Abstract
Duchenne muscular dystrophy (DMD) is an X-linked recessive genetic disease caused by mutations in the gene coding for the protein dystrophin. Recent work demonstrates that dystrophin is also found in the vasculature and its absence results in vascular deficiency and abnormal blood flow. This induces a state of ischemia further aggravating the muscular dystrophy pathogenesis. For an effective form of therapy of DMD, both the muscle and the vasculature need to be addressed. To reveal the developmental relationship between muscular dystrophy and vasculature, mdx mice, an animal model for DMD, were crossed with Flt-1 gene knockout mice to create a model with increased vasculature. Flt-1 is a decoy receptor for vascular endothelial growth factor, and therefore both homozygous (Flt-1−/−) and heterozygous (Flt-1+/−) Flt-1 gene knockout mice display increased endothelial cell proliferation and vascular density during embryogenesis. Here, we show that Flt-1+/− and mdx:Flt-1+/− adult mice also display a developmentally increased vascular density in skeletal muscle compared with the wild-type and mdx mice, respectively. The mdx:Flt-1+/− mice show improved muscle histology compared with the mdx mice with decreased fibrosis, calcification and membrane permeability. Functionally, the mdx:Flt-1+/− mice have an increase in muscle blood flow and force production, compared with the mdx mice. Consequently, the mdx:utrophin−/−:Flt-1+/− mice display improved muscle histology and significantly higher survival rates compared with the mdx:utrophin−/− mice, which show more severe muscle phenotypes than the mdx mice. These data suggest that increasing the vasculature in DMD may ameliorate the histological and functional phenotypes associated with this disease.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked muscle disease affecting one in 3000 children where the gene that codes for the protein dystrophin is mutated (1). Dystrophin is a membrane-stabilizing protein that is part of the dystrophin-associated protein complex which protects the membrane integrity in response to contraction-induced damage (2). In dystrophic muscle where this linkage is disrupted, muscle fibers develop normally but are easily damaged. Damaged muscle fibers degenerate, and new fibers, recruited from satellite cells, regenerate in their place. However, regeneration is inefficient, so successive rounds of degeneration lead to a gradual replacement of muscle by connective tissue.
Abnormal blood flow is expected to induce muscle damage as first demonstrated by Mendell et al. (3). This group showed that ischemia through microvascular infarction produces a grouping of necrotic fibers, a phenotype routinely observed in DMD-afflicted muscle. Recent studies demonstrate that dystrophin is also detected in vascular smooth muscle of mice (4,5). The absence of dystrophin in the vasculatures of mdx mice (6), an animal model for DMD, results in the vascular abnormalities that may impair blood flow. This is through lower nitric oxide (NO)-dependent flow (shear stress)-induced endothelium-dependent dilation, endothelial NO synthase and neuronal NO synthase expression, as well as decreased vascular density (7,8). In addition, utrophin, a dystrophin homologue, expression in endothelium was also reported (9). Furthermore, disruption of the sarcoglycan complex, which is associated with dystrophin in vascular smooth muscle, perturbs vascular function. This initiates cardiomyopathy and exacerbates muscular dystrophy (10). Therefore, blood flow regulation might be disturbed in DMD, possibly increasing muscle damage. Recent work elegantly demonstrates the importance of dystrophin expression in vascular smooth muscle for muscle function of mdx mice. Ito et al. (5) generated smooth muscle-specific dystrophin transgenic mdx mice (SMTg/mdx) expressing dystrophin only in the smooth muscle driven by a smooth muscle-specific promoter. SMTg/mdx mice showed restoration of the NO-dependent modulation of α-adrenergic vasoconstriction and a partially improved muscle phenotype. Taken together, these reports suggest that impaired vascular function is associated with muscular pathology in DMD. Therefore, DMD is characterized by increased muscle damage and an abnormal blood flow after muscle contraction. This is termed the state of functional ischemia.
A two-hit hypothesis is proposed for pathogenic defects in the dystrophin–glycoprotein complex in muscular dystrophy (11): the first hit is a reduction in NO-mediated protection against ischemia in dystrophic muscle, and the second hit is an increase in cellular susceptibility to metabolic stress. Until now, the cause–effect relationship between the pathogenesis of DMD and functional ischemia has been unclear. Recent work demonstrates that the vasoactive drug tadalafil, a phosphodiesterase 5 inhibitor, administered to mdx mice ameliorated muscle damage, strongly indicating that functional ischemia is necessary to cause contraction-induced muscle fiber damage (12). However, the developmental relationship between muscular dystrophy and angiogenesis has yet to be discovered. Definitive treatment for muscular dystrophies will likely require that the dystrophin protein complex is restored in all affected muscle groups as well as vasculature to improve muscle function.
Vascular endothelial growth factor (VEGF) regulates angiogenesis through the promotion of endothelial cell growth, survival and migration. VEGF interacts with its receptors VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1), which are expressed in hemangioblasts and endothelial cell lineages during developmental stage and tissue regeneration (13,14). Flt-1 is a typical tyrosine kinase receptor, and the tyrosine kinase domain of Flt-1 possesses much weaker activity than that of Flk-1. In addition to the full-length receptor, a soluble form of Flt-1 is produced via alternative splicing. Both the full-length and soluble form of Flt-1 possess strong binding affinity for VEGF (15). Mice lacking Flt-1 gene display early embryonic lethality due to an overgrowth of endothelial cells and a disorganization of blood vessels (13–15). In contrast, heterozygous Flt-1 gene knockout mice survive until adulthood and display relatively normal development. Kearney et al. (16) have shown that both heterozygous and homozygous Flt-1 gene knockout mice display increased endothelial cell differentiation in embryonic stem cell cultures. These observations suggested that Flt-1 is a negative regulator for endothelial growth and differentiation during development. Several works demonstrate that administration of VEGF facilitates angiogenesis in muscle after ischemia (17). In addition, administration of VEGF can promote the growth of myogenic fibers, protect myogenic cells from apoptosis and eventually stimulate muscle regeneration (18). In mdx mice, administration of VEGF can improve both muscle histology and function (19). However, it remains unclear whether developmentally upregulated angiogenesis affects muscular dystrophy phenotype.
To address whether we could compensate for the state of functional ischemia found in the mdx mouse muscle by developmentally increasing the vascular density, we crossed mdx mice with the heterozygous Flt-1 gene knockout mice (Flt-1+/−) in which vascular density is increased. We then compared the dystrophic phenotypes between the mdx:Flt-1+/− and the control mdx:Flt-1+/+ mice.
RESULTS
Heterozygous Flt-1 gene knockout mice show an increase in vascular density
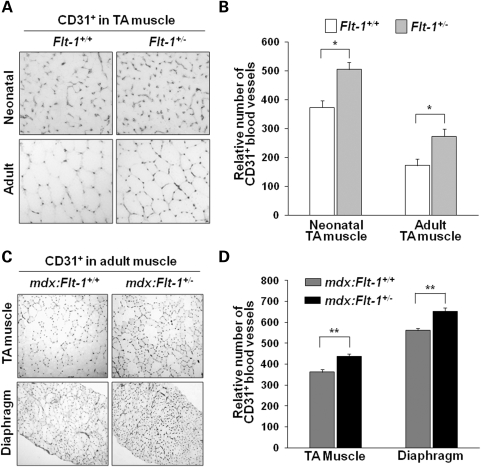
Mice lacking Flt-1 gene by the insertion of β-galactosidase gene (Flt-1−/−) display early embryonic lethality due to an overgrowth of endothelial cells and a disorganization of blood vessels (13–15). Current work showed that heterozygous Flt-1 gene knockout (Flt-1+/−) mice survive until adulthood and display no obvious phenotype. We compared vascular density between the wild-type (Flt-1+/+) and heterozygous Flt-1 knockout (Flt-1+/−) new born and adult mice. Both Flt-1+/− new born and adult mice (505.5 ± 24.5 and 273.7 ± 14.4, respectively) clearly showed increased vascular density in the tibialis anterior (TA) muscle compared with their wild-type litter mates (Flt-1+/+) (373.5 ± 23.5 and 172.6 ± 13.3, respectively) as expressed by the endothelial cell marker CD31 in cross-section (Fig. 1A and B). Fluorescence-activated cell sorting (FACS) analysis also confirmed the increased CD31+ endothelial cells in the TA muscle of Flt-1+/− mice compared with the wild-type Flt-1+/+ mice (Fig. 2A and B). These results suggest that Flt-1+/− mice display increased angiogenesis during postnatal muscle development. This observation is consistent with the published work that vasculogenesis is increased in Flt-1+/− embryonic stem cell cultures and that Flt-1 negatively regulates angiogenesis during development (16).
Figure 1.
Increase in blood vessel density in the Flt-1+/− mouse background. Blood vessel density was measured by anti-CD31 antibody staining on cryosections. (A) Representative images of CD31-stained cryosections from the neonatal and adult TA muscle of the wild-type (Flt-1+/+) and Flt-1+/− mice. (B) Relative number of CD31-positive blood vessels per view field in cross-sections of the TA muscle of neonatal (n = 4 each) and adult wild-type (Flt-1+/+) and Flt-1+/− mice (n = 6 each). (C) Representative images of anti-CD31-stained cryosections of the control mdx:Flt-1+/+ and mdx:Flt-1+/− mice. (D) Relative number of CD31-positive blood vessels per view-field in cross-sections of TA and diaphragm muscle of the mdx:Flt-1+/+ and mdx:Flt-1+/− mice (n = 12 each). Values are mean ± SEM. Asterisks or double asterisks indicate experimental pairs where differences between the compared values were statistically significant (P < 0.05 or P < 0.01, respectively).
Figure 2.
Increase in CD31+ endothelial cells in the Flt-1+/− mouse background. (A) FACS analysis shows that the dissociated cells from the TA muscle of the Flt-1+/− and mdx:Flt-1+/− mice contained increased amount of CD45-PE− CD31-APC+ endothelial cells in the gated areas compared with the wild-type (Flt-1+/+) and mdx:Flt-1+/+ mice, respectively. Control indicates the FACS profile for the control antibodies. (B) Total number of CD45− CD31+ endothelial cells per TA muscle was estimated by data from the FACS analysis. Values are mean ± SEM (n = 3). Asterisks indicate experimental pairs where differences between the compared values were statistically significant (P < 0.05).
mdx mice are an animal homologue for DMD and display continuous degeneration and regeneration with subsequent calcium deposits and fibrosis in their muscle (20,21). It is important to determine whether angiogenesis affects DMD muscle pathology and function. For this purpose, we created double-mutant mdx mice with the heterozygous allele for Flt-1 (mdx:Flt-1+/−) and compared them with their litter mates of mdx mice with the wild-type Flt-1 alleles (mdx:Flt-1+/+). The control mdx:Flt-1+/+ mice had an average of 362 ± 12 and 562 ± 12 capillaries per view-field for the TA muscle and diaphragm, respectively. The mdx:Flt-1+/− mice had higher averages of 438 ± 10 and 653 ± 17 for the TA muscle and diaphragm, respectively (Fig. 1C and D). Similar increases in CD31+ endothelial cells in the mdx:Flt-1+/− mice compared with the control mdx:Flt-1+/+ mice were also confirmed by FACS analysis of the TA muscle (Fig. 2A and B). The relatively frequent occurrence of dystrophin-positive revertant muscle fibers has been observed in mdx mice. The mdx-5cv mice used for this study have ∼10-fold fewer revertants than the original mdx mice (22). Western blot analysis and immunostaining were performed on TA muscle extracts and sections to confirm that the expression of dystrophin and dystrophin-positive revertant fibers were not altered between mdx:Flt-1+/+ and mdx:Flt1+/− mice (Fig. 3A–D).
Figure 3.
No change in dystrophin or utrophin expression in the TA muscle of the mdx:Flt-1+/− mice. (A) Representative images from TA muscle sections of the control mdx:Flt-1+/+ and mdx:Flt-1+/− mice stained with anti-dystrophin antibody to detect revertant fibers. Arrows indicate dystrophin+ revertant fibers and DAPI staining indicates nuclei (blue). (B) Quantification of dystrophin-positive revertant fibers in the TA muscle of the control mdx:Flt-1+/+ (n = 4) and mdx:Flt-1+/− (n = 3) mice. (C) Representative western blotting to detect dystrophin and utrophin proteins in the wild-type (Flt-1+/+), Flt-1+/−, mdx:Flt-1+/+ and mdx:Flt-1+/− mice. β-tubulin was used for loading control. (D) Expression of dystrophin was unchanged in Flt-1+/− compared with the wild-type (Flt-1+/+) mice (n = 3 each). Dystrophin is absent in the mdx:Flt-1+/+ and mdx:Flt-1+/− mice (n = 3 each). (E) Expression of utrophin was unchanged by the Flt-1+/− background (n = 3 each). Values are mean ± SEM.
It is known that, in the absence of dystrophin, the expression of utrophin, a dystrophin homologue, is enhanced to compensate for the membrane instability in mdx mice (23). The expression of utrophin was evaluated by western blot of the TA muscle extract. As expected, the expression of utrophin was upregulated in mdx:Flt-1+/+ and mdx:Flt1+/− mice compared with the wild-type and Flt1+/− mice but was not altered between the wild-type and Flt1+/− mice or between the mdx:Flt-1+/+ and mdx:Flt1+/− mice (Fig. 3C and E). Therefore, the mdx:Flt-1+/− mice and the control mdx:Flt-1+/+ mice were utilized for further comparison to reveal the relationship between muscular dystrophy and angiogenesis in mdx mice.
Muscle morphology and histology were improved in mdx:Flt-1+/− mice
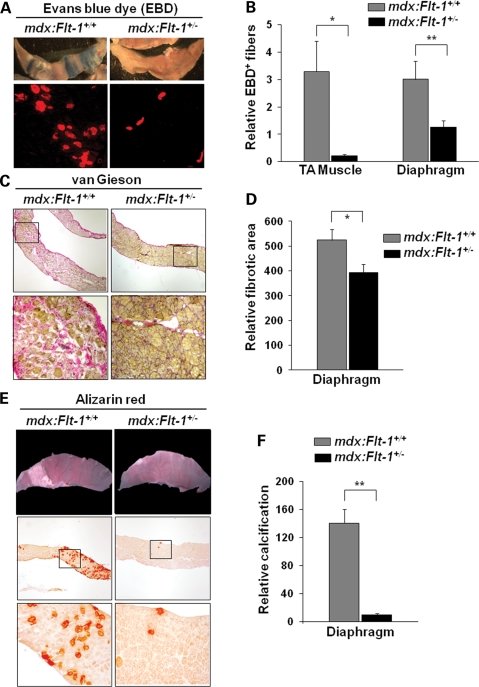
Evans blue dye (EBD) uptake is used as a measure of membrane permeability (24,25). The mdx:Flt-1+/+ mice clearly showed some EBD-positive degenerating muscle fibers in the TA muscle (3.30% ± 1.11) and in the diaphragm (3.02% ± 0.66) (Fig. 4A and B). On the other hand, the mdx:Flt-1+/− mice showed lower EBD-positive muscle fibers in the TA muscle (0.021% ± 0.05) and in the diaphragm (1.25% ± 0.25). These data strongly suggest that muscle fibers in the mdx:Flt-1+/− mice are more stable and less degenerative than the control mdx:Flt-1+/+ mice.
Figure 4.
mdx:Flt-1+/− double-mutant mice display improved muscle pathology. (A) Representative images from whole diaphragm muscle of the control mdx:Flt-1+/+ and mdx:Flt-1+/− mice injected with EBD. Blue staining denotes EBD uptake (upper panels). Cross-sections show EBD uptake in damaged TA muscle fibers of the mdx:Flt-1+/+ and mdx:Flt-1+/− mice (lower panels: red fluorescence). (B) Quantification of EBD-positive fibers normalized by muscle area. mdx:Flt-1+/− double-mutant mice display decreased membrane damage in both the TA muscle and the diaphragm (n = 9 each) compared with the control mdx:Flt-1+/+ (n = 7 each). (C) van Gieson staining on diaphragm cross-sections shows fibrosis (pink color) in the mdx:Flt-1+/+ and mdx:Flt-1+/− mice (upper panels). Lower panels show magnified views from upper panels. (D) Quantification of relative fibrotic area in the diaphragm of mdx:Flt-1+/+ and mdx:Flt-1+/− (n = 5 each) mice as evaluated by van Gieson stain. (E) Whole diaphragm shows calcium deposits on the muscle fibers of the mdx:Flt-1+/+ and mdx:Flt-1+/− mice (white color: upper panels). Cryosections stained using Alizarin red for calcification (red color: middle panels). (F) Quantification of the relative calcified area of diaphragm of the mdx:Flt-1+/+ and mdx:Flt-1+/− mice (n = 6 each) as evaluated by Alizarin red staining (lower panels). Values are mean ± SEM. Asterisks or double asterisks indicate experimental pairs where differences between the compared values were statistically significant (P < 0.05 or P < 0.01, respectively).
Histological phenotypes commonly associated with DMD include excessive fibrosis and calcification. Fibrosis is the replacement of muscle fibers by fibrous connective tissue, which not only decreases the amount of force generators but also impedes the force generated by other muscle due to its inelastic properties (21,26). The diaphragm showed improvements for fibrosis in the mdx:Flt-1+/− mice (524.8 ± 43.4) compared with the control mdx:Flt-1+/+ mice (372.4 ± 34.7) (Fig. 4C and D). In addition, the diaphragm in the control mdx:Flt-1+/+ mice showed a large amount of calcium accumulation that can be seen macroscopically although being mostly absent in the mdx:Flt-1+/− mice (Fig. 4E and F). Alizarin red staining confirmed that the mdx:Flt-1+/− had far fewer calcium deposits, which were also generally smaller in size compared with the control mdx:Flt-1+/+ mice. In contrast, as reported previously (21), fibrosis and calcification were not as prevalent in the TA muscle of the control mdx:Flt-1+/+ mice (data not shown).
Centrally located nuclei (CLN) in muscle fibers are a typical phenotype associated with many different types of muscular dystrophies (21). The mdx:Flt1+/− mice displayed 50.00% ± 1.05 of fibers as having CLN compared with the control mdx:Flt1+/+ mice, which showed 66.16% ± 2.06 of fibers with CLN (Fig. 5A and B). This value is similar to what has been found by others for the mdx mice (27). The drop in CLN in the mdx:Flt1+/− mice was also seen in the diaphragm compared with the control mdx:Flt1+/+ mice (31.97 ± 0.77 versus 38.01 ± 0.50). Therefore, lower CLN in the mdx:Flt1+/− mice is indicative of the decreased fiber turnover and increased muscle fiber stability.
Figure 5.
mdx:Flt-1+/− double-mutant mice display increased muscle fiber stability. (A) HE staining of the TA muscle and diaphragm cross-sections of the mdx:Flt-1+/+ and mdx:Flt-1+/− mice. Arrows indicate muscle fibers with CLN. (B) Quantification of the percent of CLN in the TA muscle and diaphragm in the mdx:Flt-1+/+ (n = 4 each) and mdx:Flt-1+/− mice (n = 6 and 4), respectively. (C) NADH-TR staining was used to distinguish oxidative (dark color), oxidative-glycolytic (intermediate color) and glycolytic (unstained or lightly stained) muscle fibers in the TA muscle of the mdx:Flt-1+/+ and mdx:Flt-1+/− mice. (D) Fiber type distribution shows the mdx:Flt-1+/− mice (n = 4) had higher oxidative and oxidative-glycolytic fibers and fewer glycolytic fibers compared with the mdx:Flt-1+/+ mice (n = 4). Wild-type (Flt-1+/+) mice (n = 4) display the highest oxidative fiber distribution. Values are mean ± SEM. Asterisks or double asterisks indicate experimental pairs where differences between the compared values were statistically significant (P < 0.05 or P < 0.01, respectively).
Muscle fiber type changes and hypertrophy development have been reported in mdx mouse muscle as compensation for the loss of force-generating capacity of the existing muscle. To determine the oxidative profile of the muscle, sections were stained for NADH-tetrazolium reductase (TR). The mdx:Flt1+/− mice (oxidative: 9.8 ± 2.6, oxidative-glycolytic: 56.9 ± 0.4, glycolytic: 33.2 ± 2.5) showed an increase in the amount of oxidative and oxidative-glycolytic fibers and a decrease in glycolytic fibers compared with the control mdx:Flt1+/+ mice (oxidative: 6.58 ± 0.5, oxidative-glycolytic: 50.5 ± 1.3, glycolytic: 42.8 ± 1.7) (Fig. 5C and D). Immunostaining using antibodies against the slow isoform of myosin heavy chain (MHC) (28,29) revealed an increase in the oxidative type I muscle fibers (mdx:Flt1+/−: 1.74 ± 0.4, mdx:Flt1+/+: 0.6 ± 0.2) (Supplementary Material, Fig. S1A and B). We also found that the mean fiber diameter size distribution in the mdx:Flt1+/− mice is skewed towards the smaller fiber size (Supplementary Material, Fig. S1C and D). This decrease in size may be due to a fiber type switch from glycolytic to oxidative type since oxidative fibers tend to be smaller than the glycolytic fibers. Alternatively, muscle in the mdx:Flt1+/− mice may contain reduced hypertrophic muscle compared with the control mdx:Flt1+/− mice.
mdx:Flt1+/− mice display increased blood perfusion
To evaluate whether the increase in vascular density also caused an increase in perfusion to the skeletal muscle, we measured the laser Doppler flow (LDF) in the TA muscle. The mean amount of flux is shown in perfusion units (PU) as per the manufacturer's recommendation (Fig. 6A). The Flt-1+/− (54.8 ± 1.2) and mdx:Flt-1+/− (62.6 ± 4.7) showed increased red blood cell (RBC) flux compared with the wild-type Flt-1+/+ (46.1 ± 1.1) and mdx:Flt-1+/+ (48.1 ± 3.3), respectively. This is consistent with the increase in blood vessel density seen in the TA muscle and may compensate for the functional ischemic phenotype observed in the mdx mice.
Figure 6.
mdx:Flt-1+/− display improved blood flow and muscle function. (A) The Flt+/− and mdx:Flt-1+/− mice (n = 5 each) display increased amounts of blood flow as evaluated by LDF compared with the wild-type (Flt-1+/+) and mdx:Flt-1+/+ mice (n = 8 each). (B) The Flt-1+/− and wild-type (Flt-1+/+) mice had no difference in the maximum isometric torque produced by the anterior crural muscles (n = 5 each). However, the mdx:Flt-1+/− mice had higher maximum isometric torque production compared with the mdx:Flt-1+/+ mice (n = 8 each). (C and D) Whole-body tension assay shows that the mdx:Flt-1+/− mice (n = 18) had increased force production compared with the control mdx:Flt-1+/+ mice (n = 15). There was no difference between the wild-type (Flt-1+/+) and Flt-1+/− mice (n = 5 each). Values are mean ± SEM. Asterisks indicate experimental pairs where differences between the compared values were statistically significant (P < 0.05).
mdx:Flt-1+/− mice display improved muscle contractile function
To examine whether the histological improvements translated into a functional improvement, local force production was measured in the hind limb. Maximum isometric torque was measured by quantifying the torque produced when the peroneal nerve was stimulated to cause a contraction of the anterior crural muscle. The maximum torque generated was normalized by the body mass of the mouse. Although there was no significant difference between the Flt-1+/− (101.0 ± 7.2) and the wild-type Flt-1+/+ mice (100.3 ± 4.9), the mdx:Flt-1+/− mice (71.7 ± 4.3) generated more torque compared with the control mdx:Flt-1+/+ mice (58.5 ± 4.7) (Fig. 6B). To evaluate systemic force production, whole-body tension analysis was conducted on the wild-type Flt-1+/+, Flt-1+/−, mdx:Flt-1+/+ and mdx:Flt-1+/− mice. The mdx:Flt-1+/− mice showed an improvement in both maximum pulling force and average pulling force (maximum: 73.49 ± 4.85 and average: 63.47 ± 4.00) compared with the control mdx:Flt-1+/+ mice (maximum: 62.83 ± 8.88 and average: 52.52 ± 6.74) (Fig. 6C and D). However, this improvement was only modest in comparison with the force produced by the wild-type Flt-1+/+ mice.
Evaluation of mdx:utrn−/−:Flt-1+/− triple-mutant mice
Although the mdx mice are genetically similar to DMD, they do not exhibit the pathology to the severity of DMD. Additionally, mdx mice show no significant difference in their lifespan compared with the wild-type mice. One of the reasons for the relatively mild phenotype of mdx mice is the upregulation of utrophin protein which can compensate for the lacking function of dystrophin. mdx mice carrying a utrophin gene mutation (utrn−/−) display a more severe muscle phenotype than the mdx mice (23,30). These mice display a marked kyphosis and usually die within 5 months. Therefore, they serve as a better animal model for DMD. Since we found no difference in the amount of utrophin between mdx:Flt-1+/− and mdx:Flt-1+/+ mice by western blotting (Fig. 3C and E), we proceeded to create triple-mutant mdx:utrn−/−:Flt-1+/− and the control mdx:utrn−/−:Flt-1+/+ mice. mdx:utrn−/−:Flt-1+/− mice were viable, and progenies were found to be in the expected Mendelian ratio (data not shown). The loss of both the dystrophin and utrophin results in a very severe muscle phenotype with a very high fiber turnover. However, histological assessment of the TA muscle showed decreased amounts of CLN in the mdx:utrn−/−:Flt-1+/− mice (73.28 ± 1.94) compared with the control mdx:utrn−/−:Flt-1+/+ (85.09 ± 1.99) littermates (Fig. 7A and B). The mdx:utrn−/−:Flt-1+/− mice also displayed a significant increase in the body mass compared with the control mdx:utrn−/−:Flt-1+/+ mice (Fig. 7C).
Figure 7.
mdx:utrn−/−:Flt-1+/− triple-mutant mice display an improved phenotype compared with the mdx:utrn−/−:Flt-1+/+ mice. (A) Representative images from HE staining of the TA muscle of the mdx:utrn−/−:Flt-1+/− and the control mdx:utrn−/−:Flt-1+/+ mice. (B) The mdx:utrn−/−:Flt-1+/− mice (n = 6) contain less muscle fibers with CLN compared with the mdx:utrn−/−:Flt-1+/+ mice (n = 5). (C) The mdx:utrn−/−:Flt+/− mice (n = 18) show increased body mass compared with mdx:utrn−/−:Flt-1+/+ mice (n = 20). (D) A Kaplan–Meier survival test shows the mdx:utrn−/−:Flt-1+/− mice (n = 33) have a longer survival probability and higher average survival time (E) compared with the mdx:utrn−/−:Flt-1+/+ mice (n = 36). Values are mean ± SEM. Asterisks or double asterisks indicate experimental pairs where differences between the compared values were statistically significant (P < 0.05 or P < 0.01, respectively).
Importantly, the average lifespan of mdx:utrn−/−:Flt-1+/− mice was significantly extended (177.88 days ± 17.60) compared with the control mdx:utrn−/−:Flt-1+/+ mice (91.76 days ± 10.22) (Fig. 7D and E). Both genotypes are stable for around 30–50 days, after which the mdx:utrn−/−:Flt-1+/+ mice show a sharp decline in viability compared with a more gradual lethality seen in the mdx:utrn−/−:Flt-1+/− mice. A Kaplan–Meier survival test showed there to be a significant difference between the two genotypes (Fig. 7D). These data strongly suggest that Flt-1+/− background attenuated the muscular dystrophy phenotype of the mdx:utrn−/− double-mutant mice to extend their lifespan, possibly through increased vascular density. Taken together, these data show that developmentally increasing angiogenesis and vascular density can partially rescue the dystrophic phenotype in DMD model mice.
No change in inflammatory response in the mdx:Flt-1+/− mice
Inflammation is known to contribute to muscle healing and regeneration. In addition, it is reported that Flt-1 is also expressed in macrophages (31). Therefore, we compared the degree of inflammation in the TA muscle isolated from the mdx:Flt-1+/− mice and the control mdx:Flt-1+/+ mice. Immunostaining for Mac-1 (a marker for monocytes and macrophages) and Gr-1 (a marker for monocytes and granulocytes) showed there was no significant difference in the number of inflammatory cells that were observed in the TA muscle between the mdx:Flt-1+/− mice and the control mdx:Flt-1+/+ (Supplementary Material, Fig. S2).
Satellite cells are negative for VEGF receptors Flt-1 and Flk-1
Several reports have implicated that satellite cell proliferation and migration are influenced by VEGF, possibly through its receptors, Flk-1 and Flt-1 (15). To elucidate whether satellite cells express Flt-1 and Flk-1 and to examine for any potential cell autonomous effects, we first characterized lacZ expression in the TA muscle of Flt−1+/− (Flt-1-lacZ) (14), Flk-1-lacZ (32) and Myf5+/nLacZ knock-in mice (33,34). These lacZ expressions recapitulate endogenous Flt-1, Flk-1 and Myf5 expression. Clearly, lacZ-positive cells were detected around vasculatures located outside of laminin-positive basal lamina of muscle fibers in both Flt-1-lacZ and Flk-1-lacZ mice (Supplementary Material, Fig. S3A). In contrast, lacZ-positive satellite cells were detected within laminin-positive basal lamina of muscle fibers in Myf5+/nLacZ mice, indicating that quiescent satellite cells and muscle fibers do not express Flt-1 or Flk-1 (Supplementary Material, Fig. S3A). Next, we isolated single-muscle fibers from both Flt-1-lacZ and Flk-1-lacZ mice along with control Myf5+/nLacZ mice (Supplementary Material, Fig. S3B). As expected, the satellite cells started proliferating around day 2, and by day 5, a large amount of satellite cell-derived myoblasts had started dividing outside the muscle fibers (35). However, none of the cultures from Flt-1-lacZ or Flk-1-lacZ mice were stained positive for lacZ, indicating that neither quiescent satellite cells nor satellite cell-derived myoblasts express Flt-1 or Flk-1. In addition, none of the myotubes differentiated from myoblasts isolated from Flt-1-lacZ and Flk-1-lacZ mice were stained positive for lacZ. In contrast, satellite cells and satellite cell-derived myoblasts were clearly positive for lacZ in cultures from Myf5+/nLacZ mice. In addition, immunostaining clearly showed that desmin-positive myoblasts are negative for Flt-1 or Flk-1, whereas human umbilical vein endothelial cells (HUVECs) are positive for both receptors (Supplementary Material, Fig. S3C). Therefore, these results strongly indicated that non-cell autonomous effects improved muscle phenotype in the mdx:Flt-1+/− mice.
Increased proliferation of myogenic precursor cells by increased vascular niche
The single-muscle fiber experiments ruled out the possibility that heterozygous Flt-1 gene knockout cell autonomously affects satellite cell-derived myogenic precursor cell proliferation or differentiation. The muscle in mdx mice is able to regenerate effectively in early stages due to the proliferation of the satellite cell pool, which fuses with the existing muscle fibers to repair the damaged tissue. However, as the muscle ages, the satellite cell number decreases, resulting in impaired muscle regeneration (28). To examine whether the number of satellite cells and myogenic precursor cells was affected in the mdx:Flt-1+/− mice compared with the control mdx:Flt-1+/+ mice, we performed FACS analysis on dissociated cells from the TA muscle and characterized them using a satellite cell-specific antibody, SM/C-2.6 (36) (Fig. 8A and B). Interestingly, although the total number of SM/C-2.6+ satellite cells and myogenic precursor cells isolated from the TA muscle of Flt-1+/− mice showed no significant difference compared with those of the wild-type Flt-1+/+ mice, these cell populations were markedly increased in the mdx:Flt-1+/− mice compared with the control mdx:Flt-1+/+ mice. The increased number of Pax7 + satellite cells located underneath basal lamina (laminin+) in the mdx:Flt-1+/− mice were also detected in TA muscle sections (Fig. 8C and D). Therefore, it is possible that increased vascular niche might promote myogenic precursor cell proliferation and/or survival.
Figure 8.
Increased myogenic cells in the increased vascular niche. (A) FACS analysis shows that the dissociated cells from the TA muscle of the mdx:Flt-1+/− mice contained increased amount of CD45-PE− CD31-PE− Sca-1-PE− SM/C-2.6-APC+ satellite cells and myogenic precursor cells in the gated areas, compared with the mdx:Flt-1+/+ mice. Control indicates the FACS profile for the control antibodies. (B) Total number of CD45− CD31− Sca-1− SM/C-2.6+ myogenic cells per TA muscle was estimated by multiplying the percent from the FACS analysis (n = 6 each) with the total number of cells obtained from the TA muscle. (C) Representative images from TA muscle sections of the control mdx:Flt-1+/+ and mdx:Flt-1+/− mice stained with anti-Pax7 antibody (green) to detect satellite cells (arrows) and anti-laminin antibody (red) to detect basal lamina. Nuclei were counterstained with DAPI (blue). (D) Quantification of Pax7+ myogenic cells in a TA muscle of the control mdx:Flt-1+/+ (n = 4) and mdx:Flt-1+/− (n = 3) mice. Relative amount of Pax7+ cells counted in a view field. (E) Desmin staining on myoblast co-culture with fibroblasts (10T1/2) cell line (Fb) or endothelial (b.End3) cell line (EC). (F) Quantification of the proliferation of myoblast as evaluated by the number of desmin-positive cells in culture (n = 3 each). Values are mean ± SEM. Asterisks or double asterisks indicate experimental pairs where differences between the compared values were statistically significant (P < 0.05 or P < 0.01, respectively).
To test for possible interaction between endothelial cells and myogenic precursor cells, satellite cell-derived primary myoblasts were cultured by themselves or co-cultured with either mouse 10T1/2 fibroblasts or mouse brain-derived endothelial (b.End3) cell lines (Fig. 8E and F). The cells were grown in myoblast growth media for 3 days and then stained for the muscle marker desmin. Myoblasts co-cultured with b.End3 cells displayed more desmin-positive cells than myoblasts alone or myoblasts co-cultured with 10T1/2 cells, indicating that co-culture with endothelial cells but not with fibroblasts promotes myoblast proliferation and/or survival. Taken together, these results strongly suggest that the increased levels of endothelial cells seen in mdx:Flt-1+/− muscle and the associated increased vascular niche may promote satellite cell-derived myogenic precursor cell proliferation. It is likely that this occurs by secreted factors from endothelial cells or direct interaction between endothelial cells and satellite cells or myogenic precursor cells. This may be important for the suppression of fibrosis and calcification along with the protection of muscle damage in dystrophic muscle, thus improving muscle contractile function.
DISCUSSION
Although there is currently no effective therapeutic options for DMD, there are many promising active areas of research (37,38). Most of these options focus on repairing the mechanoinstability of the muscle through cell therapy, gene therapy or a combination of the two. Recently, it has been shown that alleviating the problems associated with vasculature seen in mdx mice can drastically improve the diseased phenotype in mdx mice (5,12,19). However, the developmental relationship between angiogenesis and muscular dystrophy phenotype has remained to be elucidated.
In this report, we sought to compensate for the functional ischemia in the muscle of DMD model mice by developmentally increasing the vascular density. We found that the Flt-1+/− mice as well as the mdx:Flt-1+/− mice show increased blood vessel density in the muscle compared with the control Flt-1+/+ or mdx:Flt-1+/+ mouse counterparts. The increase in blood vessel density translates to an increase in blood perfusion to the muscle and better histological muscle phenotype as improved muscle contractile function. Concomitantly, it also led to an increase in survival rates and to improvement of muscle histology in the mdx:utrn−/−:Flt-1+/− combined triple-mutant mice compared with the control mdx:utrn−/−:Flt-1+/+ double-mutant mice. To our knowledge, this study is the first showing that developmentally increasing angiogenesis and thereby increasing vascular density can partially rescue the dystrophic phenotype in DMD model mice. Potential mechanisms include (i) decreased inflammatory response, (ii) cell autonomous effects, (iii) reducing ischemia by increasing tissue perfusion or (iv) increased vascular-derived interaction to increase satellite cell proliferation and/or protect muscle damage.
It is reported that to make room for new muscle fiber, muscle damage is followed by recruitment of macrophages to clear away the cellular debris left by damaged muscle tissue (39–42). However, we found no significant difference in the numbers of Mac-1+- or Gr-1+-infiltrated cells in the muscle of mdx:Flt-1+/− compared with the control mdx:Flt-1+/+ mice. Several reports have shown that VEGF has an effect on muscle satellite cell-derived myogenic precursor cells for cell proliferation and survival, mediated through its two major receptors, Flt-1 and Flk-1 (35,43). Since our system modulates the expression of Flt-1, it is important to examine cell autonomous effect on the muscle fibers and myogenic precursor cells. First, the muscle sectioning demonstrated that Flt-1- or Flk-1-expressing cells are located outside of basal lamina of muscle fibers. We also chose to examine the expression in satellite cell and satellite cell-derived myoblasts by single-muscle fiber cultures since it is possible to obtain the purest satellite cell population using this method. By immunological detection and lacZ knock-in mice, our data clearly demonstrate that muscle fibers, satellite cells and satellite cell-derived myoblasts do not express Flt-1 and Flk-1. This suggests that there are no cell autonomous effects on the muscle fibers or myogenic precursor cells through these receptors in our system.
Importantly, the increase in the blood vessel density in the Flt-1+/− and mdx:Flt-1+/− mice also translated into an increase in LDF at rest. During rest, the skeletal muscle lacks complete perfusion due to the absence of complete recruitment of the microvasculature (44). This recruitment of the microvasculature has been examined during exercise as well as during insulin infusion. During muscle activity, NOS-mediated vasodilation can increase the passive pressure gradient in the capillary beds, thereby recruiting more microvasculature and increasing tissue perfusion (45,46). The ischemic state created due to the lack of such vasodilation during contraction is a direct cause of muscle fiber damage in vivo in mdx mice (12). Therefore, the increase in basal blood flow detected in our muscular dystrophy model mice may offer a protective mechanism for the contraction-induced damage seen in the DMD (43).
Increased endothelial cell production in the muscle can have many different beneficial effects on dystrophic muscle through paracrine stimulation by protecting muscle fiber damage and activating myogenic precursor cells as a vascular niche. Numerous reports have shown cross-talk between endothelial cells and satellite cells (19,47,48). Our results as well as others have shown that endothelial cells and factors produced by them can mediate the response on satellite cell survival and proliferation (18,47,48). This is in accordance to our co-culture experiments which show increased proliferation of satellite cell-derived myoblasts when co-cultured with endothelial cells. Recently, Christov et al. (47) have shown that satellite cells are preferentially located next to capillaries. Since our model increases the amount of capillaries found in the muscle, it may effectively increase the amount of vascular niches that house the satellite cell compartment in the muscle. In this report we show that the number of satellite cells and myogenic precursor cells is markedly increased in the mdx:Flt-1+/− mice compared with the control mdx:Flt-1+/+ mice. This increase in the number of satellite cells and myogenic precursor cells may be responsible for the improved phenotype seen in the mdx:Flt-1+/− mice.
The present study shows that increased angiogenesis may be a novel avenue to improve some of the dystropathologies and functional parameters associated with DMD that can be used in conjuncture with other treatment strategies. For example, intramuscular injection of VEGF-containing recombinant adeno-associated viral vectors has been shown to make both functional and histological improvements in the both ischemic muscle and mdx muscle (19,49,50). This improvement is seen in combination with an increase in angiogenesis in the muscle. Ito et al. (5) also showed the importance of the lack of dystrophin in the vascular smooth muscle. They showed that expression of smooth-muscle-specific dystrophin in mdx mice resulted in partially improved muscle phenotype.
It is important to understand how vasculatures interact with muscle fibers and muscle stem cells to provide necessary oxygen and nutrition, protect from cell death and provide a vascular niche. Further studies are required to ascertain the exact mechanism and the long-term effects of increasing the vasculature. From a therapeutic point of view, neovascularization could be induced in dystrophic muscle using the administration of angiogenic factors, such as VEGF protein (19,49,50).
MATERIALS AND METHODS
Animals
DMD model mdx-5cv mice (B6Ros.Cg-Dmdmdx-5Cv/J) were obtained from the Jackson Laboratory (22). Heterozygous Flt-1 gene knockout (Flt-1+/− or Flt-1-lacZ) (13), Flk-1-lacZ (32) and Myf5+/nLacZ (33) knock-in mice were provided by Drs Guo-Hua Fong, Masatsugu Ema and Shahragim Tajbakhsh, respectively. mdx-5cv:Flt-1+/− (double-mutant) mice and mdx−5cv:Flt-1+/+ (control) mice were established by crossing mdx-5cv with Flt-1+/− mice. Heterozygous knockout mice for the utrophin+/− (utrn+/−) mice were obtained from Dr Robert W. Grange (30). mdx-5cv:utrn−/−:Flt-1+/− (triple-mutant) mice and mdx-5cv:utrn−/−:Flt-1+/+ (control) mice were established by crossing mdx-5cv:Flt-1+/− with utrn+/− mice. Age-matched litter mate mice (2–3 months old) were examined for experiments. Genotyping to detect the mutated alleles of mdx-5cv and utrophin was performed by PCR using the following primers: For mdx-5cv forward primer (no. 0981), 5′-GAAGCTCCCAGAGACAAGTC-3′; and reverse primer (no. 0982), 5′-TCATGAGCATGAAACTGTTCTT-3′. The PCR product DNA was digested with DraIII restriction enzyme (New England Biolabs). Wild-type allele generated 180 bp and mutant allele generated 50 and 130 bp bands. For the genotyping of utrophin, forward primer for utrophin knockout (no. 22803), 5′-TGCCAAGTTCTAATTCCATCAGAAGCTG-3′; forward primer for wild-type utrophin (no. 553), 5′-TGCAGTGTCTCCCAATAAGGTATGAAC-3′; and a common reverse primer (no. 554), 5′-CTGAGTCAAACAGCTTGGAAGCCTCC-3. For the genotyping of Flt-1+/− (Flt-1-lacZ), Flk-1-lacZ and Myf5+/nLacZ mice, small pieces of ear or tail tissue were stained with X-gal (Sigma-Aldrich) solution for lacZ expression as described previously (51). The animals were housed in an SPF environment and were monitored by the Research Animal Resources (RAR) of the University of Minnesota. All protocols were approved by the Institutional Animal Care and Usage Committee (IACUC) of the University of Minnesota.
Histological analysis
Freshly dissected tissues were embedded and frozen in OCT tissue freezing medium (Fisher Scientific) and sectioned at 8 µm thickness by cryostat. Sections were routinely stained with hematoxlyn and eosin (HE), and age-matched samples were used to quantify both CLN and fiber diameter. Sections were also stained with Alizarin red (Sigma-Aldrich), Oil red O (Sigma-Aldrich), van Gieson (elastic stain kit: Sigma-Aldrich) and NADH-TR (Sigma-Aldrich) for the assessment of calcification, adipose, fibrosis and fiber type, respectively. To access membrane permeability, EBD was used as described previously (24,25). Briefly, a solution of 1% Evans blue (Fluka), 0.1 ml/10 g of body mass, was injected in the intraperitoneal cavity. After 16–20 h, the animals were euthanized and their tissues were harvested. All analysis was performed manually using the ImageJ software from the NIH (52).
Immunostaining
Eight micron thick transverse cryosections were used with the Vectastain Elite ABC Kit (Vector Laboratories) for immunohistochemistry according to the manufacturer's instructions. Sections were labeled with anti-CD31 (eBioscience) followed by biotin-conjugated anti-mouse IgG secondary antibody. Sections were also labeled with biotin-conjugated anti-Mac-1 (eBioscience) and biotin-conjugated anti-Gr-1 antibodies (eBioscience). Sections were colorized using 3-amino-9-ethylcarbazole (AEC) (Sigma-Aldrich). Sections were also labeled using anti-slow MHC antibody (Sigma-Aldrich), anti-dystrophin antibody (Sigma-Aldrich) or anti-Pax7 antibody (R & D Systems) and anti-laminin antibody (Sigma-Aldrich) followed by Alexa 488-conjugated anti-mouse IgG and Alexa 596-conjugated anti-rabbit IgG secondary antibodies. Cell cultures were stained with anti-desmin antibody (Dako: D33), anti-Flt-1 antibody (Santa Cruz) or anti-Flk-1 antibody (BD Biosciences), followed by Alexa 488-conjugated anti-mouse IgG, Alexa 488-conjugated anti-goat IgG, Alexa 488-conjugated anti-rat IgG secondary antibody, respectively. Cell cultures were also stained by the Vectastain Elite ABC Kit, followed by AEC. LacZ expression in muscle was detected by X-gal staining overnight as described previously (51). Anti-laminin antibody (Sigma-Aldrich) was used to detect muscle basal lamina in X-gal-stained muscle sections. Sections and cell cultures were also nuclear stained with DAPI (4′,6-diamidino-2-phenylindole) (Sigma-Aldrich) and mounted with Dako fluorescence mounting media (Dako). Microscopic images were captured by a DP-1 digital camera attached to BX51 fluorescence microscope with 20× and 40× UPlanFLN objectives (all from Olympus). Photoshop C2 (Adobe Systems) was used for image processing.
In vivo force measurements
Maximal isometric torque of the hind limb dorsiflexors was assessed as described previously (53). Briefly, mice were anesthetized with a cocktail of fentanyl citrate (10 mg/kg body weight), droperidol (0.2 mg/kg body weight) and diazepam (5 mg/kg body weight). The left hind limb was then shaved, aseptically prepared, and each mouse was positioned on a heated platform, with its left foot placed in a metal foot plate attached to the shaft of a servomotor (model 300B-LR, Aurora Scientific, Aurora, Ontario, Canada). Two platinum electrodes (model E2-12, Grass Technologies, West Warwick, RI, USA) were inserted subcutaneously on either side of the peroneal nerve. A stimulator and stimulus isolation unit (models S48 and SIU5, respectively, Grass Technologies) stimulated the peroneal nerve via the platinum electrodes to induce a contraction of the anterior crural muscles. The parameters for stimulation were set at a 200 ms contraction duration consisting of 0.5 ms square-wave pulses at 300 Hz. The voltage was adjusted from 3.0 to 9.0 v until maximal isometric torque was achieved. Isometric torque results are presented per kilogram of body mass.
Whole-body tension assay
Muscle force production was measured by the whole-body ‘escape’ test as reported previously (54). Wild-type Flt-1+/+, Flt-1+/−, mdx:Flt-1+/+ and mdx:Flt-1+/− mice were placed between parallel wooden barriers to allow for forward movement only. The tails were attached to a fixed range force transducer (BioPac Systems). They were given a light pinch every 15 s and the force was measured for 5 min. The force values were normalized against the mouse body mass.
Laser Doppler flow
RBC flux was evaluated using the moorLab™ LDF meter with the MP7a probe that allows for collecting light from a deeper tissue level than standard probes according to the manufacturer's instructions (Moor Instruments, Millwey, Axminster, UK) (55). The fur from the right hind leg was removed using a chemical depilatory. Readings were taken using the probe from at least 10 different spots on the TA muscle. The PU was determined as the mean PU-value of the stabilized plateau.
Fluorescence-activated cell sorting
Dissociated cells were prepared from the TA muscle of 2-month-old mice after digestion of the muscle with collagenase type B and dispase II (Roche Diagnostics) (56). FACS analysis was performed on a FACS caliber equipped with double lasers (BD Biosciences). The following antibodies were used for FACS analysis: SM/C-2.6, kindly provided by Dr S. Fukada (36) with Alexa 488-labeled anti-mouse IgG, allophycocyanin (APC)-labeled CD31, phycoerythrin (PE)-labeled CD31, PE-labeled CD45, PE-labeled Sca-1 (all from PharMingen). Mouse and rat normal IgG conjugated with APC, PE and FITC (PharMingen) were used in the control experiment. Alexa 488/FITC and PE were excited by a 488 nm argon laser and their fluorescence was detected with an FL1 (530/30) and an FL2 (576/26) filter, respectively. APC was excited by a 633 nm red diode laser for the detection with an FL4 filter (620/20). Gates were strictly defined on the basis of single-antibody-stained control cells, as well as the forward scatter and side scatter patterns of the cells.
Single-muscle fiber culture
Single-muscle fibers were isolated from extensor digitorum longus (EDL) muscle of Flt-1-lacZ, Flk-1-lacZ and Myf5+/nLacZ mice, as reported previously (34,35). EDL muscle was dissected tendon to tendon. The dissected muscle was incubated in 0.2% collagenase type I (Sigma-Aldrich) at 37°C for 45–60 min. Heat-polished pipettes of subsequently smaller bore sizes were used gently to triturate and release the muscle fibers. Single-muscle fibers were picked up and transferred to Matrigel (BD Biosciences)-coated plates. The culture was maintained for up to 5 days at 37°C and 5% CO2 incubator. Plates were fixed for X-gal staining at appropriate time intervals, as reported previously (57).
Cell culture
Satellite cell-derived primary myoblasts were isolated from adult lower hind limb muscle from 2-month-old mice as described previously (58,59). Cells were maintained on collagen-coated dishes in myoblast growth medium consisting of HAM's F-10 medium supplemented with 20% FBS and 10 ng/ml basic FGF (R&D Systems). C3H10T1/2 fibroblast cell line and b.End3 cells were obtained from ATCC and maintained in DME medium supplemented with 10% FBS. Human umbilical vein endothelial cells (HUVEC) were kindly obtained from Dr Gregory M. Vercellotti and maintained on 1% gelatin-coated dishes in medium 199 supplemented with 10% FBS. Myoblasts were co-cultured with equal numbers of either 10T1/2 cells or b.End3 cells in myoblast growth medium. Myoblast proliferation was quantified by a number of desmin-positive cells 3 days after co-culture. At least 100 desmin-positive myoblasts were counted for each experiment.
Western blotting
Muscle proteins were isolated following the previously published protocols (60). The protein concentration of the fractions was determined by Micro BCA Protein Assay Reagent Kit (Pierce). Utrophin and dystrophin proteins were detected by western blotting with anti-utrophin antibody (Developmental Study Hybridoma Bank), or anti-dystrophin antibody (Sigma-Aldrich), followed by anti-mouse IgG-HRP (Bio-Rad). To verify equal loading proteins, the same blots were stripped and re-probed with anti-β-tubulin antibody (Sigma-Aldrich), followed by anti-mouse IgG-HRP (Bio-Rad). The reaction was developed using SuperSignal West Femto chemiluminescent substrate (Pierce) in accordance with the manufacturer's instructions.
Statistics
All data are presented as mean ± SEM. Comparison between groups was done by Student's t-test using a two-tailed distribution with two-sample equal variance unless otherwise stated. Data were considered statistically significant at P < 0.05. *P < 0.05 and **P < 0.01.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the Undergraduate Research Opportunity Program (637-5904 to M.V.); the Gregory Marzolf Muscular Dystrophy Training Grant (06378016 to M.V.); NIH P30 grant (P30AR057220 to D.A.L.) for supplying the mdx:utrn−/− mice, and the Muscular Dystrophy Association (MDA69856 to A.A.).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Drs James Ervasti and Kurt Prins for technical assistance in the whole-body tension assay and constructive comments, Dr LaDora V. Thompson for technical assistance in the western blotting, Dr Walter Low for the use of the MoorLab Laser Doppler, Dr Gregory M. Vercellotti for providing HUVECs, Dr So-ichiro Fukada for providing SM/C-2.6 antibody, Dr Robert W. Grange for providing mdx:utrn+/− mice and Dr Shahragim Tajbakhsh for providing Myf5+/nLacZ mice. FACS analysis was performed in the flow cytometry facility of the Stem Cell Institute, University of Minnesota Medical School.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Dalkilic I., Kunkel L.M. Muscular dystrophies: genes to pathogenesis. Curr. Opin. Genet. Dev. 2003;13:231–238. doi: 10.1016/s0959-437x(03)00048-0. doi:10.1016/S0959-437X(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 2.Davies K.E., Nowak K.J. Molecular mechanisms of muscular dystrophies: old and new players. Nat. Rev. Mol. Cell Biol. 2006;7:762–773. doi: 10.1038/nrm2024. doi:10.1038/nrm2024. [DOI] [PubMed] [Google Scholar]
- 3.Mendell J.R., Engel W.K., Derrer E.C. Duchenne muscular dystrophy: functional ischemia reproduces its characteristic lesions. Science. 1971;172:1143–1145. doi: 10.1126/science.172.3988.1143. doi:10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- 4.Miyatake M., Miike T., Zhao J., Yoshioka K., Uchino M., Usuku G. Possible systemic smooth muscle layer dysfunction due to a deficiency of dystrophin in duchenne muscular dystrophy. J. Neurol. Sci. 1989;93:11–17. doi: 10.1016/0022-510x(89)90157-3. doi:10.1016/0022-510X(89)90157-3. [DOI] [PubMed] [Google Scholar]
- 5.Ito K., Kimura S., Ozasa S., Matsukura M., Ikezawa M., Yoshioka K., Ueno H., Suzuki M., Araki K., Yamamura K., et al. Smooth muscle-specific dystrophin expression improves aberrant vasoregulation in mdx mice. Hum. Mol. Genet. 2006;15:2266–2275. doi: 10.1093/hmg/ddl151. doi:10.1093/hmg/ddl151. [DOI] [PubMed] [Google Scholar]
- 6.Cullen M.J., Jaros E. Ultrastructure of the skeletal muscle in the X chromosome-linked dystrophic (mdx) mouse: comparison with duchenne muscular dystrophy. Acta Neuropathol. 1988;77:69–81. doi: 10.1007/BF00688245. doi:10.1007/BF00688245. [DOI] [PubMed] [Google Scholar]
- 7.Loufrani L., Levy B.I., Henrion D. Defect in microvascular adaptation to chronic changes in blood flow in mice lacking the gene encoding for dystrophin. Circ. Res. 2002;91:1183–1189. doi: 10.1161/01.res.0000047505.11002.81. doi:10.1161/01.RES.0000047505.11002.81. [DOI] [PubMed] [Google Scholar]
- 8.Loufrani L., Matrougui K., Gorny D., Duriez M., Blanc I., Levy B.I., Henrion D. Flow (shear stress)-induced endothelium-dependent dilation is altered in mice lacking the gene encoding for dystrophin. Circulation. 2001;103:864–870. doi: 10.1161/01.cir.103.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weir A.P., Burton E.A., Harrod G., Davies K.E. A- and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle. J. Biol. Chem. 2002;277:45285–45290. doi: 10.1074/jbc.M205177200. doi:10.1074/jbc.M205177200. [DOI] [PubMed] [Google Scholar]
- 10.Coral-Vazquez R., Cohn R.D., Moore S.A., Hill J.A., Weiss R.M., Davisson R.L., Straub V., Barresi R., Bansal D., Hrstka R.F., et al. Disruption of the sarcoglycan–sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. doi:10.1016/S0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 11.Rando T.A. Role of nitric oxide in the pathogenesis of muscular dystrophies: a ‘two hit’ hypothesis of the cause of muscle necrosis. Microsc. Res. Tech. 2001;55:223–235. doi: 10.1002/jemt.1172. doi:10.1002/jemt.1172. [DOI] [PubMed] [Google Scholar]
- 12.Asai A., Sahani N., Kaneki M., Ouchi Y., Martyn J.A., Yasuhara S.E. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE. 2007;2:e806. doi: 10.1371/journal.pone.0000806. doi:10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong G.H., Rossant J., Gertsenstein M., Breitman M.L. Role of the flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. doi:10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 14.Fong G.H., Zhang L., Bryce D.M., Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. doi:10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 16.Kearney J.B., Ambler C.A., Monaco K.A., Johnson N., Rapoport R.G., Bautch V.L. Vascular endothelial growth factor receptor flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–2407. doi: 10.1182/blood.v99.7.2397. doi:10.1182/blood.V99.7.2397. [DOI] [PubMed] [Google Scholar]
- 17.Springer M.L., Ozawa C.R., Banfi A., Kraft P.E., Ip T.K., Brazelton T.R., Blau H.M. Localized arteriole formation directly adjacent to the site of VEGF-induced angiogenesis in muscle. Mol. Ther. 2003;7:441–449. doi: 10.1016/s1525-0016(03)00010-8. doi:10.1016/S1525-0016(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 18.Arsic N., Zacchigna S., Zentilin L., Ramirez-Correa G., Pattarini L., Salvi A., Sinagra G., Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. doi:10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Messina S., Mazzeo A., Bitto A., Aguennouz M., Migliorato A., De Pasquale M.G., Minutoli L., Altavilla D., Zentilin L., Giacca M., et al. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007;21:3737–3746. doi: 10.1096/fj.07-8459com. doi:10.1096/fj.07-8459com. [DOI] [PubMed] [Google Scholar]
- 20.Coulton G.R., Curtin N.A., Morgan J.E., Partridge T.A. The mdx mouse skeletal muscle myopathy. II. Contractile properties. Neuropathol. Appl. Neurobiol. 1988;14:299–314. doi: 10.1111/j.1365-2990.1988.tb00890.x. doi:10.1111/j.1365-2990.1988.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 21.Coulton G.R., Morgan J.E., Partridge T.A., Sloper J.C. The mdx mouse skeletal muscle myopathy. I. A histological, morphometric and biochemical investigation. Neuropathol. Appl. Neurobiol. 1988;14:53–70. doi: 10.1111/j.1365-2990.1988.tb00866.x. doi:10.1111/j.1365-2990.1988.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 22.Danko I., Chapman V., Wolff J.A. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr. Res. 1992;32:128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Deconinck A.E., Rafael J.A., Skinner J.A., Brown S.C., Potter A.C., Metzinger L., Watt D.J., Dickson J.G., Tinsley J.M., Davies K.E. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. doi:10.1016/S0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda R., Nishikawa A., Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J. Biochem. (Tokyo) 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- 25.Hamer P.W., McGeachie J.M., Davies M.J., Grounds M.D. Evans blue dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J. Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. doi:10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suelves M., Vidal B., Serrano A.L., Tjwa M., Roma J., Lopez-Alemany R., Luttun A., de Lagran M.M., Diaz-Ramos A., Jardi M., et al. uPA deficiency exacerbates muscular dystrophy in MDX mice. J. Cell Biol. 2007;178:1039–1051. doi: 10.1083/jcb.200705127. doi:10.1083/jcb.200705127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastoret C., Sebille A. Mdx mice show progressive weakness and muscle deterioration with age. J. Neurol. Sci. 1995;129:97–105. doi: 10.1016/0022-510x(94)00276-t. doi:10.1016/0022-510X(94)00276-T. [DOI] [PubMed] [Google Scholar]
- 28.Brack A.S., Bildsoe H., Hughes S.M. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell. Sci. 2005;118:4813–4821. doi: 10.1242/jcs.02602. doi:10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- 29.Maggs A.M., Taylor-Harris P., Peckham M., Hughes S.M. Evidence for differential post-translational modifications of slow myosin heavy chain during murine skeletal muscle development. J. Muscle Res. Cell. Motil. 2000;21:101–113. doi: 10.1023/a:1005639229497. doi:10.1023/A:1005639229497. [DOI] [PubMed] [Google Scholar]
- 30.Grady R.M., Teng H., Nichol M.C., Cunningham J.C., Wilkinson R.S., Sanes J.R. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. doi:10.1016/S0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 31.Sawano A., Iwai S., Sakurai Y., Ito M., Shitara K., Nakahata T., Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. doi:10.1182/blood.V97.3.785. [DOI] [PubMed] [Google Scholar]
- 32.Ema M., Takahashi S., Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. doi:10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 33.Tajbakhsh S., Rocancourt D., Buckingham M. Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in myf-5 null mice. Nature. 1996;384:266–270. doi: 10.1038/384266a0. doi:10.1038/384266a0. [DOI] [PubMed] [Google Scholar]
- 34.Beauchamp J.R., Heslop L., Yu D.S., Tajbakhsh S., Kelly R.G., Wernig A., Buckingham M.E., Partridge T.A., Zammit P.S. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. doi:10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shefer G., Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol. Biol. 2005;290:281–304. doi: 10.1385/1-59259-838-2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukada S., Higuchi S., Segawa M., Koda K., Yamamoto Y., Tsujikawa K., Kohama Y., Uezumi A., Imamura M., Miyagoe-Suzuki Y., et al. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp. Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. doi:10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Cossu G., Sampaolesi M. New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol. Med. 2007;13:520–526. doi: 10.1016/j.molmed.2007.10.003. doi:10.1016/j.molmed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien K.F., Kunkel L.M. Dystrophin and muscular dystrophy: past, present, and future. Mol. Genet. Metab. 2001;74:75–88. doi: 10.1006/mgme.2001.3220. doi:10.1006/mgme.2001.3220. [DOI] [PubMed] [Google Scholar]
- 39.Sato K., Yokota T., Ichioka S., Shibata M., Takeda S. Vasodilation of intramuscular arterioles under shear stress in dystrophin-deficient skeletal muscle is impaired through decreased nNOS expression. Acta Myol. 2008;27:30–36. [PMC free article] [PubMed] [Google Scholar]
- 40.Wehling M., Spencer M.J., Tidball J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. doi:10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehling-Henricks M., Lee J.J., Tidball J.G. Prednisolone decreases cellular adhesion molecules required for inflammatory cell infiltration in dystrophin-deficient skeletal muscle. Neuromuscul. Disord. 2004;14:483–490. doi: 10.1016/j.nmd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Barleon B., Sozzani S., Zhou D., Weich H.A., Mantovani A., Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 43.Deasy B.M., Feduska J.M., Payne T.R., Li Y., Ambrosio F., Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol. Ther. 2009;17:1788–1798. doi: 10.1038/mt.2009.136. doi:10.1038/mt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawson D., Vincent M.A., Barrett E.J., Kaul S., Clark A., Leong-Poi H., Lindner J.R. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am. J. Physiol. Endocrinol. Metab. 2002;282:E714–E720. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- 45.Lindbom L. Microvascular blood flow distribution in skeletal muscle. An intravital microscopic study in the rabbit. Acta Physiol. Scand. Suppl. 1983;525:1–40. [PubMed] [Google Scholar]
- 46.Crosbie R.H. NO vascular control in Duchenne muscular dystrophy. Nat. Med. 2001;7:27–29. doi: 10.1038/83309. doi:10.1038/83309. [DOI] [PubMed] [Google Scholar]
- 47.Christov C., Chretien F., Abou-Khalil R., Bassez G., Vallet G., Authier F.J., Bassaglia Y., Shinin V., Tajbakhsh S., Chazaud B., et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. doi:10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Germani A., Di Carlo A., Mangoni A., Straino S., Giacinti C., Turrini P., Biglioli P., Capogrossi M.C. Vascular endothelial growth factor modulates skeletal myoblast function. Am. J. Pathol. 2003;163:1417–1428. doi: 10.1016/S0002-9440(10)63499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan H., Guo Y., Zhang P., Zu L., Dong X., Chen L., Tian J., Fan X., Wang N., Wu X., et al. Superior neovascularization and muscle regeneration in ischemic skeletal muscles following VEGF gene transfer by rAAV1 pseudotyped vectors. Biochem. Biophys. Res. Commun. 2005;336:287–298. doi: 10.1016/j.bbrc.2005.08.066. doi:10.1016/j.bbrc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 50.Borselli C., Storrie H., Benesch-Lee F., Shvartsman D., Cezar C., Lichtman J.W., Vandenburgh H.H., Mooney D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl Acad. Sci. USA. 2005;107:3287–3292. doi: 10.1073/pnas.0903875106. doi:10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asakura A., Lyons G.E., Tapscott S.J. The regulation of MyoD gene expression: conserved elements mediate expression in embryonic axial muscle. Dev. Biol. 1995;171:386–398. doi: 10.1006/dbio.1995.1290. doi:10.1006/dbio.1995.1290. [DOI] [PubMed] [Google Scholar]
- 52.Abramoff M.D., Magelhaes P.J., Ram S.J. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 53.Baltgalvis K.A., Call J.A., Nikas J.B., Lowe D.A. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–454. doi: 10.1002/mus.21327. doi:10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnemann K.J., Fitzsimons D.P., Patel J.R., Liu Y., Schneider M.F., Moss R.L., Ervasti J.M. Cytoplasmic gamma-actin is not required for skeletal muscle development but its absence leads to a progressive myopathy. Dev. Cell. 2006;11:387–397. doi: 10.1016/j.devcel.2006.07.001. doi:10.1016/j.devcel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Clark A.D., Youd J.M., Rattigan S., Barrett E.J., Clark M.G. Heterogeneity of laser Doppler flowmetry in perfused muscle indicative of nutritive and nonnutritive flow. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1324–H1333. doi: 10.1152/ajpheart.2001.280.3.H1324. [DOI] [PubMed] [Google Scholar]
- 56.Asakura A., Komaki M., Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. doi:10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 57.Asakura A., Rudnicki M.A. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp. Hematol. 2002;30:1339–1345. doi: 10.1016/s0301-472x(02)00954-2. doi:10.1016/S0301-472X(02)00954-2. [DOI] [PubMed] [Google Scholar]
- 58.Asakura A., Hirai H., Kablar B., Morita S., Ishibashi J., Piras B.A., Christ A.J., Verma M., Vineretsky K.A., Rudnicki M.A. Increased survival of muscle stem cells lacking the MyoD gene after transplantation into regenerating skeletal muscle. Proc. Natl Acad. Sci. USA. 2007;104:16552–16557. doi: 10.1073/pnas.0708145104. doi:10.1073/pnas.0708145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabourin L.A., Girgis-Gabardo A., Seale P., Asakura A., Rudnicki M.A. Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J. Cell Biol. 1999;144:631–643. doi: 10.1083/jcb.144.4.631. doi:10.1083/jcb.144.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson L.V., Durand D., Fugere N.A., Ferrington D.A. Myosin and actin expression and oxidation in aging muscle. J. Appl. Physiol. 2006;101:1581–1587. doi: 10.1152/japplphysiol.00426.2006. doi:10.1152/japplphysiol.00426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.