Abstract
Spasmodic dysphonia (SD) is a task-specific focal dystonia of unknown pathophysiology, characterized by involuntary spasms in the laryngeal muscles during speaking. Our aim was to identify symptom-specific functional brain activation abnormalities in adductor spasmodic dysphonia (ADSD) and abductor spasmodic dysphonia (ABSD). Both SD groups showed increased activation extent in the primary sensorimotor cortex, insula, and superior temporal gyrus during symptomatic and asymptomatic tasks and decreased activation extent in the basal ganglia, thalamus, and cerebellum during asymptomatic tasks. Increased activation intensity in SD patients was found only in the primary somatosensory cortex during symptomatic voice production, which showed a tendency for correlation with ADSD symptoms. Both SD groups had lower correlation of activation intensities between the primary motor and sensory cortices and additional correlations between the basal ganglia, thalamus, and cerebellum during symptomatic and asymptomatic tasks. Compared with ADSD patients, ABSD patients had larger activation extent in the primary sensorimotor cortex and ventral thalamus during symptomatic task and in the inferior temporal cortex and cerebellum during symptomatic and asymptomatic voice production. The primary somatosensory cortex shows consistent abnormalities in activation extent, intensity, correlation with other brain regions, and symptom severity in SD patients and, therefore, may be involved in the pathophysiology of SD.
Keywords: laryngeal dystonia, neuroimaging, voice production
Introduction
Spasmodic dysphonia (SD) is a primary focal dystonia characterized by involuntary spasms in the laryngeal muscles during voice production for speech but not during emotional vocal expressions, such as laughter and cry (Bloch et al. 1985). The task specificity of SD symptoms suggests that the abnormalities in SD involve the central pathways required for control of learned voice production (i.e., laryngeal sensorimotor cortex, basal ganglia, thalamus, and cerebellum), whereas the pathways controlling innate nonverbal vocalizations (i.e., anterior cingulate cortex and periaqueductal gray) (Jurgens 2002) remain unaffected. The most common form of SD is the adductor type (adductor spasmodic dysphonia, ADSD) characterized by voice breaks occurring during vowels (Nash and Ludlow 1996). The less common form of SD is the abductor type (abductor spasmodic dysphonia, ABSD), which is characterized by intermittent breathy voice breaks prolonging voiceless consonants (Edgar et al. 2001).
Although the etiology and pathophysiology of the primary focal dystonias remain unclear (Defazio et al. 2007), several neuroimaging studies have identified abnormal patterns of brain activation in these disorders. In patients with task-specific focal hand dystonia, functional magnetic resonance (MR) imaging (fMRI) studies during dystonia-inducing tasks have commonly found overactivation of the primary sensorimotor cortex, supplementary motor area (SMA), cerebellum (Pujol et al. 2000; Preibisch et al. 2001), basal ganglia, and thalamus (Peller et al. 2006). Similarly, an fMRI study in patients with blepharospasm and Meige's syndrome found overactivation of the primary somatosensory cortex and SMA during affected orofacial tasks in both forms of dystonia and deficient activation within the mouth region of the primary motor and premotor cortex only in patients with Meige's syndrome (Dresel et al. 2006).
Recently, different functional brain abnormalities have been reported in patients with SD (Haslinger et al. 2005; Ali et al. 2006). An fMRI study of prolonged vowel production found decreased activation in the ventral primary sensorimotor and premotor cortices, SMA, and anterior cingulate and sensory association cortices in ADSD patients compared with healthy subjects (Haslinger et al. 2005). On the other hand, a positron emission tomography (PET) study of narrative speech production identified increased activation in the ventral sensorimotor, auditory and anterior cingulate cortices, insula, and cerebellum and decreased activation in the SMA, posterior supramarginal and posterior middle temporal gyri, and the periaqueductal gray in ADSD patients compared with healthy subjects (Ali et al. 2006). Brain functional abnormalities during asymptomatic whisper of a prolonged vowel (Haslinger et al. 2005) and whispered speech production (Ali et al. 2006) were similar to the abnormalities found during voiced vowel and speech narration, respectively, in the same ADSD patients. When examining the effects of botulinum toxin treatment on brain activity during voice production in ADSD patients, these studies also had different findings. One study found no effects of treatment on cortical activation but decreased subcortical activation within the thalamus and basal ganglia during prolonged vowel production (Haslinger et al. 2005), whereas the other study found decreased activation in the motor cortex, cerebellum, thalamus and basal ganglia and increased activation in the sensory association areas, operculum, and midbrain after treatment with botulinum toxin (Ali et al. 2006).
The discrepancies between the findings of these two studies may be explained by the differences in the experimental tasks used to elicit SD symptoms. While the fMRI study examined brain activation during production of a prolonged vowel (Haslinger et al. 2005), which is a relatively asymptomatic task for ADSD patients (Sapienza et al. 2000; Erickson 2003), the PET study reported brain activation during production of symptomatic narrative speech. A common disadvantage of both studies was the use of whisper as an asymptomatic task in these patients. Although SD symptoms are not apparent during production of whispered vowels or whispered speech, this may be because no voicing occurs during whisper, and laryngeal muscle spasms may occur but go unrecognized. This may explain why functional activation differences between voiced and whispered tasks were not observed in either study (Haslinger et al. 2005; Ali et al. 2006). A better approach to investigating functional brain abnormalities in SD would be to examine brain activation during both voiced symptomatic tasks, such as repetitive syllables or continuous speech production, and voiced asymptomatic tasks, such as whimper (a soft cry), when the absence of voice breaks can be assured.
Recently, structural brain abnormalities have also been reported in SD patients (Simonyan et al. 2008). Using diffusion tensor imaging (DTI), decreased axonal density and coherence have been identified in the right genu of the internal capsule in SD patients. A postmortem examination of brain tissue sampled from the same region in one ADSD patient has substantiated these findings revealing focal axonal degeneration and demyelination in the same region. Further, water diffusivity has been increased along the corticobulbar/corticospinal tract, in the lentiform nucleus, ventral thalamus and cerebellum bilaterally in SD patients compared with controls, while clusters of mineral accumulations in the ventral thalamus and cerebellum have been found in the postmortem case (Simonyan et al. 2008). These structural brain abnormalities in SD may alter functional interactions between cortical and subcortical regions that are essential for voluntary voice control for speech production, possibly explaining the task specificity of SD symptoms.
A common drawback of neuroimaging studies in SD is that either only ADSD patients (Haslinger et al. 2005; Ali et al. 2006) or combined ADSD and ABSD patient groups (Simonyan et al. 2008) were included. No data are available, to date, on brain activation abnormalities in ABSD patients as a separate group, although ABSD affects a different set of laryngeal musculature and has different clinical characteristics and treatment outcomes.
The aims of the present study were to 1) determine brain activation differences during voiced symptomatic and voiced asymptomatic tasks in two separate groups of patients, one with ADSD and the other with ABSD, compared with healthy controls and 2) identify differences in brain activation between ADSD and ABSD patients.
To control for additional vocal quality abnormalities present in SD patients during symptomatic voice production, we trained healthy volunteers (HV) to produce syllables with “pressed voice” but not with SD-specific voice breaks. This experimental design was used as an attempt to separate brain activation abnormalities associated with the SD-specific symptoms, that is, voice breaks, from possible compensatory voice quality abnormalities. To investigate brain abnormalities in the absence of SD symptoms, we examined functional activation during voiced asymptomatic tasks, such as whimpering, coughing, and voluntary breathing, in SD patients compared with healthy subjects.
Based on our previous findings of structural brain abnormalities in SD (Simonyan et al. 2008), we hypothesized that brain activation differences between SD patients and controls would be present in some regions of the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuits regardless of the type of task production, while activation in some other regions of these circuits involved in speech control would be abnormal only during symptomatic voice production in SD patients. We also hypothesized that abnormalities of brain activation will be reflected in the functional relationships between the sensorimotor brain regions involved in the control of voice production in SD patients. We further expected that different patterns of brain activation abnormalities may be present in ADSD and ABSD patients because of their symptom differences.
Material and Methods
Study Participants
Eleven patients with ADSD (age 50.6 ± 10.9 years, mean ± standard deviation; 8 females/3 males), 11 patients with ABSD (age 56.5 ± 8.7 years, mean ± standard deviation; 5 females/6 males), and 11 HV (age 55.7 ± 9.2 years, mean ± standard deviation; 4 females/7 males) participated in the study (Table 1). All subjects were right handed on the Edinburgh Handedness Inventory (Oldfield 1971) and monolingual native English speakers. Physical examination was normal in all participants; none had a history of neurological disorders (other than SD in the patient groups) and psychiatric or otolaryngological problems. No other forms of primary or secondary dystonia were present in the patients based on patients’ medical histories. None of the HV had voice or speech disorders when examined by an otolaryngologist and a speech–language pathologist.
Table 1.
Demographic and clinical data
| Characteristic | ADSD patients | ABSD patients | Control |
| No. of subjects | 11 | 11 | 11 |
| Gender (female/male) | 8/3 | 5/6 | 4/7 |
| Mean age ± SD (years) | 50.6 ± 10.9 | 56.5 ± 8.7 | 55.7 ± 9.2 |
| Handedness (right/left) | 11/0 | 11/0 | 11/0 |
| Duration of disorder (mean ± SD, years) | 15.1 ± 7.7 | 15.1 ± 12.1 | n/a |
| Botulinum toxin injection (number of patients) | 10 | 9 | n/a |
| Last injection received (range) | 4 months to 3 years | 9 months to 20 years | n/a |
| Number of voice breaks (mean ± SD) | 26.9 ± 14.3 | 58.8 ± 23.9 | n/a |
| Harshness (in ADSD) and breathiness (in ABSD) during speaking (in mm, mean ± SD) | 38.3 ± 29.4 | 20.8 ± 22.5 | n/a |
| Effort during speaking (in mm, mean ± SD) | 6.2 ± 2.4 | 6.7 ± 2.4 | n/a |
Note: n/a, not available; SD, standard deviation.
The mean duration of SD was 15.1 years, ranging from 4 to 45 years. Sixteen patients had regularly received botulinum toxin injections into their laryngeal muscles for SD symptom control. Those patients had elected not to receive botulinum toxin injections for symptom control for at least 4 months prior to participation in the study as only symptomatic patients were included at the time of testing. The median interval between the last botulinum toxin treatment and scanning session was 24.7 months, ranging from 4 months to 20 years. One ADSD and 2 ABSD patients had not previously been treated with botulinum toxin. All subjects underwent video fiber-optic nasolaryngoscopy to confirm the diagnosis of SD as well as normal laryngeal function in healthy subjects. During fiber-optic nasolaryngoscopy, subjects were asked to produce different laryngeal tasks, including some of those they performed in the scanner. Diagnostic criteria for SD included intermittent hyperadduction of the vocal folds during voice breaks on vowels for ADSD and prolonged vocal fold opening during breathy breaks on voiceless consonants for ABSD (Ludlow et al. 2008). Patients were asymptomatic during breathing, sniffing, and singing. All HV had normal laryngeal anatomy and function for voice production.
All participants provided written informed consent before taking part in the study, which was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Experimental Tasks
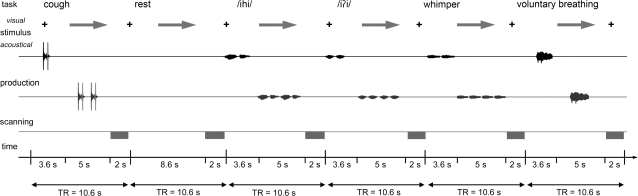
The experimental tasks included: 1) symptomatic voice production (2 repetitions of a syllable /i i/, which was symptomatic in ADSD patients, and 2 repetitions of a syllable /ihi/, which was symptomatic in ABSD patients), 2) asymptomatic voice production (whimpering), 3) asymptomatic laryngeal tasks (voluntary breathing and coughing), and 4) silent fixation as a resting baseline condition. Prior to the scanning, all patients and healthy subjects were trained on task production outside of the magnet for 15 min. Subjects were instructed to listen to the recorded examples of the task (sound of a person producing syllables, whimpering, coughing, or breathing) and to reproduce it (Fig. 1). Stimuli productions were recorded from a female native English speaker for this study.
i/, which was symptomatic in ADSD patients, and 2 repetitions of a syllable /ihi/, which was symptomatic in ABSD patients), 2) asymptomatic voice production (whimpering), 3) asymptomatic laryngeal tasks (voluntary breathing and coughing), and 4) silent fixation as a resting baseline condition. Prior to the scanning, all patients and healthy subjects were trained on task production outside of the magnet for 15 min. Subjects were instructed to listen to the recorded examples of the task (sound of a person producing syllables, whimpering, coughing, or breathing) and to reproduce it (Fig. 1). Stimuli productions were recorded from a female native English speaker for this study.
Figure 1.
Schematic illustration of the experimental design. The subject fixated on the black cross and listened to the acoustically presented sample task for a 3.6-s period. Samples were pseudorandomized and presented as pairs of voluntary coughing, syllable and whimper production, and a single voluntary breathing. No stimulus was presented for resting control condition, during which the subject maintained normally paced breathing. A green arrow cued the subject to initiate the task production within a 5-s period, which was followed by a 2-s period of image acquisition.
For symptomatic voice production, subjects were instructed to produce 2 pairs of syllables. To account for increased brain activation due to voice quality abnormalities during symptomatic voice production in SD patients, all healthy subjects were trained to perform syllable production while using “pressed” voice to imitate the voice strain and effort (but not voice breaks) of symptomatic SD patients (see Supplementary Material for audio samples). Pressed voice was produced by applying additional abdominal pressure while hyperadducting the vocal folds. After the 15-min training session, this task became automatic in healthy subjects. Comparative whole-brain analysis of functional activation during normal and pressed voice production in healthy subjects can be found in Supplementary Materials (Supplementary Fig. 1). Training of healthy subjects immediately before scanning ensured the correct performance of pressed voice task during the experiment. The limitation of this design is that the HV had experienced the effects of pressed voice production for only few hours during training and scanning in contrast to its long-term effects in the patient population (e.g., several years). For whimper production, subjects were instructed to produce 2 repetitions of soft cries with their mouth closed. For coughing, subjects produced 2 pairs of coughs through the mouth, each containing a brief inhalation followed by forced exhalation, similar to a spontaneous cough. For voluntary breathing, participants were instructed to produce a prolonged inhalation followed by a prolonged exhalation through the mouth. A resting condition of silent fixation at the cross on the screen without any auditory stimuli served as a control.
Experimental Design
To minimize scanning artifacts due to orofacial movements (Birn et al. 1999) and to neutralize the scanner noise interference with acoustic stimulus presentation, an event-related sparse sampling design was used. To additionally restrain head movements during task production, the subjects’ head was comfortably immobilized using a vacuum pillow. As shown on Figure 1, during scanning session, the subjects were instructed to fixate their attention on the black cross while listening to the auditory example of the task delivered through the MR-compatible headphones (Silent Scan Audio Systems; Avotec Inc.) within a period of 3.6 s (see Supplementary Material for audio samples). A visual cue (arrow) then instructed the subjects to reproduce the same task within a 5-s interval, which was followed by a 2-s period of image acquisition while subjects silently fixated their attention on the black cross. Because auditory stimulus delivery and motor task production by subjects occurred only during the silent period (8.6 s) of the sparse sampling design, it did not interfere with image acquisition and, therefore, minimized task-induced signal artifacts. Six scanning sessions were acquired, containing a total of 36 trials of each experimental task and 24 resting baseline conditions. All tasks were pseudorandomized between sessions and subjects.
Whole-brain functional images were acquired with a gradient-weighted echo planar imaging (EPI) pulse sequence (echo time [TE] = 30 ms; time repetition = 2 s per volume, 10.6 s between volumes; flip angle = 90°; field of view (FOV) = 240 × 240 mm; matrix 64 × 64 mm; in-plane resolution 3.75 mm; 35 sagittal slices; slice thickness 4 mm without gap) using blood oxygenation level–dependent (BOLD) contrast on a 3-T GE Signa scanner equipped with a quadrature birdcage radio frequency head coil (GE Signa). High-order shimming prior to the EPI acquisition optimized the homogeneity of the magnetic field across the brain and minimized EPI distortions. A high-resolution T1-weighted anatomical image was collected using 3D magnetization prepared rapid acquisition gradient echo sequence (3D magnetization prepared rapid gradient echo: inversion time [TI] = 450 ms; TE = 3.0 ms; flip angle = 10°; bandwidth = 31.25 mm; FOV = 240 × 240 mm; matrix 256 × 256 mm; 128 axial slices; slice thickness 1.3 mm).
Data Analysis
Functional imaging data were analyzed using the AFNI software package (Cox 1996). For each subject, the first 2 volumes in each series, collected before equilibrium magnetization was reached, were discarded. The EPI volumes were registered to the volume collected closest in time to the high-resolution anatomical scan using heptic polynomial interpolation and spatially smoothed with a 4-mm Gaussian filter. Voxelwise normalization to percent signal change was performed and further applied individually to each voxel in the whole brain. The task-related responses were analyzed using multiple linear regression with a single regressor for each task convolved with a canonical hemodynamic response function. Baseline drifts were modeled using quadratic polynomials in time for each separate imaging run, and motion parameter estimates were used as additional regressors of no interest in the multiple regression analysis. The correction for multiple comparisons was performed using Monte Carlo simulations (Forman et al. 1995) that resulted in the identification of a minimum cluster size of 450 mm3 at a voxelwise threshold of 0.005 at overall significance level of a corrected P ≤ 0.05.
Group Comparisons
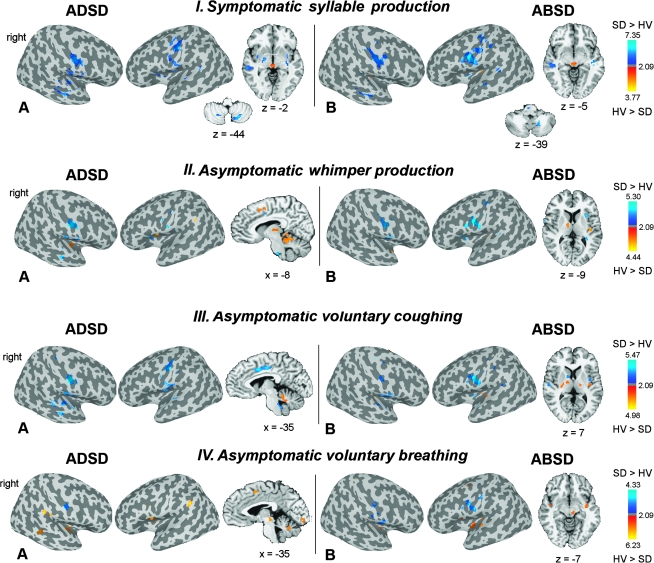
For group analysis, the 3D anatomical datasets of each subject were spatially normalized and converted to the standard anatomical space of Talairach and Tournoux (1988). The resulting normalization parameters were applied to the 4D time series datasets, which were transformed into standard stereotaxic space. To estimate the main effect of each task per group, the group analysis was carried out using a 3-way analysis of variance (ANOVA) with subject as a random factor and the task and group as fixed factors (P ≤ 0.05, corrected). Group statistical parametric maps, which were already corrected at a voxelwise threshold of 0.005, were additionally thresholded using minimal cluster volume of 450 mm3 at corrected P of 0.0125 (P = 0.05/4 task in each subject group). These statistical parametric maps represented statistically significant and corrected maps of brain activation extent in each group during each task production (see Supplementary Fig. 2 and Supplementary Tables 1–4). Statistical differences in brain activation extent (minimal cluster volume of 450 mm3 at a corrected P ≤ 0.05) during each task between ADSD and ABSD patients separately and healthy subjects and between ADSD and ABSD patients were assessed using the subtraction method within the 3-way ANOVA design. These results are reported as the maps of statistically significant differences in brain activation extent between the groups (see Fig. 2).
Figure 2.
(A,B) Significant differences in brain activation extent during production of symptomatic voice and asymptomatic laryngeal tasks in ADSD (IA, IIA, IIIA, and IVA) and ABSD (IB, IIB, IIIB, and IVB) patient groups compared with HV are presented on the inflated cortical surfaces; differences in the activation in the subcortical regions and cerebellum are shown in the series of axial or sagittal brain images of a single subject in the Talairach–Tournoux standard space (cluster size threshold ≥ 450 mm3 at a voxelwise threshold of P = 0.005). The color bar represents t-values (10 degrees of freedom) and reflects the significance of greater activation extent in SD patients compared with HV (SD > HV; dark blue to light blue) and the significance of greater activation extent in HV compared with SD patients (HV > SD; red to yellow). The coordinates are given in the RAI (right-anterior-inferior) conversion.
A Priori Region of Interest Analyses
To quantify the differences in activation intensity (i.e., the magnitude of activation) in the sensorimotor brain regions involved in the control of voice production in SD patients and healthy subjects, a priori region of interest (ROI) analyses were performed using mean percent BOLD signal changes in the primary motor cortex (areas 4a and 4p) (Geyer et al. 1996), primary somatosensory cortex (areas 3a, 3b, 1, and 2) (Geyer et al. 1999, 2000; Grefkes et al. 2001), ventral thalamus, putamen, globus pallidus, and cerebellum (IV–V and VI) (Eickhoff et al. 2005). The choice of ROIs was based on our previous studies in SD reporting structural abnormalities within the corticobulbar tract, basal ganglia-thalamo-cortical, and cerebello-thalamo-cortical circuits (Simonyan and Ludlow 2008; Simonyan et al. 2008). Anatomical parcellations of the ROIs were based on the maximum probability and macrolabel maps (Eickhoff et al. 2005). For each subject, significantly activated voxels within each ROI were identified using minimal cluster volume of 450 mm3 at a corrected P of 0.0125. Values of mean percent BOLD signal change during each task were extracted per ROI in all subjects within patient and control groups.
An initial ANOVA contrasted the three groups (HV, ADSD, and ABSD patients) to examine group (independent) effect and group interactions with ROI (repeated), task (repeated) and hemispheric (repeated) differences in mean percent signal change (P ≤ 0.05). If the group effect or its interactions were statistically significant, post hoc tests were performed to determine which brain regions differed during symptomatic versus asymptomatic tasks between the control and patient groups and between the ADSD and ABSD patients at P < 0.025.
In addition, to identify the pattern of functional correlations within the sensorimotor brain regions involved in the control of voluntary voice production in SD patients and healthy subjects, Pearson's correlation coefficients were computed to examine the relationships between the mean percent signal changes in 6 ROIs (primary motor cortex [including both areas 4a and 4p], primary somatosensory cortex [including areas 3a, 3b, 1, and 2], ventral thalamus, putamen, globus pallidus, and cerebellum) during symptomatic syllable and asymptomatic whimper production within each group. The alpha level was set at 0.003 (0.05/15) to correct for computing 15 correlations within a condition and only identified correlations that could account for more than 50% of the variance, that is, where the r value was greater than 0.70.
Speech Symptom Recording and Measurement
Before scanning, speech of 17 patients (8 ADSD and 9 ABSD patients) was recorded digitally while they repeated 2 sets of sentences: 20 sentences containing a high number of glottal stops before the vowels to elicit symptoms of ADSD and 20 sentences containing a high number of voiceless consonants (f/s/h/p/t/k) to elicit symptoms of ABSD (Ludlow et al. 2008). In the remaining 5 patients, recordings were not obtained because of the recording hardware malfunction. All recorded samples were randomly ordered and anonymized before symptom measurements were made by a speech–language pathologist. Because the most important criterion for SD diagnosis is the presence of voice breaks during speech production (Ludlow et al. 2008), the severity of symptoms was measured by quantifying the number of voice breaks in sentences during vowels in ADSD patients and during voiceless consonants in ABSD patients (Barkmeier et al. 2001; Edgar et al. 2001; Ludlow et al. 2008). In addition, patients’ voice quality abnormalities (i.e., harshness in 8 ADSD patients and breathiness in 9 ABSD patients) during speech production were rated using Consensus Auditory-Perceptual Evaluation of Voice (CAPE-V) (Karnell et al. 2007); patients’ self-ratings of vocal effort during speaking were obtained using visual analog scale in 11 ADSD patients and 7 ABSD patients. Pearson's correlation coefficients were computed to determine the relationships of voice breaks, quality abnormalities, effort, and duration of disorder with mean percent signal change in the ROIs within each SD group (P ≤ 0.01). One of the limitations of this study is that the poor quality of the audio recording system in the scanner prohibited us from counting voice breaks and measuring the vocal quality abnormalities during performance of symptomatic syllables. Therefore, we were not able to conduct direct correlations between brain activation and symptom severity in SD patients.
Results
In this study, we report two different measures of brain functional activation in SD patients and HV. Based on the whole-brain analyses, we report the statistically significant extent of activation (minimal cluster volume of 450 mm3 at a corrected P ≤ 0.05) between patient and control groups. Based on the ROI analyses, we report statistical differences in the intensity of activation derived from the measures of mean percent signal change in each ROI in each group. The results derived from both measures complement each other in providing information about the brain activation differences during performance of symptomatic and asymptomatic tasks in SD patients compared with controls and between ADSD and ABSD patients.
Brain Activation Differences in ADSD and ABSD Patients Compared with Healthy Subjects
Whole-brain analyses
In both patient and healthy subject groups, symptomatic (syllable production) and asymptomatic (whimpering, coughing, and voluntary breathing) tasks elicited bilateral activation in the brain regions typically active during production of laryngeal tasks (Supplementary Materials, Supplementary Fig. 2). A large cluster of activation was located along the Sylvian fissure, including the precentral (areas 4a, 4p, and 6) and postcentral (areas 3a, 3b, 1, and 2) gyri, extending ventrally to the insula and parietal operculum (OP 1–4), caudally to the superior and middle temporal gyri, and medially to the SMA and cingulate cortex. Subcortical activation was found in the thalamus, putamen, globus pallidus, and midbrain. Cerebellar activation was spread over both hemispheres, involving predominantly IV–V and VI lobules (coordinates of the peaks of activation are provided in Supplementary Materials, Supplementary Tables 1–4).
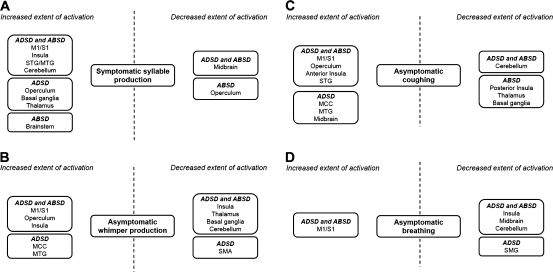
Although the localization of brain activation during laryngeal task production was similar in patients and control groups, statistically significant differences in the extent of brain activation were found during both symptomatic and asymptomatic tasks in both ADSD and ABSD groups compared with healthy subjects (Fig. 2). During symptomatic syllable production, both SD groups compared with controls had increased extent of brain activation in the primary motor and somatosensory cortices, insula, superior and medial temporal gyri, and cerebellum (IV–V and VI) and decreased extent of activation in the midbrain (Figs 2-IA,IB and 3A). In addition, ADSD patients compared with controls showed larger extent of activation in the operculum, putamen, globus pallidus, and thalamus (Figs 2-IA and 3A), whereas ABSD patients had increased extent of activation in the brainstem and decreased extent of activation in the posterior parietal operculum (Figs 2-IB and 3A).
Figure 3.
Block diagrams depicting abnormalities in brain activation extent that are common and distinct in ADSD and ABSD patients compared with healthy subjects during production of symptomatic syllable (A) and asymptomatic whimper (B), coughing (C), and breathing (D). M1/S1, ventral primary sensorimotor cortex; STG/MTG, superior and middle temporal gyrus; MCC, middle cingulate cortex; SMG, supramarginal gyrus.
During asymptomatic whimper production, brain activation extent was again significantly increased in the bilateral ventrolateral sensorimotor cortex, parietal operculum, and anterior insula but significantly decreased in the posterior insula, ventral thalamus, basal ganglia, and cerebellum (IV–V and VI) in both ADSD and ABSD patients compared with healthy subjects (Figs 2-IIA,IIB and 3B). Further, activation extent was increased in the middle cingulate cortex and middle temporal gyrus and decreased in the SMA in ADSD patients compared with controls (Figs 2-IIA and 3B).
During coughing, significantly increased extent of brain activation was found in the bilateral ventral sensorimotor cortex, parietal operculum, anterior insula, and superior temporal gyrus, whereas decreased extent of activation was observed in the cerebellum (IV–V and VI) in both ADSD and ABSD patients compared with healthy subjects (Figs 2-IIIA,IIIB and 3C). ADSD patients had also increased activation extent in the middle cingulate cortex, middle temporal gyrus, and midbrain (Figs 2-IIIA and 3C). In ABSD patients compared with controls, decreased extent of brain activation was found in the posterior insula, ventral thalamus, putamen, and globus pallidus (Figs 2-IIIB and 3C).
During voluntary breathing, a significant increase of brain activation extent was found in the left primary sensorimotor cortex in ADSD patients and in the bilateral primary sensorimotor cortex in ABSD patients, while significant decreases in activation extent were observed in the insula, midbrain, and cerebellum (IV–V and VI) in both ADSD and ABSD patients and in the supramarginal gyrus in ADSD patients compared with healthy subjects (Figs 2-IVA,IVB and 3D).
In summary, compared with healthy subjects, brain activation extent in ADSD and ABSD patients was commonly increased in the primary motor and somatosensory cortices during production of both symptomatic and asymptomatic tasks and decreased in the basal ganglia, thalamus, and cerebellum during asymptomatic tasks.
ROI analyses
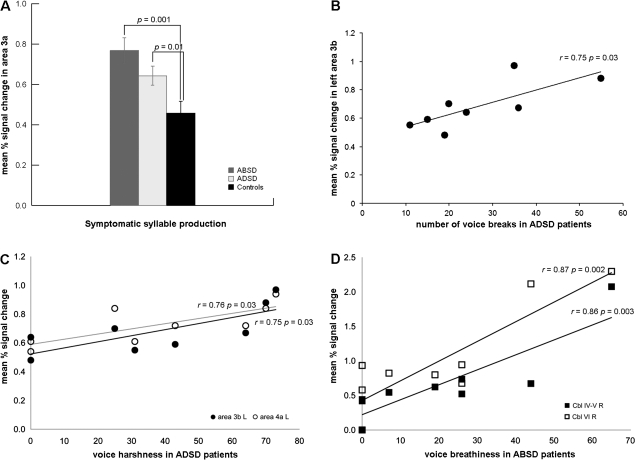
The initial overall ANOVA of mean percent signal change in the a priori ROIs during symptomatic and asymptomatic tasks between the three groups of subjects (HV, ADSD, and ABSD patients) found a significant interaction between the group, task, and ROI (F = 2.65, P = 0.03). Because no significant interaction of group with hemispheric side (F = 1.34, P = 0.15) was found, the post hoc tests examined regional differences between tasks without separating for right or left hemispheric effects. Because our whole-brain analyses determined that the primary motor and somatosensory cortices showed overall increased extent of activation during both symptomatic and asymptomatic tasks in SD patients, whereas the ventral thalamus, basal ganglia, and cerebellum showed increased activation extent during symptomatic voice production and decreased activation extent during asymptomatic tasks, we conducted 2 separate sets of post hoc analyses comparing symptomatic and asymptomatic tasks in the cortical ROIs (areas 4a, 4p, 3a, 3b, 1, and 2) and in the subcortical ROIs (ventral thalamus, putamen, globus pallidus, and cerebellum) between the 3 groups of subjects (P ≤ 0.025). Post hoc ANOVA on cortical ROIs found significant interaction of group with tasks (F = 4.87, P = 0.015), while post hoc t-tests showed significantly increased mean percent signal change in the primary somatosensory cortex (area 3a) during symptomatic voice production in SD patients compared with controls (ADSD: P = 0.01; ABSD: P = 0.001) (Fig. 4A). Post hoc ANOVA comparing symptomatic and asymptomatic tasks on subcortical ROIs did not find any significant group effects (F = 0.93, P = 0.41); therefore, no further analysis was conducted on the subset of subcortical structures.
Figure 4.
(A) Bar graphs depicting significant differences in brain activation intensity in the primary somatosensory cortex (area 3a) during symptomatic syllable production between controls and patients with ADSD and ABSD. No significant differences in the intensity of activation between groups were observed during production of asymptomatic tasks. Error bars show standard error. (B) A trend toward a positive correlation between the mean percent signal change in the left primary somatosensory cortex (area 3b) and symptom severity (i.e., number of voice breaks) in ADSD patients. (C) Trends toward a positive correlation between voice harshness during symptomatic speech production and the mean percent signal change in the left primary somatosensory cortex (area 3b) and left primary motor cortex (area 4a) in ADSD patients. (D) Significant positive relationship between the mean percent signal change in the right cerebellum (IV–V and VI) and voice breathiness during symptomatic speech production in ABSD patients. R, right; L, left; Cbl, cerebellum.
Functional correlations between sensorimotor brain regions during symptomatic and asymptomatic voice production
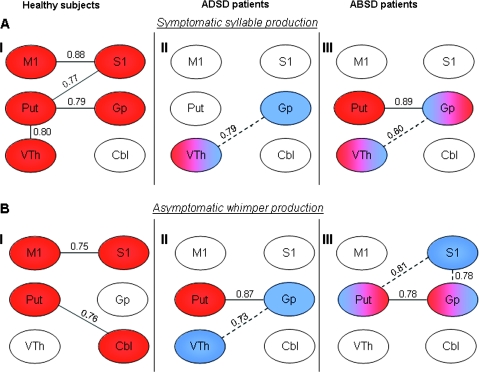
In healthy subjects, during syllable production, activation intensity in the primary motor cortex was significantly correlated with activation intensity in the primary somatosensory cortex (r = 0.88, P = 0.0005); the primary somatosensory cortex tended to correlate with the putamen (r = 0.77, P = 0.006), while the putamen showed correlation with the ventral thalamus (r = 0.80, P = 0.003) and a tendency for a correlation with the globus pallidus (r = 0.79, P = 0.004) (Fig. 5AI).
Figure 5.
Functional correlations within the sensorimotor cortical and subcortical regions involved in the control of voluntary voice production during symptomatic syllable and asymptomatic whimper production in healthy subjects (AI and BI), ADSD (AII and BII) and ABSD (AIII and BIII) patients. Red, significant correlations; blue, correlations not found in healthy subjects but significant in the patient groups. Solid lines show the statistically significant correlations present in all groups; dotted lines show statistically significant correlations present only in patients. M1, primary motor cortex; S1, primary somatosensory cortex; Put, putamen; VTh, ventral thalamus; Gp, globus pallidus; Cbl, cerebellum.
In both ADSD and ABSD patients during “symptomatic syllable production,” correlations of activation intensity were not significant between the primary motor cortex and primary somatosensory cortex (r ≤ 0.17, P ≥ 0.05) and between the putamen and ventral thalamus (r ≤ 0.68, P ≥ 0.02). Further, correlation between the putamen and globus pallidus was not significant in the ADSD group (r = 0.39, P = 0.23) but was significant in the ABSD group (r = 0.89, P = 0.0005). However, an additional significant positive correlation occurred between the ventral thalamus and globus pallidus (ADSD: r = 0.79, P = 0.004; ABSD: r = 0.80, P = 0.003) in both patient groups that was absent in healthy subjects during syllable production (Fig. 5AII,AIII).
During whimper production in healthy subjects, significant positive correlation of activation intensity was found between the primary motor cortex and primary sensory cortex (r = 0.75, P = 0.008), while the putamen tended to correlate with cerebellum (r = 0.76, P = 0.007) (Fig. 5BI).
During asymptomatic whimper production in both ADSD and ABSD patients, the correlations of activation intensity were not significant between the primary motor and primary somatosensory cortices (r ≤ 0.60, P ≥ 0.05) or between the putamen and cerebellum (r ≤ 0.46, P ≥ 0.15) but were significant between the putamen and globus pallidus (ADSD: r = 0.87, P = 0.0005; ABSD: r = 0.78, P = 0.005). In contrast to healthy subjects, a tendency to correlation was found between the globus pallidus and ventral thalamus (r = 0.73, P = 0.01) in ADSD patients, and additional significant relationship of the primary sensory cortex was observed with the putamen (r = 0.81, P = 0.003) and globus pallidus (r = 0.78, P = 0.004) in ABSD patients (Fig. 5BII,BIII).
Brain Activation Differences between ADSD and ABSD Groups
Whole-brain analysis
During symptomatic syllable production, ABSD patients showed larger extent of activation compared with ADSD patients in the left ventral sensorimotor cortex, inferior temporal cortex, ventral thalamus, and the right cerebellar vermis (Fig. 6A). During asymptomatic whimper, ABSD patients had increased extent of activation in the left inferior temporal cortex and bilateral cerebellum compared with ADSD patients (Fig. 6B). No significant differences in the extent of brain activation were found during production of asymptomatic coughing and breathing between ADSD and ABSD patients.
Figure 6.
Significant differences in brain activation extent during production of symptomatic syllable (A) and asymptomatic whimper (B) in ABSD patients compared with ADSD patients are shown in the series of axial or sagittal brain images of a single subject in the Talairach–Tournoux standard space (cluster size threshold ≥ 450 mm3 at a voxelwise threshold of P = 0.005). The color bar indicates the t-values (10 degrees of freedom). The coordinates are given in the RAI (right-anterior-inferior) conversion.
ROI analysis
When comparing the intensity of activation in the sensorimotor ROIs between ADSD and ABSD groups, no significant group differences (F = 2.46, P = 0.13) were found; therefore, no further analysis was conducted on the subset of cortical and subcortical structures.
Symptom Correlations with Brain Activation
During symptomatic speech production in ADSD patients, mean percent signal change in the left primary somatosensory cortex (area 3b) showed trends toward a positive correlation with number of voice breaks (r = 0.75, P = 0.03) (Fig. 4B). Voice harshness in ADSD patients also tended to correlate with increased activation intensity in the left primary somatosensory cortex (area 3b; r = 0.75, P = 0.03) and left primary motor cortex (area 4a; r = 0.76, P = 0.03) during symptomatic speech production (Fig. 4C). In ABSD patients, voice breathiness was positively correlated with mean percent signal change in the right cerebellum (IV–V, r = 0.86, P = 0.003; VI, r = 0.87, P = 0.002) during symptomatic tasks (Fig. 4D). The duration of disorder and vocal effort during symptom production did not relate to mean percent signal change in the ROIs in either patient group.
Discussion
In this study, we found significantly greater extent of brain activation in the primary motor and somatosensory cortices during production of all symptomatic and asymptomatic tasks in both ADSD and ABSD groups compared with healthy subjects. However, the intensity of activation was significantly increased only in the primary somatosensory cortex during symptomatic syllable but not asymptomatic task production in SD patients compared with controls. Furthermore, the extent of activation in the basal ganglia, thalamus, and cerebellum was increased during the symptomatic task and decreased during asymptomatic tasks.
When comparing 2 groups of patients, increased activation extent was found in the primary sensorimotor cortex, inferior temporal cortex, ventral thalamus, and cerebellum during symptomatic syllable production and in the inferior temporal cortex and cerebellum during asymptomatic whimpering in the ABSD patients compared with the ADSD patients. No differences in brain activation intensity were found between ADSD and ABSD patients.
ADSD patients showed trends toward a positive correlation of their symptom severity (i.e., number of voice breaks) and voice quality abnormalities (i.e., voice harshness during symptomatic speech) with increased activation intensity in the left primary sensorimotor cortex, whereas ABSD patients showed a positive correlation between voice quality abnormalities (i.e., voice breathiness during symptomatic speech) and increased activation intensity in the right cerebellum.
Primary Somatosensory Cortex
Previous studies in other forms of primary focal dystonia have reported abnormalities at the various levels of the sensory system, suggesting their important role in the development of motor control dysfunction in this disorder (Tempel and Perlmutter 1990; Hallett 1995; Kaji et al. 1995). Neuroimaging studies have found overactivity of the primary and secondary somatosensory cortices during dystonia-inducing tasks in patients with focal hand dystonia (Ceballos-Baumann et al. 1997; Lerner et al. 2004). On the other hand, increased sensory input resulted in deficient activation in the primary sensorimotor (Tempel and Perlmutter 1990) and secondary sensory cortices (Butterworth et al. 2003) in these patients. Further, a recent fMRI study found reduced activation in the parietal cortex during both execution and imagining of unaffected hand movements in patients with cervical dystonia (de Vries et al. 2008), suggesting an impaired processing of sensory information and abnormal coupling between sensory input and motor output. Although these studies have examined brain activation during either only symptom-inducing tasks or motor tasks involving other unaffected body parts, they have clearly indicated that the abnormal activation of the sensory cortex is a common feature of dystonia. This is in agreement with our present findings of commonly increased activation in the sensorimotor cortex during both symptomatic and asymptomatic task production, suggesting that abnormally increased extent and intensity of activation in this region may represent a disorder-specific phenomenon. Our results are also in agreement with those of Ali et al. (2006), who found increased activation in the sensorimotor cortex during narrative speech production in ADSD patients, but differ from those of Haslinger et al. (2005), who reported decreased activation in this region during prolonged vowel production in ADSD patients. These differences in findings most likely are due to few symptoms occurring during production of a prolonged vowel, which is usually asymptomatic in SD (Sapienza et al. 2000; Erickson 2003). In contrast, in this study we used syllables known to elicit symptoms, and the study by Ali et al. used narrative speech also known to elicit symptoms in SD patients. However, it is not entirely clear whether the abnormalities of the somatosensory cortex play a role in the development of symptoms in SD or are a result of the patients’ disorder and their attempts to compensate for it.
Despite increased activation extent in the sensorimotor cortex in SD patients, we found a lack of correlation in brain activation intensity between the primary motor and somatosensory cortices, which points to an impaired link between these two regions. As the sensory system plays an important role in driving the motor system (Defazio et al. 2007), abnormal functional activation and correlations of the primary somatosensory cortex with other brains regions in SD patients may reflect the altered processing of sensory feedback, which, in turn, may affect motor control and sensorimotor functional integration during voice production in these patients. This may have led to further modulations within the cortico-subcortical sensorimotor network with the establishment of additional relationships between the basal ganglia and ventral thalamus in SD patients that were not observed in healthy subjects (Fig. 5). Activation abnormalities in the basal ganglia and the somatosensory cortex might have also been associated with brainstem dysfunction in SD patients (Simonyan et al. 2010); however, fMRI is not adequate to study brainstem function, particularly during breathing and speech.
Basal Ganglia, Thalamus and Cerebellum
Dysfunctions of the basal ganglia, thalamus, and cerebellum have long been postulated as key components contributing to the pathophysiology of primary focal dystonias. Specifically, it has been suggested that striatal dysfunction with decreased pallidal inhibition of the thalamus may contribute to increased excitability of the primary motor cortex (Ridding, Sheean et al. 1995; Ridding, Taylor et al. 1995; Chen et al. 1997; Filipovic et al. 1997; Lenz et al. 1998), that increased excitation or atrophy of the cerebellum may be a source of dystonic movements (Lee and Marsden 1994; Campbell and Hess 1998; Fletcher et al. 1988; Pizoli et al. 2002; Jinnah and Hess 2006; Le Ber et al. 2006; Neychev et al. 2008), and that lesions in the cerebellar and pallidal relay nuclei of the ventral thalamus can relieve dystonia (Laitinen 1970; Cooper 1976; Gros et al. 1976; Andrew et al. 1983; Tasker et al. 1988; Cardoso et al. 1995).
Functional abnormalities in the basal ganglia, thalamus, and cerebellum are commonly reported neuroimaging findings in patients with primary focal dystonias, including SD (Galardi et al. 1996; Ceballos-Baumann et al. 1997; Black et al. 1998; Odergren et al. 1998; Ibanez et al. 1999; Hutchinson et al. 2000; Preibisch et al. 2001; Oga et al. 2002; Draganski et al. 2003; Blood et al. 2004; Lerner et al. 2004; Ali et al. 2006; Peller et al. 2006). Our recent DTI study in SD patients identified structural abnormalities in the putamen, globus pallidus, ventral thalamus, and cerebellum (Simonyan et al. 2008). Here, we found that functional abnormalities of the cerebellum have closely followed the pattern of altered activation in its main output region, the ventral thalamus, which, in turn, may have received an abnormal input from the globus pallidus. The pattern of similar abnormal activation in these brain regions may explain the recent finding of a better improvement of symptoms in another type of task-specific primary focal dystonia, writer's cramp, during deep brain stimulation of the cerebellar relay nucleus of the ventral thalamus compared with the stimulation of the pallidal relay nucleus of the thalamus (Fukaya et al. 2007). Observation of the similar direction of activation abnormalities in the basal ganglia, thalamus, and cerebellum in SD during symptomatic versus asymptomatic tasks may be based on functional reciprocity between these regions, suggesting disorganization of both basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuits in SD patients.
Differences between ADSD and ABSD Patients
When comparing brain activation in ADSD and ABSD patients, we hypothesized that different patterns of brain activation may be present in these patients because of their symptom differences. Differences were detected only in the extent of brain activation in ABSD patients compared with ADSD patients. The increased extent of activation in the primary sensorimotor cortex and ventral thalamus during symptom production may reflect the presence of more severe symptom expression (i.e., greater number of voice breaks) in ABSD patients (see clinical characteristics, Table 1). On the other hand, extent of activation in the cerebellum was increased in ABSD patients compared with ADSD patients during symptomatic and asymptomatic voice production but not during other asymptomatic laryngeal tasks (e.g., coughing and breathing). Furthermore, increased intensity of activation in the cerebellum was positively correlated with voice quality abnormalities during symptomatic speech production in ABSD patients. This may reflect the important role of the cerebellar modulatory control for learned voice production (Arbib 1981; Wildgruber et al. 2001; Guenther et al. 2006).
Although ABSD patients showed a larger extent of brain activation differences compared with ADSD patients, only the latter group had a tendency toward a positive correlation of their symptom severity (i.e., number of voice breaks) with increased activation intensity in the left primary somatosensory cortex, suggesting that greater intensity of activation in this region occurred in more severely affected ADSD patients.
Overall, our results indicate that the primary somatosensory cortex showed consistent abnormalities in activation extent, intensity, and correlations with other brain regions during both symptomatic and asymptomatic tasks in SD patients compared with healthy subjects and, therefore, may be involved in the pathophysiology of this disorder.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Intramural Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke (Z01NS00298 to C.L.L); National Institute on Deafness and Other Communication Disorders (R00DC009620 to K.S.).
Supplementary Material
Acknowledgments
We thank Sandra B. Martin, MS, for help with patient recruitment, Pamela R. Kearney, MD, for participants’ medical screening, and Kimberly Finnegan, MS, for rating the voice and speech samples. Conflict of Interest: None declared.
References
- Ali SO, Thomassen M, Schulz GM, Hosey LA, Varga M, Ludlow CL, Braun AR. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res. 2006;49:1127–1146. doi: 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- Andrew J, Fowler CJ, Harrison MJ. Stereotaxic thalamotomy in 55 cases of dystonia. Brain. 1983;106(Pt 4):981–1000. doi: 10.1093/brain/106.4.981. [DOI] [PubMed] [Google Scholar]
- Arbib M. Preceptual structures and distributed motor control. In: Brookhart JMMV, editor. Handbook of physiology. Motor control. Bethesda, MD: Americal Physiological Society; 1981. pp. 1449–1480. [Google Scholar]
- Barkmeier JM, Case JL, Ludlow CL. Identification of symptoms for spasmodic dysphonia and vocal tremor: a comparison of expert and nonexpert judges. J Commun Disord. 2001;34:21–37. doi: 10.1016/s0021-9924(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp. 1999;7:106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KJ, Ongur D, Perlmutter JS. Putamen volume in idiopathic focal dystonia. Neurology. 1998;51:819–824. doi: 10.1212/wnl.51.3.819. [DOI] [PubMed] [Google Scholar]
- Bloch CS, Hirano M, Gould WJ. Symptom improvement of spastic dysphonia in response to phonatory tasks. Ann Otol Rhinol Laryngol. 1985;94:51–54. doi: 10.1177/000348948509400111. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Flaherty AW, Choi JK, Hochberg FH, Greve DN, Bonmassar G, Rosen BR, Jenkins BG. Basal ganglia activity remains elevated after movement in focal hand dystonia. Ann Neurol. 2004;55:744–748. doi: 10.1002/ana.20108. [DOI] [PubMed] [Google Scholar]
- Butterworth S, Francis S, Kelly E, McGlone F, Bowtell R, Sawle GV. Abnormal cortical sensory activation in dystonia: an fMRI study. Mov Disord. 2003;18:673–682. doi: 10.1002/mds.10416. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Hess EJ. Cerebellar circuitry is activated during convulsive episodes in the tottering (tg/tg) mutant mouse. Neuroscience. 1998;85:773–783. doi: 10.1016/s0306-4522(97)00672-6. [DOI] [PubMed] [Google Scholar]
- Cardoso F, Jankovic J, Grossman RG, Hamilton WJ. Outcome after stereotactic thalamotomy for dystonia and hemiballismus. Neurosurgery. 1995;36:501–507. doi: 10.1227/00006123-199503000-00009. discussion 507–508. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer's cramp. A PET study. Brain. 1997;120(Pt 4):571–582. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Canos M, Hallett M. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Cooper IS. 20-year followup study of the neurosurgical treatment of dystonia musculorum deformans. Adv Neurol. 1976;14:423–452. [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Vries PM, Johnson KA, de Jong BM, Gieteling EW, Bohning DE, George MS, Leenders KL. Changed patterns of cerebral activation related to clinically normal hand movement in cervical dystonia. Clin Neurol Neurosurg. 2008;110:120–128. doi: 10.1016/j.clineuro.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–1193. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos-Baumann AO. Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain. 2006;129:36–46. doi: 10.1093/brain/awh665. [DOI] [PubMed] [Google Scholar]
- Edgar JD, Sapienza CM, Bidus K, Ludlow CL. Acoustic measures of symptoms in abductor spasmodic dysphonia. J Voice. 2001;15:362–372. doi: 10.1016/S0892-1997(01)00038-8. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Erickson ML. Effects of voicing and syntactic complexity on sign expression in adductor spasmodic dysphonia. Am J Speech Lang Pathol. 2003;12:416–424. doi: 10.1044/1058-0360(2003/087). [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Ljubisavljevic M, Svetel M, Milanovic S, Kacar A, Kostic VS. Impairment of cortical inhibition in writer's cramp as revealed by changes in electromyographic silent period after transcranial magnetic stimulation. Neurosci Lett. 1997;222:167–170. doi: 10.1016/s0304-3940(97)13370-5. [DOI] [PubMed] [Google Scholar]
- Fletcher NA, Stell R, Harding AE, Marsden CD. Degenerative cerebellar ataxia and focal dystonia. Mov Disord. 1988;3:336–342. doi: 10.1002/mds.870030410. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fukaya C, Katayama Y, Kano T, Nagaoka T, Kobayashi K, Oshima H, Yamamoto T. Thalamic deep brain stimulation for writer's cramp. J Neurosurg. 2007;107:977–982. doi: 10.3171/JNS-07/11/0977. [DOI] [PubMed] [Google Scholar]
- Galardi G, Perani D, Grassi F, Bressi S, Amadio S, Antoni M, Comi GC, Canal N, Fazio F. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand. 1996;94:172–176. doi: 10.1111/j.1600-0404.1996.tb07049.x. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Gros C, Frerebeau P, Perez-Dominguez E, Bazin M, Privat JM. Long term results of stereotaxic surgery for infantile dystonia and dyskinesia. Neurochirurgia (Stuttg) 1976;19:171–178. doi: 10.1055/s-0028-1090408. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Is dystonia a sensory disorder? Ann Neurol. 1995;38:139–140. doi: 10.1002/ana.410380203. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. "Silent event-related" fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–1569. doi: 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, Eidelberg D. The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology. 2000;55:673–677. doi: 10.1212/wnl.55.5.673. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer's cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kaji R, Rothwell JC, Katayama M, Ikeda T, Kubori T, Kohara N, Mezaki T, Shibasaki H, Kimura J. Tonic vibration reflex and muscle afferent block in writer's cramp. Ann Neurol. 1995;38:155–162. doi: 10.1002/ana.410380206. [DOI] [PubMed] [Google Scholar]
- Karnell MP, Melton SD, Childes JM, Coleman TC, Dailey SA, Hoffman HT. Reliability of clinician-based (GRBAS and CAPE-V) and patient-based (V-RQOL and IPVI) documentation of voice disorders. J Voice. 2007;21:576–590. doi: 10.1016/j.jvoice.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Laitinen LV. Neurosurgery in cerebral palsy. J Neurol Neurosurg Psychiatry. 1970;33:513–518. doi: 10.1136/jnnp.33.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Clot F, Vercueil L, Camuzat A, Viemont M, Benamar N, De Liege P, Ouvrard-Hernandez AM, Pollak P, Stevanin G, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67:1769–1773. doi: 10.1212/01.wnl.0000244484.60489.50. [DOI] [PubMed] [Google Scholar]
- Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord. 1994;9:493–507. doi: 10.1002/mds.870090502. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Suarez JI, Metman LV, Reich SG, Karp BI, Hallett M, Rowland LH, Dougherty PM. Pallidal activity during dystonia: somatosensory reorganisation and changes with severity. J Neurol Neurosurg Psychiatry. 1998;65:767–770. doi: 10.1136/jnnp.65.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, Hallett M. Regional cerebral blood flow correlates of the severity of writer's cramp symptoms. Neuroimage. 2004;21:904–913. doi: 10.1016/j.neuroimage.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Adler CH, Berke GS, Bielamowicz SA, Blitzer A, Bressman SB, Hallett M, Jinnah HA, Juergens U, Martin SB, et al. Research priorities in spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash EA, Ludlow CL. Laryngeal muscle activity during speech breaks in adductor spasmodic dysphonia. Laryngoscope. 1996;106:484–489. doi: 10.1097/00005537-199604000-00017. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odergren T, Stone-Elander S, Ingvar M. Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer's cramp. Mov Disord. 1998;13:497–508. doi: 10.1002/mds.870130321. [DOI] [PubMed] [Google Scholar]
- Oga T, Honda M, Toma K, Murase N, Okada T, Hanakawa T, Sawamoto N, Nagamine T, Konishi J, Fukuyama H, et al. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer's cramp: an fMRI study. Brain. 2002;125:895–903. doi: 10.1093/brain/awf083. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peller M, Zeuner KE, Munchau A, Quartarone A, Weiss M, Knutzen A, Hallett M, Deuschl G, Siebner HR. The basal ganglia are hyperactive during the discrimination of tactile stimuli in writer's cramp. Brain. 2006;129:2697–2708. doi: 10.1093/brain/awl181. [DOI] [PubMed] [Google Scholar]
- Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M. Cerebral activation patterns in patients with writer's cramp: a functional magnetic resonance imaging study. J Neurol. 2001;248:10–17. doi: 10.1007/s004150170263. [DOI] [PubMed] [Google Scholar]
- Pujol J, Roset-Llobet J, Rosines-Cubells D, Deus J, Narberhaus B, Valls-Sole J, Capdevila A, Pascual-Leone A. Brain cortical activation during guitar-induced hand dystonia studied by functional MRI. Neuroimage. 2000;12:257–267. doi: 10.1006/nimg.2000.0615. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry. 1995;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487(Pt 2):541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza CM, Walton S, Murry T. Adductor spasmodic dysphonia and muscular tension dysphonia: acoustic analysis of sustained phonation and reading. J Voice. 2000;14:502–520. doi: 10.1016/s0892-1997(00)80008-9. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Movement disorders. Chicago: Wiley-Liss; 2008. Gray matter abnormalities in spasmodic dysphonia; pp. S159–S160. [Google Scholar]
- Simonyan K, Ludlow CL, Vortmeyer AO. Brainstem pathology in spasmodic dysphonia. Laryngoscope. 2010;120:121–124. doi: 10.1002/lary.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planer stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tasker RR, Doorly T, Yamashiro K. Thalamotomy in generalized dystonia. Adv Neurol. 1988;50:615–631. [PubMed] [Google Scholar]
- Tempel LW, Perlmutter JS. Abnormal vibration-induced cerebral blood flow responses in idiopathic dystonia. Brain. 1990;113(Pt 3):691–707. doi: 10.1093/brain/113.3.691. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. Neuroimage. 2001;13:101–109. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.