‘………and a tone arises from its measured motion’
P.B. Shelley: Stanzas Written in Dejection near Naples
Introduction
Because movement sets the tone for animal behavior, nervous systems need to devote a considerable fraction of their computational power to the task of activating muscles in precise temporal and spatial patterns that drive coordinated motor activity. Even the simplest of motor tasks, in the most primitive of animals, demands the integrated activity of a diverse set of neural circuits. In vertebrates, the planning of movement prior to overt muscle activity involves the recruitment of many supraspinal networks. But the executive element of motor control – the task of determining which muscles are to be activated, how intensely, and for how long – has been assigned to neural circuits located within the spinal cord.
At the core of the spinal motor system are sets of local interneurons which assemble themselves into ordered networks capable of controlling the activity and output of spinal motor neurons. These networks are usually referred to as central pattern generators (CPGs). While CPGs possess a high degree of autonomy in output, their activation depends on input from supraspinal command centers, notably those in the mesencephalon and caudal diencephalon. Sensory feedback pathways that report on the state of muscle activity also have critical roles in refining the pattern of locomotor output in each movement cycle, permitting the core CPG network to adapt itself to the many obstacles and uncertainties that confront animals during their ambulatory excursions (1). Defining the intrinsic logic of spinal CPG networks, and the way in which they integrate descending commands and sensory feedback information, remains one of the fundamental challenge in the broader field of motor systems neuroscience.
Over the past few decades, progress in clarifying principles of spinal motor control has benefitted from a wide range of ideas and approaches -- cellular neurophysiology and anatomy, consideration of the biomechanics of limb movement, as well as computational descriptions of simulated motor behavior (see 2, 3, 4). Building on this foundation, there has been a gradual broadening of interest in the organization and function of spinal motor systems, driven in part by advances in three additional areas. Biophysical studies have provided an increasingly quantitative account of the membrane, synaptic and integrative features of identified neurons within spinal motor networks, especially so in aquatic vertebrates (5, 6). Molecular genetic strategies have progressed to the point that it is now feasible to manipulate identified spinal neurons with unprecedented specificity and to test the impact of such perturbations on circuit function and motor behavior (7, 3, 8). There has also been an increasing interest in comparative aspects of spinal motor control – probing key differences in the organization of locomotor networks that permit animals to execute motor programs that are fitted to the specialized needs of their physical environment (9).
In this brief review, we set out to highlight some of the recent advances in these research areas, while pointing out the many puzzles and uncertainties that still confound the goal of arriving at a ‘simple’ set of rules that govern the organization and function of spinal motor networks.
From lamprey to mammals: evolving patterns of vertebrate motor coordination
The first vertebrates to emerge, some 500 millions year ago, are today represented by lampreys and hagfish, two phylogenetically distinct and conserved agnathan (jawless) groups that lack paired appendages. The lamprey locomotor network, arguably the ancestral doyen of vertebrate locomotor systems, generates a pronounced left-right alternation of motor output in each segment, while imposing a segmental phase lag that results in the propagation of an undulatory wave of motor activity along the body axis, from head to tail (5). This basic motor strategy is evident in most fish, in the larval stages of amphibia, and in a more limited form, in some mammals (8, 10, 6, 11). Some 100 million years after the emergence of agnathans, the acquisition of pectoral fins – paired appendages that represent the precursors of the tetrapod limb – permitted aquatic vertebrates to use them for steering and move them in either alternating or synchronous modes during locomotion. Mammals, comparative newcomers that appeared on the scene only ∼130 million years ago, have retained and embellished this versatile appendicular addition to the motor repertoire. In mammals, each of the four limbs is controlled by a separate network which, when needed, can generate limb-specific motor patterns. Individual limb patterns can also be combined to produce higher order mammalian gait patterns that underlie the transition from walk, to trot, to gallop. The limbs of course need to remain coordinated with the trunk, although distinctly different patterns of coordination can be used, such as in a walk as compared to a gallop. In the salamander the trunk movements in the transition from swimming to walking have been reported. Interestingly it swims with a lamprey-like undulatory wave with the limbs held to the body, but when switching to walking instead a standing wave appears, coordinated with the four limbs (12). The neural origin of this switch has been approached through modeling (13, 14)
This evolutionary perspective raises the question of whether and how spinal pattern generator circuits equipped to coordinate muscle activity in limbs and other paired appendages emerged from a presumed ancestral network devoted to the generation of segmentally phased trunk movements. The analysis of neuromuscular activity patterns in frog embryos before, during, and after metamorphosis represents a particularly promising approach to this issue since, here, motor output can be followed longitudinally in a single animal over the period that motor behavior switches from a segmental to a limb-based strategy (Fig 1). Recordings from motor nerves at prospective limb levels of Xenopus tadpoles have provided evidence that prior to metamorphosis the spinal pattern generator network is initially active in phase with the trunk CPG, but that with the formation of the limb bud, motor output gradually gains independence from the trunk CPG and begins to exhibit primitive limb motor neuron bursting patterns (15, 16). These observations suggest that during the early peri-metamorphic phase the interneurons that control the developing limb are still tightly linked to the activity of the trunk CPG, and only later gain independence from their segmental shackles and begin to devote themselves to the job of activating specific limb muscles.
Figure 1. Amphibian metamorphosis is accompanied by a change in spinal locomotor pattern.
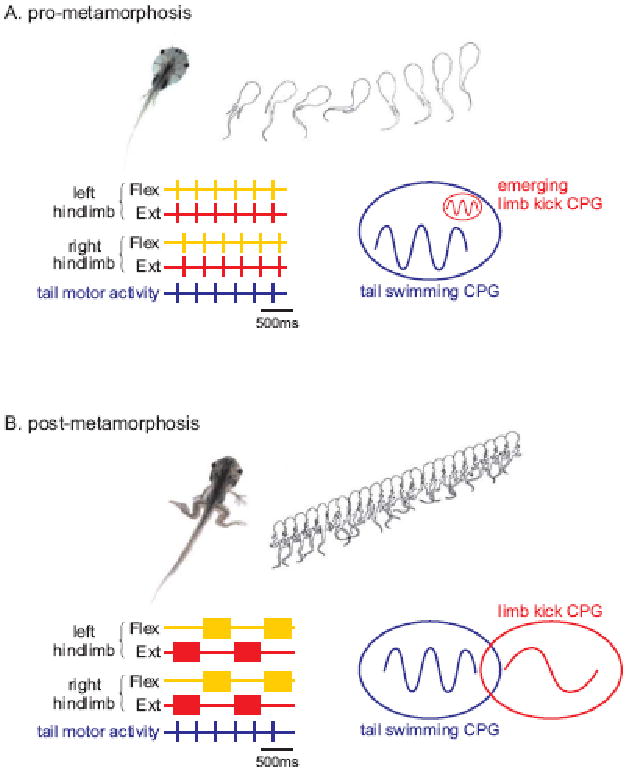
(A) Tail-based swimming in pre-metamorphic (<stage 53) Xenopus larvae. Undulatory propulsive movements are generated by alternate bilateral contraction of axial myotomes, and are associated with a rostro-caudal phase delay (upper panel). Schematic representation of extracellular ventral root (VR) recordings from the isolated brain stem/spinal cord (lower) showing spinal network motor output. This pattern is characterised by left-right alternating bursts of motor activity (see left and right VR1) that propagate rostro-caudally (see dotted line).
(B) Limb-based swimming in post-metamorphic (>stage 64) froglets. By this time, the tail has been resorbed and swimming is now produced by slower, bilaterally-synchronous cycles of hindlimb extension and flexion (upper panel). The isolated spinal cord/brain stem generates a fictive locomotor pattern in which left and right limb extensor motoneurons produce coincident bursts that alternate with bursts in left and right hindlimb flexor motoneurons.
Adapted from ref. 16.
How this fundamental switch in the pattern of locomotor output is achieved remains unclear. One possibility is that this trick is performed by a common and invariant group of spinal interneurons that manage to rewire themselves during metamorphosis, so as to accommodate the appearance of the limbs and their diversified musculature. But we know that the set of motor neurons destined to innervate newly-generated limb muscle targets is actually born during the metamorphic period (17). This finding raises the possibility that a corresponding set of interneurons is generated over this transitional period. In this scenario, the switch in spinal network pattern could be directed by the superimposition of a new set of interneurons to a cohort of pre-metamorphic CPG interneurons, so embellishing the network and reconfiguring its output.
The developmental assembly of the mammalian spinal pattern network for limb movement also proceeds in progressive phases. Initially, all hind-limb muscle groups burst in synchrony, followed by a phase in which left-right alternation of the two limbs becomes evident, proceeding finally to a more mature pattern of flexor-extensor alternation within individual limbs (18). In part, this phasic progression appears to reflect a switch in signaling sign of the GABAergic and glycinergic interneurons responsible for patterning the interneuronal and motor output that directs left-right and flexor-extensor alternation. Early in development, the profile of expression of chloride co-transporters favors steady-state chloride influx, and thus ionotropic GABAergic and glycinergic signalling drives chloride efflux and depolarization of post-synaptic neurons. But later, after the onset of K+/Cl- co-transporter (KCC2) expression, the switch in chloride equilibrium potential favors steady-state chloride efflux such that GABAergic and glycinergic signalling now produces chloride influx and hyperpolarizing responses in target neurons (19). Intriguingly, in rodents certain phases in the timing of the switch in the co-transporter expression, and thus target neuron response, are reported to be hormone-regulated (10), raising the possibility that the change in locomotor pattern and strategy during amphibian metamorphosis reflects, in part, a thyroxine-induced switch in neuronal chloride equilibrium potential in newly-generated neurons.
Insight into the steps that directed the assembly of spinal pattern generator networks during vertebrate evolution has also come from comparative anatomical and molecular studies of motor neuron diversification. The spinal motor repertoire of ancestral aquatic vertebrates such as the lamprey appears to be dominated by a segmentally-iterated set of motor neurons that are destined to innervate distinct axial muscle domains (20). These motor neurons share organizational and molecular features with the set of median motor column (MMC) neurons present in birds and mammals (8; 21). By inference, the elaboration of a more complex set of peripheral target muscles in the limb and body wall presumably demanded a corresponding program of motor neuron diversification. In birds and mice, the specification of spinal motor neuron columnar and pool subtype identities has been shown to depend on extrinsic patterning signals supplied by members of the Wnt, FGF and retinoid families (22, 23, 24). These graded signalling factors exert their actions by inducing and imposing spatial and temporal patterns of expression of transcription factors, notably the Hox homeodomain proteins (23, 25). These findings form the basis of a model (Figure 2) which suggest that the initiating evolutionary step in spinal motor neuron subtype diversification was the attenuation of an MMC inductive program of non-canonical Wnt signaling, so diverting neurons from an MMC fate and permitting the emergence of a parallel set of ‘primed’ hypaxial motor column (HMC) neurons (26). Subsequently, the idea goes, graded FGF and retinoid signals acted on this ‘primed’ set of competent HMC motor neurons, imposing an intricate rostrocaudal profile of Hox protein expression which directs the generation of lateral motor column (LMC) neurons, and their resident motor pools.
Figure 2. A model for the diversification of spinal motor neuron subtypes during vertebrate evolution.
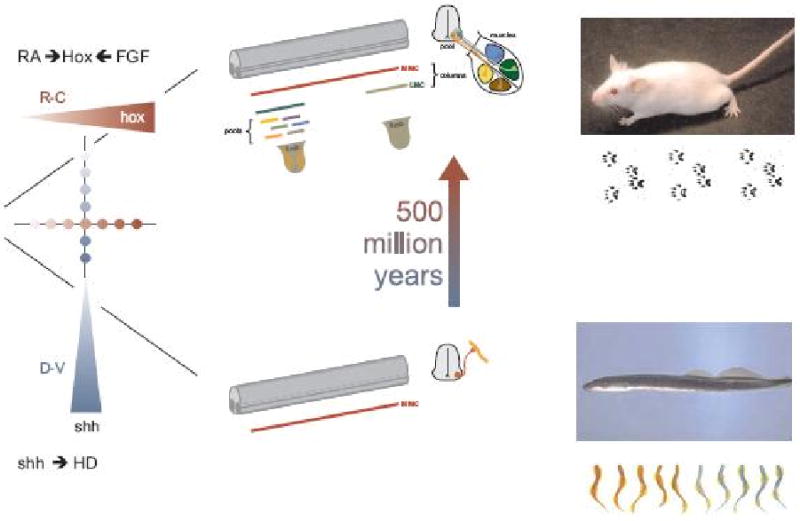
Lower branch. Inductive signaling along the dorsoventral axis of the neural tube involves the graded actions of sonic hedgehog (Shh) and Wnt proteins (not shown) secreted by the floor plate and adjacent ventral cells, and the patterned expression of homeodomain (HD) proteins by ventral progenitor cells and post-mitotic neurons. This dorso-ventral patterning pathway recruits expression of the LIM homeodomain proteins Lhx3 and Lhx4 by newly generated motor neurons, assigning a median motor column (MMC) – like identity. In primitive ancestral vertebrates such as the lamprey and hagfish the dominance of this inductive pathway confers all motor neurons with a MMC fate, providing the neural substrate for undulatory locomotor behaviors.
Upper branch. Over the course of ∼500 million years of evolution, rostro-caudal inductive signals mediated by retinoids (RA) and fibroblast growth factors (FGF) establish the patterned expression of Hox homeodomain proteins in motor neuron progenitors and post-mitotic motor neurons. The engagement of Hox transcription factors, and an essential Hox accessory factor, FoxP1, drives motor neuron columnar and pool diversification, resulting in the formation of lateral motor column (LMC) neurons and their resident motor pools. This diversification process permits the spinal motor system to innervate the more complex set of peripheral motor targets that characterizes higher vertebrates and underlies intricate tetrapod gait patterns.
The key intermediate step in this diversification process is presumed to involve the attenuation of non-canonical Wnt signaling, permitting the generation of motor neurons that lack Lhx3 and Lhx4 expression and progress to a hypaxial motor columnar (HMC) fate (not shown). This ground-state HMC-like column provides the neuronal substrate for the actions of the Hox/FoxP1 transcriptional program. For details, see text and ref 26.
Designing a direct test of this, or indeed any alternative, evolutionary model is clearly problematic. Nevertheless, a potential way forward has been suggested by the finding that the activity and output of the entire spinal motor neuron Hox repertoire depends on a single Hox transcriptional co-factor, FoxP1 (26). In mice lacking FoxP1 activity, motor neurons fail to acquire LMC columnar and pool identities, and instead acquire an HMC-like identity, in essence reverting the mammalian spinal motor system to an ancestral state that more closely resembles that evident in primitive aquatic vertebrates (26, 27) During vertebrate evolution, the diversification of the spinal motor system may therefore have involved the regulated expression and activity of Hox proteins and their key FoxP co-factors. When extended to amphibia, this model suggests that the activation of the Hox/FoxP transcriptional program underlies the addition of LMC neurons to pre-existing MMC- and HMC-like motor neurons. And since many spinal interneurons express Hox and FoxP proteins (28, 29), it is conceivable that the diversification of interneuron patterning networks in vertebrates is guided by parallel Hox/FoxP programs.
It is striking that aspects of the molecular program of vertebrate motor neuron specification are also evident in arthropods and annelids. Embryonic motor neurons in Drosophila express close counterparts of many of the transcription factors that specify the generic identity of vertebrate motor neurons (30, 31), suggesting that phasic undulatory movements in radically different animals might be governed by a conserved program of motor neuron specification. Even more remarkably, the dorso-ventral patterning program that characterizes motor neuron and interneuron generation in the spinal cord of vertebrates is largely preserved in the bristleworm Playtnereis dumerlii, a creature argued to represent the closest living relative of a now extinct common ancestor to animals with bilateral symmetry (32). Given this unanticipated molecular conservation, it is not unreasonable to consider whether superficial parallels in the neural strategies for walking in insects and terrestrial vertebrates (33), both of which rely on networks of sensory neurons, interneurons and motor neurons assembled into circuits with common design features (34), actually reflect a conserved genetic wiring program – despite the striking difference in anatomical organization and neuronal design.
A physiological framework for spinal motor control networks
Evolutionary considerations aside, just how near are we to defining a canonical spinal circuit for vertebrate locomotion? And what has the advent of molecular genetic methods for spinal network analysis added to facts and principles gleaned from tried and true physiological approaches? We first discuss the physiological canon that provides the underpinnings of current views of the spinal locomotor network, and then assess the impact of molecular genetic approaches.
Like all circuits, spinal locomotor networks are constructed from core sets of excitatory glutamatergic, and inhibitory glycinergic and/or GABAergic, interneurons (Figure 3). Considerable effort has been devoted to defining the respective contributions of these two fundamental neuronal sets to the establishment of motor pattern. Studies in aquatic and terrestrial vertebrates have shown that pharmacological blockade of glycinergic and GABAergic inhibitory transmission fails to abolish rhythmic burst activity (35, 36, 37, 38, 39), a finding that also applies to other networks like the respiratory CPG (40, 11). These and other observations have provided strong evidence that a core network of excitatory glutamatergic interneurons, acting via AMPA and NMDA receptors, is sufficient to establish rhythmic motor burst activity in groups of synergistic motor neurons. In lamprey and tadpole, intrinsic synaptic excitation within pools of excitatory premotor interneurons at the segmental level (Figure 3) can account for the burst generation in combination with their membrane properties (41, 5, 6). Gap junctional communication has recently also been reported within this pool of excitatory interneurons, providing the potential for further amplification of network activity (42). The termination of motor bursts results primarily from an activity-induced increase of intracellular Ca2+ and Na+ that in turn, activates KCa and KNa channels that will drive the membrane potential towards the equilibrium potential for K+ (43, 5), and also enhance the post-spike after-hyperpolarisation that contributes to the spike frequency adaptation (Figure 3B).
Fig. 3. Locomotor network of the lamprey.
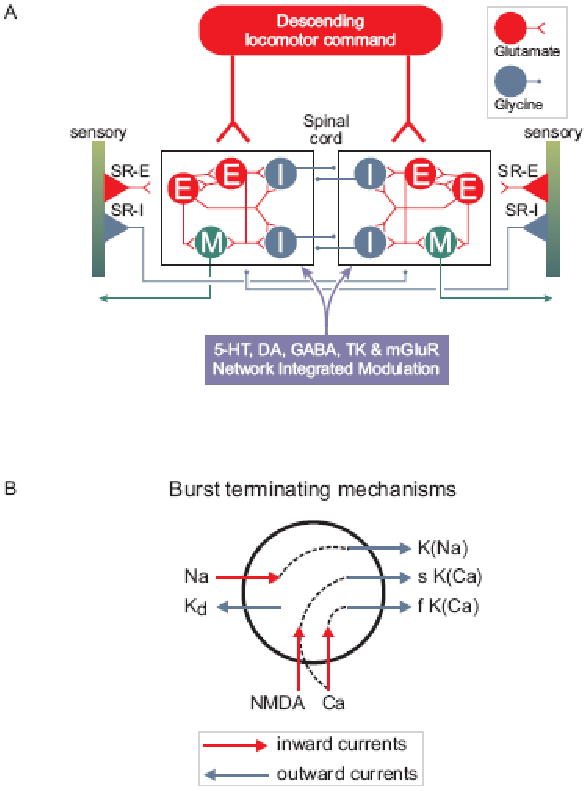
Diagrams show schematic representation of the brainstem and spinal components of the neural circuitry that generates rhythmic locomotor activity.
A. All neuron symbols denote populations rather than single cells. Descending commands exerted through glutamatergic neurons excite all classes of spinal interneurons and motoneurons. The excitatory interneurons (E) excite all types of spinal neurons, i.e. the inhibitory glycinergic interneurons (I) that cross the midline to inhibit all neuron types on the contralateral side and motoneurons (M). Stretch receptor neurons are of two types; one excitatory (SR-E), which excites ipsilateral neurons and one inhibitory (SR-I), which crosses the midline to inhibit contralateral neurons and provide sensory feedback during actual swimming. In addition, metabotropic receptors are activated during locomotion and are an integral part of the network as indicated in the box below the network (5-HT, dopamine (DA), GABA, tachykinins (TK) and mGluRs).
B. Cellular mechanisms contributing to burst termination. During a burst, Ca2+ ions entering via NMDA and voltage-dependent channels activate Ca-activated K+ channels (KCa) that will have a hyperpolarising effect with different time courses (fast (f) and slow (s)). Similarly the entry of Na+ ions (Na) during the burst will activate sodium-dependent K+ channels (KNa). Voltage-dependent K+ channels activated during the action potential are grouped and indicated as (KD).
By extension, spinal inhibitory interneurons have been argued to have a role in coordinating the activity of different sets of “excitatory core burst generators” dedicated to coordination of flexor and extensor motor output and to flexor output from different joint systems – hip and knee flexors for example (1). Thus, motoneurons from lamprey and zebrafish, to amphibians and mammals have been shown to receive phasic excitatory input during the depolarizing phase of the movement cycle, followed by phasic postsynaptic inhibition in the next phase (44, 36, 45, 46, 1). This core excitatory push -inhibitory pull arrangement has long been considered to operate through each motor cycle. It is with reference to this established view that three recent sets of findings merit discussion.
The drive for motoneuron firing – law and order or anarchy?
One recent set of observations (47) appears starkly at odds with consensus views. Analysis of motor neuron activity in turtle spinal cord during induction of the scratch reflex reported that hip motor neurons received a continuous, and mixed, barrage of excitation and inhibition. Moreover, these inhibitory and excitatory phases were observed to peak in register with overall neuronal conductance phase, during the depolarizing waves of scratch episodes. Finally, spike activity was reported to be driven by depolarizing synaptic transients. Based on these observations, Berg and colleagues argued that coincident, rather than sequential, phases of excitation and inhibition underlie locomotor rhythm and pattern.
Do these findings call for a fundamental reassessment of the origins of locomotor pattern? Studies in mammalian spinal cord using identical methodology have not found evidence for coincidence of excitatory and inhibitory synaptic input, and instead have corroborated earlier reports of alternating excitatory and inhibitory drive to most motoneurons (48). The results of Berg et al. (47) could have their basis in the specifics of experimental design, since in their studies recordings were obtained from motoneurons close to the cut surface of the spinal cord which are likely to lack many of their normal synaptic inputs. Whatever the reason, the heterodox view suggested by Berg and colleagues for the turtle scratch is not generalizable to other well-characterized locomotor networks.
Paradoxical persistence of rhythmic locomotor pattern after vGluT2 inactivation
A key tenet of the ‘core excitatory kernel’ view of motor burst initiation is that glutamatergic signaling between excitatory interneurons is needed for the establishment of motor pattern. It is puzzling therefore that attenuation of glutamatergic transmission in mouse spinal interneurons, through inactivation of the gene that encodes the synaptic vesicular glutamate transporter (vGluT2) fails to perturb spinal locomotor pattern when assessed in vitro during fictive locomotion (49). Nevertheless, respiratory pattern is severely disrupted, implying that the preservation of spinal motor pattern does not have its basis in a general failure of vGluT2 elimination to impact glutamatergic transmission in motor circuits.
What could underlie this unanticipated finding? One possibility is that there is deregulation or redundancy in the actions of the vGluT transporter family, a situation that has been considered in other regions of the CNS (50). It is also conceivable that neurons deprived of the opportunity for glutamatergic transmission rely on parallel cholinergic transmitter signalling. There are indeed now reports in mammals of dual expression of cholinergic and glutamatergic characters by some spinal neurons (51, 52, 53, 54). Co-release of glutamate and acetylcholine can also occur in the developing tadpole, and fast nicotinic receptors can be activated in concert with the glutamate receptors (52, 55). Moreover, developing motoneurons stripped of their capacity for acetylcholine synthesis by inactivation of the gene encoding choline acetyl transferase switch to the use of glutamate as excitatory transmitter (56).
Another plausible class of explanation emerges from consideration of the fact that synaptic interactions among inhibitory interneurons, and with motoneurons, remain intact in vGluT2 mutant animals. Thus, despite the elimination of endogenous excitatory drive, exogenous application of glutamate agonists in isolated spinal cord preparations will still trigger motor neuron firing that terminates under the influence of intrinsic ion conductances, resulting in burst-like activity. These drug-induced bursts could then be co-ordinated by inhibitory interneurons, to generate a quasi-normal pattern of motor activity. This scenario implies the existence of mechanisms able to synchronize groups of inhibitory interneurons with synergistic functions. Gap junctions represent one of several possible mechanisms that could contribute to such an effect, like in other regions of the CNS (57). Known instances of gap junctional communication in spinal cord (58, 59, 42, 60) could therefore contribute to the coordination of inhibitory network function and motor bursting in the absence of glutamatergic signaling.
An excitatory influence of GABAergic cells lining the central canal
A recent elegant study in larval zebrafish has shown, unexpectedly, that activation of a set of GABAergic ‘Kolmer-Agduhr’ cells in the larval zebrafish elicits a prominent facilitatory influence on locomotor network activity (61). These unusual GABAergic cells extend cilia into the central canal of the spinal cord yet also project into the spinal neuropil, and in most species reach the lateral margin of the spinal cord. In genetically modified zebrafish, photo-activation of these cells results in enhanced locomotor activity. In other vertebrate species including the lamprey, the corresponding ciliated neurons project to the lateral margin of the spinal cord, where they inhibit stretch-receptor neurons that provide movement-related feedback to the locomotor network (62). Defining the precise function of these cells, which appear conserved throughout much of vertebrate evolution, may add an important component to the logic of spinal locomotor network organization and function.
Spinal interneuron subtypes: molecular diversity in search of functional correlates
Physiological and anatomical data that document the functional diversity of interneurons involved in regulating motor output have been augmented more recently by molecular descriptions of interneuron subtype, revealed most clearly by profiles of transcription factor expression (63, 7). Interneurons that settle in the ventral spinal cord with presumed roles in the control of motor pattern derive from four progenitor populations, which give rise at cell cycle exit to four cardinal interneuron classes, termed V0, V1, V2 and V3 neurons (Figure 4), with each progenitor and post-mitotic set marked by its own distinctive set of progenitor and post-mitotic transcription factors (63). This interneuron transcriptional code is conserved across vertebrate species – the same four basic neuronal sets can be delineated in rodents, chicks, frogs and fish (64).
Figure 4. Molecular programs of interneuron diversity in the ventral spinal cord.
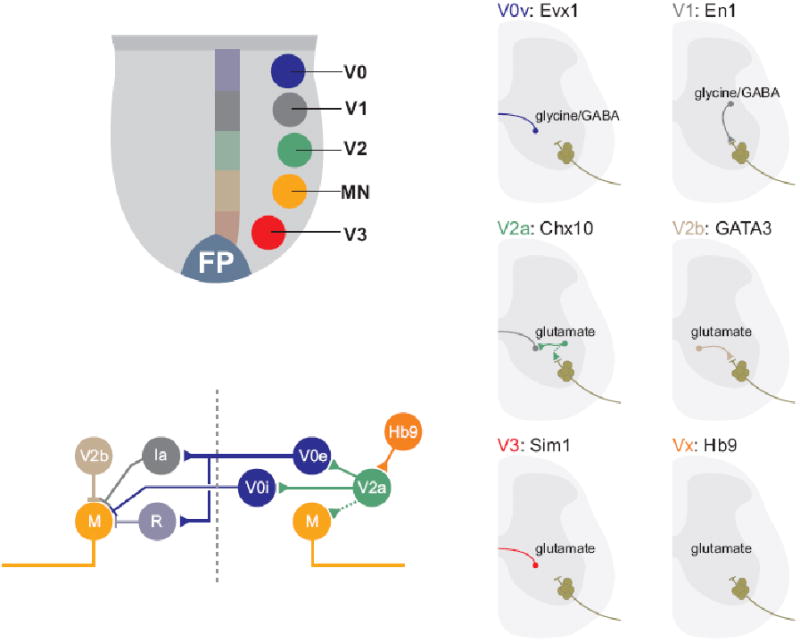
A. The position of origin of motor neurons (MN) and the four cardinal interneuron populations V0-V3) in the ventral spinal cord.
B. Representative examples of interneuron classes derived from distinct ventral progenitor domains. Many of these interneuron subtypes can be distinguished by post-mitotic transcription factor expression, axonal trajectories and transmitter phenotype.
C. Schematic diagram depicting proposed circuitry of distinct ventral interneuron classes, defined by transcriptional identity and physiological properties. Although many details of the link between molecular identity, network organization and physiological properties remain unclear, emerging evidence supports the view that molecular determinants of neuronal identity contribute to the assembly of spinal interneuron networks.
For details, and references, see text.
Within the framework of this molecular classification scheme, the task has been to determine the relationship of defined neuronal subtypes to the organizational logic of interneuron circuitry, and to ask whether molecular interventions that target individual neuronal subsets can reveal new aspects of the logic of interneuron networks in spinal motor control. Studies over the past few years have begun to link basic interneuron subtypes to network organization and function, yet suggest the existence of a far greater degree of molecular complexity and functional diversity.
The elusive transcriptional signature of rhythmogenic interneurons
Although there is now persuasive evidence that core elements of spinal locomotor rhythm are directed by a network of excitatory, glutamatergic, interneurons, the molecular identity of these interneurons has remained elusive. Several recent studies have attempted to identify core rhythmogenic interneurons by monitoring and manipulating the activity of transcriptionally defined sets of glutamatergic interneurons (Figure 4). Two major classes of glutamatergic interneurons are generated in the ventral spinal cord, from the p2 and p3 progenitor domains (65). V2a interneurons, defined by Chx10 expression, represent a class of ipsilaterally projecting neurons (66, 67), whereas most V3 neurons have contralateral projections (68). Inactivation of each set individually, by complete elimination of the neurons, or by tetanus toxin mediated blockade of neurotransmitter release, has little impact on the core locomotor rhythm revealed in isolated spinal cord preparations (68, 69). Thus each interneuron class, alone, is dispensable for rhythm generation, emphasizing the presence of overlapping neuronal mechanisms that ascertain motor pattern generation. In the absence of one neuronal set, spinal networks could undergo homeostatic compensation to scale the remaining connections, re-establishing aspects of loconmotor output.
Attention has also focused on a third, minor, set of glutamatergic interneurons of unknown progenitor provenance, marked by the transcription factor Hb9 (70, 71). In the mouse, these interneurons exhibit pronounced membrane potential oscillations during locomotor activity. Moreover, the rhythmic membrane depolarization of Hb9 interneurons occurs in register with ventral root motor burst pattern, raising the possibility that they constitute an integral component of the rhythm-generating networks (72). More recent two-photon calcium imaging studies in isolated mouse spinal cord preparations, however, reveal only sparse activity of Hb9 interneurons during locomotor episodes, and show that the onset of neuronal activity lags behind ventral root bursting (73). These findings question the idea that Hb9 interneurons are the sole intrasegmental rhythm-generating elements in the mammalian CPG. Since selective inactivation of Hb9 interneurons has not yet been achieved, their contribution to the establishment of locomotor rhythm in vivo remains uncertain. Thus, transcriptionally-based identification strategies have not succeeded, so far, in pinpointing the specific excitatory interneuronal populations that lie at the core of rhythmogenicity.
Interneuronal substrates of left-right motor alternation
More progress has been made in defining elemental circuits that underlie the alternating phasic relationship of motor output on the left and right sides of the spinal cord. In rodents, physiological and anatomical studies have implicated sets of commissural interneurons that, once activated by ipsilateral motor burst activity, project to and inhibit contralateral motor neurons (11, 74, 75). Many of these commissural interneurons (Figure 4) correspond to V0 interneurons that can be marked by expression of the transcription factor Dbx1 (76). Moreover, in Dbx1 mutant mice which lack V0 interneurons, there is a marked increase in incidence of co-bursting of left and right flexor/extensor motor neurons during drug-induced locomotion in vitro (77). Thus, V0 commissural inhibitory and excitatory interneurons appear to be a core component of spinal locomotor circuits that control stepping movements in mammals.
These findings posed the question of the organization of the excitatory neural inputs to V0 commissural interneurons involved in activating commissural pathways. A set of V2a neurons appear to serve this role. V2a interneurons, defined by Chx10 and Sox14 expression, are glutamatergic neurons with ipsilateral projections towards V0 commissural interneurons (66, 69). Genetic elimination of these neurons, through Chx10-directed diphtheria toxin expression, results in a marked erosion of the fidelity of left-right alternation of motor bursts in isolated spinal cord preparations (69). Thus one neural substrate for left-right coordination involves V0 commissural neurons and their excitatory glutamatergic input from ipsilateral V2a interneurons. Recent studies have indicated considerable diversity in the identity and connectivity of commissural interneurons (78) supporting the existence of V0-independent populations of commissural interneurons, and posing the question of whether V2a neurons provide input to all classes of commissural inhibitory interneurons.
Analysis of the role of V2a interneurons in spinal motor behavior in vivo (79) has raised the possibility that this set of neurons contributes to the fidelity of gait pattern at different speeds – an intriguing, but poorly resolved aspect of locomotor control. When challenged with locomotor tasks that require different speeds of movement, wild type mice typically employ a single trot-like gait pattern. In contrast, mice lacking V2a interneurons often switch from a trotting to galloping gait as the required speed increases. Moreover, a speed-dependent loss of left-right alternation can also be revealed during drug-induced fictive locomotion in spinal cords isolated from neonatal V2a interneuron-deficient mice. These findings suggest that V2a interneurons help to maintain left-right alternation at high speeds of locomotion, and by inference that a distinct circuit operates at lower speeds. In many ways, the left-right alternation defects observed in V2a interneuron-deficient mice are reminiscent of those observed in mice lacking the function of the EphA4 signaling system, in which a set of excitatory ipsilateral interneurons are thought to project aberrantly across the midline, to innervate contralateral neuronal targets (80). Nevertheless, despite their evident EphA4 expression, V2a interneurons, appear not to represent the population that switches from an ipsilateral to contralateral trajectory in EphA4 mutants (66), implicating another, as yet unidentified excitatory interneuron class, perhaps contained within the V3 interneuron group.
Studies on speed-dependent circuits for locomotor control in mice have an intriguing parallel in the zebrafish spinal cord (81, 82, 83, 121). At low swimming frequencies, ventrally positioned commissural excitatory interneurons are rhythmically active, whereas at higher swimming frequencies, more dorsal excitatory neurons are recruited (81). Laser ablations of ventral excitatory interneurons perturb slow movements, supporting a behavioral role for position-dependent recruitment of interneurons. The excitatory ipsilateral interneurons that are recruited during somewhat faster swimming appear to represent the counterparts to mammalian V2a neurons, in that they express the zebrafish Chx10 homolog, Alx (80, 122). Alx interneurons are glutamatergic, are activated during rapid swimming, and make frequent monosynaptic excitatory connections onto motoneurons (85). Within the Alx neuronal population, early-born neurons are active during fast swimming at frequencies normally seen in the embryo, whereas later-born neurons are preferentially recruited during the slower, sustained, swimming episodes typical of larger animals (81). At these slow speeds, manoevering usually involves the pectoral fins as well as slow body undulations, raising the issue of whether or not the activity of these ventral interneurons is also linked to pectoral fin motor programs (86). The pectoral fin CPG has received only modest attention, and further studies are clearly warranted, given that it is represents a precursor of the tetrapod limb CPG.
Taken together, these studies suggest an evolutionary conservation in the transcriptional and functional identity of glutamatergic interneurons that participate in circuits involved in the control of control of locomotor speed. In mice, current emphasis on V2a neurons has focused on their connections with commissural interneurons, whereas in zebrafish the connectivity of Alx neurons with motor neurons has been stressed. It seems plausible that Chx10/Alx neurons coordinately regulate ipsilateral motor output through direct connections with motor neurons, and contralateral motor output via commissural inhibitory pathways. More generally, these studies have placed the V2a interneuronal system at a key position in the excitatory control of locomotor pattern. They have also raised the issue of whether the variant connectivity of V2a neurons normally contributes to the selection of locomotor gait in different vertebrates (80).
Ipsilateral inhibitory interneurons and flexor-extensor coordination
Inhibitory interneurons have diverse roles in shaping locomotor pattern. Viewed from the perspective of CPG networks, they have been implicated in providing the coordination of flexor and extensor output across the same or different joints in a limb. Yet in a parallel world of sensory-motor transformation (87), inhibitory interneurons have been implicated in reciprocal (Ia inhibitory neurons), feedback (Renshaw cells) and feed-forward (pre-synaptic sensory) inhibition (88). The transition from stance to swing of the CPG is subject to a clear-cut modulation by sensory afferents signalling the end of stance (1, 89), and thus it seems likely that many classes of spinal inhibitory interneurons contribute to both intrinsic CPG and sensory-evoked motor output. Integrated and crucial information about the overall position of the limb in relation to the body, as well as the length of the limb is available at the spinal level at different parts of the step cycle. Such information can only be obtained by convergent input from many types of sensory afferents (90). Attempts to define spinal interneuron subtypes through profiles of transcription factor expression may therefore have broader relevance to the understanding of regulatory constraints on spinal motor output
In the ventral spinal cord, most GABAergic and glycinergic inhibitory interneurons with ipsilateral projections derive from the p1 or p2 progenitor domains. The cardinal V1 interneuron class, marked by expression of the transcription factor En1, innervates motor neurons and probably other ventral interneurons (91). V2b interneurons represent the inhibitory subset of p2 progenitor derived interneurons, are marked by GATA2/3 expression (66, 92), and appear to send local projections to spinal motor neurons. Anatomical and molecular studies indicate that the cardinal V1 set comprises several distinct subsets of inhibitory interneurons, including Renshaw cells and possibly Ia inhibitory interneurons (7). Complete elimination of V1 interneurons, achieved through En1-dependent toxin targeting or inactivation of the Pax6 gene, abolishes Renshaw cell-mediated recurrent inhibition (93, 94). Moreover, glycinergic En1 neurons in zebrafish and tadpole contribute to both sensory gating and motor pattern generation (95, 96) But in both mutant conditions the phasing of flexor and extensor output, as well as more direct measurement of reciprocal inhibitory connections, is preserved (97). Thus V1 interneurons are either not involved, or function redundantly with a distinct population of inhibitory interneurons. V2b neurons seem the most likely candidates for this parallel population. The inactivation of V1 output does, nevertheless, markedly reduce the speed of the locomotor step cycle (94). The precise subclass of V1 neurons involved in speed control, and how they relate to the V2a excitatory interneuronal system discussed above remains unclear.
Specialized interneurons for presynaptic inhibition of sensory-motor transmission
The transfer of sensory signals to motor neurons and interneurons is also filtered by subgroups of GABAergic interneurons that act pre-synaptically to inhibit sensory transmitter release (98). In all vertebrates examined, from lamprey to carnivores (99, 100), pre-synaptic inhibition is phase-dependent, with the consequence that the influence of sensory afferent input on locomotor programs varies according to the phase of the locomotor cycle (86). These presynaptic GABAergic terminals have been established ultrastructurally (101, 102). Recent studies have provided molecular insight into the origin and development of this set of inhibitory interneurons.
In contrast to the ventral origin of most inhibitory interneurons with motor and ventral interneuron targets, the GABAergic interneurons that mediate pre-synaptic inhibition of proprioceptive sensory input derive from a distinct dorsal (dI4) progenitor domain, and can be defined by expression of the transcription factor Ptf1a (103, 104). Moreover, the connectivity and synaptic differentiation of GABAergic interneurons that mediate pre-synaptic inhibition is directed by their sensory targets. In the absence of sensory terminals these GABAergic neurons shun other available targets, fail to undergo presynaptic differentiation, and withdraw their axons from the ventral spinal cord. Thus, the connectivity and synaptic organization of this transcriptionally defined set of GABAergic interneurons are controlled by a stringent program of sensory terminal recognition and retrograde inductive signaling. The molecular identification of a distinct set of pre-synaptic inhibitory interneurons should make it possible to eliminate the output of these neurons and assess the consequences for motor programming.
Locomotor network properties tuned by slow synaptic actions
Although the core features of locomotor network organization reflect the circuitry and actions of interneurons that mediate fast synaptic responses, more refined aspects of circuit function and motor output are controlled by the influence of neuromodulators released during activation of the core locomotor network. These modulatory signaling systems elicit slower synaptic actions, usually through activation of G-protein coupled receptors (GPCRs; Figure 5). Typically, GPCR activation regulates ion channels in the somatodendritic membrane that modify cellular properties (e.g. frequency adaptation) or modify synaptic efficacy at a pre- or postsynaptic level. Together, these modulatory systems help to sculpt the fine details of CPG output, permitting versatility in network function without the requirement for structural changes in circuit organization.
Figure 5. Regulation of cellular and locomotor network properties by endogenous neuromodulators.
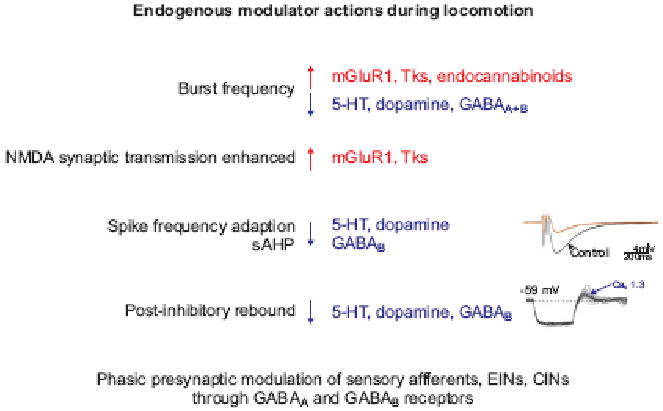
In this schematic diagram, upward red arrow indicates enhanced activity, and downward blue arrow a reduction or depression in activity. Diverse modulatory effects include regulation of burst frequency, NMDA receptor dependent synaptic transmission, spike frequency adaptation, post-inhibitory rebound and phase-dependent presynaptic inhibition.
Recent studies in lamprey, tadpole, and to some degree in rodents have provided an early glimpse of the logic of action and interaction of different spinal modulatory systems (105, 16, 40, 51, 106) (Figure 5). Overall locomotor frequency is enhanced by engagement of metabotropic glutamate and tachykinin receptors, and reduced by activation of serotonergic and GABAergic receptors. In addition to its fast synaptic action, glutamate activates a diverse array of metabotropic glutamate receptors (mGluRs). In conjunction with ionotropic excitatory network drive, activation of mGluR1 receptors enhances motor bursting by modulating leak currents and enhancing NMDA receptor elicited depolarisation (107, 108). In addition, mGluR activation triggers retrograde endocannabinoid signalling which exerts a pre-synaptic depression at inhibitory synapses. In parallel, mGluR activation evokes the release of nitric oxide (NO) which facilitates glutamatergic synaptic transmission (109, 110) and enhances overall network activity (111). The release of tachykinins elicits a similar range of synaptic effects, in part again through retrograde endocannabinoid signaling (112, 113).
Serotonin and acetylcholine also have pronounced modulatory effects on spinal motor networks. Serotonin (5-HT) serves as pivotal modulator of spinal motor networks, stabilizing locomotor rhythm and promoting a decrease in burst frequency. These serotonergic actions reflect the influence of descending brain stem pathways, and in some species, of intraspinal serotonergic neurons. Serotonin mediates these actions by engaging diverse 5-HT receptor subtypes expressed both pre- and post-synaptically, and through distinct ionic mechanisms (114, 115, 116, 51). Activation of muscarinic receptors also exerts a profound influence on motor output. Most notably, in rodents the activation of m2 muscarinic receptors, which are prominently expressed on the somata and proximal dendrites of motor neurons, elicits a marked facilitation of motor neuron firing frequency, mediated by blockade of spike after-hyperpolarization (117).
What next in the measurement of motion?
The logic of neuronal networks and their link to physiological function, whether in the context of movement itself or other behaviors, is likely to emerge only when sufficiently detailed information is available on the identity and membrane properties of individual neuronal components of the network, the synaptic interactions that connect and coordinate these neurons, and the adaptive properties of these networks under different behavioral constraints. In the case of the spinal networks that direct locomotor behavior, we are approaching this level of understanding only for aquatic vertebrates that rely to a large extent on undulatory locomotor strategies.
But even in these relatively well-studied systems we still have only a limited insight into the way in which specific locomotor tasks recruit distinct modulatory systems, to fine-tune the motor program in order to achieve a particular type of adaptation. In the lamprey and other aquatic animals, attention is beginning to turn to the problem of how visual and motor systems are integrated in the cause of goal-directed movements and the role of forebrain structures like the basal ganglia in the dynamic selection of motor programs.
In fish with paired appendages, the circuitry that controls and coordinates the motility of pectoral and pelvic fins is still largely uncharted territory, despite the evident relevance of this issue to the control of tetrapod limbs. In mammals, limb CPGs control diverse muscle groups with complex activation patterns that demand considerably greater flexibility than simple flexor-extensor coordination. By virtue of classical studies in the cat, we now have highly detailed physiological and anatomical accounts of the repertoire of identified interneuron subtypes that contribute to motor output, and quantitative models of network function (87), but still insufficient experimental control to bind these interneuronal components into testable schemes of spinal motor control.
Studies of spinal motor control in rodents, and especially in mice, offer the possibility of linking the traditions and insights of cat physiology with the emerging potential for precise genetic manipulation of spinal interneuron network function. Many recent studies, some discussed here, have provided initial accounts of changes in local circuitry and locomotor pattern after genetically programmed manipulation of molecularly defined interneuron sets. Although rapid progress has been made, the grain of current molecular insight remains coarse. It is likely that each cardinal ventral progenitor domain gives rise to multiple distinct interneuron subtypes, in much the same way that close to a dozen different motor neuron columnar and pool subtypes can differentiate from a single motor neuron progenitor domain. By analogy, each interneuron domain might generate half a dozen distinct interneuron subtypes, implying the existence of two dozen or more molecularly defined ventral interneuron classes. Matching this expanded molecular diversity to physiologically-defined neuronal subtypes and their connections will be a major task, but could help to reveal new principles of spinal network organization. And given the extensive evidence for dynamic, state-dependent, interactions between the neurons of spinal networks, the outcome of molecular interventions will need to be tied closely to, and eventually used to refine, predictive models of spinal network function.
Ultimately, studies on spinal locomotor networks will have to inform us about the logic of specific motor behaviors in an animals' normal environment. Towards this goal, the analysis of diverse motor behaviors needs to be combined with physiological studies on reduced in vitro preparations and biophysical descriptions of neuronal excitability and output. In mouse and zebrafish, there are the beginnings of a combined genetic, cellular and in vivo behavioral assault on the problem of spinal motor control (7, 10, 118, 119, 120). Along the way, it may be worth ensuring that the acceleration of technologies in genetic manipulation of defined neuronal subtypes is accompanied by the design and implementation of more refined and informative assays of motor behavior.
Acknowledgments
We thank the Kavli Foundation for providing a venue that led to initial discussions on aspects of spinal motor control included in this review, and Abdel El Manira and Ole Kiehn for comments and suggestions. We are also grateful to Ira Schieren and Kathy MacArthur for help in preparing the figures and text. TMJ is supported by grants from ProjectALS and NINDS and is an investigator of the Howard Hughes Medical Institute. SG is supported by The Swedish Research Council (M; NT), the European Commission (projects: Spinal Cord Repair, SelectandAct, Lampetra and Cortex) and Stockholm Brain Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grillner S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Tunstall MJ, Roberts A, Soffe SR. Modelling inter-segmental coordination of neuronal oscillators: synaptic mechanisms for uni-directional coupling during swimming in Xenopus tadpoles. J Neurosci. 2002;13:143–158. doi: 10.1023/a:1020114324350. [DOI] [PubMed] [Google Scholar]
- 3.Grillner S, Kozlov A, Dario P, Stefanini C, Menciassi A, Lansner A, Hellgren Kotaleski J. Modeling a vertebrate motor system: pattern generation, steering and control of body orientation. Prog Brain Rev. 2007;165:221–234. doi: 10.1016/S0079-6123(06)65014-0. [DOI] [PubMed] [Google Scholar]
- **4.Kozlov A, Huss M, Lanner A, Hellgren Kotaleski J, Grillner S. Simple cellular and network control principles govern complex patterns of motor behaviour. Proc Natl Acad Sci USA. (in press). This modeling study employs large scale simulation of the locomotor system from the basal ganglia to the spinal networks with realistic number of neurons, to produce a motor pattern that resembles, in many features, that observed in vivo. [Google Scholar]
- 5.Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nature Reviews Neuroscience. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 6.Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res Rev. 2008;57:22–8. doi: 10.1016/j.brainresrev.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–18. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fetcho JR. The spinal motor system in early vertebrates and some of its evolutionary changes. Brain Behav Evol. 1992;40:82–97. doi: 10.1159/000113905. [DOI] [PubMed] [Google Scholar]
- 9.Biewener A. Animal Locomotion. Oxford University Press; p. 285. [Google Scholar]
- 10.Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hübner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 11.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–230. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 12.Chevallier S, Jan Ijspeert A, Ryczko D, Nagy F, Cabelguen JM. Organisation of the spinal central pattern generators for locomotion in the salamander: biology and modelling. Brain Res Rev. 2008;57:147–161. doi: 10.1016/j.brainresrev.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Ijspeert AJ, Crespi A, Ryczko D, Cabelguen JM. From swimming to walking with a salamander robot driven by a spinal cord model. Science. 2007;315:1416–1420. doi: 10.1126/science.1138353. [DOI] [PubMed] [Google Scholar]
- 14.Bem T, Cabelguen JM, Ekeberg O, Grillner S. From swimming to walking: a single basic network for two different behaviors. Biol Cybern. 2003;88:79–90. doi: 10.1007/s00422-002-0340-3. [DOI] [PubMed] [Google Scholar]
- 15.Combes D, Merrywest SD, Simmers J, Sillar KT. Developmental segregation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing Xenopus laevis. J Physiol. 2004;559:17–24. doi: 10.1113/jphysiol.2004.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res Rev. 2008;57:94–102. doi: 10.1016/j.brainresrev.2007.07.018. This informative article outlines the way in which locomotor networks and output make the transition from a purely trunk CPG to a limb CPG. The study of locomotor pattern during amphibian metamorphosis provides a potential link between molecular evolutionary and functional analyses of vertebrate locomotion. [DOI] [PubMed] [Google Scholar]
- 17.Schlosser G. Mosaic evolution of neural development in anurans: acceleration of spinal cord development in the direct developing frog Eleutherodactylus coqui. Anat Embryol (Berl) 2003;206:215–227. doi: 10.1007/s00429-002-0291-4. [DOI] [PubMed] [Google Scholar]
- 18.Kudo N, Nishimary H, Nakayama K. Developmental changes in rhythmic spinal neuronal activity in the rat fetus. Prog Brian Res. 2004;143:49–55. doi: 10.1016/s0079-6123(03)43005-7. [DOI] [PubMed] [Google Scholar]
- 19.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Wallén P, Grillner S, Feldman JL, Bergelt S. Dorsal and ventral myotome motoneurons and their input during fictive locomotion in lamprey. J Neurosci. 1985;5:654–661. doi: 10.1523/JNEUROSCI.05-03-00654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 22.Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c protein expression by FGFs, Gdf11 and Retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- 23.Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- 24.Agalliu D, Takada S, Agalliu I, McMahon AP, Jessell TM. Motor neurons with axial muscle projections specified by Wnt4/5 signaling. Neuron. 2009;61:708–720. doi: 10.1016/j.neuron.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- **26.Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires formotor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. This paper, together with ref 27, demonstrate the involvement of FoxP1 as a Hox accessory factor. Dasen et al., also provide an evolutionary perspective on the origins of spinal motor neuron diversity. [DOI] [PubMed] [Google Scholar]
- 27.Rousso DL, Gaber ZB, Wellik D, Morrisey EE, Novitch BG. Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron. 2008;59:226–240. doi: 10.1016/j.neuron.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morikawa Y, Hisaoka T, Senba E. Characterization of Foxp2-expressing cells in the developing spinal cord. Neuroscience. 2009;162:1150–1162. doi: 10.1016/j.neuroscience.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter EM. Hox genes and spinal cord development. Dev Neurosci. 2002;24:24–34. doi: 10.1159/000064943. [DOI] [PubMed] [Google Scholar]
- 30.Landgraf M, Thor S. Development and structure of motoneurons. Int Rev Neurobiol. 2006;75:33–53. doi: 10.1016/S0074-7742(06)75002-4. [DOI] [PubMed] [Google Scholar]
- 31.Lacin H, Zhu Y, Wilson BA, Skeath JB. dbx mediates neuronal specification and differentiation through cross-repressive, lineage-specific interactions with eve and hb9. Development. 2009;136:3257–3266. doi: 10.1242/dev.037242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denes AS, Jékely G, Steinmetz PR, Raible F, Snyman H, Prud'homme B, Ferrier DE, Balavoine G, Arendt D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–288. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- **33.Büschges A, Akay T, Gabriel JP, Schmidt J. Organizing network action for locomotion: insights from studying insect walking. Brain Res Rev. 2008;57:162–171. doi: 10.1016/j.brainresrev.2007.06.028. This article describes the organizational principles of insect walking, and parallels with locomotion in vertebrates. [DOI] [PubMed] [Google Scholar]
- 34.Burrows M. Local circuits for the control of leg movements in an insect. Trends Neurosci. 1992;15:226–232. doi: 10.1016/0166-2236(92)90040-f. [DOI] [PubMed] [Google Scholar]
- 35.Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: The lamprey hemicord. J Neuroscience. 2005;25:923–935. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabriel JP, Mahmood R, Kyriakatos A, Söll I, Hauptmann G, Calabrese RL, El Manira A. Serotonergic modulation of locomotion in zebrafish – endogenous release and synaptic mechanisms. J Neurosci. 2009;29:10387–10395. doi: 10.1523/JNEUROSCI.1978-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiehn O, Quinlan KA, Restrepo CE, Lundfald L, Borgius L, Talpalar AE, Endo T. Excitatory components of the mammalian locomotor CPG. Brain Res Rev. 2008;57:56–63. doi: 10.1016/j.brainresrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Nistri A, Ostroumov K, Sharifullina E, Taccola G. Tuning and playing a motor rhythm: how metabotropic glutamate receptors orchestrate generation of motor patterns in the mammalian central nervous system. J Physiol. 2006;572:323–334. doi: 10.1113/jphysiol.2005.100610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiehn O, Buschges A, Duch C, Grillner S, Isa T, Lansner A, Pluger HJ, Richter DW, Sillar KT, Smith JC, Sparks DL. Microcircuits The interface between neurons and Global Brain Function. In: Grillner S, Graybiel AM, editors. Microcircuits in the Motor System. MIT press; Cambridge Mass. US: 2007. pp. 77–104. [Google Scholar]
- 40.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchanan JT, Grillner S. Newly identified ‘glutamate interneurons’ and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- 42.Li WC, Roberts A, Soffe SR. Locomotor rhythm maintencance: electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J Physiol. 2009;587:1677–1693. doi: 10.1113/jphysiol.2008.166942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallén P, Robertson B, Cangiano L, Löw P, Bhattacharjee A, Kaczmarek LK, Grillner S. Sodium-dependent potassium channels of a Slack-like subtype contribute to the slow afterhyperpolarization in lamprey spinal neurons. J Physiol. 2007;585:75–90. doi: 10.1113/jphysiol.2007.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell DF, Wallén P. On the control of myotomal motoneurones during “fictive swimming” in the lamprey spinal cord in vitro. Acta Physiol Scand. 1983;117:161–70. doi: 10.1111/j.1748-1716.1983.tb07193.x. [DOI] [PubMed] [Google Scholar]
- 45.Dale N. Reciprocal inhibitory interneurones in the Xenopus embryo spinal cord. J Physiol. 1985;363:61–70. doi: 10.1113/jphysiol.1985.sp015695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pratt CA, Jordan LM. Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. J Neurophysiol. 1987;57:56–71. doi: 10.1152/jn.1987.57.1.56. [DOI] [PubMed] [Google Scholar]
- 47.Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- 48.Endo T, Kiehn O. A symmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J Neurophysiol. 2008;100:3043–3054. doi: 10.1152/jn.90729.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallén-Mackenzie Å, Gezelius H, Thoby-Brisson M, Nygård A, Enjin A, Fujiyama F, Fortin G, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 51.Liu TT, Bannatyne BA, Jankowska E, Maxwell DJ. Cholinergic terminals in the ventral horn of adult rat and cat: evidence that glutamate is a cotransmitter at putative interneuron synapses but not at central synapses of motoneurons. Neuroscience. 2009;161:111–122. doi: 10.1016/j.neuroscience.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamotte d'Incamps B, Ascher P. Four excitatory postsynaptic ionotropic receptors coactivated at the motoneuron-Renshaw cell synapse. J Neurosci. 2008;28:1421–1431. doi: 10.1523/JNEUROSCI.3311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O'Donovan MJ. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li WC, Soffe SR, Roberts A. Glutamate and acetylcholine corelease at developing synapses. PNAS. 2004;43:15488–15493. doi: 10.1073/pnas.0404864101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers CP, Lewcock JW, Hanson MG, Gosgnach S, Aimone JB, Gage FH, Lee KF, Landmesser LT, Pfaff SL. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron. 2005;46:37–49. doi: 10.1016/j.neuron.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda T. Network architecture of gap junction-coupled neuronal linkage in the striatum. J Neurosci. 2009;29:1235–1243. doi: 10.1523/JNEUROSCI.4418-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiehn O, Tresch MC. Gap junctions and motor behaviour. Trends Neurosci. 2002;25:108–115. doi: 10.1016/s0166-2236(02)02038-6. [DOI] [PubMed] [Google Scholar]
- 59.Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron. 2001;31:1035–1046. doi: 10.1016/s0896-6273(01)00416-0. [DOI] [PubMed] [Google Scholar]
- 60.Zhang HY, Li WC, Heiter WJ, Sillar KT. Electrical coupling synchronises spinal motoneuron activity during swimming in hatchling Xenopus tadpoles. J Physiol. 2009;587:4455–4466. doi: 10.1113/jphysiol.2009.173468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ***61.Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–410. doi: 10.1038/nature08323. An elegant use of targeted neuronal manipulations in zebrafish to define the organization and function of a small set of central canal GABAergic interneurons that influence locomotor output. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christenson J, Alford S, Grillner S, Hökfelt T. Co-localized GABA and somatostatin use different ionic mechanisms to hyperpolarize target neurons in the lamprey spinal cord. Neurosci Lett. 1991;134:93–97. doi: 10.1016/0304-3940(91)90516-v. [DOI] [PubMed] [Google Scholar]
- 63.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Reviews Genetics. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 64.Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci. 2006;7:103–114. doi: 10.1038/nrn1843. [DOI] [PubMed] [Google Scholar]
- 65.Stepien AE, Arber S. Probing the locomotor conundrum: descending the ‘V’ interneuron ladder. Neuron. 2008;60:1–4. doi: 10.1016/j.neuron.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 66.Lundfald L, Restrepo CE, Butt SJ, Peng CY, Droho S, Endo T, Zeilhofer HU, Sharma K, Kiehn O. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci. 2007;26:2989–3002. doi: 10.1111/j.1460-9568.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 67.Kimura Y, Satou C, Higashijima S. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135:3001–3005. doi: 10.1242/dev.024802. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- 71.Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev. 2008;57:64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwan AC, Dietz SB, Webb WW, Harris-Warrick RM. Activity of Hb9 Interneurons during Fictive Locomotion in Mouse Spinal Cord. J Neurosci. 2009;29:11601–11613. doi: 10.1523/JNEUROSCI.1612-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci. 2007;27:6521–6530. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butt S, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- 76.Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 77.Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–86. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- 78.Restrepo CE, Lundfald L, Szabó G, Erdélyi F, Zeilhofer HU, Glover JC, Kiehn O. Transmitter-phenotypes of commissural interneurons in the lumbar spinal cord of newborn mice. J Comp Neurol. 2009;517:177–192. doi: 10.1002/cne.22144. [DOI] [PubMed] [Google Scholar]
- 78.Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydström A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- 81.McLean DL, Fetcho JR. Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev Neurobiol. 2008;68:817–34. doi: 10.1002/dneu.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Rev. 2008;57:86–93. doi: 10.1016/j.brainresrev.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **83.McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225. An impressively detailed analysis of the recruitment pattern of ventral interneurons and motor neurons in zebrafish provides evidence for the presence of speed-selective interneuronal circuits. The paper also provides insight into the positional and temporal origins of interneuron subtype diversity. Not too shabby indeed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S. Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J Neurosci. 2009;29:6780–93. doi: 10.1523/JNEUROSCI.0801-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Manira A, Grillner S. Switching gears in the spinal cord. Nat Neurosci. 2008;11:1367–1368. doi: 10.1038/nn1208-1367. [DOI] [PubMed] [Google Scholar]
- 87.Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Re Bull. 2007;73:155–202. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 89.Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Res Rev. 2008;57:222–227. doi: 10.1016/j.brainresrev.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 90.Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 200(81):539–568. doi: 10.1152/physrev.2001.81.2.539. [DOI] [PubMed] [Google Scholar]
- 91.Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol. 2005;493:177–179. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng CY, Yajima H, Burns CE, Zon LI, Sisodia SS, Pfaff SL, Sharma K. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007 doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. J Neurosci. 2004;24:1255–1264. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 95.Higashijima S, Masino MA, Mandel G, Fetcho JR. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004;24:5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li WC, Soffe SR, Roberts A. Spinal inhibitory neurons that modulate cutaneous sensory pathways during locomotion in a simple vertebrate. J Neurosci. 2002;22:10924–10934. doi: 10.1523/JNEUROSCI.22-24-10924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Z, Li L, Goulding M, Frank E. Early postnatal development of reciprocal Ia inhibition in the murine spinal cord. J Neurophysiol. 2008;100:185–196. doi: 10.1152/jn.90354.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rudomin P. In search of lost presynaptic inhibition. Exp Brain Res. 2009;196:139–151. doi: 10.1007/s00221-009-1758-9. [DOI] [PubMed] [Google Scholar]
- 99.Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 100.El Manira A, Tegnér J, Grillner S. Locomotor-related presynaptic modulation of primary afferents in the lamprey. Eur J Neurosci. 1997;9:696–705. doi: 10.1111/j.1460-9568.1997.tb01418.x. [DOI] [PubMed] [Google Scholar]
- 101.Conradi S, Cullheim S, Gollvik L, Kellerth JO. Electron microscopic observations on the synaptic contacts of group Ia muscle spindle afferents in the cat lumbosacral spinal cord. Brain Res. 1983;265:31–39. doi: 10.1016/0006-8993(83)91330-6. [DOI] [PubMed] [Google Scholar]
- 102.Christenson J, Shupliakov O, Cullheim S, Grillner S. Possible morphological substrates for GABA-mediated presynaptic inhibition in the lamprey spinal cord. J Comp Neurol. 1993;328:463–472. doi: 10.1002/cne.903280402. [DOI] [PubMed] [Google Scholar]
- 103.Betley JN, Wright C, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic pre-synaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glasgow SM, Henke R, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- 105.El Manira A, Kyriakatos A, Nanou E, Mahmood R. Endocannabinoid signaling in the spinal locomotor circuitry. Brain Res Rev. 2008;57:29–36. doi: 10.1016/j.brainresrev.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 106.Liu J, Akay T, Hedlund PB, Pearson KG, Jordan LM. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J Neurophysiol. 2009;102:337–348. doi: 10.1152/jn.91239.2008. [DOI] [PubMed] [Google Scholar]
- **107.Nanou E, Kyriakatos A, Kettunen P, El Manira A. Separate signalling mechanisms underlie mGluR1 modulation of leak channels and NMDA receptors in the network underlying locomotion. J Physiol. 2009;587:3001–3008. doi: 10.1113/jphysiol.2009.172452. This work reveals important details of the integration of slow metabotropic synaptic actions with fast synaptic actions, and the link with network modulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chapman JP, Issberner P, Sillar KT. Group I mGluRs increase locomotor network excitability in Xenopus tadpoles via presynaptic inhibition of glycinergic neurotransmission. European J of Neurosci. 2008;28:903–913. doi: 10.1111/j.1460-9568.2008.06391.x. [DOI] [PubMed] [Google Scholar]
- 109.Kettunen P, Kyriakatos A, Hallén K, El Manira A. Neuromodulation via conditional release of endocannabinoids in the spinal locomotor network. Neuron. 2005;45:95–104. doi: 10.1016/j.neuron.2004.12.022. [DOI] [PubMed] [Google Scholar]
- **110.Kyriakatos A, El Manira A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J Neurosci. 2007;27:12664–12674. doi: 10.1523/JNEUROSCI.3174-07.2007. A further detailed analysis of complementary retrograde signaling pathways used in locomotor modulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mclean DL, Sillar KT. Metamodulation of a spinal locomotor network by nitric oxide. J Neurosci. 2004;24:9561–9571. doi: 10.1523/JNEUROSCI.1817-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thörn Pérez C, Hill RH, Grillner S. Endogenous tachykinin release contributes to the locomotor activity in lamprey. J Neurophysiol. 2007;97:3331–3339. doi: 10.1152/jn.01302.2006. [DOI] [PubMed] [Google Scholar]
- 113.Thörn Pérez C, Hill RH, El Manira A, Grillner S. Endocannabinoids mediate tachykinin-induced effects in the lamprey locomotor network. J Neurophysiol. 2009;102:1358–1365. doi: 10.1152/jn.00294.2009. [DOI] [PubMed] [Google Scholar]
- 114.Hill RH, Svensson E, Dewael Y, Grillner S. 5-HT inhibits N-type but not L-type Ca2+ channels via 5-HT1A receptors in lamprey spinal neurons. Eur J Neurosci. 18:2919–2924. doi: 10.1111/j.1460-9568.2003.03051.x. [DOI] [PubMed] [Google Scholar]
- 115.Wedderburn JF, Sillar KT. Modulation of rhythmic swimming activity in post-embryonic Xenopus laevis tadpoles by 5-hydroxytryptamine acting at 5HT1a receptors. Proc Biol Sci. 1994;257:59–66. doi: 10.1098/rspb.1994.0094. [DOI] [PubMed] [Google Scholar]
- 116.Photowala H, Blackmer T, Schwartz E, Hamm HE, Alford SG. protein betagamma-subunits activated by serotonin mediate presynaptic inhibition by regulating vesicle fusion properties. Proc Natl Acad Sci U S A. 2006;103:4281–4286. doi: 10.1073/pnas.0600509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akay T, Fouad K, Pearson KG. New technique for drug application to the spinal cord of walking mice. J Neurosci Methods. 2008;171:39–47. doi: 10.1016/j.jneumeth.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 119.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 121.McLean DL, Fetcho JR. Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev Neurobiol. 2008;68:817–34. doi: 10.1002/dneu.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


