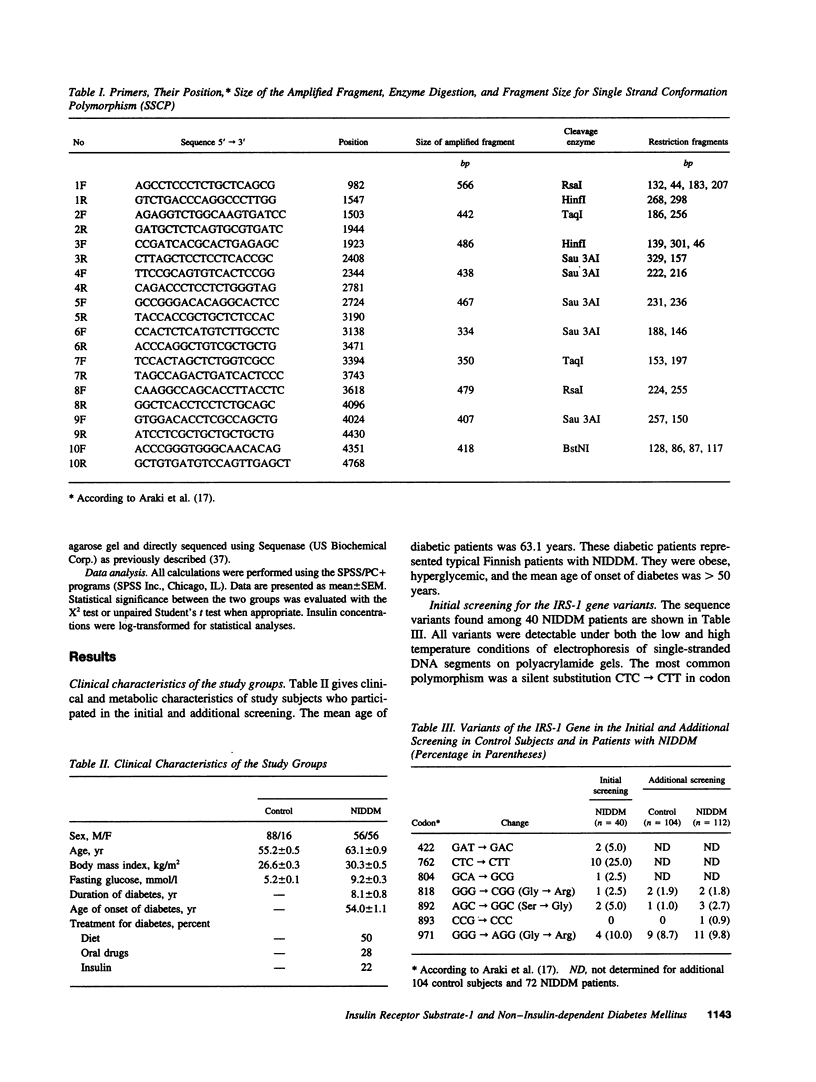
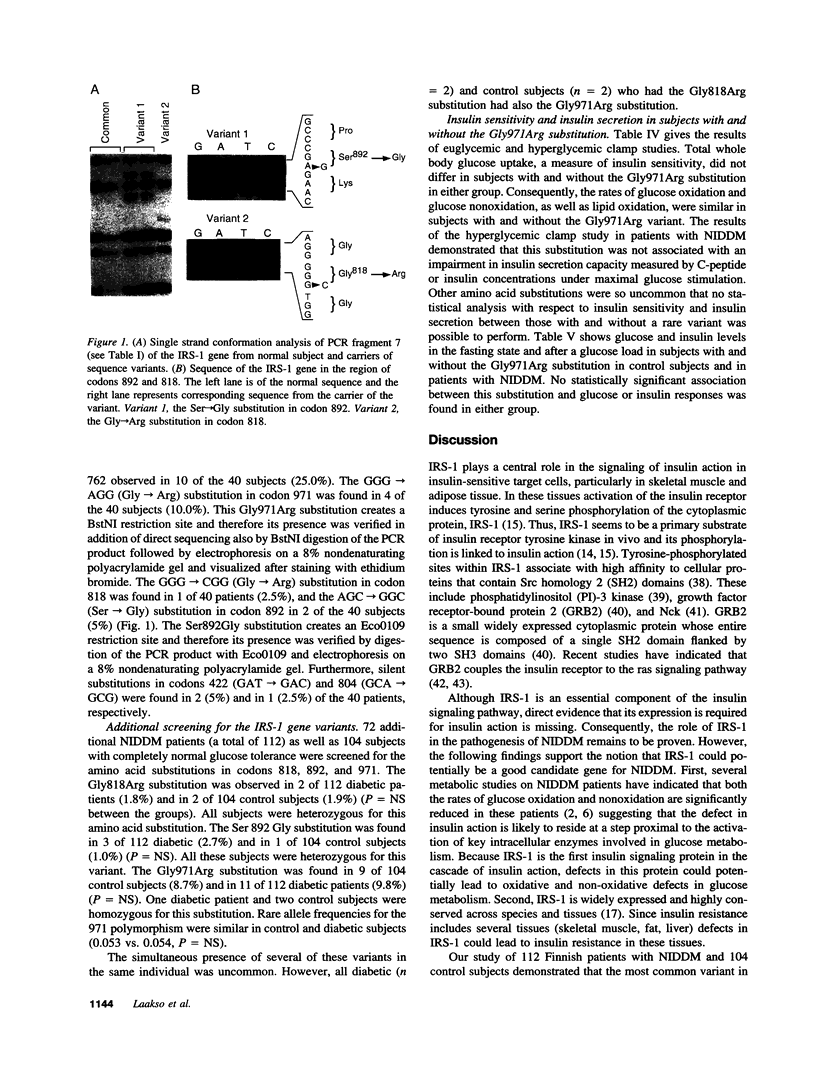
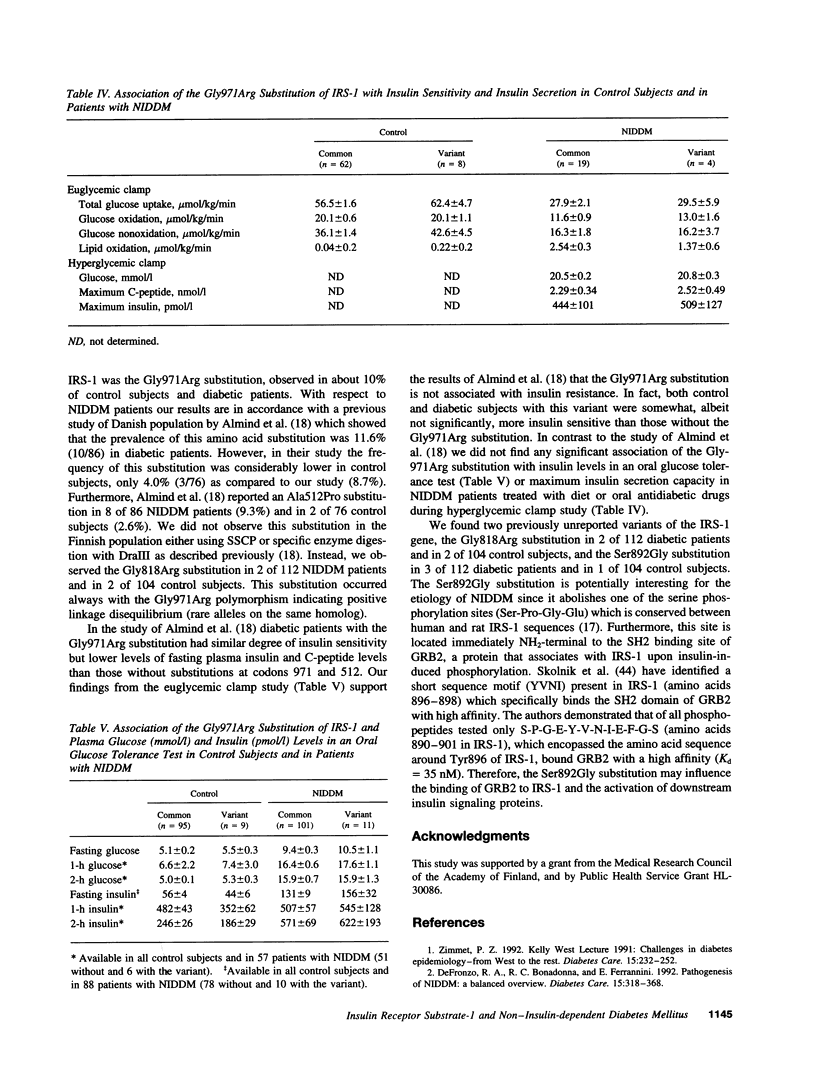
Insulin receptor substrate-1 (IRS-1) plays an important role in insulin-stimulated signaling mechanisms. Therefore, we investigated the frequency and clinical significance of variants in the coding region of this gene in patients with non-insulin-dependent diabetes (NIDDM). Initial screening included a population-based sample of 40 Finnish patients with typical NIDDM. Applying single strand conformation polymorphism analysis the following amino acid substitutions were found among the 40 NIDDM patients: Gly818-Arg, Ser892Gly, and Gly971Arg. The first two variants have not been previously reported. Additional samples of 72 patients with NIDDM and 104 healthy control subjects with completely normal oral glucose tolerance test and a negative family history of diabetes were screened. The most common polymorphism was the Gly971Arg substitution which was found in 11 (9.8%) of 112 NIDDM patients and in 9 (8.7%) of 104 control subjects. The Gly818Arg substitution was found in 2 (1.8%) of NIDDM patients and in 2 (1.9%) of control subjects, and the Ser892Gly substitution was found in 3 (2.7%) NIDDM patients and in 1 (1.0%) control subject. The Gly971Arg substitution was not associated with an impairment in insulin secretion capacity (estimated by insulin responses in an oral glucose tolerance test or by the hyperglycemic clamp) or insulin action (estimated by the euglycemic clamp). Of the three amino acid substitutions observed Ser892Gly is the most interesting one since it abolishes one of the potential serine phosphorylation sites (SPGE) which is located immediately NH2-terminal to the only SH2 binding site of growth factor receptor-bound protein (GRB2), and thus could potentially influence some aspects of signal transduction and metabolic response to insulin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almind K., Bjørbaek C., Vestergaard H., Hansen T., Echwald S., Pedersen O. Aminoacid polymorphisms of insulin receptor substrate-1 in non-insulin-dependent diabetes mellitus. Lancet. 1993 Oct 2;342(8875):828–832. doi: 10.1016/0140-6736(93)92694-o. [DOI] [PubMed] [Google Scholar]
- Araki E., Sun X. J., Haag B. L., 3rd, Chuang L. M., Zhang Y., Yang-Feng T. L., White M. F., Kahn C. R. Human skeletal muscle insulin receptor substrate-1. Characterization of the cDNA, gene, and chromosomal localization. Diabetes. 1993 Jul;42(7):1041–1054. doi: 10.2337/diab.42.7.1041. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Shoffner J. M., Hedaya E. V., Trounce I., Polak M. A., Koontz D. A., Wallace D. C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992 Apr;1(1):11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Baltensperger K., Kozma L. M., Cherniack A. D., Klarlund J. K., Chawla A., Banerjee U., Czech M. P. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993 Jun 25;260(5116):1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Barnett A. H., Eff C., Leslie R. D., Pyke D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981 Feb;20(2):87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Finegood D. T., Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985 Winter;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Del Prato S., Bonadonna R. C., Bonora E., Gulli G., Solini A., Shank M., DeFronzo R. A. Characterization of cellular defects of insulin action in type 2 (non-insulin-dependent) diabetes mellitus. J Clin Invest. 1993 Feb;91(2):484–494. doi: 10.1172/JCI116226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Folli F., Saad M. J., Backer J. M., Kahn C. R. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993 Oct;92(4):1787–1794. doi: 10.1172/JCI116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froguel P., Zouali H., Vionnet N., Velho G., Vaxillaire M., Sun F., Lesage S., Stoffel M., Takeda J., Passa P. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. N Engl J Med. 1993 Mar 11;328(10):697–702. doi: 10.1056/NEJM199303113281005. [DOI] [PubMed] [Google Scholar]
- Granner D. K., O'Brien R. M. Molecular physiology and genetics of NIDDM. Importance of metabolic staging. Diabetes Care. 1992 Mar;15(3):369–395. doi: 10.2337/diacare.15.3.369. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., White M. F. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest. 1988 Oct;82(4):1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhapä P., Uusitupa M., Voutilainen E., Laakso M. Effects of bezafibrate on insulin sensitivity and glucose tolerance in subjects with combined hyperlipidemia. Clin Pharmacol Ther. 1992 Dec;52(6):620–626. doi: 10.1038/clpt.1992.200. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kretz K. A., Carson G. S., O'Brien J. S. Direct sequencing from low-melt agarose with Sequenase. Nucleic Acids Res. 1989 Jul 25;17(14):5864–5864. doi: 10.1093/nar/17.14.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M., Rönnemaa T., Pyörälä K., Kallio V., Puukka P., Penttilä I. Atherosclerotic vascular disease and its risk factors in non-insulin-dependent diabetic and nondiabetic subjects in Finland. Diabetes Care. 1988 Jun;11(6):449–463. doi: 10.2337/diacare.11.6.449. [DOI] [PubMed] [Google Scholar]
- Laakso M., Sarlund H., Salonen R., Suhonen M., Pyörälä K., Salonen J. T., Karhapä P. Asymptomatic atherosclerosis and insulin resistance. Arterioscler Thromb. 1991 Jul-Aug;11(4):1068–1076. doi: 10.1161/01.atv.11.4.1068. [DOI] [PubMed] [Google Scholar]
- Laakso M., Uusitupa M., Takala J., Majander H., Reijonen T., Penttilä I. Effects of hypocaloric diet and insulin therapy on metabolic control and mechanisms of hyperglycemia in obese non-insulin-dependent diabetic subjects. Metabolism. 1988 Nov;37(11):1092–1100. doi: 10.1016/0026-0495(88)90074-1. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Li W., Nishimura R., Zhou M., Batzer A. G., Myers M. G., Jr, White M. F., Schlessinger J., Skolnik E. Y. Nck associates with the SH2 domain-docking protein IRS-1 in insulin-stimulated cells. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11713–11717. doi: 10.1073/pnas.90.24.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Madsbad S., Alberti K. G., Binder C., Burrin J. M., Faber O. K., Krarup T., Regeur L. Role of residual insulin secretion in protecting against ketoacidosis in insulin-dependent diabetes. Br Med J. 1979 Nov 17;2(6200):1257–1259. doi: 10.1136/bmj.2.6200.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller D. E., Flier J. S. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991 Sep 26;325(13):938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Jr, White M. F. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993 May;42(5):643–650. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Wands J. R. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1992 Feb 28;183(1):280–285. doi: 10.1016/0006-291x(92)91640-c. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Reina M., Brunzell J. D., Deeb S. S. Molecular basis of familial chylomicronemia: mutations in the lipoprotein lipase and apolipoprotein C-II genes. J Lipid Res. 1992 Dec;33(12):1823–1832. [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sarlund H., Laakso M., Voutilainen E., Penttilä I., Pyörälä K. Familial aggregation of non-insulin dependent diabetes and coronary heart disease are accompanied by different effects on serum lipids, lipoproteins and apolipoproteins. Atherosclerosis. 1991 Jan;86(1):17–29. doi: 10.1016/0021-9150(91)90095-k. [DOI] [PubMed] [Google Scholar]
- Sarlund H., Pyörälä K., Penttilä I., Laakso M. Early abnormalities in coronary heart disease risk factors in relatives of subjects with non-insulin-dependent diabetes. Arterioscler Thromb. 1992 Jun;12(6):657–663. doi: 10.1161/01.atv.12.6.657. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Batzer A., Li N., Lee C. H., Lowenstein E., Mohammadi M., Margolis B., Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993 Jun 25;260(5116):1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Tager H. S., Chan S. J., Nanjo K., Sanke T., Rubenstein A. H. Lessons learned from molecular biology of insulin-gene mutations. Diabetes Care. 1990 Jun;13(6):600–609. doi: 10.2337/diacare.13.6.600. [DOI] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Takala J., Keinänen O., Väisänen P., Kari A. Measurement of gas exchange in intensive care: laboratory and clinical validation of a new device. Crit Care Med. 1989 Oct;17(10):1041–1047. doi: 10.1097/00003246-198910000-00015. [DOI] [PubMed] [Google Scholar]
- Taylor S. I. Lilly Lecture: molecular mechanisms of insulin resistance. Lessons from patients with mutations in the insulin-receptor gene. Diabetes. 1992 Nov;41(11):1473–1490. doi: 10.2337/diab.41.11.1473. [DOI] [PubMed] [Google Scholar]
- Zimmet P. Z. Kelly West Lecture 1991. Challenges in diabetes epidemiology--from West to the rest. Diabetes Care. 1992 Feb;15(2):232–252. doi: 10.2337/diacare.15.2.232. [DOI] [PubMed] [Google Scholar]
- Zouali H., Vaxillaire M., Lesage S., Sun F., Velho G., Vionnet N., Chiu K., Passa P., Permutt A., Demenais F. Linkage analysis and molecular scanning of glucokinase gene in NIDDM families. Diabetes. 1993 Sep;42(9):1238–1245. doi: 10.2337/diab.42.9.1238. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A. Disease gene mapping in isolated human populations: the example of Finland. J Med Genet. 1993 Oct;30(10):857–865. doi: 10.1136/jmg.30.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]




