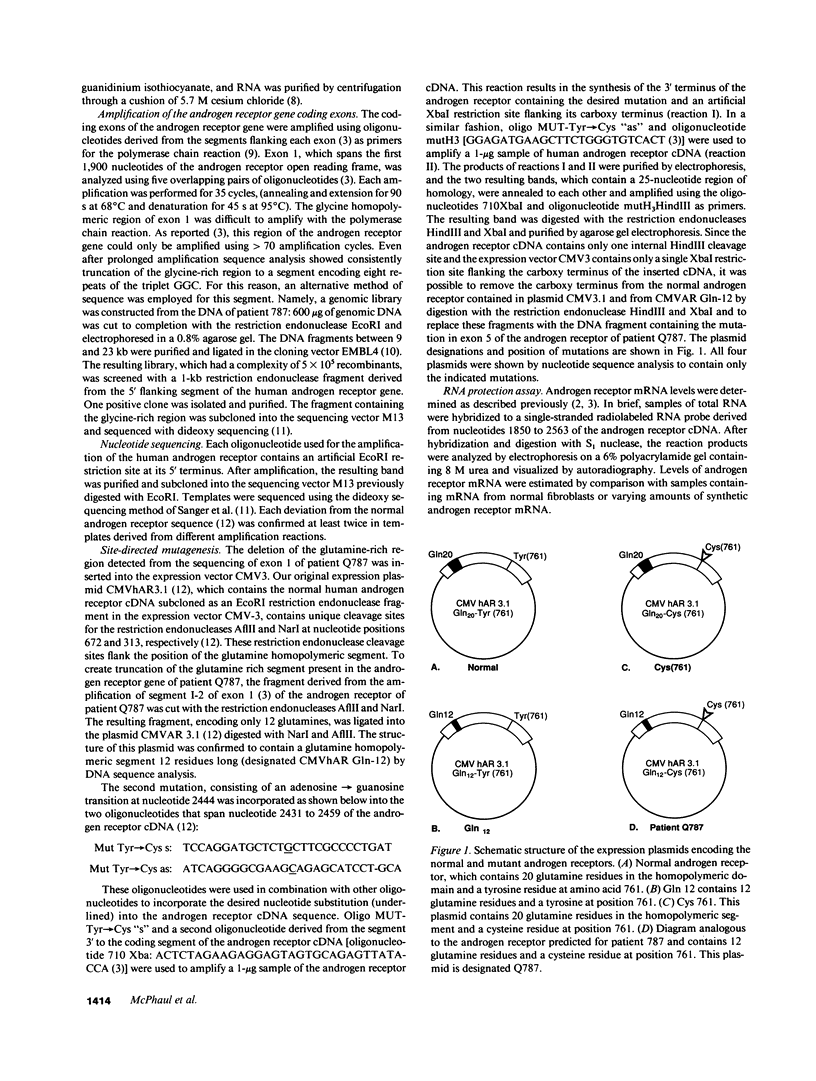
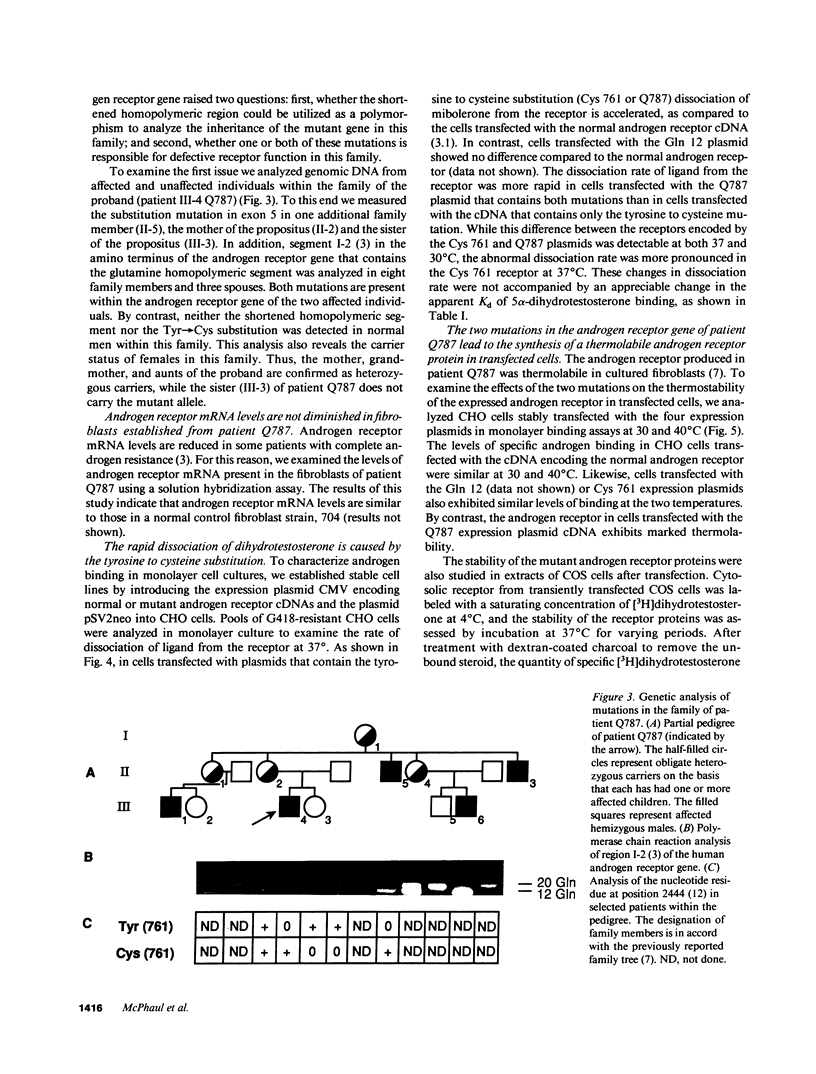
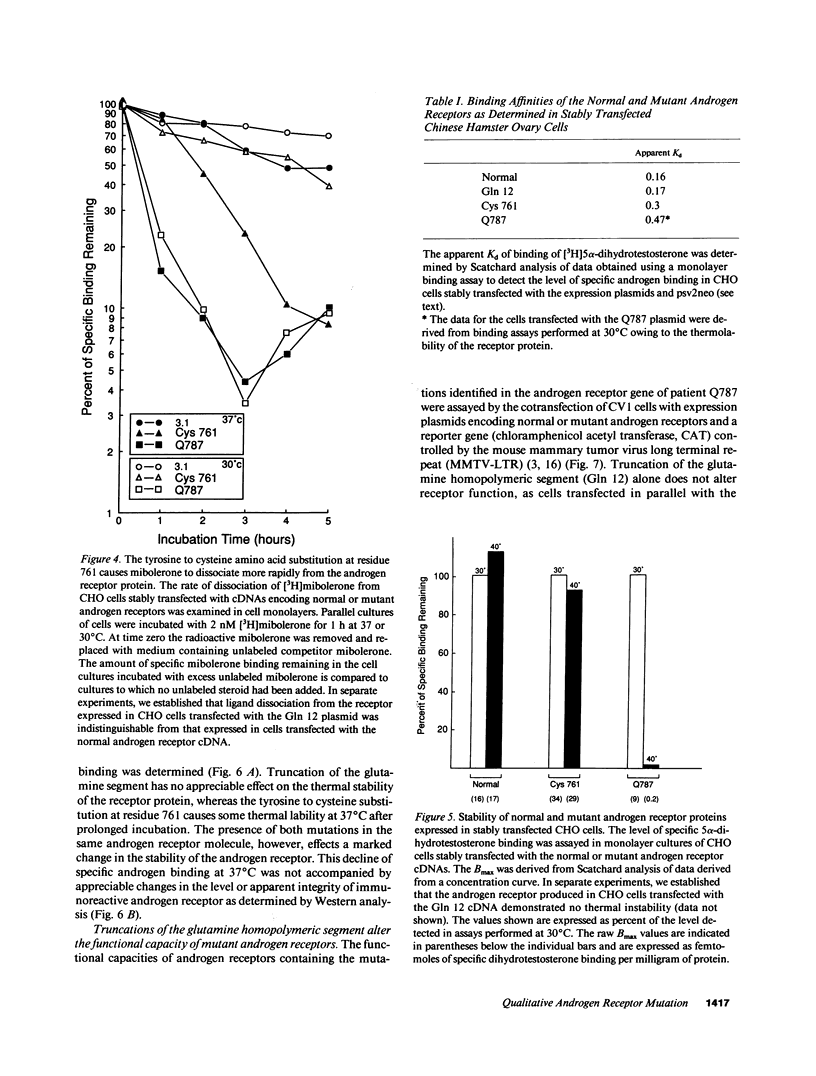
Abstract
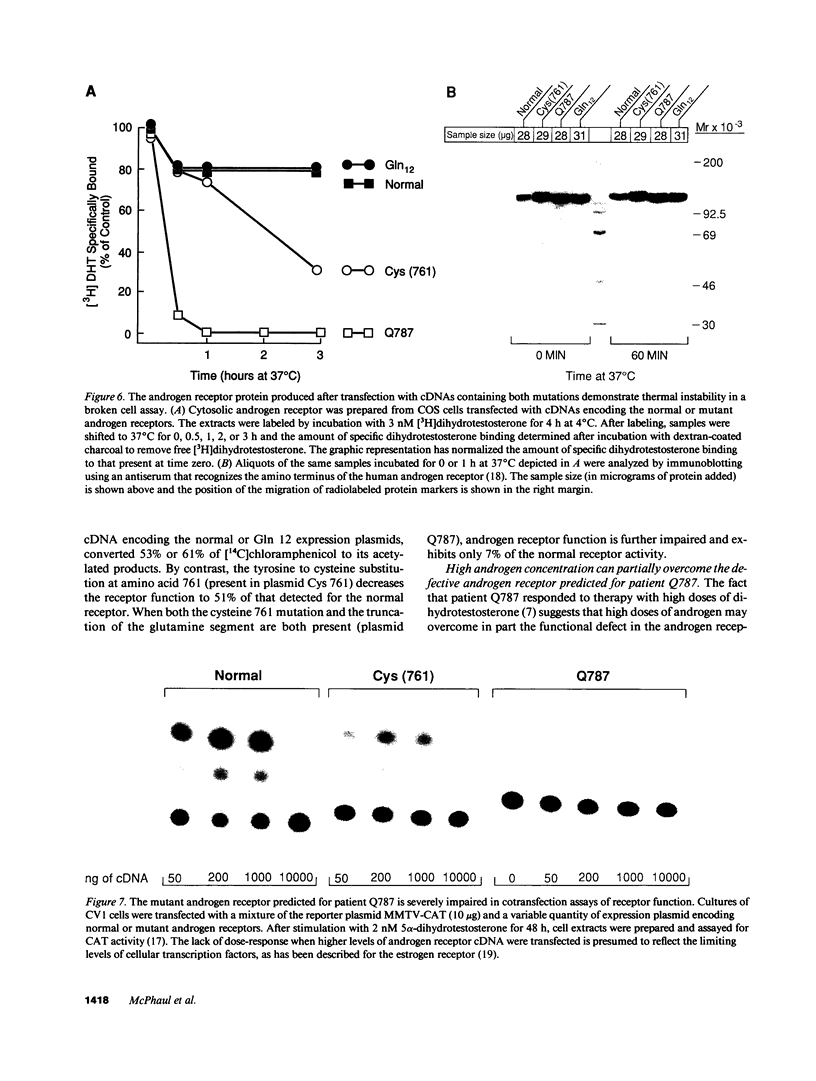
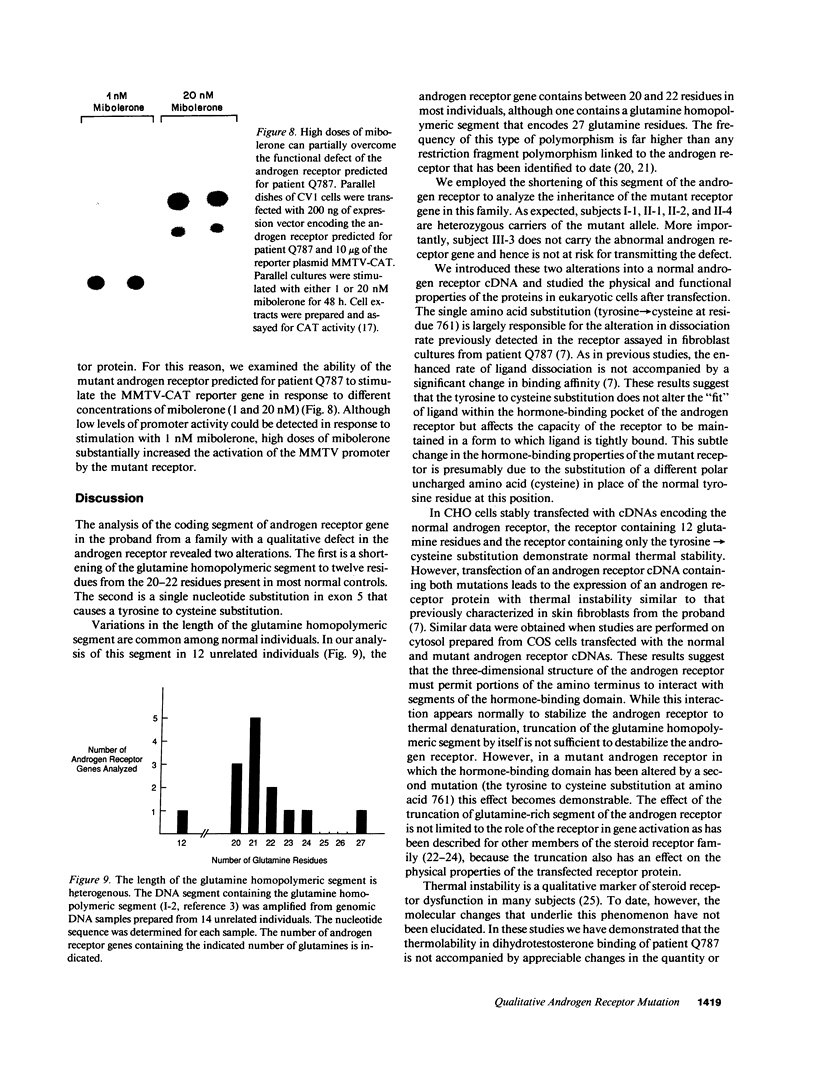
We have examined the nature of the mutant androgen receptor in a family with a severe defect in virilization associated with a qualitative defect in receptor function. The androgen receptor gene in this family contains two structural alterations: a single nucleotide substitution at position 2444 in exon 5 (adenosine----guanosine) that converts tyrosine 761 to a cysteine residue and a shortened glutamine homopolymeric segment in exon 1 that encodes 12 rather than the usual 20-22 glutamines. A family study was performed using polymerase chain reaction amplification of the glutamine-rich segment, and it was shown that the sister of the proband does not carry the mutant allele. The effects of these two mutations on the function of the androgen receptor were studied by introducing the changes, individually and in combination, into cDNAs encoding the normal human androgen receptor and analyzing the receptor protein produced after transfection of the cDNAs into eukaryotic cells. The presence of a cysteine residue at position 761 causes rapid dissociation of dihydrotestosterone from the receptor protein. Marked thermolability of the transfected receptor protein, however, was demonstrable only upon introduction of an androgen receptor cDNA containing both the partial deletion of the glutamine homopolymeric segment and a cysteine residue at position 761. Likewise, the ability of the receptor to stimulate a reporter gene is strikingly diminished only when both alterations are present, suggesting that the shortened glutamine homopolymeric segment amplifies the impairment of receptor function caused by the tyrosine to cysteine substitution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocquel M. T., Kumar V., Stricker C., Chambon P., Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989 Apr 11;17(7):2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. J., Goss S. J., Lubahn D. B., Joseph D. R., Wilson E. M., French F. S., Willard H. F. Androgen receptor locus on the human X chromosome: regional localization to Xq11-12 and description of a DNA polymorphism. Am J Hum Genet. 1989 Feb;44(2):264–269. [PMC free article] [PubMed] [Google Scholar]
- Brown T. R., Lubahn D. B., Wilson E. M., Joseph D. R., French F. S., Migeon C. J. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8151–8155. doi: 10.1073/pnas.85.21.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Malloy P. J. Hereditary 1,25-dihydroxyvitamin D resistant rickets: molecular basis and implications for the role of 1,25(OH) 2D3 in normal physiology. Mol Cell Endocrinol. 1990 Sep 10;72(3):C57–C62. doi: 10.1016/0303-7207(90)90137-w. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Picard D., Yamamoto K. R. Signal transduction and transcriptional regulation by glucocorticoid receptor-LexA fusion proteins. Science. 1988 Aug 12;241(4867):812–816. doi: 10.1126/science.3043662. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Griffin J. E. Testicular feminization associated with a thermolabile androgen receptor in culutred human fibroblasts. J Clin Invest. 1979 Dec;64(6):1624–1631. doi: 10.1172/JCI109624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. Studies on the pathogenesis of the incomplete forms of androgen resistance in man. J Clin Endocrinol Metab. 1977 Dec;45(6):1137–1143. doi: 10.1210/jcem-45-6-1137. [DOI] [PubMed] [Google Scholar]
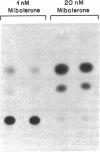
- Grino P. B., Isidro-Gutierrez R. F., Griffin J. E., Wilson J. D. Androgen resistance associated with a qualitative abnormality of the androgen receptor and responsive to high dose androgen therapy. J Clin Endocrinol Metab. 1989 Mar;68(3):578–584. doi: 10.1210/jcem-68-3-578. [DOI] [PubMed] [Google Scholar]
- Husmann D. A., Wilson C. M., McPhaul M. J., Tilley W. D., Wilson J. D. Antipeptide antibodies to two distinct regions of the androgen receptor localize the receptor protein to the nuclei of target cells in the rat and human prostate. Endocrinology. 1990 May;126(5):2359–2368. doi: 10.1210/endo-126-5-2359. [DOI] [PubMed] [Google Scholar]
- Kaufman M., Pinsky L., Feder-Hollander R. Defective up-regulation of the androgen receptor in human androgen insensitivity. Nature. 1981 Oct 29;293(5835):735–737. doi: 10.1038/293735a0. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn D. B., Brown T. R., Simental J. A., Higgs H. N., Migeon C. J., Wilson E. M., French F. S. Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9534–9538. doi: 10.1073/pnas.86.23.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Griffin J. E., Wilson J. D., McPhaul M. J. Definition of the human androgen receptor gene structure permits the identification of mutations that cause androgen resistance: premature termination of the receptor protein at amino acid residue 588 causes complete androgen resistance. Mol Endocrinol. 1990 Aug;4(8):1105–1116. doi: 10.1210/mend-4-8-1105. [DOI] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Wilson J. D., Griffin J. E., McPhaul M. J. A single nucleotide substitution introduces a premature termination codon into the androgen receptor gene of a patient with receptor-negative androgen resistance. J Clin Invest. 1990 May;85(5):1522–1528. doi: 10.1172/JCI114599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai T. J., Seino S., Chang C. S., Trifiro M., Pinsky L., Mhatre A., Kaufman M., Lambert B., Trapman J., Brinkmann A. O. An exonic point mutation of the androgen receptor gene in a family with complete androgen insensitivity. Am J Hum Genet. 1990 Jun;46(6):1095–1100. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Takeda K., Ain K., Ceccarelli P., Nakai A., Seino S., Bell G. I., Refetoff S., DeGroot L. J. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor beta. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8977–8981. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster N. J., Green S., Tasset D., Ponglikitmongkol M., Chambon P. The transcriptional activation function located in the hormone-binding domain of the human oestrogen receptor is not encoded in a single exon. EMBO J. 1989 May;8(5):1441–1446. doi: 10.1002/j.1460-2075.1989.tb03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieacker P., Griffin J. E., Wienker T., Lopez J. M., Wilson J. D., Breckwoldt M. Linkage analysis with RFLPs in families with androgen resistance syndromes: evidence for close linkage between the androgen receptor locus and the DXS1 segment. Hum Genet. 1987 Jul;76(3):248–252. doi: 10.1007/BF00283617. [DOI] [PubMed] [Google Scholar]