Abstract
The cholinergic basal forebrain projects throughout the neocortex, exerting a critical role in modulating plasticity associated with normal learning. Cholinergic modulation of cortical plasticity could arise from 3 distinct mechanisms by 1) “direct” modulation via cholinergic inputs to regions undergoing plasticity, 2) “indirect” modulation via cholinergic projections to anterior, prefrontal attentional systems, or 3) modulating more global aspects of processing via distributed inputs throughout the cortex. To segregate these potential mechanisms, we investigated cholinergic-dependent reorganization of cortical motor representations in rats undergoing skilled motor learning. Behavioral and electrophysiological consequences of depleting cholinergic inputs to either motor cortex, prefrontal cortex, or globally, were compared. We find that local depletion of cholinergic afferents to motor cortex significantly disrupts map plasticity and skilled motor behavior, whereas prefrontal cholinergic depletion has no effect on these measures. Global cholinergic depletion perturbs map plasticity comparable with motor cortex depletions but results in significantly greater impairments in skilled motor acquisition. These findings indicate that local cholinergic activation within motor cortex, as opposed to indirect regulation of prefrontal systems, modulate cortical map plasticity and motor learning. More globally acting cholinergic mechanisms provide additional support for the acquisition of skilled motor behaviors, beyond those associated with cortical map reorganization.
Keywords: acetylcholine, attention, attentional mechanisms, cortical reorganization, experience-dependent plasticity, immunotoxin, learning, motor cortex, motor learning, nucleus basalis, prefrontal cortex, saporin
Introduction
Numerous studies implicate the basal forebrain cholinergic system as a key substrate associated with cortical map reorganization. The basal forebrain cholinergic system is essential for mediating the reorganization of cortical motor representations in association with skilled motor learning (Conner et al. 2003; Ramanathan et al. 2009) and with recovery of motor function following cortical injury (Conner et al. 2005). Specifically, global depletion of corticopetal cholinergic innervation abolishes behaviorally mediated cortical map reorganization and impairs acquisition of skilled motor behaviors (Conner et al. 2003; Ramanathan et al. 2009). Cholinergic mechanisms also modulate sensory cortical plasticity associated with whisker pairing (Baskerville et al. 1997; Maalouf et al. 1998; Sachdev et al. 1998; Zhu and Waite 1998) and discriminatory olfactory conditioning (Linster et al. 2001). Within auditory cortex (AUD), map reorganization is enabled by nucleus basalis activity (Bakin and Weinberger 1996; Bjordahl et al. 1998; Kilgard and Merzenich 1998; Dimyan and Weinberger 1999), and learning-induced auditory plasticity and associated memory formation is blocked by pharmacological disruption of muscarinic signaling (Miasnikov et al. 2001, 2008). Together, these prior studies clearly indicate an essential role for cholinergic systems in mediating plasticity associated with cortical processing. However, prior studies have not identified the specific locus of cholinergic action mediating these effects.
In the case of skilled motor learning, it is postulated that cholinergic systems could potentially regulate motor cortex plasticity and learning via 3 distinct mechanisms: 1) through direct projections to the cortical region undergoing plasticity (motor cortex), 2) via modulation of prefrontal attentional systems that, in turn, project to the cortical region undergoing plasticity, or 3) through cholinergic actions in targets (e.g., sensory cortex or visual cortex) distinct from either the prefrontal cortex (PFC) or motor cortex that in turn converge on motor cortex to influence plasticity and motor learning. Prior experiments provide some support for each of these possibilities.
The dorsolateral PFC is strongly activated during the initial phase of learning a complex motor task in humans (Debaere et al. 2004), presumably due to high attentional demands in initial learning (Puttemans et al. 2005). PFC ablation in rats causes chronic impairments in coordinated forelimb activity (Kolb and Whishaw 1983). Behaviors that tax attention increase acetylcholine (AChE) release within PFC (McGaughy and Sarter 1998; Dalley et al. 2001; Himmelheber et al. 2001), and removal of prefrontal cholinergic inputs impairs performance in tasks requiring high attentional demand (Gill et al. 2000; Dalley et al. 2004). In addition, sensory stimulation induces cortical map reorganization only in the context of sensory-guided behavioral learning (Blake et al. 2006), likely requiring attentional mechanisms (Recanzone et al. 1992). Together, these data argue that prefrontal attentional systems, and the cholinergic modulation of attentional mechanisms, may facilitate skilled motor learning and enable associated cortical map reorganization.
There is also evidence that AChE, acting locally within cortical regions undergoing plasticity, can directly modify cortical processing and plasticity. Iontophoretic application of AChE directly within the cortex may facilitate processing of sensory information as reflected by potentiating sensory evoked responses (Donoghue and Carroll 1987; Metherate et al. 1988; Rasmusson and Dykes 1988), increasing neuronal firing rates (Metherate et al. 1988), producing shifts in neuronal receptive fields (Metherate and Weinberger 1989), and reducing activation thresholds (Metherate et al. 1990). Importantly, AChE release is selectively increased within activated sensory areas (Fournier et al. 2004), under the control of prefrontal mechanisms (Rasmusson et al. 2007). Moreover, cortical AChE release is significantly increased in association with new learning, in comparison to sensory experience devoid of learning (Butt et al. 2009). Thus, cholinergic mechanisms, acting locally within sensory and motor cortices, may enable cortical reorganization and behavioral performance during motor learning.
Finally, some evidence supports a role for more global and diverse cholinergic projections in collectively supporting cortically mediated plasticity and learning. Skilled motor learning is presumed to occur through the integration of information arising from distributed cortical systems, including visual and somatosensory regions (Pavlides et al. 1993; Shadmehr and Krakauer 2008). It has been postulated that cholinergic mechanisms act globally to facilitate the integration of distributed information by increasing coherence across distributed networks (Holschneider et al. 1998; Xiang et al. 1998; Borgers et al. 2008).
To gain insight into the parcellation of cholinergic contributions to cortical plasticity, we selectively depleted cholinergic inputs to either the motor cortex, the PFC, or global cholinergic projections, in rats learning a skilled forelimb reaching task. We then examined resulting effects on cortical map plasticity and behavior. Comparison was made with rats that underwent selective cholinergic denervation of a cortical region presumably unrelated to skilled motor acquisition, the AUD. We now demonstrate that direct cholinergic inputs to motor cortex are essential for enabling cortical map plasticity and facilitating the acquisition of a skilled motor behavior.
Materials and Methods
Experimental Design
To determine which aspects of cortical cholinergic innervation mediate cortical map reorganization and improvements in performance during acquisition of a skilled reaching behavior, site-specific cholinergic lesions were performed by direct intraparenchymal injection of the immunotoxin 192-IgG-saporin (SAP; Holley et al. 1994) into adult male F344 rats (∼275 g). Focal lesions were targeted to either motor cortex (n = 10), dorsolateral PFC (n = 10), or AUD (as a control irrelevant to motor function; n = 10). In addition, 10 animals were given global cholinergic lesions by injecting the immunotoxin directly within the nucleus basalis/substantia inominata as previously described (Conner et al. 2003, 2005), thereby eliminating cholinergic projections throughout most of the cortical mantle (Berger-Sweeney et al. 1994; Conner et al. 2003). To control for potential injury resulting from the injection procedure alone, additional rats received vehicle (artificial cerebral spinal fluid) injections into either the prefrontal (n = 3), motor (n = 7), or auditory (n = 8) cortices. Two weeks following SAP or vehicle injections, rats underwent a 3-week period of training in a skilled forelimb reaching task (Whishaw 2000; Conner et al. 2003), and reaching performance was assessed daily. Following behavioral testing, intracortical microstimulation (ICMS) was used to assess plasticity of motor representations as a consequence of training, comparing ICMS-derived maps from groups of trained animals with those obtained from untrained controls. Immediately following ICMS, rats were perfused and a quantitative assessment of cholinergic lesion effectiveness was made using established histological approaches (AChE staining) (Conner et al. 2001). Quantitative results throughout the study are expressed as the mean ± standard error of the mean.
Behavioral Training
All procedures and animal care adhered strictly to institutional guidelines for experimental animal health, safety, and comfort. Motor training was carried out using single pellet retrieval boxes as previously described (Whishaw 2000; Conner et al. 2003). All rats underwent food restriction to increase motivation to perform the task. Food was restricted for a period of 5 days before training, bringing animals to a weight of 80–85% of their free-feeding body weight. Once animals began forelimb reach training, their weights were increased back to 90–95% free-feeding weight for the duration of the study. During acquisition training, rats performed 60 reaches per day, 5 days per week, for 3 weeks. On the first 2 testing days, rats were taught to orient to the slot and were encouraged to reach for pellets by adding a small amount of peanut butter to the surface of pellets placed on the tray. Thus, accuracy measures in the skilled reaching task were first recorded on the third training day. Each day, rats were placed into the test box for 10 min or until the rat had made 60 reaches. A “reach” was scored when the rat extended its forelimb through the slot. A “hit” was scored if the rat successfully brought the pellet back to his mouth. The time to complete all 60 trials and the limb used by each animal was recorded each session. The order of testing was randomized each day.
Basal Forebrain Cholinergic Depletion
Specific destruction of the basal forebrain cholinergic neurons innervating the cortex was achieved using the SAP (Advanced Targeting Systems, San Diego, CA) (Torres et al. 1994; McGaughy et al. 2002; Conner et al. 2003). “Global” lesions of the corticopetal cholinergic system were achieved by injecting the immunotoxin directly within the nucleus basalis/substantia innominata using a Hamilton syringe equipped with a 33 gauge blunt needle (WPI, Sarasota, FL) as previously described (Conner et al. 2003, 2005). For global cholinergic lesions, the immunotoxin was injected at a concentration of 0.375 μg/μL at 2 rostrocaudal locations site #1 (0.3μL)—R/C = −1.4 mm, M/L = ± 2.5 mm, and D/V = −8.0 mm; site #2 (0.2 μL)—R/C = −2.6 mm, M/L = ± 4.0 mm, and D/V = −7.0 mm. Injections were made at 0.1 μL/min, and the needle remained in place for 4 min after each injection to allow for diffusion of the injected fluid into the parenchyma. Local depletion of discrete cortical areas was made using procedures similar to those described by others (Holley et al. 1994; McGaughy and Sarter 1998). For focal cortical depletion, the immunotoxin was injected at a concentration of 0.075 μg/μL into the following sites: “prefrontal depletion”: site #1 (0.25 μL)—R/C = +3.7 mm, M/L = ± 0.5 mm, and D/V = −3.5 mm; site #2 (0.25 μL)—R/C = +2.2 mm, M/L = ± 0.5 mm, and D/V = −4.0 mm; “motor cortex depletion”: site #1 (0.25 μL)—R/C = 0 mm, M/L = ± 3.5 mm, and D/V = −1.5 mm; site #2 (0.25 μL)—R/C = +1.5 mm, M/L = ± 3.5 mm, and D/V = −1.5 mm; “AUD depletion”: (0.5 μL)—R/C = −4.8 mm, M/L = ± 4.5 mm, and D/V = −3.0 mm from cortical surface with the electrode carrier set 28° from vertical. In all cases, cholinergic depletions were carried out bilaterally.
Electrophysiology
Standard microelectrode stimulation techniques were used to derive maps of the motor cortex both ipsilateral and contralateral to the trained paw (see Nudo et al. 1996; Kleim et al. 1998 for further details). The side of cortex mapped first was randomly determined without prior knowledge of the animal's paw preference during behavioral training. Because untrained animals did not have a “preferred paw,” the size of the forepaw representation obtained from both hemispheres was averaged. Pulled glass electrodes (input impedance ∼0.5 M-Ohm at 300 Hz), filled with 3 M NaCl, and containing a 125-μm chlorided silver wire, were used. Microelectrode penetrations were made at 500-μm intervals at a depth of ∼1700 μm (corresponding to cortical layers V and VI). Stimulation consisted of a 30-ms train of 200-μs duration monophasic cathodal pulses delivered at 333 Hz from an electrically isolated, constant current stimulator (AMPI, Jerusalem, Israel) under the control of a programmable pulse generator (AMPI). Two pulse trains were delivered 1.2-s apart, with additional pulse trains delivered as needed to assess body movements evoked by the stimulation. Evoked movements were examined with the animal maintained in a prone position and the limbs supported in a consistent manner. At each penetration site, the stimulating current was gradually increased until a movement could be detected (threshold current). If no movement was detected at 200 μA, the site was defined as “nonresponsive.” The size of the forelimb representation for each animal was determined by multiplying the number of responsive sites evoking a movement of the forelimb by 0.25 mm2.
Histology
At the end of behavioral and/or electrophysiological testing, rats were perfused with 75 mL phosphate-buffered saline and 250 mL 4% paraformaldehyde in 0.1 M phosphate buffer. Coronal sections (40 μm) were cut on a sliding microtome and a series of sections, 240 μm apart, were processed for AChE using a modified Tago method (Di Patre et al. 1993), and an adjacent series of sections (also 240 μm apart) were processed for the p75 receptor using the 192-IgG monoclonal antibody (Taniuchi and Johnson 1985) according to previously described methods (Conner et al. 1992). In brief, sections were rinsed for 30 min in 0.1 M Tris-buffered saline (TBS), blocked for 60 min in TBS containing 5% normal horse serum and incubated for 40 h (at 4 °C) in primary antibody (2.5 μg/mL for the 192 IgG). Bound antibodies were detected by sequentially incubating sections for 3 h in 1.5 μg/mL biotinylated horse antimouse IgG (Vector Labs, Burlingame, CA) and for 90 min in an avidin–biotin peroxidase reagent (1:250 dilution ABC Elite, Vector Labs). Sections were rinsed and treated with a solution containing 0.04% diaminobenzidine tetrahydrachloride, 0.06% nickel chloride, and 0.06% hydrogen peroxide in 0.1 M Tris–HCl buffer (pH = 7.4). AChE-stained and p75-labeled sections were mounted on to gel subbed slides, dehydrated in a graded series of alcohols, cleared, and coverslipped. To assess p75 immunoreactive fibers within the PFC, the immunohistochemical protocol was further modified to include a tyramide signal amplification step (TSA–biotin system; Perkin Elmer, Wellesley, MA) between processing with the secondary antibody and the ABC complex.
Assessment of Cortical Cholinergic Depletion
Histological assessment of cortical cholinergic innervation was carried out using previously described, unbiased quantitative methods (Conner et al. 2003). Sampling sites for prefrontal, motor, and auditory cortices are illustrated in Figure 4. For each cortical region, quantitative analysis was carried out within layers II/III, in 2 different brain sections spaced 240 μm apart. In addition, a qualitative assessment of the extent of cortical volume depleted by each lesion condition was made. In all cases, the experimenter conducting the analysis was blinded to group identity.
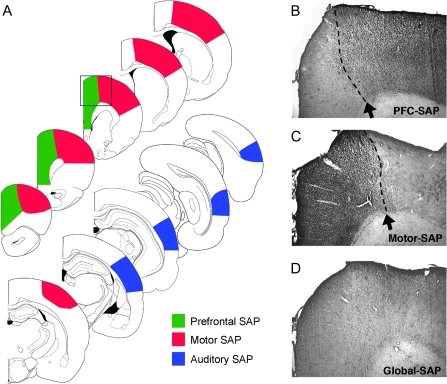
Figure 4.
Histological analysis of site-specific cholinergic lesions. (A) Series of line drawings demonstrating the extent of cholinergic depletion following focal intraparenchymal injections of the immunotoxin SAP into either the PFC, motor cortex, or AUD. Quantitative analysis of the extent of loss in innervation is presented in Table 1. Site-specific lesions depleted cholinergic inputs to the targeted region, creating a well-defined demarcation with adjacent cortical structures. For instance, injections of the immunotoxin into the dorsolateral PFC resulted in near complete loss of cholinergic fibers from within the prelimbic and cingulated cortices but did not affect innervation of the adjacent motor cortex (B). Following focal injections of the immunotoxin into motor cortex, a sharp demarcation was observed between the depleted motor cortex and the unaffected PFC (C). Global cholinergic lesions, generated by injecting the immunotoxin directly within the nucleus basalis/substantia innominata, depleted cholinergic innervation to both the prefrontal and motor cortices (D). Panels B–D were taken at the level indicated by the box in the line drawings in panel (A).
Results
Effects of Site-Specific Cortical Cholinergic Depletion on Skilled Motor Performance
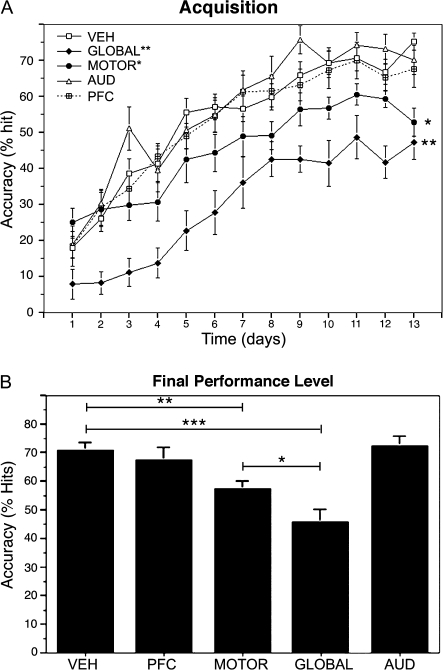
Animals receiving vehicle injections into either the PFC, motor cortex, or AUD did not differ significantly from one another in behavioral performance, ICMS maps, or cortical cholinergic innervation density, and these groups were combined into a single “vehicle” group (n = 18) for further analyses. Animals that received vehicle injections exhibited significant improvements in reaching performance over the 3-week training period, comparable with levels of performance observed previously in unoperated animals (Conner et al. 2003, 2005; Ramanathan et al. 2009). Across treatment groups, significant differences in performance were not found on the first day of testing (P = 0.1, analysis of variance [ANOVA]; Fig. 1) but were apparent across most of the subsequent 3-week training period. Significant group differences were observed in overall acquisition of the skilled motor task over 3 weeks of testing (P = 0.008; repeated measures ANOVA, group × time; Fig. 1A), and in the final asymptotic level of reaching performance, measured as the average accuracy over the final 3 testing days (P < 0.0001; ANOVA; Fig. 1B). Consistent with previous reports (Conner et al. 2003; Ramanathan et al. 2009), animals with global cholinergic depletion exhibited significant impairments in acquiring the skilled motor task relative to vehicle-injected rats (P < 0.0001; post hoc Fisher's; Fig. 1A) and were significantly impaired in their final level of reaching performance (P < 0.0001; post hoc Fisher's; Fig. 1B). Acquisition of the skilled motor task was not impaired following site-specific depletion of cholinergic inputs to either the auditory (P = 0.6, post hoc Fisher's compared with vehicle-treated rats) or the prefrontal (P = 0.9, post hoc Fisher's compared with vehicle-treated rats) cortices. Similarly, the final asymptotic level of performance assessed over the last 3 days of testing was not altered following cholinergic depletion of either auditory (P = 0.7, post hoc Fisher's compared with vehicle-treated rats) or prefrontal (P = 0.5, post hoc Fisher's compared with vehicle-treated rats) cortices. Site-specific of depletion of cholinergic inputs to motor cortex, however, resulted in significant impairments in both task acquisition (P < 0.05; post hoc Fisher's; Fig. 1A) and in final levels of reaching performance (P < 0.005; post hoc Fisher's; Fig. 1B) compared with vehicle-treated animals. Animals with global cholinergic lesions exhibited the greatest impairment in behavior, demonstrating significantly poorer performance relative to all other groups, including subjects with motor cortex cholinergic depletion (“acquisition”: P < 0.005; post hoc Fisher's comparing motor-depleted and globally depleted animals; “asymptotic performance”: P < 0.05; post hoc Fisher's comparing motor-depleted and global-depleted animals; Fig. 1). Additional between group comparisons are reported in supplementary Table 1.
Figure 1.
Effects of site-specific depletion of basal forebrain cholinergic corticopetal inputs on skilled motor learning. (A) Global depletion of cortical cholinergic innervation (filled diamonds) following 192-SAP injections into the nucleus basalis/substantia innominata significantly impaired acquisition of a skilled forelimb reaching behavior (P < 0.0001, overall repeated measures ANOVA; P < 0.0001 post hoc Fisher's comparing global SAP-lesioned animals to vehicle-injected controls). Focal depletion of cholinergic inputs to the motor cortex (filled circles) also resulted in significant impairments in task acquisition relative to vehicle-treated controls (P < 0.05 post hoc Fisher's). However, animals with selective depletion of the motor cortex performed significantly better than animals with global cholinergic depletions (P < 0.005 post hoc Fisher's). (B) Final levels of reaching performance, determined as the average reaching accuracy over the last 3 days of training, were also significantly reduced in rats with either global cholinergic lesions (P < 0.0001, overall ANOVA; P < 0.0001 post hoc Fisher's) or cholinergic depletion of the motor cortex (P < 0.005, post hoc Fisher's) relative to vehicle-treated controls. Animals with global depletion of cortical cholinergic innervation performed significantly worse than animals with focal cholinergic depletion of the motor cortex (P < 0.05, post hoc Fisher's). Focal cholinergic depletion of either PFC or AUD had no significant effect on either skilled motor acquisition (A) or final reaching performance (B) relative to vehicle-treated animals.
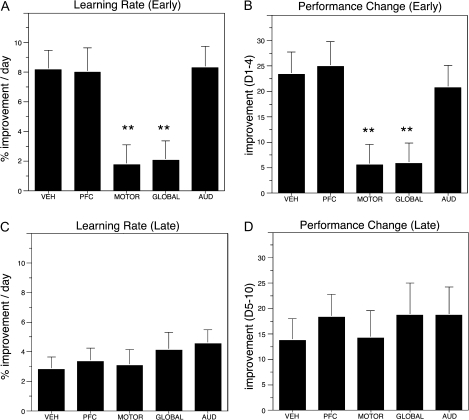
To further explore possible mechanisms contributing to differences in overall skilled motor performance among treatment groups, we subsequently analyzed motor learning (rates of task acquisition) across distinct phases of training. Prior studies have suggested that skilled motor learning is associated with 2 distinct phases; an early phase in which rapid improvements in reaching performance are typically observed and a late phase whereby more subtle refinements in distal forelimb movements take place (Karni et al. 1998; Kleim et al. 2004). Rates of motor learning were calculated in 2 ways: either by determining the slope of the acquisition performance curve over a specified time period or by determining the absolute change in performance over a given period. We separately analyzed learning (rates of task acquisition) as a function of regional cholinergic depletion during “early” (days 1–4) and “late” (days 5–10) phases of skilled motor acquisition (days 11–13 of training were typically associated with asymptotic levels reaching performance). Regardless of the method of analysis, animals with cholinergic lesions of either the PFC or AUD were indistinguishable from vehicle-treated controls in both early and late phases of motor learning (Figs. 1 and 2). In contrast, rates of learning during the early phase of acquisition were significantly altered in animals with either selective motor depletions or global cholinergic lesions (Fig. 2A,B). Learning rates during later phases of skilled motor acquisition did not differ across treatment groups (Fig. 2C,D). In spite of comparable rates of learning between the motor-lesioned and global-lesioned groups during both early and late phases of acquisition, animals with global cholinergic lesions performed significantly poorer than motor cortex lesioned animals throughout the acquisition period (P = 0.003 post hoc Fisher comparing global and motor cortex lesioned animals, overall ANOVA P < 0.0001) and had significantly poorer levels of asymptotic performance over the final 3 testing days. Taken together, these findings suggest that cholinergic depletion of motor cortex results in significant deficits in final asymptotic levels of reaching performance, possibly by impairing learning during the early phase of acquisition. Additional impairments in skilled reaching performance are attributable to more globally acting cholinergic mechanisms.
Figure 2.
Effects of site-specific depletion of basal forebrain cholinergic corticopetal inputs to early and late phases of skilled motor learning. Motor learning (rates of task acquisition) were analyzed across 2 distinct phases; an “early” phase (days 1–4) in which rapid improvements in reaching performance are typically observed, and a late phase (days 5–10) whereby more subtle refinements in performance occur. Rates of motor learning were calculated either by determining the slope of the acquisition performance curve (A,C), or by determining the absolute change in performance over the given analyzed period (B,D). Animals with cholinergic lesions targeting either PFC or AUD had no significant effect on early or late phases of learning relative to vehicle-treated controls (P > 0.7 for all post hoc comparisons). In contrast, rates of learning during the early phase of acquisition were significantly altered in animals with either selective motor depletions or global cholinergic lesions (A,B; **P < 0.005 in all cases). Animals subjected to selective cholinergic depletion of the motor cortex exhibited comparable deficits in the rate of learning during early phase acquisition relative to global cholinergic lesioned animals (post hoc Fisher's, P = 0.89 [for slope] and P = 0.96 [for absolute change in performance] comparing motor-lesioned and global-lesioned animals). Learning rates during late phase skilled motor acquisition did not differ across treatment groups (C,D, P = 0.68 overall ANOVA for “slope” and P = 0.91 overall ANOVA for absolute change in performance).
Effects of Site-Specific Cortical Cholinergic Depletion on Cortical Plasticity
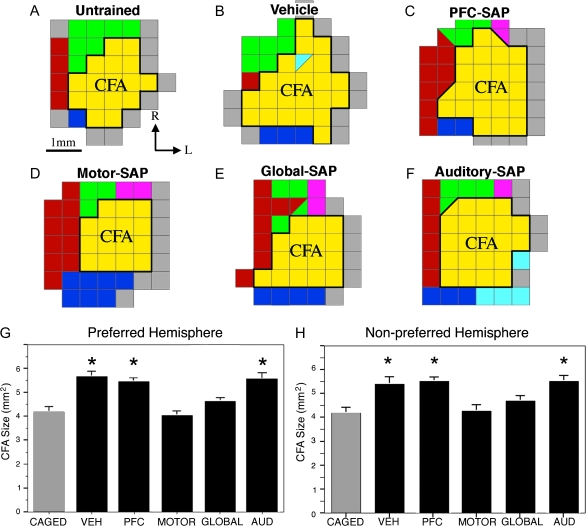
Prior studies have indicated that skilled motor learning is associated with significant plasticity of cortical motor representations (Nudo et al. 1996; Kleim et al. 1998; Conner et al. 2003). Consistent with prior reports, skilled forelimb training in vehicle-treated animals resulted in a significant expansion of the caudal forelimb representation (P < 0.0001, overall ANOVA; P < 0.0001, post hoc Fisher's comparing trained, vehicle-injected rats to caged, untrained animals [n = 6]; Fig. 3). Moreover, the present results confirm prior reports (Conner et al. 2003; Ramanathan et al. 2009) indicating that global depletion of cholinergic corticopetal innervation completely blocks the training-mediated expansion of forelimb representations (P < 0.0005, post hoc Fisher's comparing global-depleted and vehicle-injected trained animals). Depletion of cholinergic innervation to either the AUD or PFC did not significantly affect plasticity of the forelimb representation associated with skilled motor training (p = 0.7, post hoc Fisher's comparing auditory-SAP and vehicle-injected groups; P = 0.4, post hoc Fisher's comparing prefrontal-SAP and vehicle-injected groups; Fig. 3). Depleting cholinergic afferents specifically to the motor cortex completely blocked learning-mediated cortical plasticity (P < 0.0001 post hoc Fisher's comparing motor-SAP and vehicle-injected trained animals), thereby eliminating any differences in cortical representations from untrained animals (P = 0.7 post hoc Fisher's comparing motor-SAP and untrained animals Fig. 3). The loss of learning-mediated cortical motor plasticity was equivalent in subjects with local motor cortex cholinergic lesions and global cholinergic lesions (Fig. 3). Analysis of the cortical forelimb representation was also carried out for the cortical hemisphere associated with the untrained forelimb (Fig. 3H). Prior studies have demonstrated that unilateral skilled forelimb training can impart bilateral increases in dendritic complexity (Withers and Greenough 1989) and bilateral expansion of forelimb motor representations (Conner et al. 2003). Although the basis for bilateral plasticity following unilateral motor training is not known, it is postulated that information related to plastic changes induced in one hemisphere may be transferred to the contralateral hemisphere by way of extensive callosal projections between hemispheres of the rat primary motor cortex (Donoghue and Parham 1983). The present results confirm prior reports demonstrating significant group differences in the size of the caudal forelimb representation of the untrained hemisphere (ANOVA P = 0.0008; Fig. 3H). Training resulted in a significant increase in the size of the caudal forelimb representation of the untrained hemisphere in vehicle-injected animals and in rats with local cholinergic depletion of either the PFC or AUD. Plasticity of the caudal forelimb representation in the untrained hemisphere was completely and significantly blocked in rats with either global cholinergic depletion or selective depletion of the motor cortex (Fig. 3H). Cortical representations did not differ in size, comparing the trained and untrained hemispheres within any treatment group (P > 0.05 in all cases, paired t-test). Taken together, these results indicate that the reorganization of motor representations observed in association with skilled motor training can be accounted for by cholinergic mechanisms acting directly within the motor cortex.
Figure 3.
Effects of site-specific depletion of basal forebrain cholinergic corticopetal inputs on behaviorally mediated cortical map reorganization. (A–F) Representative ICMS-derived motor maps demonstrating the effects of site-specific cholinergic lesions on plasticity of the caudal forelimb representation of the preferred hemisphere (CFA). Skilled forelimb training in vehicle-treated animals (B) resulted in the expansion of the caudal forelimb representation (yellow) relative to untrained controls (A). Focal depletion of cholinergic inputs to the motor cortex (D) completely blocked the behaviorally mediated expansion of the CFA, to the same extent as was observed following global cholinergic lesions (E). Focal depletion of either the PFC (C) or AUD (F) had no effect on the behaviorally mediated plasticity of the CFA. (G) Quantitative analysis of cortical plasticity across all animals confirms that training-mediated plasticity in the preferred hemisphere is blocked by either global cholinergic depletion or site-specific depletion of cholinergic inputs to the motor cortex, but behaviorally mediated plasticity was not altered by site-specific cholinergic depletion of PFC or AUD (overall ANOVA P < 0.0001; * indicated P < 0.001 relative to caged controls). (H) Skilled forelimb training in vehicle-treated animals also led to an expansion of the caudal forelimb area “within the primary motor cortex associated with the untrained limb (nonpreferred hemisphere).” As observed in the preferred hemisphere, plasticity is completely blocked following either global cholinergic depletion (P = 0.25 relative to caged controls) or site-specific depletion of cholinergic inputs to the motor cortex (P = 0.72 relative to caged controls), but behaviorally mediated plasticity was not altered by site-specific cholinergic depletion of PFC or AUD (overall ANOVA P < 0.001; * indicates P < 0.005 relative to caged controls). Yellow—forelimb; Red—vibrissa; Green—neck; Pink— jaw/tongue; Purple—hindlimb; Blue—shoulder; and Gray— unresponsive.
Even though expansion of the caudal forelimb area with training was completely blocked by either global cholinergic depletion or by selective depletion of motor cortex, it is possible that more subtle changes may have occurred specifically with respect to cortical areas associated with control of fine distal forelimb movements. Prior studies have indicated that skilled forelimb training can selectively increase the proportion of distal forelimb movements, even when overall expansion of the caudal forelimb area does not occur (Kleim et al. 1998). To examine this possibility, the percentage of the entire caudal forelimb representation associated with distal movements was compared across treatment groups. No significant group differences were observed (P = 0.35, ANOVA) indicating that cholinergic lesions did not selectively affect distal forelimb representations.
Quantitative Assessment of Focal Immunotoxic Lesions
To verify the effectiveness of focal intraparenchymal SAP infusions for selectively depleting cholinergic innervation to distinct cortical sites, unbiased sampling techniques (Conner et al. 2003) were used to quantify the density of cholinergic innervation to motor, prefrontal, and auditory cortices (Fig. 4). AChE histochemistry was used to identify cholinergic terminals within motor and auditory cortices. p75 immunohistochemistry, which selectively labels cholinergic terminals arising from basal forebrain cholinergic afferents to the cortex, was used to identify cholinergic innervation to the PFC. p75 labeling was necessary due to high levels of cellular and background AChE staining present within the PFC, which prevented clear visualization of individual AChE- positive fibers in this region. Cholinergic innervation density in control, vehicle-injected animals was 412 ± 18 fibers/mm2 in PFC, 370 ± 19 fibers/mm2 in AUD, and 110 ± 13 fibers/mm2 in sensorimotor cortex. As seen in Table 1 and Figs. 4 and 5, SAP injections into the nucleus basalis/substantia innominata resulted in a complete loss of cholinergic innervation to both motor cortex and AUD, and a 85.3 ± 2.4% loss of innervation to the PFC. Direct intraparenchymal injection of the SAP immunotoxin into distinct cortical sites depleted cholinergic innervation to the targeted regions to levels comparable with those seen following global cholinergic depletions (Table 1 and Fig. 5) and did not affect nontargeted cortical sites (Table 1 and Fig. 5). For instance, SAP injections into the motor cortex depleted cholinergic innervation within motor cortex by >98%, but immunotoxin injections into either the PFC or AUD failed to reduce cholinergic innervation to motor cortex (Table 1 and Fig. 5). Following injections of the SAP immunotoxin into the motor cortex, cholinergic fibers were completely eliminated from a region extending rostrally +3.5 mm from bregma and caudally –1.0 mm from bregma and extending medially 1.5–2.0 mm from the midline and laterally 4.5–6.0 mm from the midline. Thus, immunotoxic lesions targeting the motor cortex completely depleted cholinergic innervation from the entire forelimb, vibrissa, jaw, and neck region and most of the hindlimb region of primary motor cortex. These results indicate that targeted immunotoxic lesions produced complete and highly site-specific cholinergic depletion of distinct cortical regions.
Table 1.
Quantitative analysis of cortical cholinergic depletion following site-specific cholinergic lesions
| Cholinergic depletion (% loss relative to intact) |
||||
| PFC | Motor cortex | AUD | ||
| Targeted lesion site | PFC | 93.7 ± 3.0*** | 7.5 ± 9.1 | ND |
| Motor cortex | 9.5 ± 5.5 | 98.7 ± 0.6*** | 20.2 ± 7.6* | |
| AUD | 14.0 ± 3.6* | −4.9 ± 4.3 | 98.1 ± 1.2*** | |
| Global | 85.3 ± 2.4*** | 99.7 ± 0.3*** | 100 ± 0.0*** | |
Note: Values in the table represent the percentage loss in innervation relative to vehicle-treated (intact) controls. Site-specific injections of the immunotoxin within the cortical parenchyma reduce cholinergic innervation to a level comparable with that seen following global depletion but only within the region targeted by the lesion. In most cases, cortical sites not targeted by the lesion showed no significant loss of cholinergic innervation density. *P < 0.05; ***P < 0.001; ND: not determined.
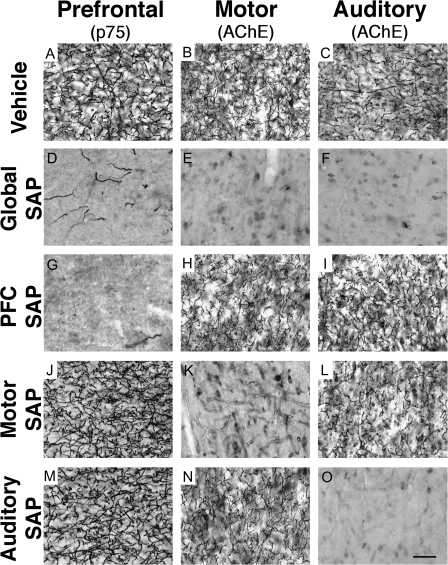
Figure 5.
Site-specific cholinergic lesions result in restricted depletion of cortical cholinergic innervation. Normal patterns of cholinergic innervation to PFC (A), motor cortex (B), and AUD (C). Injections of the SAP immunotoxin directly within the nucleus basalis/substantia innominata depletes cholinergic innervation to all 3 regions (D–F). Focal injections of the immunotoxin into either the PFC (G–I), motor cortex (J–L), or AUD (M–O) selectively depleted cholinergic innervation to the targeted region but did not affect innervation to nontargeted regions. Scale bar in (O) = 50 μm and applies to all panels.
Discussion
The present findings demonstrate that cholinergic inputs directly to motor cortex enable local cortical plasticity and modulate performance on a skilled motor task. Although previous studies clearly implicate a possible role for prefrontal attentional systems in modulating cortical plasticity and resulting behaviors, it appears that dynamic cholinergic modulation of prefrontal function is not a necessary feature for enabling the efficacy of these attentional mechanisms in the context of skilled motor learning. The present results also suggest that functional improvements made during early phases of skilled motor learning are required to optimize final levels of performance on a skilled motor task. Thus, the loci of cholinergic modulation of cortical plasticity and motor learning are intrinsic to the motor cortex (to generate map plasticity and optimal performance), although cholinergic mechanisms extrinsic to the motor cortex appear necessary to support additional aspects of behavioral performance.
Interestingly, although cholinergic inputs to motor cortex are required for normal motor map plasticity and for optimal acquisition of skilled motor learning, partial learning can still occur when these forms of plasticity are abolished. This is consistent with a previous suggestion by Kargo and Nitz (2003, 2004) that a significant portion of skilled motor learning takes place independent of the motor cortex and involves the selection and adaptation of existing motor synergies. It is postulated that motor map reorganization, which is mediated by cholinergic mechanisms acting locally within the region undergoing synaptic reorganization, serves to optimize acquisition of skilled motor learning (Karni et al. 1998; Kleim et al. 2004) but is not required for some degree of learning to occur.
The present findings also demonstrate that global cholinergic lesions, which blocked plasticity to the same extent as motor cortex depletions, result in significantly greater deficits in functional performance compared with motor cortex depletions alone. An analysis of behavioral performance indicates that rate of learning was comparable among animals with motor cortex cholinergic depletion and global cholinergic depletion but that the final performance level of globally lesioned animals was inferior to animals with cholinergic lesions restricted to the motor cortex. The physiological basis for this relative deficit in globally lesioned animals is not known, but the results suggest that cholinergic mechanisms, acting outside the motor cortex and independent of cortical map reorganization, contribute significantly to aspects of skilled motor performance. One possible explanation for the significant differences in behavioral performance between motor cortex–lesioned and global-lesioned animals is that local injections of the immunotoxin failed to deplete cholinergic innervation over the full extent of the motor cortex, resulting in only partial behavioral deficits. This explanation is unlikely, however, because histological evaluations demonstrated that focal immunotoxin injections resulted in a complete loss of cholinergic fibers (>98%) across a region that would typically encompass the full extent of the forelimb motor representation and most of the adjacent vibrissa, neck, jaw, and hindlimb representations. Furthermore, motor cortex cholinergic lesions abolished cortical plasticity to the same extent as global cholinergic lesions. In addition, although the present results clearly indicate that cholinergic projections to prefrontal attentional systems do not modulate either motor map plasticity or skilled motor learning, cholinergic mechanisms acting in other cortical regions, such as the posterior parietal cortex, could influence motor learning by disrupting attentional mechanisms. Posterior parietal mechanisms are not associated directly with sensory or motor deficits, but damage to this cortical region can result in sensory neglect across multiple modalities (Bucci 2009). In addition, selective cholinergic depletion of posterior parietal cortex can impose deficits in incremental attentional processing of sensory information (Bucci et al. 1998). Thus, although prior studies have indicated that global cholinergic lesions do not impair basic sensorimotor coordination per se (Conner et al. 2003), such lesions may impair higher order processing involving the integration of multimodal information relevant for skilled motor learning (such as the integration of visual and somatosensory information; Pavlides et al. 1993; Shadmehr and Krakauer 2008).
In the present study, cholinergic depletion of motor cortex disrupted early phases of motor learning and resulted in persistent deficits in skilled motor performance. This finding is intriguing in light of the fact that cortical reorganization, which is dependent upon cholinergic mechanisms acting locally within motor cortex, has been shown to occur during later phases of skilled motor acquisition (Kleim et al. 2004). Previous investigators have suggested that the appearance of cortical reorganization at later stages of learning, following synaptic (Rioult-Pedotti et al. 2007) and structural (Kleim et al. 2004) modifications, may indicate that this aspect of plasticity is involved in encoding the learned behavior rather than serving as a substrate for enabling refinements to occur. Based upon this interpretation, the present result would suggest that aspects of early phase learning serve as a critical substrate for later cortical map reorganization and optimal acquisition of skilled motor performance.
In summary, site-specific depletion of cholinergic inputs to the motor cortex abolishes learning-mediated plasticity of cortical motor maps and impairs skilled motor learning. Global depletion of cortical cholinergic inputs further impairs skilled motor performance through mechanisms that are likely extrinsic to motor cortex and independent of cortical map reorganization. Dynamic cholinergic modulation of prefrontal attentional mechanisms is not required either to acquire a new skilled motor behavior or to elicit plasticity of cortical motor representations. These findings contribute to our understanding of the parcellation of effects of neuromodulatory systems in support of cortical plasticity and learning.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
NIH (AG10435), the Veterans Administration, the Alzheier's Association, and the Dr Miriam and Sheldon G. Adelson Medical Research Foundation.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville KA, Schweitzer JB, Herron P. Effects of cholinergic depletion on experience-dependent plascticity in the cortex of the rat. Neuroscience. 1997;80(4):1159–1169. doi: 10.1016/s0306-4522(97)00064-x. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Heckers S, Mesulam M-M, Wiley RG, Lappi DA, Sharma M. Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. J Neurosci. 1994;14(7):4507–4519. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgers C, Epstein S, Kopell NJ. Gamma oscillations mediate stimulus competition and attentional selection in a cortical network model. Proc Natl Acad Sci U S A. 2008;105:18023–18028. doi: 10.1073/pnas.0809511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ. Posterior parietal cortex: an interface between attention and learning? Neurobiol Learn Mem. 2009;91:114–120. doi: 10.1016/j.nlm.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AE, Chavez CM, Flesher MM, Kinney-Hurd BL, Araujo GC, Miasnikov AA, Weinberger NM. Association learning-dependent increases in acetylcholine release in the rat auditory cortex during auditory classical conditioning. Neurobiol Learn Mem. 2009;92:400–409. doi: 10.1016/j.nlm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Conner JM, Darracq MA, Roberts J, Tuszynski MH. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci U S A. 2001;98:1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Muir D, Varon S, Hagg T, Manthorpe M. The localization of nerve growth factor-like immunoreactivity in the adult rat basal forebrain and hippocampal formation. J Comp Neurol. 1992;319:454–462. doi: 10.1002/cne.903190310. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex. 2004;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Changes in brain activation during the acquisition of a new bimanual coordination task. Neuropsychologia. 2004;42:855–867. doi: 10.1016/j.neuropsychologia.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Di Patre PL, Mathes CW, Butcher LL. Differential visualization of cholinesterasic neuronal somata and fibers by use of modifications of acetylcholinesterase pharmacohistochemistry. J Histochem Cytochem. 1993;41:129–135. doi: 10.1177/41.1.7678024. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behav Neurosci. 1999;113:691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Carroll KL. Cholinergic modulation of sensory responses in rat primary somatic sensory cortex. Brain Res. 1987;408:367–371. doi: 10.1016/0006-8993(87)90407-0. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Parham C. Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 1983;217:390–404. doi: 10.1002/cne.902170404. [DOI] [PubMed] [Google Scholar]
- Fournier GN, Semba K, Rasmusson DD. Modality- and region-specific acetylcholine release in the rat neocortex. Neuroscience. 2004;126:257–262. doi: 10.1016/j.neuroscience.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. The effects of manipulations of attentional demand on cortical acetylcholine release. Brain Res Cogn Brain Res. 2001;12:353–370. doi: 10.1016/s0926-6410(01)00064-7. [DOI] [PubMed] [Google Scholar]
- Holley LA, Wiley RG, Lappi DA, Sarter M. Cortical cholinergic deafferentation following the intracortical infusion of 192 IgG-saporin: a quantitative histochemical study. Brain Res. 1994;663:277–286. doi: 10.1016/0006-8993(94)91274-2. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Leuchter AF, Scremin OU, Treiman DM, Walton NY. Effects of cholinergic deafferentation and NGF on brain electrical coherence. Brain Res Bull. 1998;45:531–541. doi: 10.1016/s0361-9230(97)00446-2. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Nitz DA. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J Neurosci. 2003;23:11255–11269. doi: 10.1523/JNEUROSCI.23-35-11255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J Neurosci. 2004;24:5560–5569. doi: 10.1523/JNEUROSCI.0562-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Dissociation of the contributions of the prefrontal, motor, and parietal cortex to the control of movement in the rat: an experimental review. Can J Psychol. 1983;37:211–232. doi: 10.1037/h0080724. [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001;115:826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Miasnikov AA, Dykes RW. Blockade of cholinergic receptors in rat barrel cortex prevents long-term changes in the evoked potential during sensory preconditioning. J Neurophysiol. 1998;80:529–545. doi: 10.1152/jn.1998.80.2.529. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav Neurosci. 1998;112:1519–1525. doi: 10.1037//0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH, Weinberger NM. Acetylcholine modifies neuronal acoustic rate-level functions in guinea pig auditory cortex by an action at muscarinic receptors. Synapse. 1990;6:364–368. doi: 10.1002/syn.890060409. [DOI] [PubMed] [Google Scholar]
- Metherate R, Tremblay N, Dykes RW. The effects of acetylcholine on response properties of cat somatosensory cortical neurons. J Neurophysiol. 1988;59:1231–1252. doi: 10.1152/jn.1988.59.4.1231. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Acetylcholine produces stimulus-specific receptive field alterations in cat auditory cortex. Brain Res. 1989;480:372–377. doi: 10.1016/0006-8993(89)90210-2. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiol Learn Mem. 2008;90:443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, McLin D, 3rd, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. Neuroreport. 2001;12:1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophysiol. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- Puttemans V, Wenderoth N, Swinnen SP. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J Neurosci. 2005;25:4270–4278. doi: 10.1523/JNEUROSCI.3866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D, Tuszynski MH, Conner JM. The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. J Neurosci. 2009;29:5992–6000. doi: 10.1523/JNEUROSCI.0230-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson DD, Dykes RW. Long-term enhancement of evoked potentials in cat somatosensory cortex produced by co-activation of the basal forebrain and cutaneous receptors. Exp Brain Res. 1988;70:276–286. doi: 10.1007/BF00248353. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Smith SA, Semba K. Inactivation of prefrontal cortex abolishes cortical acetylcholine release evoked by sensory or sensory pathway stimulation in the rat. Neuroscience. 2007;149:232–241. doi: 10.1016/j.neuroscience.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Donoghue JP, Dunaevsky A. Plasticity of the synaptic modification range. J Neurophysiol. 2007;98:3688–3695. doi: 10.1152/jn.00164.2007. [DOI] [PubMed] [Google Scholar]
- Sachdev RNS, Lu S-M, Wiley RG, Ebner FF. Role of basal forebrain cholinergic projection in somatosensory cortical plasticity. J Neurophysiol. 1998;79:3216–3228. doi: 10.1152/jn.1998.79.6.3216. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Johnson EM. Characterization of the binding properties and retrograde axonal transport of a monoclonal antibody directed against the rat nerve growth factor receptor. J Cell Biol. 1985;101:1100–1106. doi: 10.1083/jcb.101.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres EM, Perry TA, Blokland A, Wilkinson LS, Wiley RG, Lappi DA, Dunnett SB. Behavioral, histochemical and biochemical consequences of selective immunolesions in discrete regions of the basal forebrain cholinergic system. Neuroscience. 1994;63(1):95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Zhu XO, Waite PM. Cholinergic depletion reduces plasticity of barrel field cortex. Cereb Cortex. 1998;8:63–72. doi: 10.1093/cercor/8.1.63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.