Abstract
We have recently reported in male rats that medial prefrontal cortex (mPFC) neurons that project to the basolateral nucleus of the amygdala (BLA) are resilient to stress-induced dendritic remodeling. The present study investigated whether this also occurs in female rats. This pathway was identified using the retrograde tracer Fast Blue injected into the BLA of ovariectomized female rats with estrogen replacement (OVX + E) and without (OVX + veh). Animals were exposed for 10 days either to 2-h immobilization stress or to home cage rest, after which layer III mPFC neurons that were either retrogradely labeled by Fast Blue or unlabeled were filled with Lucifer Yellow and analyzed for apical dendritic length and spine density. No dendritic remodeling occurred in unlabeled neurons from OVX + veh or OVX + E animals. In BLA-projecting neurons, however, stress had no effect on length in OVX + veh animals, but stressed OVX + E females showed greater dendritic length than controls at intermediate branches. Stress also caused an increase in spine density in all neurons in OVX + veh animals and a spine density increase in BLA-projecting neurons in OVX + E females. Estrogen also increased spine density on BLA-projecting neurons in unstressed animals. These data demonstrate both independent effects of estrogen on pyramidal cell morphology and effects that are interactive with stress, with the BLA-projecting neurons being sensitive to both kinds of effects.
Keywords: connectivity, dendritic arborization, medial prefrontal cortex, neural plasticity, sex difference
Introduction
It is well documented that women are twice as likely as men to develop both major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) (Weissman et al. 1996), a phenomenon that cannot be accounted for by cultural and social factors alone. This gender discrepancy arises after puberty, persists through the childbearing years, and disappears after menopause (Bebbington et al. 2003), suggesting that circulating estrogen may play a role in rendering women more vulnerable to stress-related disorders. These disorders are often brought on or worsened by exposure to a stressful event, and sex differences in the biochemical, cellular, and behavioral effects of stress in animal models have been demonstrated (Shors et al. 2001; Mitsushima et al. 2003; Shansky et al. 2004), offering compelling evidence that ovarian hormones can interact with stress systems to produce a unique, often enhanced, response in females.
The symptoms of MDD and PTSD suggest a dysfunction of the connection between the medial prefrontal cortex (mPFC) and the amygdala (Baxter et al. 1989; Drevets et al. 1997), which together form a network that governs learning, memory, and behavior based on emotionally salient information (reviewed in Arnsten 1998 and Maren and Quirk 2004). The amygdala is known to regulate the processing of, memory of, and response to negative stimuli (Hendler et al. 2003; Wright et al. 2003), while the mPFC integrates information from multiple brain areas to regulate behavior, thought, and affect (Arnsten 1998). Accordingly, MDD and PTSD are often characterized by an overactive amygdala (reviewed in Drevets 2003) and a decrease in mPFC activity (Liberzon and Phan 2003; Pizzagalli et al. 2004). This intriguing relationship has prompted speculations that a deregulation of the amygdala by the mPFC promotes rumination and the recurring negative thoughts that are commonly observed in patients afflicted by these disorders.
Postmortem studies have revealed morphological abnormalities in both the mPFC (Rajkowska et al. 2001) and the amygdala of suicide patients (Frodl et al. 2003), and morphological effects of stress on these brain regions have been reported in animal models. Specifically, chronic stress has been shown to induce dendritic retraction in the anterior cingulate (ACg), prelimbic (Radley et al. 2004), and infralimbic (IL; Shansky et al., 2009) regions of the mPFC but an expansion of the dendritic arbor in the amygdala (Vyas et al. 2003). We have recently reported, however, that in male rats, IL neurons that project to the basolateral nucleus of the amygdala (BLA) are particularly resilient against the morphological effects of stress (Shansky et al. 2009), suggesting that stress-related remodeling in the brain may be circuit specific.
To date, neither the circuit-specific effects nor the general morphological effects of stress have been studied in the mPFC of female rats. Moreover, the effects of estrogen on dendritic morphology in this region have yet to be comprehensively explored. Recent work suggests, however, that estrogen can modulate spine density in the dorsolateral prefrontal cortex (PFC) of macaque monkeys (Hao et al. 2007) as well as in the rat hippocampus (Woolley et al. 1990) and mPFC (Shors et al. 2001; Luine et al. 2006). The current study sought to add to this literature by extending the scope of analysis to the level of the circuit. Specifically, it was hypothesized that ovariectomized (OVX) female rats with estrogen treatment would demonstrate a heightened sensitivity to stress-related dendritic remodeling in mPFC neurons that project to the BLA. We report here that stress causes an unexpected increase in dendritic arborization and spine density in this subset of neurons in estrogen-treated females only, indicating that estrogen and stress can interact at the level of this circuit to produce a unique response to stress in females.
Methods
Subjects
Adult female Sprague Dawley rats (n = 46), weighing 200–250 g, were used in this study. Animals were housed at the Rockefeller University Laboratory Research Center in a normal 14:10-h light:dark cycle. All procedures were conducted during the light phase in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Rockefeller University and Mount Sinai School of Medicine.
Ovariectomy Surgery
Female rats were anesthetized with a ketamine (90 mg/kg) and xylazine (4 mg/kg) cocktail and laid on their abdomen on a heating pad covered with sterile bench coat. A dorsal incision was made into the dermal layer using surgical scissors. Through this incision, 2 incisions were made into the right and left sides of the cavity. The ovary-containing fat sacs were pulled through these incisions, and the ovaries were tied and resected using surgical scissors. The fat sacs were then returned to the cavity. Half of the animals were implanted subcutaneously under the nape of the neck with a 1-cm silastic capsule of 10% 17β-estradiol and 90% cholesterol to obtain high but physiological estrogen (E) levels, mimicking those during proestrus (McGinnis et al. 1981; Adams et al. 2001) (OVX + E), while the remaining animals received a placebo capsule containing cholesterol (OVX + veh). The wound was then closed using VetBond surgical glue (3M, St. Paul, MN). All animals received an intramuscular Buprenex injection following surgery and were monitored for healthy eating, drinking, and grooming habits. Animals were allowed to rest for 1 week before stereotaxic surgery was undertaken.
Retrograde Labeling
Stereotaxic surgery for retrograde tracer injection was done identically to that described previously (Shansky et al. 2009). Animals were anesthetized using ketamine (90 mg/kg) and xylazine (4 mg/kg). Each animal was secured in a Kopf stereotaxic apparatus (David Kopf Instruments, Tujunga, CA), the skull exposed, and bilateral holes drilled above the BLA (AP −3 mm, ML ± 5.0 mm, and DV −8.0 mm). A solution of the retrograde tracer Fast Blue (Sigma Aldrich, St Louis, MO; 5%, 200 nL) was deposited through pressure injection using a 1-μL Hamilton microsyringe (Hamilton Co, Reno, NV) held by a stereotaxic attachment. The injection was done at a speed of approximately 20 nL per minute, and the apparatus was left undisturbed for 10 min before syringe removal to allow for diffusion. The incision was sealed using VetBond, and all animals were given an intramuscular Buprenex injection following surgery and monitored for healthy behavior for several days after surgery. Animals were allowed to recover for 7 days before beginning the stress regimen.
Stress Exposure and Euthanasia
The rats in each “stress” group were placed in plastic rodent immobilization bags for 2 h/day for 10 days, as described in Vyas et al. (2002), and then returned to their home cages, while control animals remained in their home cages in a separate room. This regimen has been shown to produce dendritic remodeling in the hippocampus and amygdala comparable to 21 days of chronic restraint stress (Vyas et al. 2002). Twenty-four hours after cessation of the final stressor, animals were deeply anesthetized with 30% chloral hydrate and perfused transcardially with 1% paraformaldehyde phosphate buffer (pH 7.4), followed by 4% paraformaldehyde and 0.125% glutaraldehyde in phosphate buffer.
Tissue Preparation, Cell Loading, Reconstruction, and Analysis
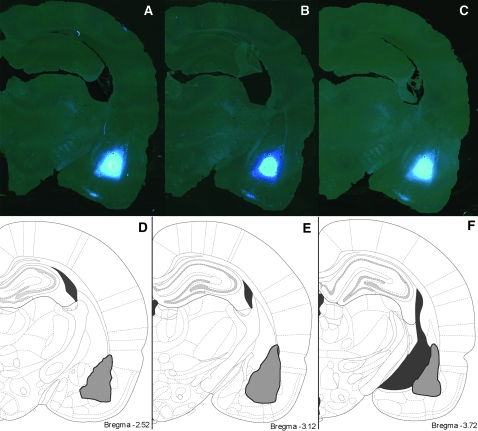
After perfusion, the brains were removed and the IL region of the mPFC was dissected (2.0–3.5 mm rostral from bregma), postfixed, and serial 250-μm-thick sections were collected using a Vibratome (Leica, Vienna, Austria). Sections were mounted on nitrocellulose filter paper and submerged in phosphate buffer. To evaluate accuracy of retrograde tracer injections, 50-μm-thick coronal sections were collected from the injection area. These sections were mounted, coverslipped, and examined using a light microscope with a ultraviolet (UV) filter. Figure 1 shows representative injection site and Fast Blue diffusion; brains in which the injection did not successfully reach the BLA were removed from the study.
Figure 1.
Retrograde tracer injection sites. Representative serial sections showing sites of retrograde tracer injection into the BLA are shown in (A–C). Schematic sections showing maximal diffusion of tracer are shown in (D–F).
In order to remain consistent with prior work (Radley et al. 2004, 2008) and as a way to compare the effects of stress in the IL-BLA pathway to its effects in other IL neurons, both labeled and unlabeled cells in each brain were loaded with Lucifer Yellow. It is of course impossible to know that every mPFC neuron that projects to the BLA was labeled, and thus, the unlabeled cells may include a few BLA-projecting neurons. However, as the IL-BLA pathway only constitutes approximately 10% of all neurons in the IL (Takagishi and Chiba 1991), it is unlikely that BLA-projecting neurons make up even a moderately substantial proportion of the unlabeled neuron population included in the study.
After neurons that did not meet the criteria for inclusion in the analysis (see below) were removed, each group included 5–8 animals and a range of 2–8 neurons per animal were analyzed (see Table 1 for cells per animal per group). As in previous studies (Radley et al. 2004, 2005, 2008), the hemisphere from which each neuron was taken was not noted.
Table 1.
Summary of number of neurons used in each experiment per experimental group
| Treatment | OVX + veh control | OVX + veh stress | OVX + E control | OVX + E stress |
| Unlabelled | ||||
| Number of animals | 8 | 7 | 8 | 7 |
| Number of neurons | 37 (5,3,4,2,5,5,6,7) | 36 (4,5,3,5,7,5,7) | 30 (5,3,2,2,3,8,4,6) | 37 (7,5,4,6,7,4,4) |
| BLA projecting | ||||
| Number of animals | 7 | 5 | 7 | 6 |
| Number of neurons | 27 (2,3,3,5,3,6,5) | 25 (3,6,6,4,6) | 33 (3,6,5,3,6,6,4) | 29 (3,8,5,4,5,4) |
Note: Numbers in parentheses represent the number of cells included from each animal in a group.
Fast Blue–labeled neurons were identified using epifluorescence with a UV filter. Both Fast Blue–labeled neurons and unlabeled neurons were loaded with iontophoretic injections of 5% Lucifer Yellow (Molecular Probes, Eugene, OR) using a DC current of 1–6 nA for 5–10 min until distal processes are filled with dye and no further loading can be observed. Sections were then mounted and coverslipped under PermaFluor (Thermo Fisher Scientific, Fremont, CA). Neurons were traced and 3-dimensionally reconstructed at ×400 using a Zeiss Axiophot 2 microscope equipped with a motorized stage, video camera system, and Neurolucida morphometry software (MBF Bioscience, Williston, VT). Criteria for a neuron to be included in analysis were as follows: 1) It must be within layer III of the IL region; 2) the dendritic tree must be completely filled; 3) it must have intact primary and secondary branches; and 4) it must have intact tertiary branches. Three-dimensional Sholl analyses (Sholl 1953) were prepared for each neuron with NeuroExplorer software (MBF Biosciences). Results were expressed in terms of total apical dendritic length, total branch number on the apical dendrite, and apical dendritic length per radial distance from the soma in 30-μm increments (Radley et al. 2004).
The method for sampling apical dendritic branches for spine density (i.e., spines per micrometer dendritic length) was performed as described previously (Radley et al. 2006). The selection of a particular branch for optical imaging had to satisfy the following criteria: 1) The entire segment had to fall within a depth of 50 μm owing to the working distance of the lens; 2) it had to be either parallel or at acute angles to the coronal surface of the section; and 3) it did not show overlap with other branches that would obscure visualization of spines. Segments were selected with a systematic random design at 50, 100, 150, and 200 μm from the soma for digital reconstruction. In total, approximately 600 reconstructed dendritic segments were analyzed (approximately 8 segments per neuron). Dendritic segment and spine reconstructions were performed using a Zeiss 410 confocal laser scanning microscope using a 488 nm excitation wavelength at a magnification of ×100 and a zoom of 5. After gain and offset settings were optimized, segments were digitally reconstructed at 0.1-μm increments throughout the entire z-axis of the branch. The digitized optical stacks were then deconvolved with AutoDeblur (v. 8.0.2; Media Cybernetics, Bethesda, MD) and analyzed for spine number and length using NeuronStudio software (Radley et al. 2008; Rodriguez et al. 2008), after which they were manually verified. Values for each branch segment were expressed as spine number per micrometer. The average dendritic segment was approximately 30 μm in length.
Statistical Analysis
The values shown in Figures 2, 3, 5 and 6 represent means ± SEM for each group. For each measure, values for each animal were averaged and then formal analyses were done by animal to ensure that each animal contributed equally to the results. Analyses for effects of stress and estrogen on weight gain, branch points, and dendritic length were performed separately for each cell group (unlabeled or BLA projecting) using 2-way analysis of variance (ANOVA) with variables of stress and estrogen. A mixed model for repeated measures ANOVA with Bonferroni post hoc test was used to test the effect of stress on dendritic length across the apical dendritic trees (Sholl analysis). Spine density analysis was done using a mixed model F-test using randomly selected data points (up to 12 observations per animal) to balance samples from each animal. N represents the number of animals for all analyses (see summary in Table 1). The statistical significance level was set at α = 0.05.
Figure 2.
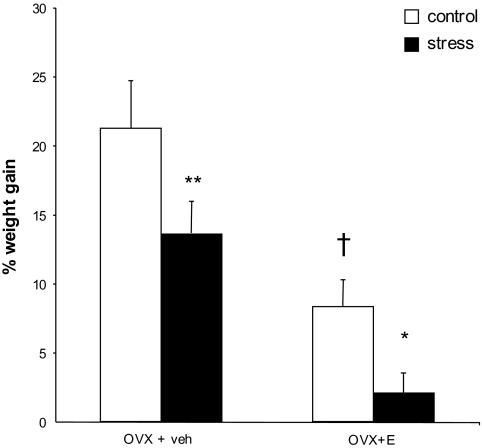
Stress reduces weight gain. Stressed animals gained less weight than their controls. Estrogen-treated animals also gained less weight than OVX + veh animals. Data are displayed as mean percent weight gain ± SEM. *P < 0.05 compared to same-group control; **P < 0.01 compared to same-group control; †P < 0.0001 compared to OVX + veh control. N = 8, 7, 8, and 7 for OVX + veh control, stress, and OVX + E control, stress, respectively.
Figure 3.
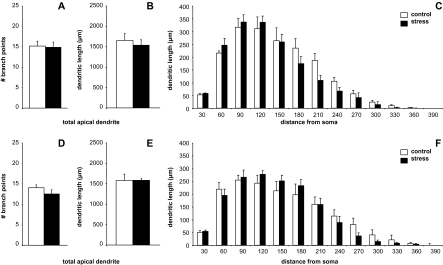
Unlabeled neurons in female IL show no stress-induced remodeling. In OVX + veh animals, neither mean branch point number (A), total dendritic length (B) nor Sholl Analysis comparisons (C) were different between control and stress groups (P > 0.05 for all comparisons). Furthermore, no stress-related changes were seen in OVX + E animals in these measures (D–F). Data are displayed as mean dendritic length ± SEM. N = 8, 7, 8, and 7 for OVX + veh control, stress, and OVX + E control, stress, respectively.
Figure 5.
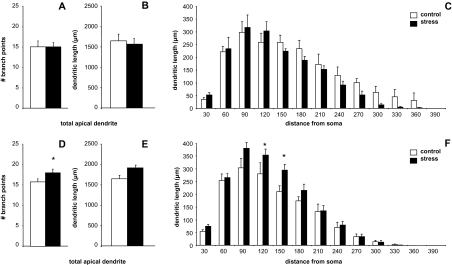
BLA-projecting IL neurons in ovariectomized animals show stress-induced remodeling in estrogen-treated females only. In OVX + veh animals, neither mean branch point number (A), total dendritic length (B) nor Sholl Analysis comparisons (C) were different between control and stress groups (P > 0.05 for all comparisons). In OVX + E animals, while stress had a significant effect on branch point number (D), apical dendrites did not show an overall stress-induced increase in dendritic length (E). However, significant changes were observed at intermediate branches (F). *P < 0.05. Data are displayed as mean dendritic length ± SEM. N = 7, 5, 7, and 6 for OVX + veh control, stress, and OVX + E control, stress, respectively.
Figure 6.
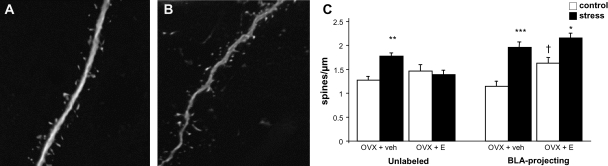
Effects of estrogen and stress on spine density. Representative deconvolved images of dendrite segments from unlabeled neurons from OVX + veh animals in control (A) and stress (B) conditions. Stress caused an increase in spine density in OVX + veh animals regardless of neuron set and an increase in spine density in BLA-projecting neurons from OVX + E rats. In addition, estrogen treatment increased spine density in BLA-projecting neurons of control animals (C). *P < 0.05 compared to same-group control; **P < 0.01 compared to same-group control; ***P < 0.001 compared to same-group control; †P < 0.05 compared to OVX + veh control animals. Data are displayed as mean spine density ± SEM. N = 3 for all groups except N = 4 for unlabeled OVX + veh, control, and stress groups.
Results
Weight Gain
A 2-way ANOVA revealed significant effects of both estrogen and stress on percent weight gain (OVX + veh control vs. stress: 21.2 ± 1.96% vs. 13.6 ± 1.46%; OVX + E control vs. stress: 8.33 ± 1.69% vs. 2.08 ± 0.93%. Effect of estrogen: F1,23 = 67.28, P < 0.0001; effect of stress: F1,23 = 21.56, P = 0.0001; Fig. 2).
Dendritic Parameters: “Unlabeled” Neurons
Mean branch points, overall apical dendritic length, and Sholl analyses are shown for OVX + veh and OVX + E groups in Figure 3A–C and D–F, respectively. Two-way ANOVAs revealed no significant effects of stress or estrogen on unlabeled neurons’ branch points or on total apical dendritic length, nor were there any estrogen–stress interactions. Bonferroni post hoc tests did not reveal any localized effects of stress at any distance from the soma in either group. N = 8, 7 and 8, 7 for OVX + veh control, stress, and OVX + E control, stress, respectively.
Dendritic Parameters: BLA-Projecting Neurons
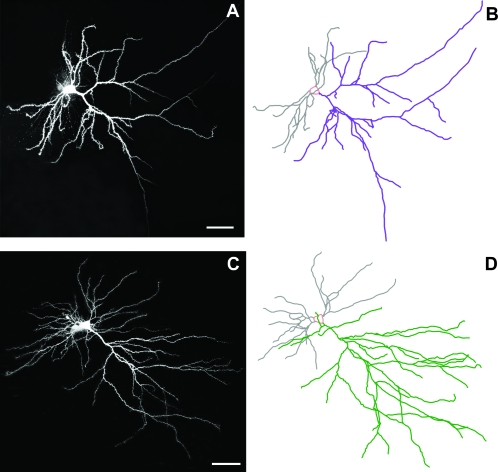
Images of representative Neurons and Neurolucida tracings of BLA-projecting IL neurons from the OVX + E control and stress groups are shown in Figure 4A and B, respectively. Mean branch points, overall apical dendritic length, and Sholl analyses are shown for OVX + veh and OVX + E groups in Figure 5C and F, respectively. Two-way ANOVAs of total branch points did not reveal a significant interaction of estrogen and stress, but Bonferroni post hoc tests showed a significant stress-related increase in branch points in OVX + E group only (control vs. stress: 15.4 ± 0.58 vs. 18.1 ± 0.93, P < 0.02). Two-way ANOVAs of total apical dendritic length did not reveal a significant interaction of estrogen and stress. However, post hoc tests revealed a near-significant effect of stress in OVX + E group only (control vs. stress: 1697.0 ± 52.9 vs. 1921.5 ± 105.0, P = 0.06), with stress causing a 13% increase in overall apical dendritic length in this group. Sholl analysis revealed no localized effects of stress for OVX + veh group, but OVX + E group showed a significant stress × distance interaction (F1,11 = 1.935, P = 0.04), with Bonferroni post hoc tests revealing significant effects of stress at 120 and 150 μm from the soma (control vs. stress: 283.9 ± 14.8 μm vs. 351.4 ± 16.8 μm, P < 0.05; 220.7 ± 7.5 μm vs. 284.2 ± 34.4 μm, P < 0.05). N = 7, 5, 7, and 6 for OVX + veh control, stress, and OVX + E control, stress, respectively.
Figure 4.
Visualization and analysis of neurons. Representative Lucifer Yellow-filled BLA-projecting neurons and corresponding Neurolucida tracings from OVX + E stressed (A and B) and control (C and D) animals. Scale bar = 50 μm.
Spine Density
Representative deconvolved images of dendrite segments from OVX + veh control and stress groups are shown in Figure 6A and B, respectively. In unlabeled neurons, a mixed model F-test revealed an overall interaction of stress and estrogen on spine density (OVX + veh control vs. stress: 1.29 ± 0.13 vs. 1.84 ± 0.13 spines per micrometer, OVX + E control vs. stress: 1.48 ± 0.15 vs. 1.41 ± 0.19 spines per micrometer; F1,9 = 7.18; P < 0.03), with stress causing a 42% increase in spine density in OVX + veh group (post hoc test, P < 0.01) but no change in OVX + E group (Fig. 5C).
In BLA-projecting neurons (Fig. 5C), F-test revealed significant effects of both estrogen and stress on spine density but no interaction (OVX + veh control vs. stress: 1.31 ± 0.16 vs. 2.20 ± 0.15 spines per micrometer, OVX + E control vs. stress: 1.71 ± 0.16 vs. 2.19 ± −0.08 spines per micrometer. Effect of estrogen, F1,8 = 5.52, P < 0.05; effect of stress, F1,8 = 10.56, P = 0.01). Post hoc tests revealed significant effects of stress in both OVX + veh (69% increase in spine density, P < 0.001) and OVX + E (29% increase in spine density, P < 0.05) groups as well as a significant effect of estrogen in control animals (31% increase in spine density, P < 0.05).
In control animals, no differences were seen between BLA-projecting and unlabeled neurons in any of the parameters (F1,64 = 0.005, P = 0.942), suggesting that there are no underlying baseline morphological differences between these 2 neuron populations. A summary of all findings combined with recent findings in male rats (Shansky et al. 2009) is shown in Table 2.
Table 2.
Summary of morphological effects of stress effects in IL neurons in males and OVX + veh and OVX + E animals
| Unlabeled neurons |
BLA-projecting neurons |
|||||
| Males | OVX + veh | OVX + E | Males | OVX + veh | OVX + E | |
| Dendrites | Retraction | No change | No change | No change | No change | Expansion |
| Spine density | No change | Increase | No change | No change | Increase | Increase |
Note: In both unlabeled and BLA-projecting neurons, the morphological effects of stress differ between males and females, suggesting both sex- and hormone-regulated responses to stress
The neurons used in the spine analysis represent a subset of the total neurons used for the dendritic arbor analyses, and thus, there is a smaller number (3–4 animals per group). However, the results are in many cases highly significant.
Discussion
The current studies reveal several novel findings. Most notably, this is the first report of morphological effects of stress in the IL of female rats. We have recently reported that in male rats, unlabeled IL neurons show robust dendritic retraction with stress (Shansky et al. 2009), while BLA-projecting IL neurons are unaffected by stress. In contrast, both OVX + veh and OVX + E females showed no remodeling in unlabeled neurons, suggesting that overall, there may be a fundamental sex-determined resistance to stress-related dendritic changes. In BLA-projecting neurons, however, OVX + veh animals again showed no stress-related dendritic retraction, but OVX + E animals showed stress-induced dendritic “growth.” Thus, although estrogen alone did not affect dendritic arborization, estrogen in combination with stress led to a significant change in branching. These results suggest that although most IL neurons may be protected from stress-related remodeling in females, estrogen may render the IL-BLA pathway particularly sensitive to stress (for comparison with males, see Table 2).
These studies also confirm previous findings that estrogen treatment can restore mPFC dendritic spine loss observed after OVX (Luine et al. 2006). In the present studies, this was found to be the case only in BLA-projecting neurons. Although control estrogen-treated unlabeled neurons had, on average, 16% greater spine density than OVX + veh controls, this difference was not significant. However, in both neuron sets, it was found that stress could restore spine density in OVX + veh animals to the same levels as with estrogen treatment. Finally, OVX + E animals were again observed to have a circuit-specific response to stress: that is, spine density in BLA-projecting neurons (but not unlabeled neurons) “increased” with stress. This neuronal population then not only has a more complex dendritic arbor but also more spines on these dendrites, suggesting a robust increase in spines per neuron.
Together, these data indicate that stress affects brain morphology with remarkable specificity, causing remodeling in some, but not all, neurons and interacting with ovarian hormones to produce a distinct morphological profile in the IL of females. Moreover, that estrogen effects were seen only in BLA-projecting neurons suggests that the neurons in this pathway are particularly sensitive to estrogen’s actions. Although how these effects might contribute to the development of behavioral pathology remains to be determined, the presence of sex differences in these processes is a provoking addition to stress literature in males and females.
Estrogen, Stress, and Neuronal Morphology
As described above, estrogen is a potent modulator of structural changes in neurons. It has been demonstrated in both nonhuman primates and rodents that estrogen treatment in OVX animals increases spine and synapse density in the CA1 region of the hippocampus (Adams et al. 2001; Hao et al. 2003; reviewed in Woolley 1998), an effect that has also been demonstrated in the monkey dorsolateral PFC (Tang et al. 2004; Hao et al. 2007). Further support is derived from evidence that hippocampal spine density is highest during proestrus and then drops substantially during estrus (Woolley et al. 1990; reviewed in McEwen 2002). Although the estrus cycle has been reported to have no effect on spine density or dendritic arborization in the ACg, both parameters were significantly higher in young male rats than in female rats (Markham and Juraska 2002), suggesting overall sex differences and hence possible organizational effects of estrogen during development.
Estrogen–stress interactions have been explored experimentally on many levels, with varying, and at times conflicting, results. Behavioral results appear to be task specific. For example, animals with high estrogen levels (either during proestrus or OVX + E) have been shown to be particularly sensitive to acute stress-induced impairments in working memory (Shansky et al. 2004) and eyeblink conditioning (Wood and Shors 1998) but resistant to the effects of stress on hippocampus-dependent tasks like the Y-maze and radial arm maze (Bowman et al. 2002; Conrad et al. 2004). From a morphological standpoint, females have been shown to be protected from the CA3 apical dendritic remodeling seen in males after exposure to chronic stress (Galea et al. 1997), consistent with the observed lack of effect on spatial memory in females.
We have recently observed that while male rats exhibit dendritic retraction in the IL with exposure to stress, the BLA-projecting neurons in this region are resilient against this effect, suggesting that this pathway is protected from at least one of the consequences of repeated stress (Shansky et al. 2009). Our estrogen-treated females showed the exact opposite pattern, appearing resistant to stress-induced dendritic changes in general but sensitive to the effects of stress in BLA-projecting neurons. Thus, there are sex differences in the effects of stress in the mPFC as in the hippocampus, although the direction of these differences varies depending on brain region. These diverging effects of stress in males and females may hold important implications for the nature of sex differences in stress-related mental illnesses, but the functional consequences of these morphological changes have yet to be thoroughly investigated.
The current observation of stress-induced increase in dendritic arbor and spine density in estrogen-treated BLA-projecting IL neurons is the first report of IL dendritic expansion with stress, and the implications of such an effect are unclear. Conventional thinking would seem to dictate that retraction and spine loss be regarded as maladaptive responses; insofar as stress-induced neuronal atrophy in hippocampus or mPFC (in male rats) is accompanied by a corresponding impaired functioning of each area (Luine et al. 1994; Conrad 2006; Cerqueira et al. 2007). An increase in branching could thus be envisioned as an adaptive mechanism, in that greater arborization might enhance connectivity and function. However, this is not necessarily the case, nor is it consistent with reports from the human population that stress-related disorders are more prevalent in women than in men (Weissman et al. 1996). Hypertrophy may render neurons vulnerable to overstimulation and thus to dysfunction or death. Since excess catecholamine activity in the mPFC can cause working memory impairments (Arnsten 1997), females’ sensitivity to stress may be in part due to a stress-induced increase in receptivity to catecholamine stimulation. This possibility will be investigated in future studies.
Functional Implications of Circuit-Specific Stress Effects
The mPFC–amygdala connections are well defined (McDonald et al. 1996) and have been shown in physiological studies to be functionally significant, most often reported in the context of fear conditioning (Garcia et al. 1999; Rosenkranz et al. 2003). While the amygdala is crucial in mediating the acquisition and expression of conditioned fear, as well as the acquisition of extinction (Kapp et al. 1979; Iwata et al. 1986; Rogan et al. 1997), the mPFC—and particularly the IL—has been shown in both lesion and physiology studies to be necessary for the “retrieval” of extinction (Morgan et al. 1993; Milad and Quirk 2002; Lebron et al. 2004), consistent with its role in suppressing inappropriate responses. Additional support is drawn from reports that mPFC stimulation results in reduced amygdala response to a conditioned stimulus (Quirk et al. 2003; Rosenkranz et al. 2003).
To date, these physiological studies have not been performed in female rats, and the limited work that has been done with female rats in fear conditioning paradigms is inconsistent in their findings. Sex differences have been reported in the acquisition (Maren et al. 1994) and extinction (Gupta et al. 2001) of contextual fear, but the role of estrogen in mediating these differences is unclear. It has recently been observed that estrogen-treated females are impaired at extinguishing a previously conditioned fear response (Toufexis et al. 2007), an effect that has also been reported in cycling women (Milad et al. 2006). Whether this modulation of emotional learning also occurs with respect to retrieval of extinguished fear remains to be thoroughly tested. Such investigations will make an important contribution to the understanding of estrogen’s modulation of this pathway and thus its potential for mediating sex differences in the development of stress-related disorders.
Funding
National Institutes of Health (MH58911 to J.H.M., P.R.H., and B.S.M. and MH075506 to R.M.S.).
Acknowledgments
The authors would like to thank William Janssen, Deena Goldwater, Bridget Wicinski, Melinda Miller, and Gwendolyn Wood for contributing their knowledge and technical assistance and Alfredo Rodriguez, Douglas Ehlenberger, and Dr Susan L. Wearne for developing and providing access to the NeuronStudio software. Conflict of Interest: None declared.
References
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci USA. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacology. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, Meltzer H. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Int Rev Psychiatry. 2003;15:74–83. doi: 10.1080/0954026021000045976. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol, Biochem Behav. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, et al. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, Malach R, Bleich A. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr. 2003;8:641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23:579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Steroid receptor levels in intact and ovariectomized estrogen-treated rats: an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981;33:158–165. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Masuda J, Kimura F. Sex differences in the stress-induced release of acetylcholine in the hippocampus and corticosterone from the adrenal cortex in rats. Neuroendocrinology. 2003;78:234–240. doi: 10.1159/000073707. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:325. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS ONE. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AFT. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl D. Dendritic organization of the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neuroscience. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, et al. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]