Abstract
Combining transcranial magnetic stimulation (TMS) with concurrent functional magnetic resonance imaging (fMRI) allows study of how local brain stimulation may causally affect activity in remote brain regions. Here, we applied bursts of high- or low-intensity TMS over right posterior parietal cortex, during a task requiring sustained covert visuospatial attention to either the left or right hemifield, or in a neutral control condition, while recording blood oxygenation-level–dependent signal with a posterior MR surface coil. As expected, the active attention conditions activated components of the well-described “attention network,” as compared with the neutral baseline. Also as expected, when comparing left minus right attention, or vice versa, contralateral occipital visual cortex was activated. The critical new finding was that the impact of high- minus low-intensity parietal TMS upon these visual regions depended on the currently attended side. High- minus low-intensity parietal TMS increased the difference between contralateral versus ipsilateral attention in right extrastriate visual cortex. A related albeit less pronounced pattern was found for left extrastriate visual cortex. Our results confirm that right human parietal cortex can exert attention-dependent influences on occipital visual cortex and provide a proof of concept for the use of concurrent TMS–fMRI in studying how remote influences can vary in a purely top–down manner with attentional demands.
Keywords: concurrent TMS–fMRI, posterior parietal cortex, state-dependence, visuospatial attention
Introduction
Numerous human neuroimaging studies have now implicated a dorsal frontoparietal “attention network” in endogenous, top–down attention-related modulation of visual processing (e.g., Driver and Vuilleumier 2001; Kastner and Ungerleider 2001; Corbetta and Shulman 2002; Yantis and Serences 2003; Corbetta et al. 2008). Within this network, the posterior parietal cortex (PPC) has been suggested to play an important potential role in directing visual spatial attention (e.g., Mesulam 1981; Hopfinger et al. 2000; Bisley and Goldberg 2003) and is also implicated on clinical grounds from patient studies, especially for the right hemisphere (e.g., Mesulam 1999; Driver and Vuilleumier 2001; He et al. 2007) that has been proposed to play a role in directing attention to either side of space (e.g., Mesulam 1999).
It is now well established that regions in visual cortex can also be strongly modulated by spatial attention (e.g., Heinze et al. 1994; Kastner et al. 1998; Hopfinger et al. 2000; Yantis et al. 2002). Furthermore, it has often been suggested that parietal cortex may play a role in imposing such attentional modulations, for instance as based on clinical evidence from brain-damaged patients (e.g., Mesulam 1999; Driver and Vuilleumier 2001). However, there is a surprising lack of truly causal (i.e., interventional) evidence for modulatory influences from human parietal cortex upon visual cortex due to attention, as strictly speaking most studies typically use just correlative measures rather than causal interventional manipulations (though see Corbetta et al. 2005 and Vuilleumier et al. 2008 for recent studies of how parietal lesions in patients might affect function in remote but interconnected visual cortex).
A recent physiological study by Saalmann et al. (2007) produced some evidence for possible parietal interactions with visual cortex during spatial attention, when recording simultaneously from neurons in visual area MT and from PPC in monkeys. Increased coherence of local field potentials was observed between these areas during attentional visual tasks, with a leading phase for the PPC neurons, consistent with a possible influence from PPC upon MT. But this work falls short of an interventional causal manipulation of PPC, showing instead a relation between PPC and visual cortex for which the temporal ordering is consistent with a potentially causal influence from PPC.
A more directly causal approach is to intervene in (or “perturb”) activity for a given region, while recording the causal impact of this intervention for activity elsewhere, such as in visual cortex (see Paus 2005). This approach was recently employed in monkey studies combining microstimulation of a targeted region with neural recordings elsewhere (e.g., Moore and Armstrong 2003; Moore 2006) or during whole-brain functional magnetic resonance imaging (fMRI) (Tolias et al. 2005; Ekstrom et al. 2008). However, those existing studies typically targeted other sites (e.g., frontal eye field, FEF) rather than PPC sites in particular, to date.
While invasive microstimulation is usually not possible in humans, transcranial magnetic stimulation (TMS) is now well established as a noninvasive method for targeted brain stimulation in human studies (e.g., Pascual-Leone 2002; Walsh and Pascual-Leone 2003; Wasserman et al. 2008). TMS can affect neural activity in targeted areas (Hallett 2007) and may modulate ongoing brain rhythms (Fuggetta et al. 2008; Thut and Miniussi 2009). The exact neural consequences of TMS at the targeted site are not fully understood but remain the focus of intensive current research (see Wasserman et al. 2008). Depending on the exact TMS protocol (e.g., number of pulses, intensity, coil size and orientation, plus frequency of stimulation), TMS can either increase or decrease neuronal excitability (e.g., Pascual-Leone 2002; Walsh and Pascual-Leone 2003; Wasserman et al. 2008). Single pulses or short bursts of TMS (typically considered as “excitatory” protocols, as for the protocol used here, see below) induce electrical currents in the underlying brain tissue. These are known to be capable of inducing depolarization and action potentials in the targeted tissue (e.g., Roth, Cohen, and Hallett 1991; Roth, Saypol, et al. 1991; Rothwell 1997; Allen et al. 2007; Wagner et al. 2007; Epstein 2008; Pasley et al. 2009), with excitatory postsynaptic potentials (that may be followed by more generalized inhibitory postsynaptic potentials, see Moliadze et al. 2003). The induced magnetic field (which induces current in the brain) is inversely proportional to the square of the distance between coil and cortex (Ilmoniemi et al. 1999; Wagner et al. 2007). Appropriate coil designs can ensure that direct effects of stimulation are more or less restricted to cortex close to the outer convexity of the brain under the TMS scalp site.
The key point for present purposes is that TMS can provide a causal intervention (or “perturbation”) for a targeted brain region. As regard our present concern with possible impacts of PPC TMS on visual attention in particular, several purely behavioral TMS studies have now shown that PPC TMS can impact on visual performance (e.g., Pascual-Leone et al. 1994; Hung et al. 2005; Nyffeler et al. 2008). Furthermore, due to recent technical advances, TMS can now be combined with concurrent fMRI, applying TMS during scanning so as to assess not only local effects of TMS but also any causal influences on blood oxygen level–dependent (BOLD) signal in remote, potentially interconnected brain regions (Bohning et al. 2000; Ruff et al. 2006; Bestmann, Ruff, Blankenburg, et al. 2008; Bestmann, Swayne, et al. 2008; Blankenburg et al. 2008; Ruff et al. 2008). The technical aspects of combining TMS with fMRI have been reviewed elsewhere (e.g., Sack and Linden 2003; Paus 2005; Bestmann, Ruff, Blankenburg, et al. 2008; Bestmann, Ruff, Driver, and Blankenburg 2008). Here, we focus on use of the concurrent TMS–fMRI approach to study possible remote influences upon visual cortex in particular, testing for the first time whether any such influences can be attention dependent (i.e., varying with the “current attentional state”).
Ruff et al. (2006) recently showed that right FEF TMS could modulate BOLD in early human visual cortex at rest (or during visual stimulation) when participants had no task other than central fixation. In follow-up work, they reported that right intraparietal TMS can also have distinct effects on visual cortex that may vary with current visual input (Ruff et al. 2008, 2009). But their design did not allow any test for whether remote effects of parietal TMS on visual cortex might vary with attentional state in a purely top–down, task-related manner, when visual stimulation is held constant so that bottom–up factors do not vary. Any dependence of PPC TMS effects for BOLD signal in visual cortex upon current top–down attentional state would constitute a new form of evidence that “effective connectivity” between PPC and visual cortex may vary in a purely top–down manner, as a function of attention (see Buchel et al. 1998; Corbetta et al. 2005; Saalmann et al. 2007; Driver et al. 2009).
Here, we used a blocked visuospatial covert attention task in order to investigate any attentional-state dependence of PPC TMS effects upon early visual processing. The visual stimuli were held constant across conditions and comprised a series of bilateral checkerboards, each containing embedded small targets in the form of deviant checks (see Fig. 1). Participants had to indicate by a button press the number of small targets (2, 3, or 4) within each successive checkerboard, for the currently attended side only, which was blocked. During visual stimulation, event-related bursts of TMS with a high or low intensity were applied over right PPC. We used low-intensity TMS (expected to be neurally ineffective) as a control condition for nonspecific effects of TMS, such as the “click” sound inevitably associated with TMS delivery and/or any brain activations associated with anticipation of TMS delivery (see also Ruff et al. 2006, 2008, 2009; Bestmann, Ruff, Blankenburg, et al. 2008; Bestmann, Swayne, et al. 2008; Blankenburg et al. 2008). Please note that the comparison of different TMS intensities is better controlled in such respects than would be the case if instead comparing effective TMS to no TMS whatsoever.
Figure 1.
Schematic illustration and timeline of the sequence of events starting from the beginning of a block. Empty square placeholders were present in the left and the right upper quadrants (each square subtending 7° visual angle, centered at an eccentricity of 8.5° vertically and 6° horizontally) throughout the experiment in order to denote the locations where bilateral checkerboards could appear (8 × 8 checks each, 0.875° visual angle per check, with 2, 3, or 4 checks marked in red pseudorandomly for each checkerboard). Note along the timeline how functional image acquisition was interleaved with presentation of TMS bursts and bilateral visual displays, with the TMS bursts and bilateral displays coinciding.
The right PPC stimulation site was selected based on fMRI results from a classic visual attention study by Hopfinger et al. (2000) that reported possible involvement of this site in top–down attentional modulation of visual processing. As well as comparing left versus right attention blocks, we also implemented a more “neutral” condition that did not require judgments of one or other side (see below). To anticipate, we found that high- versus low-intensity PPC TMS affected BOLD signal in extrastriate visual cortex (likely including V4 as defined by separate anatomical information, see below) in a manner that depended strongly on current attentional state, as manipulated in a purely top–down, task-related manner.
Material and Methods
Eight participants were screened for MRI and TMS compatibility and gave written informed consent in accord with local ethics. The study was approved by the joint ethics committee of the National Hospital for Neurology and Neurosurgery (UCL Hospitals National Health Service Foundation Trust) and UCL Institute of Neurology. One subject was excluded due to poor behavioral performance (chance level) and another for technical reasons. The remaining 6 were all male right-handers, aged 24–35 years.
The Montreal Neurological Institute (MNI) coordinates for the TMS site over right PPC (x = 22, y = −60, z = 60) were derived from the peak fMRI activation in a previous study of visual attention by Hopfinger et al. (2000). The structural scans of each of our participants were normalized into MNI standard space. The inverse of this spatial mapping was then used in order to obtain the coordinates of the above mentioned location for each subject, starting from the MNI coordinate and transforming back to their individual native space. The corresponding scalp site was then localized with Brainsight, a frameless stereotaxy system (Rogue Research, Montreal, Canada), and marked on the head of the participant prior to scanning. The subject was placed into a 1.5-T whole-body scanner (Magnetom Sonata, Siemens Medical System, Erlangen, Germany) and the head fixed with vacuum pads within a custom-built visual surface MR coil (Nova Medical, Boston, MA) that had maximum sensitivity over occipital cortices extending into temporal cortices. Please note that our use of an occipital surface coil, in order to maximize sensitivity for visual cortex, inevitably meant that we could not record attention-related and/or TMS-related signals in more anterior structures, such as frontal cortex. To remind readers of this throughout, we indicate the extent of the imaged volumes for each brain figure in this paper.
The occipital surface coil was operated in receive mode only. Transmission of radiofrequency signals was performed with the scanner's inbuilt body coil. A custom-built, figure-of-8, MR-compatible TMS coil (30 mm inner diameter, 70 mm outer diameter, 15 turns each winding, 22.9 mH inductance, 4.7 kVA predicted maximal current at 100% stimulator output; from the MAGSTIM Company, Dyfed, UK) was carefully placed over the marked PPC site and fixed by means of an MR-compatible custom coil holder. The TMS coil was connected to a Magstim SuperRapid stimulator (MAGSTIM Company) that was housed in a shielded metal cabinet inside the scanner room, via a custom filter box (the MAGSTIM Company), and ferrite sleeves (Wuerth Elektronik, Waldenburg, Germany). The participant had an MR-compatible response panel in their right hand on which they could press any 1 of 3 buttons.
Functional data were acquired using a gradient echo planar imaging sequence with the following parameters: TR/TA/TE = 3000/2430/50 ms, FA = 90°, 27 axial slices, slice thickness = 2 mm, interslice gap = 1 mm, matrix size = 64 × 64, FoV = 192 × 192 mm2. The readout bandwidth was 2298 Hz per pixel, the echo spacing was 500 μs.
We used a blocked spatial attention task, comprising sustained covert visuospatial attention to checkerboards in one or other hemifield during successive bilateral stimuli or a more “neutral” baseline task (see below) during the same stimuli (see Fig. 1). Each block lasted 30 s and started with a spatial cue (fixation point turning into an arrowhead for 570 ms) indicating that attention should be covertly directed either to the right or left hemifield or maintained neutrally. Thus, our paradigm involved “sustained” attention to one or other side (or neither) during each block, rather than any shifts of attention within a block (cf. Molenberghs et al. 2007; Kelley et al. 2008). The cue was presented in the first pause (570 ms) between successive scans (27 slices acquired in 2430 ms) within a block. In the subsequent 9 pauses (each 570 ms) between successive scans (each 2430 ms) within each block, bilateral checkerboards were presented in both upper quadrants with embedded red deviants (2, 3, or 4 of these per hemifield, determined randomly and independently) for 500 ms. Following the arrowhead cue, throughout the block subjects had to count the number of “deviant” red checks within each successive black-and-white checkerboard on that side (which varied unpredictably as 2, 3, or 4 red checks), while ignoring comparable but independent numbers of red deviant checks within the concurrent checkerboard in the left hemifield. After each successive bilateral visual presentation, the subjects had to indicate by a button press how many red deviants were present (right index finger = 2 targets, middle finger = 3 targets, or ring finger = 4 targets) for the attended side only. Visual checkerboards were equivalent (and red checks fully counterbalanced) over the course of the experiment in all conditions. On the remaining one-third of “neutral” blocks, an upward central arrow cue indicated that there was no longer any requirement to direct covert spatial attention to one side or other. Equivalent bilateral checkerboards (with red deviants) were presented as before, but the instruction was now simply to press a single specific button whenever bilateral stimulation appeared, rather than concentrating on one side only for the demanding red deviant count within each checkerboard on a particular side.
In addition, a burst of 5 pulses of TMS with either high intensity (75% stimulator output) or low intensity (35% stimulator output) was applied at 10 Hz over the right parietal cortex site, concurrently with each 500-ms checkerboard display. It might be interesting to apply TMS at various time points during such attentional tasks, for instance during preparation for upcoming displays or during stimulus presentation. For this initial study, we chose to present the TMS pulses together with the checkerboard stimuli since we knew that visual attention should be engaged as instructed at these time points and could confirm that via the accuracy of the behavioral responses in the attend-right or attend-left conditions.
We note that the MR-compatible TMS coil and connecting cables produce somewhat less intense stimulation than standard TMS systems outside the scanner (see Ruff et al. 2006 for details), but nevertheless with highly reproducible intensities, and clear differences between 75% and 35% output. The latter low intensity should not be neurally effective but was intended to control for nonspecific factors such as the “click” sound associated with TMS delivery and/or expectation of TMS delivery. Each block of concurrent TMS bursts and bilateral visual stimulation was followed by 5 interblock volumes of MR acquisition, in which only the fixation cross and the empty placeholders were presented. Each of the 6 blocked conditions (attend right or attend left, during low or high TMS; or neutral attention, also with low or high TMS) was repeated 4 times in random order. Three hundred and seventy volumes were acquired in one session (6 volumes were discarded to allow for T1 saturation). The data were analyzed with SPM2 (Friston et al. 1995).
Eye position, pupil diameter, and any blinks were monitored at 60 Hz throughout scanning with an ASL 504 Remote Optics Eye tracker (Applied Science Laboratories, Bedford, MA), via the same mirror used for visual stimulus viewing.
After image reconstruction, the MR volumes were screened for any potential TMS-related artifacts (see Ruff et al. 2006, 2009; Bestmann, Swayne, et al. 2008; Blankenburg et al. 2008). A moving average of 10 successive slices was used to detect any outliers (voxels with values >2 SDs from the mean signal of the slices obtained from the moving average). The identified slices were then visually inspected and if necessary (as applied for 0.6% of all acquired slices) substituted by the mean of the preceding and succeeding slice. The data were unwarped and normalized to the MNI standard brain by coregistering the anatomical scan to the mean of the functional scan and then applying the transformation from normalization of the anatomical scan to the functional data. In addition, the data were interpolated (2 × 2 × 2 mm), detrended (Macey et al. 2004), and smoothed with an isotropic Gaussian kernel of 9 mm3. The onsets of each successive trial within a blocked condition (attend neutral TMS low; attend neutral TMS high; attend right TMS low; attend right TMS high; attend left TMS low; or attend left TMS high) were convolved with the canonical hemodynamic response function and entered into a fixed effect analysis.
In our analyses below, we do not include the main effect of high versus low TMS because that could in principle be contaminated by a potential MR artifact in the vicinity of the TMS coil (Weiskopf et al. 2009). Switching randomly between high and low TMS intensities can induce a very small leakage current in the TMS coil inside the scanner, which can change the local magnetic field properties and thereby the local MR signal intensity, for the main effect of high versus low intensity TMS, near the TMS coil. Hence, this main effect cannot be interpreted here. But importantly, any such potential artifacts could not contribute to the most critical contrasts in our study, namely, interactions between TMS effects and the currently attended location, as any TMS artifact per se could not interact with the cognitive task. Nevertheless, to provide a further check on this issue, we did confirm that there was no TMS intensity main effect within those brain regions that turned out to be affected by the critical interaction (which all fell within extrastriate visual cortex, remote from the TMS coil; see below).
Raw eye-position data were filtered to identify and exclude blinks and then transformed to degrees of visual angle. Blinks were identified as continuous losses of pupil signal for more than 5 frames (80 ms). In order to eliminate any possible visual activations induced by eye blinks or changes in pupil size, we also modeled those parameters with 2 additional regressors (see also Ruff et al. 2006, 2008), ensuring that any variance due to those eye parameters was partialed out in the GLM analysis and so could not contribute to significant results for the other regressors of interest.
As it turned out, the active experimental conditions did not affect eye position, eye position variability or pupil diameter in any case. This was confirmed by a 2 (attend left vs. right) × 2 (high vs. low TMS intensity) repeated-measures ANOVAs on the eye-position or pupil data, which found no significant effects of TMS intensity or left/right attention condition, neither main effects (all F1,5 <0.42, nonsignificant [n.s.]) nor interactions (all F1,5 <0.60, n.s.). Thus, neither eye position nor pupil diameter varied as a function of our main conditions.
As an additional parametric regressor in the SPM analysis, reaction times were also included in the model to account for any additional variance in the data. The reported activations were either familywise error (FWE) corrected for the entire brain or for a volume of interest (Worsley et al. 1996) independently defined by an orthogonal contrast, as specified below. All activations are reported in MNI space.
Results
Behavioral Data
Performance of the attention task did not differ between left and right hemifields, and importantly our TMS manipulation did not disrupt behavior (see below for statistical confirmation). Hence, the TMS influences we report for the BOLD data are uncontaminated by behavioral change, consistent with the present use of TMS here as a physiological probe for remote influences upon visual cortex, rather than as a way to disrupt behavior (see also Ruff et al. 2006, 2008; Bestmann, Swayne, et al. 2008), as we discuss later. The only behavioral effect was that responses were quicker and more accurate for the less demanding “neutral” condition, as expected. The neutral condition merely required a button to be pressed as soon as a bilateral display appeared (mean reaction time ± standard deviation of 595 ± 158 ms under low TMS, 614 ± 135 ms under high TMS), with accuracy unsurprisingly 100% due to the foreknown response in the blocked neutral condition. Reaction times were slower for the more demanding attend-left condition (815 ± 119 ms under TMS low, 824 ± 120 ms under TMS high) and attend-right condition (857 ± 140 ms under TMS low, 891 ± 166 ms under TMS high), with no difference between these (P > 0.25, n.s.). A 3 × 2 ANOVA found no effect or interaction involving TMS (P > 0.6, n.s.) but a main effect of task (P < 0.01). The task effect was due solely to the expected faster performance in the neutral condition.
A similar pattern was found for accuracy (attend-left 80% correct under low TMS and 81% correct under high TMS, attend-right 77% correct under low TMS and 80% correct under high TMS), with no impact of TMS on this (all P's > 0.7, n.s.) and no difference between attend-left and attend right (P > 0.7, n.s.). Accuracy was 100% in the neutral condition but trivially so.
fMRI Data
We initially contrasted the active attention conditions (i.e., attend-right and attend-left, considered together) minus the less demanding “neutral” condition. As expected, this revealed posterior components (recall our use of a posterior MR surface coil) of the well-described “attentional network,” plus visual areas modulated by the requirement for the deviant-check task. Thus, occipital, temporal, and some parietal regions (falling within the scanned volume) all showed more activation during the demanding attention task than in the less demanding “neutral” condition (see Fig. 2, which omits anterior brain regions beyond the scanned volume, and Table 1). This included some activation in right superior parietal cortex, although note that we had less sensitivity to BOLD signals there than for more posterior regions due to our use of a surface coil to maximize power for visual cortex.
Figure 2.
(A) Group statistical parametric T-maps shown for the contrast of active attention (red-deviant target-counting task initially considered regardless of whether this was required for the left or right visual field) versus the “neutral” attention condition (that required only a simple button press whenever bilateral visual displays appeared). The SPM for this contrast is superimposed onto the segmented and rendered brain (with the cerebellum removed plus anterior brain regions missing that were not covered by the posterior MR surface coil) of one subject (thresholded at P <0.05 FWE corrected; see Table 1 and main text for peak coordinates of activations). Wide expanses of visual cortex were modulated by the attentional task, despite equivalent visual stimuli being presented in all conditions, as was superior parietal cortex also within the scanned volume. The L/R labels in the figure identify the left versus right hemispheres, as also for panels (B) and (C). Please note that in the rotated views that reveal ventral cortex in the lower panels, posterior cortex appears upper. Anterior regions beyond the scanned volume have been removed. (B) displays the group statistical T-map of the contrast for attending left minus right, superimposed onto the rendered brain of one subject; (C) shows the reverse contrast, that is, attending right minus attending left. Thresholded at P <0.05 FWE corrected; see also Table 1 and main text. Thus, wide expanses of occipital visual cortex showed higher BOLD signal for contralateral than ipsilateral covert visual attention, as expected.
Table 1.
Main activation clusters for the contrast of active peripheral attention (to the left or to the right initially considered together) versus neutral attention; or for attention left versus right; or attention right versus left.
| Region | Left | FWE | Right | FWE |
| Active peripheral attention versus neutral attention | ||||
| IPL | −40, −38, 42 | <0.05 | 40, −40, 48 | <0.05 |
| SPL | −26, −66, 60 | <0.05 | 28, −60, 56 | <0.05 |
| MOG | −36, −84, 20 | <0.05 | 34, −76, 22 | <0.05 |
| LG/CS | −19, −74, 10 | <0.05 | 16, −70, 8 | <0.05 |
| IOG/ITG | −42, −68, −12 | <0.05 | 48, −56, −12 | <0.05 |
| Attention left versus right | ||||
| LG | 20, −76, −8 | <0.05 | ||
| MOG | 46, −76, 2 | <0.05 | ||
| SOG | 28, −84, 26 | <0.05 | ||
| Attention right versus left | ||||
| LG | −20, −72, −12 | <0.05 | ||
| MOG | −30, −84, 18 | <0.05 | ||
| MOG | −40, −82, 8 | <0.05 | ||
Note: Recall our use of a posterior occipital surface coil, with no sensitivity for more anterior structures, such as frontal cortex. IPL = inferior parietal lobule, SPL = superior parietal lobule, MOG = middle occipital gyrus, LG/CS = lingual/calcarine gyrus, IOG/ITG = inferior occipital gyrus/inferior temporal gyrus, SOG = superior occipital gyrus.
The more specific contrasts of attend-left minus attend-left, or vice versa, revealed the anticipated activations of the contralateral visual hemisphere (see Fig. 2B,C, plus Table 1).
We turn now to the crucial question of our study, which is whether high versus low TMS over right PPC had any remote impacts (e.g., on visual cortex) that might vary with the current attentional state (as manipulated here in a purely top–down manner, by the current task requirements). We specifically tested for the interactions of attending left > right (or vice versa), as a function of high > low TMS to PPC. We initially did so within brain regions that had already shown a main effect of contralateral attention overall (cf. Fig. 2B,C), correcting our interaction tests for this independently defined volume of interest. Note that for these interactions the applied high- or low-intensity TMS over the right PPC remains the same, but we now assess whether its impact on the brain varies with cognitive context in a purely top–down, task-dependent, attention-related manner.
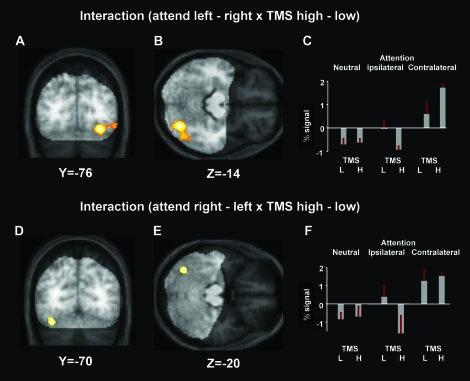
The interaction of high versus low TMS intensity with left minus right side of attention revealed a cluster (see Fig. 3A,B, which also differentiate the scanned volume from more anterior areas) in right occipital–temporal cortex (peak in right fusiform gyrus, x = 30,y = −76, z = −14) at P < 0.05 FWE corrected for the volume yielded by the orthogonal contrast of attending left minus right (cf. Fig. 2B). Thus, some regions in right occipital–temporal cortex that showed activation by contralateral attention (vs. ipsilateral) showed a more pronounced version of this attentional modulation under high- than low-intensity TMS over right PPC (see plots in Fig. 3C and the corresponding figure legend). Note that this pattern clearly reflects a modulation of remote TMS effects by current cognitive state (i.e., with the TMS effect depending on the focus of covert spatial attention toward left or right, as determined in a purely top–down manner by the current task). Moreover, exactly the same TMS manipulation (high minus low intensity) had no significant influence on these same right occipital–temporal regions during the less demanding neutral condition (see plots in Fig. 3C). Please note that signal change was defined relative to the overall session mean (i.e., “zero” on the y-axis in the plots of Fig. 3 corresponds to that overall mean value) in the plots so that negative beta values do not necessarily reflect a “deactivation.”
Figure 3.
(A, B) Interaction of high versus low TMS intensity with side of attention (left minus right), small volume corrected (at P <0.05 FWE) for the orthogonal contrast of attend left minus right (cf. Fig. 2B), projected onto the mean (A) coronal and (B) transversal structural scan of the subjects, with structural regions beyond the imaged functional volume (recall that a posterior surface MR coil was used for fMRI) shown in lower contrast. The interaction reveals that some regions in right occipital–temporal cortex that had displayed activation by contralateral attention (vs. ipsilateral, cf. Fig. 2B) showed a stronger version of this differential attentional modulation under high than low intensity TMS over right PPC. (C) plots the extracted data in percent signal change (relative to the session mean, which corresponds to “zero” along the y-axis) from the cluster, now including for neutral attention conditions also (though these did not contribute to the tested interaction). Note that high versus low TMS had no impact whatsoever under neutral attention but that high TMS specifically increased the differential effect of contralateral minus ipsilateral attention during the peripheral attention conditions. These results thus demonstrate a remote effect of right parietal TMS on visual cortex that is highly dependent on current attentional state. (D, E) Interaction of high versus low TMS intensity with side of attention (now right minus left), small volume corrected (P <0.05 FWE) for regions that also showed an effect of contralateral versus ipsilateral (here, right minus left) attention in that orthogonal contrast (cf. Fig. 2C). The SPM for this contrast is overlaid on the (D) coronal and (E) transversal slice of the averaged structural scan (with lower contrast for anterior regions beyond the volume imaged for fMRI). Thus, some regions in left occipital–temporal cortex that showed activation by contralateral attention (vs. ipsilateral) also showed enhancement of this differential attentional modulation under high- versus low-intensity TMS over right parietal cortex. (F) plots the extracted data in percent signal change (relative to the session mean) from the left occipitotemporal cluster, now including the neutral attention conditions also (although these did not contribute to the tested interaction). Note that high versus low PPC TMS had no impact whatsoever under neutral attention but that it increased the differential effect of contralateral minus ipsilateral attention during the peripheral attention conditions; see main text for discussion.
The present study did not include retinotopic mapping of specific visual areas, but we used an anatomical toolbox (Eickhoff et al. 2005) to ascertain probabilistic regional assignment of the substantial cluster in right occipital–temporal cortex that showed the critical interaction (see Fig. 3A,B). This yielded a 70% assignment to anatomically defined right V4, with the remainder of the cluster evidently extending in the right fusiform gyrus (which is not strictly defined by the anatomical toolbox).
The opposite interaction, of high versus low TMS intensity but now with right minus left side of attention, revealed instead an activation (see Fig 3D,E) in the opposite left occipital–temporal cortex (peak in left fusiform gyrus, x = −36, y = −70, z = −20), at P < 0.05 FWE corrected for the orthogonal contrast of attending right minus left (cf. Fig. 2C). Thus, analogously to the result in Figure 3A,B, some regions in left occipital–temporal cortex that showed activation due to contralateral versus ipsilateral attention also exhibited a stronger version of this attentional modulation under high- than low-intensity TMS over right parietal cortex (see Fig. 3F and its legend). Once again, this remote effect of TMS was absent during the less demanding neutral condition (Fig. 3F). The anatomical toolbox yielded a 20% assignment of this interaction cluster (Fig. 3D,E) to left V4, with the remainder clearly extending more anteriorly, including into the left fusiform gyrus.
We found no significant interactions of TMS intensity with attended side beyond these reported regions or beyond the network initially defined as in Figure 2A. But recall that our use of an occipital surface MR coil, in order to maximize power for visual cortex, will lead to less sensitivity for more anterior regions and to zero sensitivity for brain areas beyond the imaged volume. Likewise, we found no significant interactions between TMS intensity and active attention versus neutral in other regions.
Discussion
Here, we used the recently introduced approach of concurrent TMS–fMRI to assess how spatial attention can modulate the influences of TMS bursts over right PPC on processing in visual cortex. The form of TMS bursts applied here would usually be thought of as an “excitatory” intervention but is perhaps most neutrally considered as a targeted perturbation (see Bestmann, Ruff, Blankenburg, et al. 2008). For present purposes the key point was that the TMS provided a causal intervention at the targeted right PPC site. Our specific question was whether any remote impacts of high- versus low-intensity PPC TMS upon occipital visual cortex might vary with the current attentional state when attention was manipulated in a purely top–down manner, by current task demands. Our attentional manipulations were blocked, requiring either a demanding judgment to be made for the checkerboards in just the left visual field or for just those in the right visual field, during equivalent bilateral visual stimulation. We also ran a more “neutral,” less demanding baseline task, in which participants simply had to press one button whenever the bilateral visual displays appeared. Importantly, the bilateral visual stimulation itself was equivalent across all 3-blocked attention conditions (unlike the TMS–fMRI studies of Ruff et al. 2006, 2008, 2009, which had varied bottom–up visual input), while eye tracking confirmed that neither eye position, nor its variability, nor pupil dilation, differed systematically between any of our conditions. Hence, bottom–up factors were held constant here.
We factorially crossed the 3-blocked attention conditions with high- or low-intensity TMS delivered over right PPC at coordinates chosen because of their possible implication in visuospatial attention by previous fMRI work (e.g., Hopfinger et al. 2000). Our TMS manipulation did not alter behavioral performance, being used here instead as a physiological perturbation to probe whether remote influences of high versus low PPC TMS (as assessed with the concurrent fMRI) might vary with the current attentional state due to purely top–down task-related factors. The lack of TMS-induced changes in performance within our physiological perturbation approach means that the TMS effects on BOLD signals that we report are not contaminated by any associated behavioral changes that might otherwise have made them harder to interpret (see Ruff et al. 2006, 2008, 2009; Bestmann, Swayne, et al. 2008; Blankenburg et al. 2008).
Contrasting the active attention conditions (i.e., attending left or right initially considered together) versus the neutral baseline task condition revealed activation (see Fig. 2A) of extensive occipital–temporal regions (plus some parietal regions within the posteriorly imaged volume), consistent with the higher demands of the active attention task. In addition, attend left minus right, or vice versa, revealed the expected lateralized activations (see Fig. 2B,C) due to contralateral versus ipsilateral attention in occipital–temporal visual areas, consistent with much previous work (e.g., Heinze et al. 1994; Hopfinger et al. 2000; Corbetta and Shulman 2002; Yantis and Serences 2003).
Our most crucial and novel result was that the impact of high versus low right PPC TMS on remote visual cortex varied in a highly attention-dependent manner, when only the top–down task demands were changed. Specifically, the impact of high- versus low-intensity TMS on remote occipitotemporal cortex differed as a function of which hemifield was currently attended in the active attention conditions. For leftward minus rightward attention, high-intensity TMS over right PPC increased the differential effect of contralateral versus ipsilateral attention for an extensive cluster in right occipital–temporal cortex (see Fig. 3A–C). By contrast, exactly the same TMS manipulation had no significant influence on these same right occipital–temporal regions during the less demanding neutral condition (see plot in Fig. 3C). An anatomical toolbox (Eickhoff et al. 2005) assigned the critical interaction cluster (Fig. 3A,B) primarily to anatomically defined right V4, but this cluster extended into the right fusiform gyrus also.
A more restricted region of left occipital cortex (partially attributable to anatomically defined V4 but extending anteriorly into the left fusiform gyrus, see Fig. 3D,E) showed a somewhat analogous pattern. Again, high versus low PPC TMS increased the differential effect of contralateral versus ipsilateral attention but now for attending right minus left. However, this effect mainly reflected (see plot in Fig. 3F) lower BOLD signal during high TMS and leftward attention, the very same situation that also led to the highest occipital activations in the opposite right hemisphere (cf. Fig. 3A–C). The left-hemisphere BOLD decreases found for leftward attention might therefore potentially reflect interhemispheric competition with the more activated right visual hemisphere in that particular situation. But regardless of such interpretative issues, the present finding for left occipital cortex certainly shows that TMS applied to one hemisphere can have consequences for BOLD signals in the other hemisphere (see also Bestmann, Swayne, et al. 2008; Blankenburg et al. 2008; Driver et al. 2009). Once again, exactly the same TMS manipulation (of high vs. low intensity) that had significant remote effects on left occipital cortex during comparisons of attended side (see Fig. 3D–F; as also for right occipital cortex cf. Fig. 3A–C) actually had no significant effect during the less demanding neutral baseline condition (see plot in Fig. 3F).
These data provide direct evidence that influences of high versus low right PPC TMS on occipital visual cortex can vary with current attentional state, when the side of sustained spatial attention is manipulated in a purely top–down manner, while holding the visual stimuli and response requirements constant. The interaction of TMS intensity with attended side in occipital regions (likely to include V4) seems consistent with previous evidence for strong attentional modulation there (e.g., Moran and Desimone 1985; Heinze et al. 1994; Pinsk et al. 2004) and with proposals that V4 may serve as a particularly important “gateway” into further processing along the ventral stream (e.g., McAdams and Maunsell 1999; Pinsk et al. 2004; Schwartz et al. 2005), as for more anterior fusiform gyrus here.
We attribute the present condition-dependent remote effects of TMS to top–down influences from parietal cortex that vary with attentional condition in a top–down manner, since the bottom–up visual stimulation with bilateral checkerboards was constant across all the different conditions here. We acknowledge, however, that in future extensions of the paradigm introduced here it might be interesting to examine any remote effects of parietal TMS on BOLD activity not only when the bilateral visual stimulation is present, but also shortly before presentation of the visual stimuli (i.e., when anticipatory attention may already be directed to the task-relevant hemifield but in the absence of current visual input). A pioneering recent monkey study by Ekstrom et al. (2008), which combined microstimulation (rather than TMS) with fMRI in macaques, showed that some top–down influences (in their case, from FEF rather than PPC as here) can depend on the current level of bottom–up visual input.
An intriguing aspect of our present findings was that for the critical regions showing the interaction effects (see Fig. 3A/B and 3D/E) the differential effects of attending contralaterally versus ipsilaterally were actually increased rather than decreased by higher intensity TMS (see plots in Fig. 3C,F). This might initially seem counterintuitive if one supposed that high-intensity TMS should always tend to “knock out” rather than enhance particular effects, somewhat akin to a lesion or a so-called “virtual lesion.” However, as briefly mentioned in our Introduction, short bursts of TMS like those used here are often considered to provide “excitatory” protocols (see Wasserman et al. 2008; Ruff et al. 2009) that may induce depolarization and action potentials in the targeted region (Allen et al. 2007). Although BOLD signals do not of course measure such neuronal events directly, one can think of the current TMS protocol as probably inducing activity in the targeted region (here right PPC) that may then propagate to functionally interconnected regions (not necessarily only monosynaptically connected), such as occipital cortex (see Ungerleider et al. 2008) for evidence of some parietal–occipital connections in monkey). From such a perspective, our results indicate that the propagated influence of right PPC stimulation upon visual cortex (probable V4 and downstream fusiform gyrus) can vary substantially as a function of the currently attended visual hemifield, as determined in a purely top–down manner. Such attention dependence in the propogation of TMS effects may relate to attention-dependent changes in “effective connectivity” (see Buchel et al. 1998; Saalmann et al. 2007; Taylor et al. 2007; Womelsdorf and Fries 2007; Fuggetta et al. 2008; Siegel et al. 2008; Driver et al. 2009).
We note that our most general point, concerning the dependence of the observed remote effects on purely top–down attentional factors, should still stand regardless of the exact neuronal mechanisms underlying TMS action. Nevertheless, it should be acknowledged that a very different outcome might have been observed if a highly “inhibitory” TMS protocol had been used instead, say involving very extended application of TMS with the aim of producing reduced excitability at the targeted site (e.g., Huang et al. 2005). Such prolonged “off-line” TMS protocols might operate somewhat more like a “virtual lesion” than for the present online use of short TMS bursts.
Some recent studies have literally taken a lesion approach to study the possible role of parietal cortex in attentional influences upon visual cortex by using fMRI in brain-damaged patients. Vuilleumier et al. (2008) reported that 2 patients with focal damage to right parietal cortex showed pathological effects of foveal attentional load on visual responses to peripheral stimuli in right visual cortex, especially for V4. He et al. (2007) showed that right hemisphere damage can lead to pathologies in interregional coupling that can relate to the severity of clinical deficits and to recovery. Such patient fMRI work is broadly consistent with the present study, in showing the importance of remote influences from parietal areas within an extended interconnected network. But the present work differs very substantially in the particular methods used (concurrent TMS–fMRI in neurologically intact subjects vs. fMRI in lesioned patients) and also in the particular attentional manipulations (sustained attention to left or right side here vs. attentional load at fixation in Vuilleumier et al. [2008] or cued attentional shifts in Corbetta et al. [2005] and He et al. [2007]). Some other fMRI work in healthy participants indicates that further subregions of parietal cortex may be particularly involved in shifts of attention (e.g., Molenberghs et al. 2007; Kelley et al. 2008), but the present work clearly implicates a role for the targeted right PPC region in sustained covert spatial attention to one or other hemifield.
A further intriguing aspect of the present findings was that high versus low TMS over right PPC had some attention-dependent impact not only on ipsilateral right occipitotemporal cortex (Fig. 3A,B) but also (albeit to a lesser extent) on contralateral left occipitotemporal cortex (Fig. 3C,D). Moreover, not only right PPC but also left PPC was activated when comparing the left/right attention conditions jointly to the less demanding neutral task (Fig. 2A). Future variations on the paradigm introduced here could explore whether high- versus low-intensity left PPC TMS will have a similarly attention-dependent impact or whether its impact might be more restricted to ipsilateral left visual cortex. The latter outcome might be consistent with some interpretations of the clinical neglect syndrome (see Mesulam 1999), in terms of right PPC being involved in spatial attention to either side but left PPC only for attention toward the right. Alternatively, left PPC TMS might even have no impact on visual cortex at all (see Ruff et al. 2009, for TMS–fMRI evidence on this but acquired during rest rather than during active attentional tasks as here). Depending on further methodical developments, futures studies may even investigate such issue by stimulating 2 distinct TMS sites concurrently or in series, with multiple TMS coils during concurrent fMRI, to study the impact of perturbations at multiple loci during spatial attention.
In conclusion, the present concurrent TMS–fMRI study provides direct new evidence for remote causal influences of right PPC upon visual cortex. Importantly, we find that the remote effects of high versus low right PPC TMS can vary with the current attentional state, as here when attending to the left or right hemifield (or in the less demanding neutral condition), even when this is determined in a purely top–down, task-related manner. Our results add to a growing literature on possible interplay between PPC and visual cortex in humans (e.g., see Hung et al. 2005; Ruff et al. 2008; Silvanto et al. 2009) and in monkeys (see Saalmann et al. 2007). The critical new aspect of our findings lies in demonstrating that the impact of right PPC stimulation on occipitotemporal visual cortex (probable V4 and fusiform cortex) is heavily dependent on the current attentional state. Such states are now also known to affect the functional coupling, synchronization, or coherence between distinct but interconnected nodes in the attention network, as has been shown with sophisticated connectivity analyses of fMRI data, or MEG and EEG studies of synchronization and coherence between distinct sources, or with invasive recordings from multiple areas concurrently in animals (e.g., Buchel et al. 1998; Saalmann et al. 2007; Womelsdorf and Fries 2007; Siegel et al. 2008; Driver et al. 2009). We suggest that the change in remote effects of TMS with attentional state here may reflect such changes in communication between remote brain areas. Accordingly, in future research, it will be important to assess whether remote effects of TMS in fMRI, that depend on the current state of spatial attention, are predictable from corresponding changes in effective connectivity, synchronization, or coherence as assessed with other experimental approaches.
Funding
Wellcome Trust; Medical Research Council; Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung); Biotechnology and Biological Sciences Research Council; BrainSync (EU FP7 200728); Research Priority Program on the Foundations of Human Social Behavior at the University of Zurich to C.C.R.
Acknowledgments
J.D. is a Royal Society Anniversary Research Professor. We thank Nikolaus Weiskopf and all staff from the Wellcome Centre for Neuroimaging at UCL for their help and baby Jacques for inspiration. Conflict of Interest: None declared.
References
- Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Blankenburg F, Weiskopf N, Driver J, Rothwell JC. Mapping causal interregional influences with concurrent TMS-fMRI. Exp Brain Res. 2008;191:383–402. doi: 10.1007/s00221-008-1601-8. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Driver J, Blankenburg F. Concurrent TMS and functional magnetic resonance imaging: methods and current advances. In: Wasserman EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby S, editors. The Oxford handbook of transcranial stimulation. Oxford: Oxford University Press; 2008. [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Haggard P, Weiskopf N, Josephs O, Driver J, Rothwell JC, Ward NS. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex. 2008;18:1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Wassermann EM, Ziemann U, Lorberbaum JP, Nahas Z, Lomarev MP, George MS. BOLD-f MRI response to single-pulse transcranial magnetic stimulation (TMS) J Magn Reson Imaging. 2000;11:569–574. doi: 10.1002/1522-2586(200006)11:6<569::aid-jmri1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion. A functional MRI study. Brain. 1998;121(Pt 7):1281–1294. doi: 10.1093/brain/121.7.1281. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Driver J, Blankenburg F, Bestmann S, Vanduffel W, Ruff CC. Concurrent brain-stimulation and neuroimaging for studies of cognition. Trends Cogn Sci. 2009;13:319–327. doi: 10.1016/j.tics.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. 2008. TMS stimulation coils. Wassermann E, Epstein C, Ziemann U, Walsh V, Paus T, Lisanby S, editors. Oxford handbook of transcranial stimulation. 1st ed. Oxford: Oxford University Press. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Pavone EF, Fiaschi A, Manganotti P. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: a combined EEG and TMS study. Hum Brain Mapping. 2008;29:1–13. doi: 10.1002/hbm.20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H, et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hung J, Driver J, Walsh V. Visual selection and posterior parietal cortex: effects of repetitive transcranial magnetic stimulation on partial report analyzed by Bundesen's theory of visual attention. J Neurosci. 2005;25:9602–9612. doi: 10.1523/JNEUROSCI.0879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Ruohonen J, Virtanen J, Aronen HJ, Karhu J. EEG responses evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1999;51:22–29. [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cereb Cortex. 2008;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. NeuroImage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RR. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex. 2007;17:2703–2712. doi: 10.1093/cercor/bhl179. [DOI] [PubMed] [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. J Physiol. 2003;553:665–679. doi: 10.1113/jphysiol.2003.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. The neurobiology of visual attention: finding sources. Curr Opin Neurobiol. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Cazzoli D, Wurtz P, Luthi M, von Wartburg R, Chaves S, Deruaz A, Hess CW, Muri RM. Neglect-like visual exploration behaviour after theta burst transcranial magnetic stimulation of the right posterior parietal cortex. Eur J Neurosci. 2008;27:1809–1813. doi: 10.1111/j.1460-9568.2008.06154.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A. 2002. Handbook of transcranial magnetic stimulation. London: Arnold (Oxford University Press Distributor) [Google Scholar]
- Pascual-Leone A, Gomez-Tortosa E, Grafman J, Alway D, Nichelli P, Hallett M. Induction of visual extinction by rapid-rate transcranial magnetic stimulation of parietal lobe. Neurology. 1994;44:494–498. doi: 10.1212/wnl.44.3_part_1.494. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Allen EA, Freeman RD. State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron. 2009;62:291–303. doi: 10.1016/j.neuron.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images: a perturbation approach. Philos Trans R Soc Lond B Biol Sci. 2005;360:1109–1114. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, Kastner S. Push-pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol. 2004;92:622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- Roth BJ, Cohen LG, Hallett M. The electric field induced during magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;43:268–278. [PubMed] [Google Scholar]
- Roth BJ, Saypol JM, Hallett M, Cohen LG. A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:47–56. doi: 10.1016/0168-5597(91)90103-5. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS-fMRI. Cereb Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Weiskopf N, Driver J. Hemispheric differences in frontal and parietal influences on the human occipital cortex: direct confirmation with concurrent TMS-fMRI. J Cogn Neurosci. 2009;21(6):1146–1161. doi: 10.1162/jocn.2009.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Sack AT, Linden DE. Combining transcranial magnetic stimulation and functional imaging in cognitive brain research: possibilities and limitations. Brain Res. 2003;43:41–56. doi: 10.1016/s0165-0173(03)00191-7. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cereb Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60:709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Lavie N, Walsh V. The perceptual and functional consequences of parietal top-down modulation on the visual cortex. Cereb Cortex. 2009;19:327–330. doi: 10.1093/cercor/bhn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn Sci. 2009;13:182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Tolias AS, Sultan F, Augath M, Oeltermann A, Tehovnik EJ, Schiller PH, Logothetis NK. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron. 2005;48:901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18:477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Verdon V, Maravita A, Hutton C, Husain M, Driver J. Abnormal attentional modulation of retinotopic cortex in parietal patients with spatial neglect. Curr Biol. 2008;18:1525–1529. doi: 10.1016/j.cub.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation a neurochronometrics of mind. Cambridge (MA): MIT Press; 2003. [Google Scholar]
- Wasserman EM, Epstein CM, Ziemann U. The Oxford handbook of transcranial stimulation. Oxford: Oxford University Press; 2008. [Google Scholar]
- Weiskopf N, Josephs O, Ruff CC, Blankenburg F, Featherstone E, Thomas A, Bestmann S, Driver J, Deichmann R. Image artifacts in concurrent transcranial magnetic stimulation (TMS) and fMRI caused by leakage currents: modeling and compensation. J Magn Reson Imaging. 2009;29:1211–1217. doi: 10.1002/jmri.21749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol. 2007;17:154–160. doi: 10.1016/j.conb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]