Abstract
Analogical reasoning is central to learning and abstract thinking. It involves using a more familiar situation (source) to make inferences about a less familiar situation (target). According to the predominant cognitive models, analogical reasoning includes 1) generation of structured mental representations and 2) mapping based on structural similarities between them. This study used functional magnetic resonance imaging to specify the role of rostral prefrontal cortex (PFC) in these distinct processes. An experimental paradigm was designed that enabled differentiation between these processes, by temporal separation of the presentation of the source and the target. Within rostral PFC, a lateral subregion was activated by analogy task both during study of the source (before the source could be compared with a target) and when the target appeared. This may suggest that this subregion supports fundamental analogy processes such as generating structured representations of stimuli but is not specific to one particular processing stage. By contrast, a dorsomedial subregion of rostral PFC showed an interaction between task (analogy vs. control) and period (more activated when the target appeared). We propose that this region is involved in comparison or mapping processes. These results add to the growing evidence for functional differentiation between rostral PFC subregions.
Keywords: analogy, BA 10, fMRI, reasoning, rostral prefrontal
Introduction
“We are living in a world of perpetual novelty, in which no experience is ever exactly repeated. Yet paradoxically, we don't feel that each situation is new, often finding it familiar, and overall we are able to adapt our behavior to each one.” (Holyoak and Thagard 1997). This ability to adapt our behavior relies on 2 fundamental (and interacting) properties of the human brain: the capacity to build and maintain mental representations that guide our actions and the capacity to find similarities between these distinct representations, for example, between past experiences and the present situation. This ability to find similarities between distinct situations or sets of stimuli, though they are not identical, is a key component of analogical reasoning.
The use of analogical reasoning in human behavior is fundamental. Children learn new words and concepts by analogy to ones previously learned. Adults use analogies to understand, explain, or create abstract ideas and concepts. Analogical reasoning is thus central for learning and abstract thinking (Gentner 1983; Kotovsky and Gentner 1996; Gentner and Holyoak 1997; Holyoak and Thagard 1997), to solve new problems and to make predictions, to adapt our behavior to changing situations, and for creative thinking (Holyoak and Kroger 1995; French 2002; Bowdle and Gentner 2005; Geake and Hansen 2005). In this context, understanding analogical reasoning and its neural correlates has implications in understanding higher level cognitive functions and human behavioral adaptation, particularly in the fields of medicine, education and communication (Gentner and Holyoak 1997; Kolodner 1997).
In analogical reasoning, a more familiar situation (called the source) is used to make inferences about a less familiar situation (called the target). But also, establishing an analogy between 2 situations can give rise to a general schema or abstract representation that encompasses both (Gentner 1983; Gentner et al. 1993; Gentner and Markman 1997; Holyoak and Thagard 1997; Gentner and Medina 1998; Blanchette and Dunbar 2000). But how is such an analogy established? According to the predominant cognitive models, the “structure mapping theory” (Gentner 1983; Gentner et al. 1993; Gentner and Holyoak 1997) and the “multiconstraint theory” (Holyoak and Thagard 1995, 1997), analogy depends on mapping elements of the source and target. The mapping takes place not only between elements but also between relations linking these elements. In fact, these relations describe particular aspects of the “structure” of an object/situation (or how the elements are organized in the object/situation). In other words, the mapping is based on relational or structural similarity (Gentner et al. 1993; Holyoak and Thagard 1995; Gentner and Markman 1997). In this respect, analogical reasoning is a form of “relational reasoning.” Thus, analogical thinking includes conceptualization and abstract thought because of the need to generate mental representations of the structure. And it includes mapping based on relational or structural similarities (Blanchette and Dunbar 2000; Markman and Gentner 2000).
Analogical reasoning has been extensively studied in experimental psychology (Gentner 1983; Gentner et al. 1993; Kotovsky and Gentner 1996; Gentner and Holyoak 1997; Gentner and Markman 1997; Holyoak and Thagard 1997; Gentner and Medina 1998; Blanchette and Dunbar 2000; Markman and Gentner 2000; Spellman et al. 2001; Bowdle and Gentner 2005) and computational modeling (Jani and Levine 2000; French 2002), but its cerebral basis is poorly understood. The small number of imaging studies that have explored analogical reasoning have pointed to one region in particular, located in the rostral prefrontal cortex (rostral PFC), approximately Brodmann area 10 (BA10), as playing a central role in analogical reasoning and also in complex relational reasoning (Prabhakaran et al. 1997; Waltz et al. 1999; Wharton et al. 2000; Christoff et al. 2001; Kroger et al. 2002; Luo et al. 2003; Bunge, Wendelken et al. 2005; Geake and Hansen 2005; Green et al. 2006, 2008, 2010; Wendelken, Nakhabenko et al. 2008; Bunge et al. 2009; Cho et al. 2009). Studies in children have shown that the development of these abilities is associated with the maturation of this region (Dumontheil et al. 2008; Crone et al. 2009).
In other domains, functional imaging studies show that hemodynamic changes in BA10 can occur not only in many different cognitive paradigms involving high-level cognition, such as memory (Christoff and Gabrieli 2000; Simons et al. 2005, 2006; Gilbert, Spengler, Simons, Steele et al. 2006), prospective memory (Burgess et al. 2000, 2001), multitasking and task switching (Koechlin et al. 1999; Burgess et al. 2000; Braver and Bongiolatti 2002; Koechlin and Hyafil 2007), relational integration (Christoff et al. 2001; Kroger et al. 2002; Reynolds et al. 2006; De Pisapia and Braver 2008), theory of mind (Frith and Frith 2006) but also in simpler attentional tasks and even during “rest” (Gusnard and Raichle 2001; Raichle et al. 2001; Christoff et al. 2004; Mason et al. 2007). General theories about the functional role of the rostral PFC have been drawn recently from these multiple results. Most of these theories focus on the critical component that is involved in all the tasks activating rostral PFC (Bunge 2004; Courtney 2004; Ramnani and Owen 2004; Reynolds et al. 2006; Badre 2008). For some authors, the rostral PFC is associated with the processing of abstract information (Christoff et al. 2003; Christoff and Keramatian 2006) or more generally in biasing attention toward stimulus-oriented or stimulus-independent thoughts (Burgess et al. 2005, 2006; Gilbert et al. 2005; Burgess, Dumontheil, and Gilbert 2007; Burgess, Gilbert, and Dumontheil 2007; Gilbert et al. 2007). For others, rostral PFC is involved in coordinating a main goal with different subgoals or outcomes (Koechlin et al. 1999; Braver and Bongiolatti 2002; Ramnani and Owen 2004) or in relational integration (Christoff et al. 2001; Kroger et al. 2002; Reynolds et al. 2006; De Pisapia and Braver 2008). However, the large size of the rostral PFC and its activation in various studies suggest that it may be heterogeneous and involved in different processes. Indeed, a recent meta-analysis from Gilbert, Spengler, Simons, Steele et al. (2006) provided strong evidence for functional specialization within BA10.
The aim of this study was to investigate the role of the rostral PFC in distinct processes involved in analogical reasoning. Considering the predominant cognitive models (Gentner 1983; Holyoak and Thagard 1995), we explored the following questions, equally consistent with recent theories on BA10 functions: Is activation in the rostral PFC associated with the generation of an abstract representation of the relations between objects or is it associated with the mapping of different representations? Further, are these distinct processes supported by different rostral prefrontal subregions?
In order to answer these questions, we devised a novel analogy paradigm that allowed us to isolate, for the first time, the processes involved in mapping from those involved in self-generation of relational representations. For this purpose, we used 2 experimental manipulations. First, we compared an analogy task with a control task in which subjects had to match the attributes of the stimuli without generating structured representations. This comparison allowed identification of the network involved in generating and processing these representations. Second, we separated in time the presentation of the source from the presentation of the target. Thus, the comparison of the source and the target period of the analogy task allowed us to isolate the mapping processes, which only take place during target presentation, whereas generation of structured representations takes place in the source period as well.
Materials and Methods
Subjects
Sixteen right-handed healthy volunteers (6 men and 10 females; ages 22–34, mean 26.5) participated in this study. The experiment was approved by the local research ethics committee. All subjects provided written informed consent. Participants were paid £15 as approved by the local research ethics committee. No participant had a significant history of neurological or psychiatric disorders nor was he/she taking psychoactive prescription medication.
Experimental Paradigm
General Principles
Participants performed a series of trials during 4 blocks of 12 minutes each in the magnetic resonance imaging (MRI) scanner (total duration of 1 h, including functional and structural imaging), preceded by 15 min of explanation and training outside the MRI scanner and followed by 15 min of written debriefing (Fig. 1).
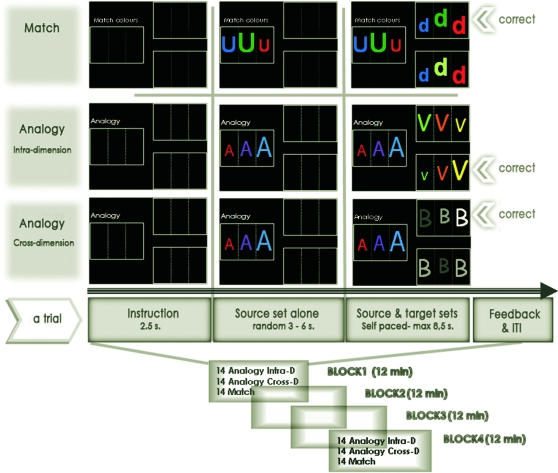
Figure 1.
Experimental design and tasks. Experimental design consisted of 2 different tasks: Match and Analogy, with the latter subdivided into crossdimension and intradimension conditions.
The tasks required participants to make comparisons between sets of stimuli and to choose which sets matched, depending on a written instruction displayed with the sets. The instruction indicated that participants should perform either analogical judgment (“analogy” task) or identity judgment (“match” task). On each trial, participants were presented with a source set and 2 target sets. Subjects were asked to decide which of the 2 target sets went with the source set. The stimuli we used for both tasks were sets of 2 or 3 letters, varying in different dimensions: size, color, texture, orientation, number, or identity. The analogy and match tasks were equated in terms of visual and temporal features.
Examining the Self-Generation of Representations with 2 Experimental Conditions: Analogy and Match
The match task required participants to judge if 2 different sets shared a particular attribute. These sets were not identical in all respects, but the instruction told the participants which attribute was relevant. Thus, there were different instructions for this task: “Match letters?” (match the identity of the letters), “Match figures?” (match the identity of the written numbers), “Match colors?,” “Match sizes?,” “Match numbers?” (match the quantity of stimuli), and “Match texture?” (match the filling of the stimuli). Thus, in the match task, participants' decisions were based on perceptual attributes of the external stimuli.
The analogy task required participants to find similarities between the structures (relationships between the constitutive stimuli) of 2 sets of stimuli. In contrast with the match task, the similarity between 2 sets did not concern a common attribute but rather a common organization—the structure—of the stimuli. Thus, in the analogy task, the decision was based on a self-generated internal representation of this structure.
In total, there were 7 different “rules” to discover, which were not used during the training. These rules were either visuospatial or mathematical. They could be verbalized as “linear increase of a feature of the 3 stimuli in the set,” “symmetry around the stimulus in the middle,” “mirror image,” “the first stimulus is different from the 2 others,” “the first plus the second gives the third stimulus,” “the first minus the second gives the third stimulus,” “the last is a multiple of the first.” Each “rule” was presented 4 times in each block and was repeated with different stimuli from block to block. There were 2 types of analogy task that used the same rules: intradimension and crossdimension analogies (see Fig. 1). In intradimension task, analogy concerned the same dimension in the source and target (e.g., increase in size of the stimuli in both the source and the target). In the crossdimension task, analogy concerned different dimensions (for instance, increase in size of the stimuli in the source and increase in color lightness of the target stimuli).
During the functional magnetic resonance imaging (fMRI) session, 14 crossdimension analogy trials, 14 intradimension analogy trials, and 14 match trials were randomly presented during each of 4 blocks.
Isolation of the Mapping Processes: Separation in Time within a Trial
Each trial began with a written instruction, followed by the display of the source set of letters alone. Following a delay of random duration (3–6 s.), 2 different target sets additionally appeared (one on the lower part, the other on the upper part of the right side of the screen). The participant then had up to 8.5 s to choose the target set that matched the source, by pressing the lower or the upper button of a response keypad held in the right hand. The button press triggered the disappearance of the sets, and the display of immediate visual feedback (random duration: 0.3–0.7 s), in the form of a green circle for correct responses and a red circle for errors. Button press and feedback constituted the response period. Immediately after this feedback, an intertrial interval (ITI) with a blank screen was presented. Its duration was automatically generated by subtracting the reaction time from a pseudorandom duration between 8.5 and 10.5 s. This allowed us to equalize mean trials durations by compensating for variations in response time across trials.
With this experimental design, subjects could not compare the source and target sets until the target set appeared. Thus, the mapping processes only took place during the target period. Before the onset of the target display (i.e., during the source period), they could only analyze the source set and generate mental representations about its structure. Thus, the separation in time of the source and the target display allowed isolation of the mapping process (only in target period) from the formation of relational representations (in both periods). However, although we modeled these periods separately and jittered durations, the target may not be fully independent from the source, because source and target always and necessarily appear in this same successive order. This is inherent of an analogy task, and altering this sequence would have engaged other cognitive processes and altered the validity of inferences. Conscious of this limitation, we did not directly compare source and target periods but only looked at task effects and at interactions between tasks and periods.
Debriefing and Evaluation
After the scanning, participants were asked to fill out a questionnaire and to perform another short task called the “evaluation task.” The questionnaire assessed the conscious strategies used by the subjects in each task and period to evaluate the difficulty of the tasks. They could also provide free comments about the experiment. The evaluation task aimed to assess whether participants were able to recognize the rules that they encountered in the analogy tasks. This part involved presenting single sets of 2 or 3 stimuli, as in the source period of the analogy task but without presenting a forthcoming target. The participants had to describe the rule underlying the organization of the stimuli in the set. They were told that this task concerned the same rules as those used in the fMRI tasks. All participants but one performed this evaluation test. To allow evaluation of whether the rules had been learned during the experiment, we also submitted this task to another group of 12 healthy volunteers who did not perform the analogy tasks.
fMRI Scan Acquisition
Visual stimuli were generated by a PC computer and projected using an active matrix video projector onto a screen positioned at the foot end of the MRI scanner bore. Participants viewed the screen through a mirror mounted on the head coil. Subjects' head motion was restricted by using cushions. Responses were given using an MRI-compatible keypad, and accuracy and response times were recorded.
Whole-brain gradient echo planar MR images were acquired using a Siemens TIM Avanto 1.5-T MRI scanner at the Birkbeck-UCL Centre for NeuroImaging, London. Functional and structural images were acquired during the same session. Functional images were T2-weighted echo planar images (a 64 × 64 matrix; 3.5 × 3.5 × 3.5-mm voxels; time echo [TE]: 40 ms; time repetition [TR]: 2500 ms; flip angle: 90) sensitive to blood oxygen level–dependent (BOLD) contrast. Each volume comprised 33 interleaved axial slices aligned to the AC–PC plane, allowing coverage of most of the brain, except for the cerebellum and inferior aspects of the occipital lobes. For each of the 4 blocks of the experiment, 288 volumes were acquired, lasting 12 min. The first 3 volumes were discarded to allow for signal equilibration effects. Structural scans were obtained with a 3D T1-weighted sequence, composed of 160 slices of 1-mm thickness with an in-plane resolution of 1 × 1 mm (256 × 224 matrix; TE: 5.6 ms; TR: 120 ms; and flip angle: 19).
Behavioral Analysis
Accuracy and response times were analyzed for all conditions (i.e., match task, intradimension, and crossdimension analogy tasks). Statistical analyses used paired t-tests to compare conditions. For the evaluation phase, the number of rules that were discovered by the fMRI group after the fMRI session was compared with another group of subjects who did not perform the experiment before, using a t-test.
fMRI Analysis
fMRI data were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). The preprocessing of functional and structural volumes was performed using the default parameters of SPM5. Functional volumes were realigned, coregistered with the structural images, normalized into the Montreal Neurological Institute (MNI) template, using 2 × 2 × 2 voxels, and smoothed with an isotropic 8-mm full-width, half-maximum Gaussian kernel.
The variance in BOLD signal of the time series was decomposed in a general linear model (Friston et al. 1995). Activation associated transiently with different stimuli was modeled using delta functions convolved with a canonical hemodynamic response function. Subject-specific models were constructed using separate regressors for each condition (match, crossdimension, and intradimension tasks, restricted to correct trials only). Each condition was modeled with 3 regressors coding for each period of the trial (source alone, source and target, and response), yielding a total of 9 regressors of interest. Three additional regressors (one for each period of a trial) coded for analogy trials where an error was made. These unsuccessful trials were modeled but not analyzed. Error trials in the match condition were not modeled because they were too rare (mean error rate: 5.5%). Another regressor was used to model the instruction period, regardless of the forthcoming task. In addition to these 13 regressors of interest, the full model included residual movement parameters and the constant mean over scans. A temporal cut-off of 128 s was applied to filter low-frequency drift. Parameter estimates for each regressor were calculated from the least-mean-squares fit of the model to the data.
Whole-Brain Analysis
To test hypotheses about regionally specific condition effects, parameter estimates were compared using linear contrasts. Separate analyses of signal change were performed for the different periods of the tasks (Friston 1997). Specifically, the analyses focused on signal change during source and target periods in analogy and match conditions. Effects of interest were then assessed at the second level in a random effects analysis. Group analyses were performed using repeated-measures analysis of variance (ANOVA) on the contrast images resulting from individual analysis (Friston 2002). ANOVA was performed to analyze not only the task effects but also the interaction between tasks and periods (source and target). Statistical maps were analyzed at an uncorrected threshold of P < 0.001 with a cluster size of 10 contiguous voxels and at a familywise error (FWE)-corrected threshold of P < 0.05.
Region of Interest (ROI) Analyses
Two sets of ROI analyses were performed. The first set was performed on ROIs identified in the whole-brain analysis. Firstly, it was aimed to investigate whether the observed differences in activation between analogy and match tasks were associated with differences in reaction times between the 2 tasks. In this set of analyses, ROIs were defined as spheres of 8-mm radius, centered on the BA10 activation peaks from the whole-brain contrasts. Parameter estimates for each regressor of interest were extracted using MARSBAR (http://marsbar.sourceforge.net/), separately for each subject. In these ROIs, we correlated the difference between mean analogy and match estimates with the difference in mean reaction times between these 2 tasks, using correlation matrices in Statistica software (STATISTICA 5.5A© StatSoft). In addition, we performed the independent comparison of crossdimension versus intradimension analogy tasks, using repeated-measures ANOVAs in Statistica software (STATISTICA 5.5A© StatSoft).
For further analyses, a second set of ROIs was defined independently from the whole-brain contrasts, in order to ensure unbiased results. Firstly, a lateral and a medial ROI were defined by averaging published MNI coordinates of rostral prefrontal activation peaks in previous analogy or relational reasoning studies. Studies were identified by searches in the PubMed database for articles that included the word ‘‘fMRI’’ along with at least one of the phrases: ‘‘analogy,’’ “analogical reasoning,” ‘‘relational reasoning,’’ or “relation integration.” Studies were included if they investigated healthy young adults, and if they reported one or more activations with peak coordinates falling within BA10, when comparing analogy or relational reasoning tasks with a control task. Of the 10 studies that fulfilled these criteria, coordinates of the most significant peak of activation within lateral and/or medial BA10 were collected, yielding a total of 8 maxima in lateral BA10 (Christoff et al. 2001; Ruff et al. 2003; Bunge, Wendelken et al. 2005; Geake and Hansen 2005; Wendelken, Nakhabenko et al. 2008; Bunge et al. 2009; Cho et al. 2009; Wendelken and Bunge 2009) and 4 in medial BA10 (Ruff et al. 2003; Green et al. 2006, 2010; Wendelken, Nakhabenko et al. 2008). Each activation peak was classified as lateral or medial depending on whether its absolute × coordinate was greater or less than the mean × coordinate of all activation peaks. Coordinates reported in Talairach space were transformed into MNI space (www.mrc-cbu.cam.ac.uk/Imaging) so that all coordinates were in a common stereotaxic framework. The mean coordinates for lateral BA10 were |x| = 39, y = 50, and z = 2, and those for the medial BA10 were |x| = 8, y = 59, and z = 19. They were used as centers of 8-mm radius spheres to define “lateral” and “dorsomedial” ROIs in both hemispheres. Superimposed on a standard template brain (ch2.nii MRI template) provided by MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/) in MNI coordinates, the lateral ROI felt into the anterior part of the middle frontal gyrus (MFG), on the border of the superior frontal sulcus and next to the frontopolar gyrus. The dorsomedial ROI was located in the medial part of the superior frontal gyrus (SFG)/frontopolar gyrus.
Secondly, although in the analogy task responses are based on self-generated and stimulus-independent representations, they are based on external stimuli in the match task. Burgess and Gilbert (Burgess et al. 2005, 2006; Gilbert et al. 2005; Gilbert, Simons, et al. 2006; Gilbert, Spengler, Simons, Frith, and Burgess 2006; Gilbert, Spengler, Simons, Steele, et al. 2006; Burgess, Dumontheil, and Gilbert 2007; Burgess, Gilbert, and Dumontheil 2007) recently demonstrated that medial rostral PFC is activated during conditions of stimulus-oriented processing when contrasted to stimulus-independent processing. As a consequence, a further ROI was defined from a previous study published by Gilbert, Simons, et al. (2006), when contrasting stimulus-oriented versus stimulus-independent thoughts and which MNI coordinates are x = 0, y = 48, and z = −16. The third ROI of this analysis, the “ventromedial” ROI was thus defined as an 8-mm sphere centered on these coordinates. This ventromedial ROI was located in the orbitofrontal cortex, rectus gyrus.
For this set of analyses, subjects' parameter estimates for each regressor were extracted using MARSBAR (http://marsbar.sourceforge.net/), from the 5 ROIs described above, respectively, the left and right lateral ROIs, the left and right dorsomedial ROIs, and the ventromedial ROI. In each ROI, the means across subjects were plotted separately for each task and each period of the trials (source and target periods). We then used repeated-measures ANOVAs in Statistica software (STATISTICA 5.5A© StatSoft) to test for “within-ROI” task by period interactions, and “between-ROI” region by task and region by period interactions.
Results
Behavioral Results
Both analogy and match tasks were performed with a high degree of accuracy (80% vs. 94.5% correct, respectively), with lower accuracy in the analogy task (paired t-test: t(15) = 8.4; P < 0.001). Within analogy task, accuracy was lower in crossdimension (73.5%) than in intradimension (86.5%) tasks (paired t-test: t(15) = 5.2; P < 0.001). Reaction times were also significantly different between analogy and match tasks (3530 ± 510 and 2205 ± 449 ms, respectively; paired t-test: t(15) = 14.4; P < 0.001), as well as between crossdimension and intradimension tasks (3771 ± 583 and 3327 ± 490 ms; paired t-test: t(15) = 6.0; P < 0.001). Both analogy tasks were significantly different from the match task in accuracy (paired t-test crossdimension vs. match: t(15) = 7.7; P < 0.001; paired t-test intradimension vs. match: t(15) = −6.3; P < 0.001) and reaction times (paired t-test crossdimension vs. match: t(15) = 13.5; P < 0.001; paired t-test intradimension vs. match: t(15) = 14.4; P < 0.001).
The debriefing task showed that abstract rules were well learned and recognized by the subjects (mean 22.8 ± 3.34 SD of 28 rules recognized in the test group vs. mean 15.2 ± 4.87 SD in the naïve group who had not encountered the analogy task before). A t-test showed a significant difference between the 2 groups (t(25) = 4.9; P < 0.001). This confirmed that participants learned explicit representations of the rules during the fMRI experiment.
fMRI Results
In order to examine the involvement of BA10 in distinct analogy processes, we looked for brain regions
1) That showed greater BOLD signal change for analogy than match tasks. This would suggest involvement in generating and organizing relational abstract representations.
2) That exhibited a greater difference between target and source for analogy than match task. Assuming that self-generated relational representations may be needed in both the source and the target period, the mapping processes could be isolated by subtracting the source activation from the target activation. A greater BOLD signal difference between target and source for the analogy than match task would more specifically reveal the neural correlates of mapping.
We also looked for regions more activated during the match compared with the analogy task, as rostral PFC has also previously been differentially associated with stimulus-oriented versus stimulus-independent processing (Gilbert et al. 2005; Gilbert, Simons et al. 2006), which is relevant to our task.
1) Lateral rostral PFC BOLD signal increases from the source period during the analogy task (Table 1; Fig. 2a)
Table 1.
MNI coordinates of significant cluster maxima (the most significant maxima are given for each gyrus and BA) in the group analysis for the task effect (analogy vs. match across periods, analogy vs. match during the source period only, and analogy vs. match during the target period only)
| Anatomical regions | BA | Left coordinates | Z scores (n voxels) | P values | Right coordinates | Z scores (n voxels) | P values |
| Analogy versus match tasks | |||||||
| MFG rostral | 10/46 | −44, 50, −4 | 5.12 (364) | <0.001*,** | |||
| MFG rostral | 47/10 | −34, 54, −6 | 4.03 (364) | <0.001* | |||
| MFG | 46 | −46, 44, 10 | 4.20 (364) | <0.001* | 44, 42, 30 | 3.56 (68) | <0.001 |
| MFG | 9 | −48, 14, 48 | 4.74 (387) | <0.001*,** | |||
| MFG | 8 | ||||||
| IFG | 44 | −52, 22, 36 | 4.28 (387) | <0.001* | 62, 16, 16 | 3.41 (11) | <0.001 |
| IFG | 45 | 52, 32, 28 | 4.61 (68) | <0.001 | |||
| IFG | 47 | −34, 32, −2 | 3.39 (16) | <0.001 | 32, 26, −4 | 4.07 (48) | <0.001 |
| 32, 46, −12 | 3.53 (38) | <0.001 | |||||
| AG | 40 | −28, −50, 30 | 4.97 (1303) | <0.001*,** | 26, −46, 32 | 4.68 (928) | <0.001* |
| IPL | 40 | −44, −50, 52 | 4.80 (1303) | <0.001*,** | 40, −52, 48 | 4.83 (928) | <0.001* |
| SPL | 7 | 24, −72, 56 | 4.65 (928) | <0.001* | |||
| LG | 19 | −26, −72, 2 | 5.58 (251) | <0.001*,** | 20, −76, 2 | 5.51 (1403) | <0.001*,** |
| MTG | 21 | −60, −48, −4 | 3.33 (350) | <0.001* | |||
| MTG | 37 | −34, −54, 0 | 3.79 (57) | <0.001 | |||
| ITG | 37/20 | −54, −56, −14 | 4.81 (350) | <0.001*,** | 56, −38, −12 | 4.03 (65) | <0.001 |
| Cuneus | 18 | 10, −92, 26 | 4.68 (1403) | <0.001* | |||
| Caudate | 18, −8, 18 | 3.67 (27) | <0.001 | ||||
| Thalamus | 0, −16, 10 | 3.95 (77) | <0.001 | ||||
| Source analogy versus source match | |||||||
| MFG rostral | 10/46 | −48, 46, 0 | 4.69 (264) | <0.001* | |||
| MFG rostral | 47/11 | 34, 44, −12 | 3.64 (21) | <0.001 | |||
| IFG | 47 | −30, 24, −4 | 4.02 (44) | <0.001 | 32, 28, −4 | 5.33 (110) | <0.001** |
| IFG | 6/44 | −42, 4, 30 | 5.20 (1270) | <0.001*,** | 44, 6, 34 | 5.03 (539) | <0.001*,** |
| IFG | 44 | −52, 12, 40 | 4.94 (1270) | <0.001*,** | |||
| IFG | 45 | −50, 28, 32 | 4.93 (1270) | <0.001*,** | 50, 36, 26 | 5.29 (539) | <0.001*,** |
| SFG | 6/8 | 32, 6, 64 | 4.25 (171) | <0.001 | |||
| SFG medial | 8/32 | −2, 28, 46 | 4.20 (404) | <0.001* | 6, 24, 44 | 3.49 (404) | <0.001* |
| SMA | 8 | 0, 18, 50 | 4.89 (404) | <0.001*,** | |||
| AG | 40 | −48, −42, 46 | 5.87 (5456) | <0.001*,** | 42, −36, 42 | 6.21 (6933) | <0.001*,** |
| SPL | 7 | −20, −60, 48 | 5.60 (5456) | <0.001*,** | 26, −56, 46 | 6.11 (6933) | <0.001*,** |
| ITG | 37 | −46, −62, −12 | 6.07 (5456) | <0.001*,** | |||
| IOG | 19 | 40, −70, −16 | 6.18 (6933) | <0.001*,** | |||
| Pallidum | −14, 2, −4 | 3.70 (49) | <0.001 | ||||
| Caudate | −10, 8, 0 | 3.45 (49) | <0.001 | 10, 12, 2 | 3.43 (15) | <0.001 | |
| 4, −16, 12 | 4.04 (108) | <0.001 | |||||
| Thalamus | 4, −28, −4 | 4.26 (194) | <0.001* | ||||
| Target analogy versus target match | |||||||
| IFG | 47 | −50, 18, −6 | 3.71 (44) | <0.001 | 52, 18, −8 | 3.48 (17) | <0.001 |
| IFG | 45/47 | 48, 20, 4 | 3.44 (13) | <0.001 | |||
| ITG | 37 | −58, −60, −14 | 4.00 (32) | <0.001 | |||
| ITG | 20 | −58, −32, −18 | 3.71 (181) | <0.001* | |||
| SMG | 40 | 58, −36, 40 | 3.68 (25) | <0.001 | |||
| AG | 39/40 | −40, −50, 32 | 3.50 (32) | <0.001 | 46, −52, 42 | 3.46 (32) | <0.001 |
| Precuneus | 7 | −6, −52, 68 | 3.54 (19) | <0.001 | 2, −60, 60 | 3.39 (13) | <0.001 |
| Postcingulate | 29 | −8, −38, 12 | 3.37 (36) | <0.001 | |||
| STG | 21/22 | 70, −24, 2 | 3.77 (37) | <0.001 | |||
| STG | 42 | −54, −30, 16 | 3.68 (49) | <0.001 | |||
| Cuneus | 18 | 8, −90, 28 | 4.97 (235) | <0.001*,** | |||
| LG | 19 | −28, −70, 2 | 4.26 (239) | <0.001* | 32, −66, 2 | 4.28 (234) | <0.001* |
| Thalamus | −22, −28, 14 | 3.37 (12) | <0.001 | ||||
| Caudate | −20, −16, 20 | 3.89 (63) | <0.001 | 18, −8, 18 | 4.16 (134) | <0.001 | |
Note: Coordinates correspond with the MNI template brain. Statistical maps were thresholded for significance at P < 0.001 uncorrected, with a cluster size of at least 10 voxels. Z scores and P values and the number of voxels in the activated clusters (in round brackets) are reported at this threshold. For all reported activations, FWE-corrected P values <0.05 at the adjusted cluster level (extent cluster correction) are indicated by * and FWE-corrected P values <0.05 at the voxel level are indicated by **. IFG: inferior frontal gyrus; IOG: inferior occipital gyrus; IPL: inferior parietal lobule; ITG: inferior temporal gyrus; LG: lingual gyrus; MTG: middle temporal gyrus; SMA: supplementary motor area; SMG: supramarginal gyrus; SPL: superior parietal lobule; STG: superior temporal gyrus.
Figure 2.
Significant signal change between conditions in 4 distinct analyses (P < 0.001 uncorrected, minimum extent: 10 voxels): (a) the contrast “analogy versus match” during source (in orange) is superimposed with the contrast analogy versus match during target (in red); (b) greater period effect (target–source) in the analogy than in the match task (obtained from a task by period interaction with exclusion of regions more activated in match than analogy task); and (c) the contrast match versus analogy.
The comparison of analogy and match tasks (Table 1) demonstrated activation in a large bilateral network, including rostral PFC (lateral BA10), dorsolateral (BA 8, 9, and 46) and ventrolateral prefrontal cortices (BA 44, 45, and 47), superior and inferior parietal cortices including the intraparietal sulcus and angular gyrus (AG; BA 7 and 40), and inferior temporal (BA 20, 21, and 37) and occipital cortices (BA 18 and 19).
A further contrast between analogy and match tasks during the source period alone revealed that lateral rostral PFC was recruited from the source period (Table 1; Fig. 2a). Many of the regions associated with the global analogy versus match comparison were also activated when this comparison was restricted to the source period only. These regions encompassed superior frontoparietal and rostral prefrontal areas (lateral BA10 bilateral with a left dominance). On the contrary, these regions were not observed at the chosen threshold during the target period only for this comparison (Table 1; Fig. 2a).
Within analogy tasks, we also contrasted cross- versus intradimension analogy tasks. In a whole-brain analysis, no region was significant at a corrected threshold. An ROI analysis performed in the previous lateral BA10 region associated with the analogy task (ROI centered on peak coordinates −44, 50, −4) showed no task effect between intra and crossdimension tasks (F(15) = 2.9; NS) nor task by period interaction (F(15) = 0.1; NS).
Finally, given the difference in reaction times between analogy and match tasks, we performed a correlation analysis between the difference in parameter estimates extracted from BA10 maxima (MNI coordinates −44, 50, −4) and the difference in reaction times between analogy and match tasks. This correlation was not significant (t(14) = 0.6; r = 0.17; NS). This suggests that BOLD increase in this rostral lateral prefrontal activation for analogy task is not simply related to an increase in time on task.
2) A dorsomedial rostral PFC signal increases during the target period in analogy tasks
As mapping is very likely to take place during the target but not during the source period, the contrast target versus source is crucial to study these mapping processes. Thus, we compared the target versus source contrast between analogy and match tasks, by running a task by period interaction analysis (Table 2; Fig. 2b). We used the contrast match versus analogy task (at a P < 0.05 uncorrected) as an exclusive mask, to exclude activation that was greater in the match than analogy condition. In other words, this analysis identified regions showing a greater period effect (target vs. source) during analogy than match task, that is, a greater signal increase from source to target for analogy than match task. The BOLD signal increase encompassed rostral PFC (medial BA 10), the superior and middle temporal gyri (BA 22, 42, 20, and 21), AG and a temporoparietal region (BA 39 and 40), a medial temporal region, the posterior cingulate and the precuneus. Activation within rostral PFC was more medial and dorsal than one found in the previous contrast (analogy vs. match tasks).
Table 2.
MNI coordinates of significant cluster maxima (the most significant maxima are given for each distinct gyrus and BA) in the group analysis for the task by period interaction ([Target Analogy − Source Analogy] − [Target Match − Source Match])
| Anatomical regions | BA | Left coordinates | Z scores (n voxels) | P values | Right coordinates | Z scores (n voxels) | P values | |
| SFG medial | 10 | 18, 62, 22 | 3.82 (16) | <0.001 | ||||
| SG | 25 | 0, 0, −8 | 3.70 (36) | <0.001 | ||||
| Midcingulate | 23/32 | −4, −26, 36 | 3.73 (28) | <0.001 | ||||
| Postcingulate | 29 | −8, −38, 12 | 4.83 (182) | <0.001* | 8, −40, 28 | 3.74 (23) | <0.001 | |
| PaC | 4/5 | −6, −48, 68 | 3.31 (40) | <0.001 | ||||
| PIns | 34, −22, 2 | 3.79 (148) | <0.001 | |||||
| SMG | 40 | 52, −32, 26 | 5.14 (1131) | <0.001*,** | ||||
| AG | 39 | −50, −56, 38 | 4.42 (455) | <0.001* | 50, −52, 32 | 4.47 (1131) | <0.001* | |
| STG/AG/TPJ | 42/39/40 | −58, −30, 14 | 5.25 (443) | <0.001*,** | ||||
| Precuneus | 7−23 | −14, −58, 30 | 3.64(15) | <0.001 | 16, −52, 32 | 4.00 (37) | <0.001 | |
| STG | 21/37 | −58, 0, 2 | 4.61 (121) | <0.001 | ||||
| MTG | 20/22 | −60, −28, −16 | 3.72 (73) | <0.001 | 44, −4, −12 | 4.28 (43) | <0.001 | |
| STS | 22 | 58, 2, 8 | 4.32 (192) | <0.001* | ||||
| Hippo. | −16, −14, −18 | 4.52 (46) | <0.001 | 30, −22, −16 | 3.89 (13) | <0.001 | ||
| PHippo. | −32, −34, −8 | 4.21 (102) | <0.001 | |||||
| Amygdala | 28, 2, −16 | 3.72 (10) | <0.001 | |||||
| Cuneus | 18 | 6, −90, 28 | 3.92 (134) | <0.001 | ||||
| Caudate | −22, 10, 26 | 4.24 (79) | <0.001 | 22, −10, 20 | 3.74 (52) | <0.001 | ||
| Putamen | −32, 6, 12 | 3.48 (19) | <0.001 | 32, −4, 2 | 3.60 (74) | <0.001 | ||
Note: Coordinates correspond with the MNI template brain. Statistical maps were thresholded for significance at P < 0.001 uncorrected, with a cluster size of at least 10 voxels. Z scores and P values and the number of voxels in the activated clusters (in round brackets) are reported at this threshold. For all reported activations, FWE−corrected P values <0.05 at the adjusted cluster level (extent cluster correction) are indicated by * and FWE−corrected P values <0.05 at the voxel level are indicated by **. Hippo: hippocampus; IFG: inferior frontal gyrus; MTG: middle temporal gyrus; PaC: paracentral region; PHippo: parahippocampus; Pins: posterior insula; SG: subgenual region; SMG: supramarginal gyrus; SPL: superior parietal lobule; STG: superior temporal gyrus; STS: superior temporal sulcus; and TPJ: temporo-parietal junction.
A within ROIs ANOVA comparing intradimension versus crossdimension analogy tasks in the BA10 region identified by this contrast (ROI centered on coordinates 18, 62, and 22) also showed a task by period interaction (F(15) = 7.6; P = 0.015) but no significant task effect (F(15) = 0.7; NS).
3) Ventromedial rostral PFC BOLD signal increases during the match, compared with the analogy task
The contrast of match versus analogy task, across both source and target periods, implicated a different network (Fig. 2c; Table 3) that included bilaterally 1) medial PFC, both ventrally and dorsally, including anterior frontal BA10 and BA11, the subgenual area (BA 25), the anterior cingulated (BA 32) and laterally the SFG (BA 9); 2) the posterior cingulate (BA 23), retrosplenial cortices, and precuneus, 3) the medial temporal cortex, including the hippocampus, and superior and middle temporal gyri in their anterior portion (BA 21, 22, 37, and 4), the occipital cortex (BA 18), and 4) the pericentral region (BA 3/4/6).
Table 3.
Significant brain activation for match versus analogy tasks
| Anatomical regions | BA | Left coordinates | Z scores (n voxels) | P values | Right coordinates | Z scores (n voxels) | P values |
| MOrbG | 11/10 | −6, 50, −10 | 5.77 (1253) | <0.001*,** | 0, 44, −16 | 5.96 (1253) | <0.001*,** |
| SG | 25 | 0, 14, −14 | 5.38 (1253) | <0.001*,** | |||
| SFG/MFG | 9 | −24, 42, 40 | 3.81 (44) | <0.001 | 30, 30, 40 | 3.76 (117) | <0.001 |
| Cing. | 23/24 | −4, −32, 52 | 5.94 (2605) | <0.001*,** | 0, −50, 24 | 4.86 (2605) | <0.001*,** |
| PaC/SMA | 4/6 | −10, −22, 68 | 4.59 (2605) | 0.001* | |||
| Pericentral | 3/4/6 | −54, −8, 50 | 3.79 (41) | <0.001 | 46, −16, 62 | 3.79 (100) | <0.001 |
| MTG | 21/38 | −54, 6, −18 | 4.26 (573) | <0.001* | 40, 4, −20 | 5.04 (495) | <0.001*,** |
| STS | 38 | 64, 0, 14 | 3.63 (30) | <0.001 | |||
| STS | 22 | 56, −2, 0 | 3.65 (31) | <0.001 | |||
| IOG/LG/FG | 18 | −28, −84, −10 | 3.99 (110) | <0.001 | 22, −86, −8 | 3.70 (20) | <0.001 |
| Hippo. | −26, −20, −18 | 4.34 (124) | <0.001 | 24, −12, −18 | 4.61 (164) | <0.001 | |
| Caudate | −18, 26, −8 | 4.01 (23) | <0.001 |
Note: The table shows the coordinates of significant cluster maxima (the most significant maxima are given for distinct gyrus/sulcus and BA) in the group analysis for match versus analogy contrast. Coordinates correspond with the MNI template brain statistical maps were thresholded for significance at P < 0.001 uncorrected, with a cluster size of at least 10 voxels. Z scores and P values and the number of voxels in the activated clusters (in round brackets) are reported at this threshold. For all reported activations, FWE−corrected P values <0.05 at the adjusted cluster level (extent cluster correction) are indicated by * and FWE-corrected P values <0.05 at the voxel level are indicated by **. Cing.: middle and posterior cingulate; FG: fusiform gyrus; Hippo.: hippocampus; IOG: inferior occipital gyrus; LG: lingual gyrus; MOG: middle occipital gyrus; MOrbG: middle orbital gyrus; MTG: middle temporal gyrus; PaC: paracentral region; Pins: posterior insula; SG: subgenual region; SMA: supplementary motor area; and STS: superior temporal sulcus.
A within-ROI ANOVA comparing intra versus crossdimension tasks in the BA10 region identified by this contrast (centered on coordinates 0, 44, −16) did not show any significant task effect (F(15) = 2.3; NS) nor a task by period interaction (F(15) = 0.1; NS).
Again, given the difference in reaction times between our 2 tasks, we performed a correlation analysis between task differences (analogy − match) in 1) parameter estimates and 2) reaction times. In medial ventral rostral PFC (ROI centered on MNI coordinates 0, 44, −16), this correlation was not significant (t(14) = −0.4; r = −0.10; NS).
In summary, these findings highlight 3 different patterns of activation within the rostral PFC (Fig. 3). First, a lateral subregion was activated as soon as the source was presented when the analogy task was compared with a simple match task. Second, a more polar and dorsomedial subregion was more active during the target than during the source phase. Third, more ventral medial subregions of rostral PFC were more activated during the match than during the analogy task.
4) Independent ROI analyses (Fig. 3)
Figure 3.
Differential activities within the rostral PFC for 3 identified subregions. On the left, activation obtained in the whole-brain analyses is superimposed on a frontal slice (y = 58) on a standard MRI brain and on a canonical surface brain in the MNI space. In orange: analogy versus match activating the lateral rostral PFC (maxima = −44, 50, −4); in blue: task by period interaction, activating a more dorsal and medial part of the rostral PFC (maxima = 18, 62, and 22); in green: match versus analogy, activating a ventral and medial frontopolar subregion (maxima = 0, 44, −16). On the right, graphs represent parameter estimates for the rostral prefrontal ROIs defined independently from the whole-brain analysis: lateral ROI (MNI coordinates = −39, 50, 2), dorsomedial ROI (MNI coordinates = 8, 59, 19), and ventromedial ROI (MNI coordinates = 0, 48, −16). A schematic representation of each of these ROIs is shown on frontal slices of a standard brain (ch2.nii template provided by MRIcron in the MNI space), at the upper part of the figure. Parameter estimates were extracted for each task (analogy and match) and period (source and target) and averaged between subjects. On the y axis are the values of the mean parameter estimates across subjects and trials. This shows qualitative differences in activation profile of the 3 rostral prefrontal regions. Stars represent significant condition by ROI interactions. In black are schematized the condition by ROI interaction for the “source analogy” versus “source match” conditions. In white are schematized the condition by ROI interaction for the “target analogy” versus “target match” conditions. In orange is schematized the condition by ROI interaction for the “target analogy” versus “source analogy” conditions.
As described in the Materials and Methods section, right and left lateral, dorsomedial and a ventromedial ROIs were defined from previous studies involving BA10, in order to examine the involvement of distinct rostral subregions in each condition.
Within ROIs: ANOVAs Comparing Analogy and Match Tasks
Within the left lateral ROI (MNI coordinates −39, 50, 2), there was a significant task effect (a higher BOLD signal during analogy than match task, F(1,15) = 16.8; P <0.001) but no task by period interaction (F(1,15) = 1.0; NS). A paired t-test performed between “target analogy” versus “target match” was significant (t(15) = 2.9; P = 0.011), as well as the comparison between “source analogy” versus “source match” (t(15) = 3.1; P = 0.008). For the right lateral ROI (coordinates 39, 50, 2), no effect was significant (task effect: F(1,15) = 0.7; NS); task by period interaction: F(1,15) = 0.01; NS). Within the right dorsomedial ROI (coordinates 8, 59, 19), there was a significant task effect (F(1,15) = 4.9; P = 0.043), and task by period interaction, with a greater difference between target and source periods in the analogy than match task (F(1,15) = 4.8; P = 0.044). For the left dorsomedial ROI (coordinates −8, 59, 19), task effect (F(1,15) = 3.5; NS) and task by period interaction (F(1,15) = 1.1; NS) were not significant. Finally, within the ventromedial ROI (coordinates 0, 48, −16), there was a significant task effect (a higher BOLD signal during match task, F(1,15) = 8.2; P = 0.012) but no task by period interaction (F(1,15) = 0.2; NS).
In sum, we found a significant task effect in the left lateral ROI (−39, 50, 2), in favor of the analogy task. This result is consistent and the region spatially close to the one found in the whole-brain analysis (Table 1; Figs. 2a and 3) when contrasting analogy and match tasks (activation peak: −44, 50, −4). In the right dorsomedial ROI (8, 59, 19), we found a task by period interaction showing that the increase of signal in this region from source to target is greater for the analogy than match task. This result is highly concordant with the task by period interaction conducted on the whole brain, which exhibited a very similar region (activation peak: 18, 62, and 22) (Table 2; Figs. 2b and 3). Finally, in the ventromedial ROI (0, 48, −16), in contrast with the dorsomedial ROI, there was a task effect but no task by period interaction. This shows that the increase of signal in this region for match relative to analogy task was not significantly modulated by source and target periods. This is convergent with the previous match versus analogy contrast, which showed an activation peak in medial BA10 (coordinates 0, 44, −16).
Between ROIs: Region by Task and Region by Period Interactions
Figure 3 suggests that the left lateral, right dorsomedial, and ventromedial ROIs within rostral PFC exhibit distinct profiles. Firstly, when comparing analogy and match tasks during the source period, only in the lateral ROI was there a significant difference. ANOVAs confirmed that during the source period, task by region interactions were significant (highlighted by black stars on Fig. 3) when comparing both lateral versus dorsomedial ROIs (F(1,15) = 29.7; P < 0.001), and lateral versus ventromedial ROIs (F(1,15) = 33.0; P < 0.001). This suggests that during source, the lateral ROI is more activated for analogy task than the other ROIs.
Secondly, when comparing analogy and match tasks during target period, there was a significant task by region interaction when testing the following ROIs (highlighted by white stars on Fig. 3): lateral versus dorsomedial (F(1,15) = 23.3; P < 0.001), lateral versus ventromedial (F(1,15) = 20.3; P < 0.001), dorsomedial versus ventromedial (F(1,15) = 8.8; P = 0.010). More precisely, there was a greater difference between “target analogy” and “target match” in the lateral than in the dorsomedial ROI.
Finally, and importantly, there was a significant period (“source analogy” vs. “target analogy”) by region interaction between the lateral and dorsomedial ROIs (F(1,15) = 14.9; P = 0.002). In other words, the difference between target and source during analogy was greater in the dorsomedial than in the lateral ROI (highlighted by an orange star in Fig. 3). Taken together with previous results, this suggests that source and target periods did not equally activate these 2 ROIs: The lateral ROI was activated during both periods, whereas the dorsomedial was more activated by the target period.
Discussion
The objective of this study was to examine the role of the rostral PFC in analogical reasoning. A novel experimental paradigm was designed to dissociate different processes that have been proposed to play a role in analogical reasoning: the generation of abstract representations and the mapping of these representations. Analyses at both the ROI and whole-brain level indicate 2 principal new findings (Fig. 3). First, within the rostral PFC, a lateral BA10 region was found to be activated during any phase of analogical reasoning, compared with a control task that involved attribute matching only. Unlike other rostral prefrontal regions, this region was activated from the time of the presentation of the source alone, in other words, before the source could be compared with a target. This may suggest that this region is involved in generating or processing abstract and structured representations, processes that are needed in both phases of analogy tasks. However, it cannot be excluded that the activation in this region during the target period reflects its involvement in additional processes, such as relational mapping, or is due to a prolonged activity related to the source processes and that is misattributed to the target period. Second, as part of a distinct network, a dorsomedial region of BA10 was specifically involved when the target appeared (i.e. during the target period) and not during the source period. This may suggest that this region is associated with comparison or mapping processes, rather than with generating structured representations. Taken as a whole, this study suggests a novel functional organization of rostral PFC, with different subregions supporting distinct cognitive processes (Fig. 3). These processes may be involved not only in analogical reasoning but may also in various other experimental paradigms that activate BA10.
First Phase of Analogical Reasoning: Exploring Source Stimuli and Making a Structured Representation
The frontoparietal network, including left lateral BA10 (Fig. 2) associated with the analogy condition, is highly consistent with the activations found in other studies of analogical reasoning. It also overlaps with the regions associated with tests of fluid intelligence and relational integration tasks (Christoff et al. 2001; Ruff et al. 2003; Bunge, Wendelken, et al. 2005; Geake and Hansen 2005; Wendelken, Nakhabenko et al. 2008; Bunge et al. 2009; Cho et al. 2009; Wendelken and Bunge 2009). Indeed, the averaged coordinates of peak activation in these studies, used to define the center of our ROIs, fall very close to our rostral PFC activation on the left hemisphere (−39, 50, 2 for the former, −44, 50, −4 for the latter). However, the fact that BOLD signal increases in this frontoparietal network during the source period in the present study (before the source could be mapped to the target) gives new indications about its role. During the source period of analogy tasks, subjects are required to generate a structured representation of the stimuli.
These observed frontoparietal regions have been associated with the manipulation of mental representations in working memory (Christoff and Gabrieli 2000; Christoff et al. 2003), the polar region being recruited as a function of the degree of abstraction of information to be processed in working memory. More recently, Wendelken, Bunge, and Carter (2008) suggested the role of such a network in organizing mental representations in working memory. These authors showed activation in a frontoparietal network comparable with our findings—including left lateral BA10—when comparing structured (organized) versus unstructured internal representations. In our analogy task, the source set had to be manipulated in order to find a structured representation of the rule that had to be compared with the target set. In our match task, there was no need to reorganize the presented information, as subjects had to focus on one given visual attribute and use it to make their decision. Concordant with this, in debriefing, all participants noted that they had attempted to find structure in the source set during analogy trials but not during match trials. In analogical reasoning, the involvement of lateral BA10 in the organization of representations has also been suggested by Geake and Hansen (2005), when using different types of analogy between strings of letters. In addition, Christoff et al. (2003, 2009) showed a hierarchical organization of representations' structure, the rostral lateral PFC being involved in the highest level of abstraction.
This interpretation is also coherent with brain anatomy. Although rostral PFC does not appear to have monosynaptic efferent pathways to parietal cortex (Petrides and Pandya 2007), coactivation between rostral PFC and parietal cortex could be mediated via their common connections with caudal lateral prefrontal regions (Petrides and Pandya 1999). The indirect connection of rostral PFC with parietal multisensory/integrative via caudal prefrontal control regions could support its role in building structured abstract representations.
How do we generate these structured representations? Subjects may generate hypotheses about the expected structure or rule to discover. Hypothesis generation and rule finding (Strange et al. 2001; Goel and Vartanian 2005; Reverberi et al. 2005) have indeed also been associated with the rostral PFC. Similarly, the lateral rostral PFC (together with the intraparietal sulcus) may specifically be implicated in exploratory phases of tasks involving rule finding (Daw et al. 2006). And how are these hypotheses generated? It could be argued that subjects retrieve the rule-based knowledge necessary to find the rules (e.g., letter identity, mathematical rules, and semantic knowledge). The retrieval of rules has been associated with anterior ventrolateral prefrontal areas and posterior middle temporal gyrus (Bunge 2004; Bunge, Wallis, et al. 2005; Donohue et al. 2005), regions that are also activated in our analogy task. This may suggest that the retrieval of rule-based knowledge could be responsible for middle temporal and anterior inferior prefrontal activation in our study.
Our study does not allow us to distinguish the specific role of each region activated during source. However, interpreted together with other findings from the literature, it suggests that the role of the rostral PFC in interaction with other regions is to generate more structured and abstract mental representations. It is more difficult to discern the role of these regions during target processing, because different cognitive processes are required during this period; Moreover, we could not exclude that some of the extended source activity might be attributed to the target period.
Second Phase of Analogical Reasoning: Comparing and Mapping
A specific network exhibited an interaction effect between period (target vs. source) and task (analogy vs. match). This target-related network during analogy includes a right dorsomedial subregion of BA10 (Fig. 2b). It is also composed of a posterior parietal activation, in the AG extending to the junction with temporal cortex (temporoparietal junction, TPJ) and activation in superior and in medial temporal regions. An independent ROI analysis (Fig. 3) converged and showed a significant task by period interaction in this region. It also showed that activity in the right dorsomedial rostral prefrontal region during the analogy task is relatively low in the source period. This suggests that unlike the lateral region, this dorsomedial region may not be involved in generating abstract relational representations.
Comparing ROIs, the region by task and region by period interactions (Fig. 3) also suggest that this dorsomedial region is activated during the “target analogy” and the “target match” conditions. In other words, this region was activated when target stimuli were compared with source stimuli. There are at least 2 ways of interpreting this result. First, it could suggest a role for dorsomedial BA10 in the detection of a similarity between dissimilar targets and sources (Gentner et al. 1993; Holyoak and Thagard 1995; Gentner and Markman 1997; Gentner and Medina 1998; Blanchette and Dunbar 2000; Markman and Gentner 2000; Pothos 2005). Second, the activation of this region could reflect the processes involved in the comparison between what is expected (expectations formed from the source set) and what is presented (the target set) (Summerfield et al. 2006).
Regarding the first possibility, studies of visual categorization argue for a role of the medial rostral PFC in detecting similarities (Vogels et al. 2002; Koenig et al. 2005; Smith and Grossman 2008). Koenig et al. (2005) observed a specific network associated with similarity-based categorization (learning or use of novel categories), encompassing similar regions to ours in medial rostral PFC and TPJ. These regions showed less activation during rule-based categorization, in which visual similarity was less important to perform the task. The TPJ is a neocortical area that is thought to integrate distinct types of information from other associative areas and has already been observed in analogy studies (Wharton et al. 2000; Luo et al. 2003). This region seems to be crucial for similarity-based categorization in neuropsychological studies (Grossman et al. 2002).
A recent study investigating analogical reasoning demonstrated the influence of semantic distance on dorsomedial BA10 activation (Green et al. 2010). More distant analogies (i.e., those with reduced similarity between source and target) were associated with greater rostral PFC activation. Although we did not entirely replicate their results in the visual domain when comparing cross and intradimension analogies, these sets of data reinforce the idea that this region plays a crucial role in detecting similarities between dissimilar items (Holyoak and Thagard 1995). In connection with this, it is important to underline that in the match task, source and target were not similar, except for the attribute to match.
Regarding the second possibility, it has been proposed that medial rostral PFC is involved in generating a template against which to match observed information in the environment. This template would allow anticipation of the forthcoming environment, thus facilitating perceptual decisions (Summerfield et al. 2006). Summerfield et al. (2006) found that the medial rostral PFC (in a location close to the present dorsomedial ROI) is more activated when perception matched subjects' expectations. In the present study, the mental representations generated during the source period would form the expectation against which to match the target set when it appears.
These hypotheses are consistent with the finding that during the target period, the dorsomedial ROI seems to be activated in both analogy (relational mapping) and match (attribute mapping) tasks (Fig. 3). Finally, we should emphasize that dorsomedial BA10 is likely to operate as part of a network, together with other regions such as the TPJ and superior temporal regions, whose respective roles in detecting similarities or storing templates will need further clarification. Anatomically, these coactivations may be supported by direct connections between rostral PFC and superior lateral temporal areas, as shown in monkeys by Petrides and Pandya (2007).
A Network More Activated during the Match Task
In both the whole brain and the ROI analyses, the ventromedial rostral prefrontal region was less activated in the analogy task than in the match task (Figs. 2c and 3). Other activations were also observed in this contrast: more ventral prefrontal regions (BA11 and BA25), posterior cingulate and retrospenial cortices, anterior lateral temporal cortex, and medial temporal region. Some of these regions are anatomically connected with the rostral PFC, via the uncinate fasciculus and the cingulate fasciculus (Petrides and Pandya 2007; Greicius et al. 2009). This replicates previous findings that medial rostral PFC activation may be observed in contrasts between stimulus-oriented conditions, where participants base their responses on perceptual information, versus stimulus-independent conditions, where participants must base their responses on internally generated information (Gilbert et al. 2005, 2007). In line with these studies, the match condition involves more stimulus-oriented attending than the analogy condition, as subjects have to focus on one visual feature displayed on the screen. Conversely, they have to generate stimulus-independent representations (representation of the structure of the displayed sets) in the analogy condition. This has been developed in a more complete theory, the “gateway hypothesis” (Burgess et al. 2005, 2006; Burgess, Dumontheil, and Gilbert 2007; Burgess, Gilbert, and Dumontheil 2007) that argues for a role of rostral PFC in attentional control between stimulus-independent and stimulus-oriented representations.
The current “match versus analogy” network, including medial rostral PFC, also broadly overlaps the so-called “default network.” The default network is composed of a set of brain regions that are more activated for low-demand tasks or rest than for high-demand tasks, across a variety of different situations (Raichle et al. 2001; Buckner et al. 2008). It is thought to reflect certain types of cognitive processes that are more common during easy tasks or passive states and that may be suspended during performance of more effortful and goal-directed tasks. The nature of these processes is still a matter of debate and beyond the scope of the present study. Previous studies have suggested that these processes include monitoring of stimuli in the external world (Gilbert et al. 2007; Buckner et al. 2008 for a review), as required in the match task, and/or task-unrelated mind wandering (Mason et al. 2007), which could also occur in the match task.
Taken as a whole, these results describe distinct roles for lateral and medial BA10 subregions in different analogy processes and add to growing evidence for the existence of at least 2 functionally distinct brain networks, one associated with lateral and the other with medial BA10 (Gilbert, Spengler, Simons, Frith, and Burgess 2006; Burgess, Dumontheil, and Gilbert 2007). Recently, examination of resting state brain fluctuations also suggests that lateral and medial BA10 are strongly connected to different brain networks (Vincent et al. 2008). These networks partly overlap with the set of brain regions observed in the present study, including lateral and medial BA10. However, although it is difficult to interpret the functional significance of spontaneous fluctuations at rest, the current work helps to identify the roles of lateral and medial BA10 in distinct cognitive processes, which might be involved in a wide variety of tasks.
Conclusion
To adapt our behavior to a changing world, we need to be able to use our experience in order to transfer known solutions to new situations. This is also how we learn, and it depends on our ability to represent these known and new situations in a structured way and to compare and detect similarity between them. We present new findings showing the neural correlates of these abilities within rostral PFC. A lateral rostral PFC subregion appears to be involved during many phases of analogical reasoning, starting with where one is required to generate structured mental representations of stimuli. A dorsomedial rostral PFC subregion seems instead to be principally involved when one is comparing and detecting similarities between 2 situations or between expected and present. These processes and their neural correlates are likely not only involved in analogical reasoning but may also relate to patterns of rostral PFC activation in various other types of experimental paradigm. This topic may have important implications for understanding patterns of deficit in patients with damage in the rostral PFC, whose problems in behavioral adaptation to ill-structured situations are not well understood (Mesulam 1986; Burgess et al. 2000; Burgess, Gilbert, and Dumontheil 2007; Burgess et al. 2009).
Funding
Wellcome Trust (grant number 061171 to P.W.B), the Royal Society (University Research Fellowship to S.J.G.), University College London (Graduate School Research Scholarship to R.B.), and the German Academic Exchange Service (grant to R.B.), and the “Fondation Bettencourt Schueller” (Young Researcher Prize to E.V.).
Acknowledgments
The authors thank the Birbeck-UCL Centre for NeuroImaging for their support of our study and thank Gil Gonen Yaacovi and Iroise Dumontheil for helpful comments and suggestions.
Conflict of Interest: None declared.
References
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Blanchette I, Dunbar K. How analogies are generated: the roles of structural and superficial similarity. Mem Cogn. 2000;28:108–124. doi: 10.3758/bf03211580. [DOI] [PubMed] [Google Scholar]
- Bowdle BF, Gentner D. The career of metaphor. Psychol Rev. 2005;112:193–216. doi: 10.1037/0033-295X.112.1.193. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Helskog EH, Wendelken C. Left, but not right, rostrolateral prefrontal cortex meets a stringent test of the relational integration hypothesis. NeuroImage. 2009;46:338–342. doi: 10.1016/j.neuroimage.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wallis JD, Parker A, Brass M, Crone EA, Hoshi E, Sakai K. Neural circuitry underlying rule use in humans and nonhuman primates. J Neurosci. 2005;25:10347–10350. doi: 10.1523/JNEUROSCI.2937-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortex. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Volle E, Benoit RG, Gilbert SJ. Mesulam's frontal lobe mystery re-examined. Restor Neurol Neurosci. 2009;27:493–506. doi: 10.3233/RNN-2009-0511. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gilbert S, Okuda J, Simons JS. Rostral prefrontal brain regions (area 10): a gateway between inner thought and the external world? In: Sebanz WPN, editor. Disorders of volition. Cambridge (MA): MIT Press; 2006. pp. 373–396. [Google Scholar]
- Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362:887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. In: Duncan J, Phillips L, McLeod P, editors. Measuring the mind: speed, control, and age. Oxford: Oxford University Press; 2005. pp. 215–246. [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Cho S, Moody TD, Fernandino L, Mumford JA, Poldrack RA, Cannon TD, Knowlton BJ, Holyoak KJ. Common and dissociable prefrontal loci associated with component mechanisms of analogical reasoning. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp121. Advance Access published on June 23; doi:10.1093/cercor/bhp121. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JD. The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Christoff K, Keramatian K. Abstraction of mental representations: theoretical considerations and neuroscientific evidence. In: Wallis S, editor. The neuroscience of rule-guided behavior. Oxford University Press; 2006. [Google Scholar]
- Christoff K, Keramatian K, Gordon AM, Smith R, Mädler B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Res. 2009;1286:94–105. doi: 10.1016/j.brainres.2009.05.096. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Gabrieli JD. Neural basis of spontaneous thought processes. Cortex. 2004;40:623–630. doi: 10.1016/s0010-9452(08)70158-8. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Christoff K, Keramatian K, Gordon AM, Smith R, Mädler B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Research. 2009;1286:94–105. doi: 10.1016/j.brainres.2009.05.096. [DOI] [PubMed] [Google Scholar]
- Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cogn Affect Behav Neurosci. 2004;4:501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, van Leijenhorst L, Honomichl RD, Christoff K, Bunge SA. Neurocognitive development of relational reasoning. Dev Sci. 2009;12:55–66. doi: 10.1111/j.1467-7687.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, O'Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pisapia N, Braver TS. Preparation for integration: the role of anterior prefrontal cortex in working memory. Neuroreport. 2008;19:15–19. doi: 10.1097/WNR.0b013e3282f31530. [DOI] [PubMed] [Google Scholar]
- Donohue SE, Wendelken C, Crone EA, Bunge SA. Retrieving rules for behavior from long-term memory. Neuroimage. 2005;26:1140–1149. doi: 10.1016/j.neuroimage.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Burgess PW, Blakemore SJ. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Dev Med Child Neurol. 2008;50:168–181. doi: 10.1111/j.1469-8749.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RM. The computational modeling of analogy-making. Trends Cogn Sci. 2002;6:200–205. doi: 10.1016/s1364-6613(02)01882-x. [DOI] [PubMed] [Google Scholar]
- Friston K. Imaging cognitive anatomy. TrendsCogn Sci. 1997;1:21–27. doi: 10.1016/S1364-6613(97)01001-2. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional integration and inference in the brain. Prog Neurobiol. 2002;68:113–143. doi: 10.1016/s0301-0082(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Geake JG, Hansen PC. Neural correlates of intelligence as revealed by fMRI of fluid analogies. Neuroimage. 2005;26:555–564. doi: 10.1016/j.neuroimage.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Gentner D. Structure-mapping: a theoretical framework for analogy. Cogn Sci. 1983;7:155–170. [Google Scholar]
- Gentner D, Holyoak KJ. Reasoning and learning by analogy. Am Psychol. 1997;52:32–34. doi: 10.1037//0003-066x.52.1.32. [DOI] [PubMed] [Google Scholar]
- Gentner D, Markman AB. Structure mapping in analogy and similarity. Am Psychol. 1997;52:45–56. [Google Scholar]
- Gentner D, Medina J. Similarity and the development of rules. Cognition. 1998;65:263–297. doi: 10.1016/s0010-0277(98)00002-x. [DOI] [PubMed] [Google Scholar]
- Gentner D, Rattermann MJ, Forbus KD. The roles of similarity in transfer: separating retrievability from inferential soundness. Cogn Psychol. 1993;25:524–575. doi: 10.1006/cogp.1993.1013. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. Eur J Neurosci. 2005;21:1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform. 2006;32:45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Frith CD, Burgess PW. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain–behavior associations. Cereb Cortex. 2006;16:1783–1789. doi: 10.1093/cercor/bhj113. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O. Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cereb Cortex. 2005;15:1170–1177. doi: 10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Dunbar KN. The micro-category account of analogy. Cognition. 2008;106:1004–1016. doi: 10.1016/j.cognition.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Green AE, Fugelsang JA, Kraemer DJ, Shamosh NA, Dunbar KN. Frontopolar cortex mediates abstract integration in analogy. Brain Res. 2006;1096:125–137. doi: 10.1016/j.brainres.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Green AE, Kraemer DJM, Fugelsang JA, Gray JR, Dunbar KN. Connecting long distance: semantic distance in analogical reasoning modulates frontopolar cortex activity. Cereb Cortex. 2010;20:70–76. doi: 10.1093/cercor/bhp081. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. The neural basis for category-specific knowledge: an fMRI study. Neuroimage. 2002;15:936–948. doi: 10.1006/nimg.2001.1028. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Holyoak KJ, Kroger JK. Forms of reasoning: insight into prefrontal functions? Ann N Y Acad Sci. 1995;769:253–263. doi: 10.1111/j.1749-6632.1995.tb38143.x. [DOI] [PubMed] [Google Scholar]
- Holyoak KJ, Thagard P. Mental leaps. Analogy in creative thought. Cambridge (MA): MIT Press; 1995. [Google Scholar]
- Holyoak KJ, Thagard P. The analogical mind. Am Psychol. 1997;52:35–44. [PubMed] [Google Scholar]
- Jani NG, Levine DS. A neural network theory of proportional analogy-making. Neural Netw. 2000;13:149–183. doi: 10.1016/s0893-6080(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Gee J, Grossman M. The neural basis for novel semantic categorization. Neuroimage. 2005;24:369–383. doi: 10.1016/j.neuroimage.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Kolodner JL. Educational implications of analogy. A view from case-based reasoning. Am Psychol. 1997;52:57–66. doi: 10.1037//0003-066x.52.1.57. [DOI] [PubMed] [Google Scholar]
- Kotovsky L, Gentner D. Comparison and categorization in the development of relational similarity. Child Dev. 1996;67:2797–2822. [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Luo Q, Perry C, Peng D, Jin Z, Xu D, Ding G, Xu S. The neural substrate of analogical reasoning: an fMRI study. Brain Res Cogn Brain Res. 2003;17:527–534. doi: 10.1016/s0926-6410(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Markman AB, Gentner D. Structure mapping in the comparison process. Am J Psychol. 2000;113:501–538. [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Frontal cortex and behavior. Ann Neurol. 1986;19:320–325. doi: 10.1002/ana.410190403. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EM. The rules versus similarity distinction. Behav Brain Sci. 2005;28:1–14. doi: 10.1017/s0140525x05000014. discussion 14–49. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven's progressive matrices test. Cogn Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Reverberi C, D'Agostini S, Skrap M, Shallice T. Generation and recognition of abstract rules in different frontal lobe subgroups. Neuropsychologia. 2005;43:1924–1937. doi: 10.1016/j.neuropsychologia.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, McDermott KB, Braver TS. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cereb Cortex. 2006;16:519–528. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Knauff M, Fangmeier T, Spreer J. Reasoning and working memory: common and distinct neuronal processes. Neuropsychologia. 2003;41:1241–1253. doi: 10.1016/s0028-3932(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Simons JS, Owen AM, Fletcher PC, Burgess PW. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Simons JS, Scholvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44:1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Smith EE, Grossman M. Multiple systems of category learning. Neurosci Biobehav Rev. 2008;32:249–264. doi: 10.1016/j.neubiorev.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman BA, Holyoak KJ, Morrison RG. Analogical priming via semantic relations. Mem Cogn. 2001;29:383–393. doi: 10.3758/bf03196389. [DOI] [PubMed] [Google Scholar]
- Strange BA, Henson RN, Friston KJ, Dolan RJ. Anterior prefrontal cortex mediates rule learning in humans. Cereb Cortex. 2001;11:1040–1046. doi: 10.1093/cercor/11.11.1040. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314:1311–1314. doi: 10.1126/science.1132028. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R, Sary G, Dupont P, Orban GA. Human brain regions involved in visual categorization. Neuroimage. 2002;16:401–414. doi: 10.1006/nimg.2002.1109. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone KB, Mishkin FS, de Menerzes Santos M, Thomas CR, Miller B. A system for relational reasoning in human prefrontal cortex. Psychol sci. 1999;10:119. [Google Scholar]
- Wendelken C, Bunge S. Transitive inference: distinct contributions of rostrolateral prefrontal cortex and the hippocampus. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21226. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA, Carter CS. Maintaining structured information: an investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia. 2008;46:665–678. doi: 10.1016/j.neuropsychologia.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. “Brain is to thought as stomach is to?”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 2008;20:682–693. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Wharton CM, Grafman J, Flitman SS, Hansen EK, Brauner J, Marks A, Honda M. Toward neuroanatomical models of analogy: a positron emission tomography study of analogical mapping. Cognit Psychol. 2000;40:173–197. doi: 10.1006/cogp.1999.0726. [DOI] [PubMed] [Google Scholar]