Abstract
Smoking promotes the development of allergic asthma and pneumonia. Chlamydophila pneumoniae lung infection is associated with an increased risk for asthma, inducing an immune response regulated by dendritic cells (DCs). This study sought to determine whether exposure to cigarette smoke modulates the functional activity of CD11c-positive DCs in the lung, with and without concomitant C. pneumoniae infection. Bone marrow–derived DCs (BMDCs) were exposed in vitro to cigarette smoke extract (CSE) and/or live C. pneumoniae (Cpn), and then adoptively transferred intratracheally into wild-type mice. Although CSE plus Cpn appeared to exert an additive effect on the production of Th2 cytokines in vitro, we did not see this effect in vivo. However, the adoptive transfer of DCs pulsed with both CSE and C. pneumoniae into the lungs of naive mice led to an influx of plasmacytoid DCs (pDCs) that suppressed the Th2 skewing ability of the transferred BMDCs. The depletion of pDCs by antibody restored the Th2 skewing ability of the BMDCs. The expression of indoleamine-2,3-dioxygenase in the lung was reduced after the depletion of pDCs, and blocking IFN-α in vitro prevented the ability of pDCs to inhibit the Th2 responses induced by myeloid DCs (mDCs), suggesting their potential involvement in the mechanism of altered polarization. In conclusion, exposure to cigarette smoke skews C. pneumoniae–induced mDCs responses toward a Th2 bias in the lung, which is prevented by pDCs. We propose that pDCs may play a major role in the immunosuppressive lung environment in smokers with C. pneumoniae infection.
Keywords: dendritic cells, Chlamydia pneumoniae, bacterial pneumonia, cigarette smoke exposure
CLINICAL RELEVANCE.
Smoking and Chlamydia infection often coexist. Understanding lung Th2 responses and allergic asthma and the role of plasmacytoid dendritic cells are important when smoking and Chlamydia infection coexist.
Smoking has profound pathophysiologic effects on the lung, leading to major health problems such as chronic obstructive disease (COPD), emphysema, and cancer. Smokers are also more vulnerable to infections and allergic airways diseases (1, 2) compared with nonsmokers. Numerous studies indicate that cigarette smoke (CS) causes an inflammatory response, similar to that evoked by pathogens, characterized by the early influx and activation of inflammatory cells and a release of chemokines (3, 4).
Chlamydophila pneumoniae is an obligate intracellular bacterium that can also exacerbate atherosclerosis and asthma (5, 6). We showed that C. pneumoniae, depending on the infective dose, can lead to either a Th1 response, or can act as an adjuvant to induce allergic sensitization and lead to Th2-like immune responses in mice (6). Specifically, a lower dose of C. pneumoniae (5 × 105 inclusion-forming units [IFUs]/ml) induced allergic sensitization to subsequent challenge with an inert antigen, 5 days after inoculation. This sensitization was characterized by pulmonary inflammation, increased eosinophils, goblet-cell hyperplasia, and antigen-specific IgE and IgG1 (6). These effects were mediated via the recognition of the pathogen by innate immune cells, such as dendritic cells (DCs), that were responsible for processing the bacteria in a myeloid differentiation factor (MyD88)-dependent manner. In contrast, a higher dose of C. pneumoniae (5 × 106 IFUs/ml) led to a more Th1-like response, which resulted in a lack of allergic sensitization.
DCs are professional antigen-presenting cells (APCs) whose influence can determine the immune host response against any type of insult. Vassallo and colleagues (7) reported that CS-treated human blood monocyte–derived DCs increased the production of cytokines and chemokines that induced the recruitment of CD4-positive lymphocytes. These data are consistent with studies in “smoking” mice, where higher numbers of macrophages in the lung were evident (8). DCs express Toll-like receptors (TLRs) and other pattern-recognition receptors that can sense pathogens and endogenous ligands, including oxidants (9). Exposure to CS can prime cells for the subsequent activation of TLR2 and TLR4, and can increase the susceptibility of smokers to infection by bacteria that are detected by TLR2 and TLR4 (10). Cigarette smoke extract (CSE) was established in several studies as a valid surrogate marker of CS for in vitro studies investigating the mechanisms of action of CS (7).
DCs play a major role in both C. pneumoniae infection models (6, 11–13) and “smoking” mice models (14). D'Hulst and colleagues described increased recruitment and activation of DCs to the lungs of CS-exposed mice (14). In this study, we investigated the functional activity and role of lung DCs (CD11c-positive cells) when bone marrow–derived DCs (BMDCs) pulsed with CS extract (CSE), with and without C. pneumoniae, were adoptively transferred into naive mice. We found that BMDCs exposed in vitro to both CSE and C. pneumoniae displayed a strong Th2 skew, as evidenced by higher levels of IL-4 and IL-5, and lower levels of IL-12. In contrast, the adoptive transfer of BMDCs pulsed ex vivo with both CSE and C. pneumoniae into the lungs of naive mice led to an increased influx of plasmacytoid DCs (pDCs), which prevented the Th2 skewing. An antibody depletion of pDCs restored the Th2-type inflammation in the lung. The expression of indoleamine-2,3-dioxygenase (IDO) in the lungs when BMDCs were adoptively transferred into mice was reduced by the depletion of pDCs, suggesting their potential involvement in the mechanism of altered polarization. Collectively, our results indicate that exposure to CSE and C. pneumoniae induces the responses of myeloid DCs (mDCs) toward a Th2 bias, which is normally prevented by pDCs. We propose that pDCs play a major role in the immunosuppressive lung environment in smokers with C. pneumoniae infection.
MATERIALS AND METHODS
Mice
Male, pathogen-free C57BL/6 mice (aged 8–12 wk) were used throughout the study. The C57BL/6 wild-type and TLR2 knockout (KO) mice were purchased from Jackson Laboratories (Bar Harbor, ME), whereas TLR4 KO mice were kindly provided by Dr. Shizuo Akira (Osaka University, Osaka, Japan). A homogenous population of these mice was established by backcrossing onto the C57BL/6 background for at least 10 generations, as previously described (5). Mice were fed a standard chow diet and housed under specific pathogen–free conditions at Cedars-Sinai Medical Center. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center.
Bone Marrow–Derived Dendritic Cells and Adoptive Transfer Experiments
Mice were anesthetized with isofluorane before adoptive-transfer experiments. The BMDCs were previously isolated from femurs and tibias, and cultured with granulocyte macrophage colony-stimulating factor (10 ng/ml; Invitrogen, Carlsbad, CA) for 6 to 7 days, as previously described (11). The purity of BMDCs, as assessed by flow cytometry, was typically greater than 95%. These cells were CD11c-positive (DC marker) and F4/80-negative (specific macrophage marker). The BMDCs were CD11c positively isolated by means of MicroBeads (Miltenyi Biotec, Auburn, CA) before any type of treatment. Cells were then pulsed overnight with CSE and/or C. pneumoniae (5 × 105 IFUs/ml) before adoptive transfer into mice. The intratracheal application of treated or PBS-treated CD11c-positive BMDCs (5 × 105 cells/mouse) was performed (100 μl) in recipient mice. The inoculation of cells (in PBS), extensively washed, was performed only once. For pDC depletions, the 120G8 antibody (120G8 Ab, 150 μg/mouse; Dendritics, Lyon, France) or IgG2a was intravenously injected 3 days after the adoptive transfer of treated BMDCs. Two days after the injection of the depleting antibody, the mice were killed. For some experiments, bronchoalveolar lavage fluid (BAL) was collected with 0.5 ml of PBS (0.5 mM EDTA in PBS).
Infection with C. pneumoniae
The C. pneumoniae strain CM-1 (ATCC, Manassas, VA) was propagated in Hep-2 cells and stored suspended in sucrose sodium phosphate glutamic acid buffer at −80°C, as previously described (6). The Hep-2 cells and C. pneumoniae stocks were determined by PCR to be free of mycoplasma contamination. The BMDCs were treated with 5 × 105 IFUs/ml of C. pneumoniae or with the buffer alone, performed as a negative control (mock treatment; data not shown).
Preparations of CSE
Cigarette smoke extract was prepared as described by Paul-Clark and colleagues (10). Briefly, the smoke from four full-strength Marlboro cigarettes (filters removed) was passed through 100 ml of RPMI 1640. The percentage of CSE was referred as to the undiluted solution, and was considered to be 100%. The CSE was tested for LPS contamination, using the Limulus assay (Sigma, St. Louis, MO), as previously described (10). The CSE was used in all cases within 30 minutes of preparation.
Preparation of Lung CD11c-Positive Dendritic Cells
Lung DCs or lung CD11c-positive cells were generated by digesting the lungs with 10 μg/ml Blendzyme 3 (Roche, Mannheim, Germany), 20 μg/ml DNaseI (Roche), and antibiotics for 45 minutes. Cell suspensions were passed through 70-μm cell strainers, and red blood cells were lysed. For some experiments, CD11c-positive (CD11c+) cells were isolated using anti-CD11c–coated microbeads (Miltenyi Biotec). Purity was checked by flow cytometry, using anti-CD11c, CD11b, F4/80, and Gr-1 antibodies (eBioscience, San Diego, CA), and was routinely approximately 85%.
Flow Cytometry Analysis
The composition of lung inflammatory cells was determined by flow cytometry, using several antibodies: CD11c-phycoerythrin (PE) or allophycocianin, CD11b-fluorescein isothiocyanate (FITC), Gr1-PE-Cy5, CD3-PE-Cy5, CD4-PE-Cy5, CD8-FITC, F4/80-PE-Cy5, and B220-PE (eBioscience).
ELISA
Interleukin-5, IL-4, IL-12p70, IL-10, monocyte chemoattractant protein-1, IgE, and IFN-γ were measured in conditioned media and BAL, using commercially available ELISAs (eBioscience and BD System, San Jose, CA). The detection of the cytokines for in vitro studies was performed after the treatment of cells with CSE (3–10%), C. pneumoniae (5 × 105 IFUs/ml), IFN-α–neutralizing antibody (2.5 ng/ml; PBL Interferon Source, Piscataway, NJ), or IgG1 isotype control. Experiments for IFN-α neutralization were performed by adding the neutralizing antibody to BMDCs treated with CSE (3–10%) and/or C. pneumoniae (5 × 105 IFUs/ml).
Immunoblot Analysis
Bone marrow–derived plasmacytoid dendritic cells (BMpDCs) were prepared by isolating bone marrow cells from tibias and femurs, as described previously. Cells were treated with FLT-3 ligand (50 ng/ml; Santa Cruz Technologies, Santa Cruz, CA) for 10 days, as described by Romani and colleagues (15). The purity of BMpDCs was analyzed in terms of CD11c+ B220+ cells, using flow cytometry. After stimulation with the various ligands, cells were lysed directly in sample buffer. Samples were subsequently analyzed by SDS-PAGE, and transferred to polyvinylidene fluoride membranes. Membranes were immunoblotted with anti-IDO antibody (Biolegend, San Diego, CA) or with an anti–glyceraldehyde 3-phosphate dehydrogenase antibody (Santa Cruz Technologies) to confirm equal protein loading.
RT-PCR and Real-Time PCR
Total RNA was isolated from BMpDCs, using the RNeasy Mini Kit (Qiagen, Valencia, CA). The cDNA was generated by reverse transcription, using random hexamers. The cDNA (5 μg/reaction) was used as a template in subsequent PCR analyses. Transcript levels were determined by real-time PCR (BioRad, Hercules, CA), using the TaqMan Master Mix Reagent Kit (Applied Biosystems, Foster City, CA). The IFN-α PCR primers were purchased from the Applied Bioscience Co. Primers were used at a concentration of 1 μM for real-time PCR. Cycling conditions for real-time PCR (with a total of 45 cycles) comprised: step 1, 15 minutes at 95°C; step 2, 25 seconds at 60°C (IFN-α) or 55°C (GAPDH), and 25 seconds at 72°C; step 3, 5 minutes at 72°C; step 4, 5 seconds transition time from 65°C to 95°C. Data from the reaction were collected and analyzed using complementary computer software (BioRad). Relative quantifications of gene expression were calculated using standard curves, and were normalized to the GAPDH.
Assessment of Cell Viability
The effect of treatments on viability was determined by measuring the mitochondrial-dependent reduction of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (Sigma, Poole, UK) to formazan, as we described previously (10). This procedure was performed after all treatments. None of these treatments significantly affected cell viability.
Immunohistochemistry and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling TUNELAssay
Left lung lobes were fixed in PBS/2% paraformaldehyde/0.2% picric acid, to assess eosinophilic airway inflammation. The 7-μm cryosections were cut, and eosinophils were detected using eosinophil peroxidase-specific staining, as described previously (6). For other experiments, formalin-fixed lung sections were stained for apoptotic cells, using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay as described in the manufacturer's instructions (R&D System), or IDO was detected using the FITC-IDO antibody (Biolegend). Data were expressed as number of eosinophils or TUNEL-positive cell/cm2 of lung section, as determined using Image Pro Plus 5.1 Software (Media Cybernetics, Bethesda, MD).
Statistical Analysis
Results are expressed as mean ± SEM. Changes observed in treatment groups compared with control cells were analyzed using one-way ANOVA and/or the Student t test. P < 0.05 was considered significant.
RESULTS
CSE Promotes C. pneumoniae–Induced DC Responses toward a Th2 Bias
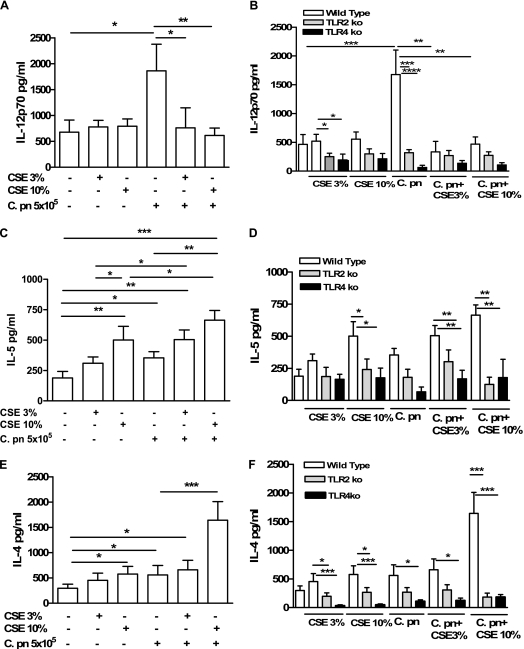
The BMDCs were treated with CSE with or without C. pneumoniae (5 × 105 IFUs/ml). Cell-free supernatants were harvested, and the expression of Th1 (IL-12p70) and Th2 (IL-4, IL-5, and IL-10) cytokines in the supernatants was assayed using ELISA. The CSE (3% and 10%) induced a predominantly Th2-type response. We observed the increased expression of IL-5 (Figure 1B), IL-4 (Figure 1C), and IL-10 (data not shown), but not IL-12p70 (Figure 1). In contrast, the C. pneumoniae infection of BMDCs resulted in a predominantly Th1-type response. We observed an ∼3-fold increase in expression of IL-12p70 compared with untreated control cells. However, we also found significant increases in expression of IL-5 and IL-4 (Figures 1A–1C). Of interest, the simultaneous treatment of BMDCs with both CSE and C. pneumoniae significantly increased the amount of Th2-driven cytokines, compared with either alone or untreated controls (IL-5 and IL-4, and IL-10; Figures 1B and 1C, and data not shown, respectively). However, the simultaneous treatment with CSE and C. pneumoniae completely abolished the increased expression of IL-12p70 caused by stimulation with C. pneumoniae alone. The concentrations of IL-12p70 with cotreatment were no different than in untreated control cells (Figure 1A). Moreover, C. pneumoniae plus CSE-treated BMDCs also secreted increased amounts of TGF-β and IL-10, compared with either treatment alone (data not shown). Collectively, these results appear most consistent with the conclusion that C. pneumoniae causes a Th1-biased response in BMDCs, whereas CSE predominantly causes a Th2 polarization. However, when BMDCs are treated with both C. pneumoniae and CSE simultaneously, the effects of CSE dominate and overcome the Th1 bias induced by C. pneumoniae alone. To exclude the possibility that the alteration in polarization toward a Th2 phenotype by CSE was not the result of the specific C. pneumoniae multiplicity of infection (MOI) used (i.e., an IFU-dependent effect), we performed similar experiments using varying MOIs (specifically, 1, 5, 10, and 50). Regardless of the MOI used, CSE shifted the usual Th1 cytokine bias caused by C. pneumoniae toward a Th2 phenotype (data not shown). The reduced cytokine secretion was not attributable to cell death, as assessed using the MTT assay (data not shown).
Figure 1.
The cigarette smoke extract (CSE) skews the C. pneumoniae–induced Th1-like response toward a Th2-like response in vitro. The bone marrow–derived dendritic cells (BMDCs) were treated with CSE alone (3% and 10%) and/or C. pneumoniae, and 24 hours later the supernatants were harvested. Concentrations of IL-12p70 (A, B), IL-5 (C, D), and IL-4 (E, F) were measured using ELISA. (A, C, E) Wild-type BMDC mice (open bars). (B, D, F) Wild-type (open bars), TLR2 KO (shaded bars), and TLR4 KO (solid bars) mice. There were 12 experiments, and each treatment was performed in triplicate. Statistically significant differences are denoted as *P < 0.05, **P < 0.01, and ***P < 0.0001, as determined by one-way ANOVA and the Student t test.
Intact TLR2 and TLR4 Signaling Is Necessary for CSE and C. pneumoniae to Cause Cytokine Release from DCs
Ligands produced by C. pneumoniae are known to activate both TLR2 and TLR4 (5, 6, 16). Although TLRs were initially thought to be pathogen-sensing receptors, TLRs are also known to recognize a wide variety of other ligands, including host-derived “danger signals” and other molecules (16). Results in the previous paragraph suggest that CSE somehow antagonizes the effects of C. pneumoniae on DCs. This could occur in a variety of ways: CSE could directly compete for TLR2 and/or TLR4, or alternately, CSE could directly or indirectly affect TLR2-dependent and/or TLR4-dependent signaling at some level downstream from the receptor–ligand interaction. To determine whether CSE might directly interact with TLR2 and/or TLR4, we obtained BMDCs from TLR2−/− or TLR4−/− mice, and stimulated them with CSE, C. pneumoniae, both, or control media vehicle only. As expected, in the absence of TLR2 or TLR4, C. pneumoniae failed to increase the expression of IL-12p70, IL-5, or IL-4 (Figures 1D–1F). In the absence of either TLR2 or TLR4, CSE also failed to induce the release of IL-5 (Figure 1E) and IL-4 (Figure 1F) from CDs. The cotreatment of TLR2-deficient or TLR4-deficient DCs with C. pneumoniae and CSE caused no significant increase in expression of any of the cytokines measured, compared with baseline untreated conditions. These results suggest a direct impact of CSE on TLR2 and TLR4 signaling pathways, raising the possibility that this interaction could occur at the receptor–ligand level, and indicating that intact TLR2 and TLR4 signaling is necessary for CSE to cause upregulation of IL-4 and IL-5 by DCs.
CSE Modulates C. pneumoniae–Induced Lung Inflammation In Vivo
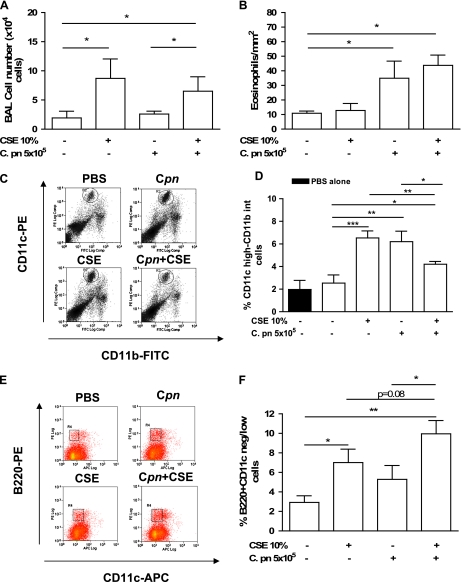
To investigate if our in vitro results would also apply to an in vivo situation, we used adoptive transfer experiments to evaluate the effect of CSE-pulsed and/or C. pneumoniae-pulsed BMDCs in the lungs of naive mice. We stimulated BMDCs with C. pneumoniae, CSE, both, or PBS (control condition) overnight, and performed adoptive transfer intratracheally into wild-type mice, specifically to investigate the instructive roles that these ex vivo pulsed DCs play. Five days later, the mice were killed and their lung inflammation was determined. The adoptive transfer of BMDCs treated with CSE alone significantly increased the BAL cell number (Figure 2A) compared with control mice that received PBS-treated BMDCs, whereas C. pneumoniae–treated BMDCs did not induce an increase in BAL cell numbers (Figure 2A), most likely because of the atypical delivery mode of C. pneumoniae. However, the adoptive transfer of DCs treated with CSE together with C. pneumoniae did not result in any greater BAL cell counts over CSE exposure alone (Figure 2A). In contrast to the BAL cell counts, the adoptive transfer of C. pneumoniae–treated BMDCs resulted in increased eosinophils in the lung, whereas CSE alone did not (Figure 2B). However, like the BAL cell counts, the combination of CSE and C. pneumoniae treatment did not result in any further increase in eosinophil counts. These results are in contrast to the in vitro data presented in Figure 1 that demonstrated increased Th2 cytokines released by BMDCs when exposed to both CSE and C. pneumoniae.
Figure 2.
The adoptive transfer of CSE-pulsed and/or C. pneumoniae–pulsed BMDCs modulates the immune response in the lung. The BMDCs were pulsed ex vivo overnight with PBS, CSE (10%), C. pneumoniae (5 × 105 IFUs/ml), or both, and then adoptively transferred (intratracheally; 5 × 105 cells/mouse) into wild-type recipient mice. Mice were killed 5 days later, and lungs were excised and properly stained for CD11c-PE and CD11b-FITC. (A) BAL cell numbers. (B) Eosinophil numbers. (C, D) Lung mDCs were expressed as percentages of CD11c highly positive and CD11b intermediately positive cells. (E, F) In the same manner, lung pDCs were determined as CD11c negative/low and B220 high. (D) Solid bar represents values of mDCs in the lungs of wild-type mice injected with 100 μl of PBS. (C, E) Experiments representative of bar graphs in D and F, respectively. Data represent mean ± SEM, n = 9/each group. Experiments were performed on 2 different days. Statistically significant differences are denoted as *P < 0.05, **P < 0.01, and ***P < 0.005, as determined by one-way ANOVA and the Student t test.
Adoptive Transfer of DCs Pretreated with Both CSE and C. pneumoniae Reduces mDC Numbers but Enhances pDC Numbers in the Lung
To understand why the BAL and eosinophil cell counts (Figures 2A and 2B) did not reflect the in vitro cytokine data (Figure 1), we sought to determine the identity and numbers of leukocytes recruited by the adoptive transfer of CSE-treated BMDCs. We digested the lungs of treated mice and performed a FACS analysis for myeloid DCs, plasmacytoid DCs, macrophages, and neutrophils. Myeloid DCs were identified as CD11c-high, CD11b-positive/intermediate, and F4/80-negative. Plasmacytoid DCs were determined as CD11c-negative/low and B220-positive, based on a negative gate for CD3, CD19, and CD11b. Numbers of pDCs were also evaluated using CD11c-positive and Gr-1–intermediate cells as identifying antigens, as previously reported (12). The adoptive transfer of CSE-pulsed BMDCs increased the numbers of both mDCs and pDCs in the lungs (Figures 2C–2F). Control mice treated with 100 μl of PBS showed similar levels of mDCs (Figure 2D) and pDCs (data not shown) as mice adoptively transferred with PBS-pulsed BMDCs, excluding any possibility that the adoptive transfer procedure itself might have affected mDC or pDC numbers. The C. pneumoniae alone resulted in an increase in mDCs (Figure 2B), whereas the increase in pDCs did not achieve significance compared with the control condition (Figure 2F). Interestingly, when mice received adoptively transferred DCs pretreated with both CSE and C. pneumoniae, the number of mDCs decreased compared with either treatment alone (Figure 2D). Furthermore, a trend toward a further increase in pDC numbers was evident, compared with mice receiving DCs treated only with CSE (Figure 2F), but this tendency did not reach statistical significance (P = 0.08). Neutrophils (Gr-1–high and CD11b-positive cells) and macrophages (F4/80-high and CD11c+ cells) were also significantly increased after CSE stimulation (data not shown). Hence, the adoptive transfer of C. pneumoniae–pulsed BMDCs increased lung mDCs, but CSE increased both mDC and pDC subpopulations. However, the adoptive transfer of DCs pretreated with both C. pneumoniae and CSE enhanced pDC numbers in the lungs over those concentrations observed after either stimulation alone. These results appear most consistent with the interpretation that in the context of C. pneumoniae infection, CSE suppresses mDC numbers, but enhances pDC numbers, in the lung.
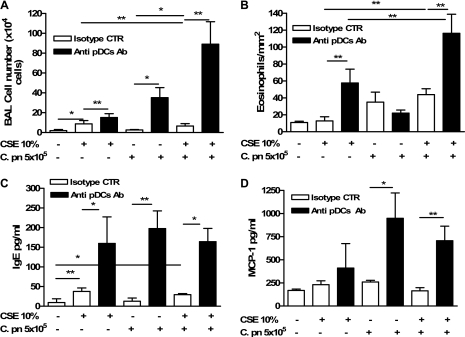
Depletion of pDCs Significantly Enhances Th2 Lung Inflammation in Mice Receiving BMDCs Pulsed with Both CSE and C. pneumoniae
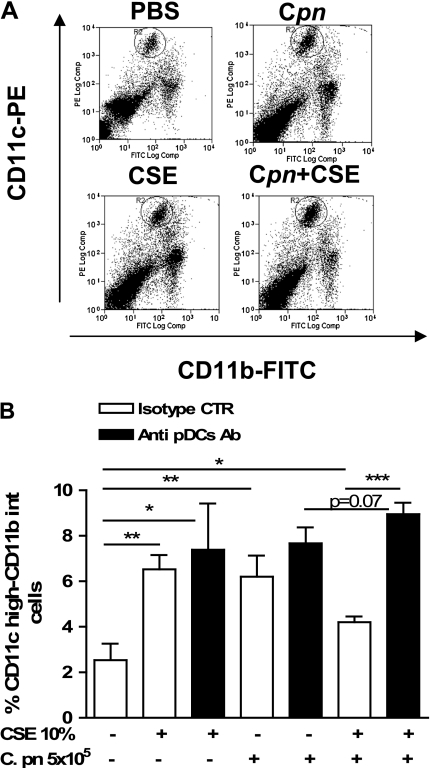
To dissect further what role pDCs may play in mDC activity, 3 days after the adoptive transfer, we injected mice intravenously with a specific pDC-depleting antibody (120G8 Ab; 150 μg/mouse) (17). The 120G8 Ab reduced the number of pDCs to ≈34% or 54% in the lung or spleen, respectively, as revealed by B220 and CD11c staining (data not shown). Five days after the adoptive transfer of BMDCs pulsed with CSE and/or C. pneumoniae (i.e., 2 d after antibody injection), the lungs were analyzed as already described. The depletion of pDCs resulted in increased BAL cell numbers after either CSE-pulsed or C. pneumoniae–pulsed DC transfer (Figure 3A). When mice received DCs pretreated with both CSE and C. pneumoniae and were then given 120G8 Ab, BAL cell numbers and eosinophils were markedly increased compared with either C. pneumoniae alone or CSE alone, and the effect was more than additive (Figures 3A and 3B). Furthermore, the depletion of pDCs resulted in significantly increased IgE (Figure 3C) and MCP-1 (Figure 3D) concentrations in CSE-instilled and/or C. pneumoniae–instilled mice, suggesting an increase in the recruitment of immunocompetent cells able to boost lung inflammation. The absence of pDCs resulted in a significant increase in the number of mDCs in the lungs of mice that received BMDCs treated with both C. pneumoniae and CSE (Figures 4A and 4B). In contrast, the adoptive transfer of BMDCs treated with CSE or C. pneumoniae alone did not show a significant increase in mDCs in the lungs after the depletion of pDCs (Figures 4A and 4B). The 120G8 Ab also did not alter mDC numbers in mice that received PBS-pulsed BMDCs (Figures 4A and 4B). Collectively, these data suggest that pDCs suppress inflammation and the infiltration of inflammatory cells, and particularly eosinophils, when the lungs are exposed to both CSE and C. pneumoniae together.
Figure 3.
Depletion of plasmacytoid dendritic cells (pDCs) results in increased Th2 inflammation after transfer of BMDCs pulsed with CSE, C. pneumoniae, or both. Mice were injected intravenously with either isotype control Ab (IgG2a, open bars), or with 120G8 Ab (solid bars), that is, the specific Ab for the depletion of pDCs. The intravenous injection of IgG2a or 120G8 Ab was performed 3 days after the adoptive transfer of CSE-pulsed and/or C. pneumoniae–pulsed BMDCs. The bronchoalveolar lavage (BAL) cell numbers (A) and eosinophil numbers (B) were evaluated as described in the text. The ELISA experiments were performed to evaluate IgE (C) and MCP-1 (D) in BAL-derived supernatants. Data represent mean ± SEM; n = 9 and n = 6 for pDC depletion experiments. Two separate experiments were performed. Statistically significant differences are denoted as *P < 0.05 and **P < 0.01, as determined by one-way ANOVA and the Student t test.
Figure 4.
Depletion of pDCs results in increased mDCs in the lung. The BMDCs were treated overnight with PBS, CSE (10%), C. pneumoniae (5 × 105 IFUs/ml), or both, and then adoptively transferred (intratracheally; 5 × 105 cells/mouse) into wild-type recipient mice. Mice were killed 5 days later, and lungs were excised and properly stained for CD11c-PE and CD11b-FITC. (A, B) Lung mDCs were expressed as percentages of CD11c highly positive CD11b intermediately positive cells. (A) An experiment representative of bar graph in B. Data represent mean ± SEM, n = 9 per group. Experiments were performed on 2 different days. Statistically significant differences are denoted as *P < 0.05, **P < 0.01, and ***P < 0.0001, as determined by one-way ANOVA and the Student t test.
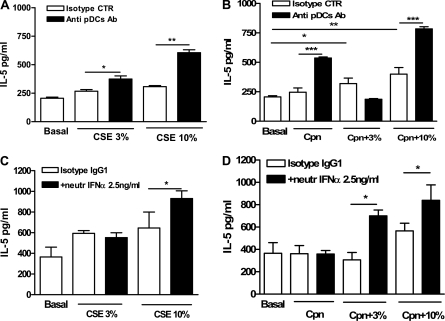
Depletion of pDCs in Mice Leads to Increased Production of IL-5 by CD11c+ Lung DCs Stimulated with Both CSE and C. pneumoniae
Our in vivo results indicate an interaction, direct or indirect, between mDCs and pDCs when both CSE-treated and C. pneumoniae–treated BMDCs were adoptively transferred into the lung. Our in vitro studies with BMDCs did not attempt to isolate the individual effects of mDCs and pDCs. We therefore repeated some of these experiments with lung mDCs purified using a cell separation kit. The addition of CSE or C. pneumoniae (Figure 5A) alone did not significantly alter the secretion of IL-5 by CD11c+ lung-derived cells, but the combination of both stimuli did significantly increase IL-5 concentrations over untreated control conditions (Figure 5B). Similar effects on the secretion of other cytokines such as TNF-α and IL-6 were also evident (data not shown). In addition, the cotreatment of cells with both CSE and C. pneumoniae reduced the expression of IL-12p70 to basal levels (data not shown). We next sought to determine how the expression of these cytokines was affected by the presence or absence of lung-derived pDCs. An analysis of CD11c+ cells obtained from wild-type, untreated mice showed that 85 to 90% of CD11c+ cells were mDCs (CD11c+ and CD11b+), and approximately 10% were pDCs (CD11c−/low and B220+) (data not shown). Wild-type naive mice were injected with the 120G8 Ab, 2 days before the isolation of lung CD11c+ cells. The depletion of pDCs by this antibody increased mDC purity to approximately 95%. These cells (i.e., CD11c+ cells depleted of the majority of pDCs) were then treated with CSE alone, C. pneumoniae alone, or both. Higher concentrations of IL-5 were evident after CSE or C. pneumoniae treatment, or after the combination of both (Figures 5A and 5B).
Figure 5.
Lung CD11c+ cell release increased IL-5 ex vivo when pDCs were depleted before purification. The BMDCs were treated overnight with PBS, CSE (10%), C. pneumoniae (5 × 105 IFUs/ml) or both, and then adoptively transferred (intratracheally; 5 × 105 cells/mouse) into wild-type recipient mice. Two days later, 120G8 Ab or isotype control were intravenously injected (150μg/ml) lung CD11c+ cells were isolated by means of a technique using Miltenyi Biotec microbeads 3 days later. (A, B) IL-5 concentrations were measured using ELISA. Neutralizing Ab for IFN-α (2.5 ng/ml) or isotype control was added to CD11c+ cells isolated from the lung, and IL-5 concentrations were measured when exposed to CSE and C. pneumoniae (C, D). Concentrations of IL-5 were analyzed using ELISA in cell-free supernatants. Data represent mean ± SEM, n = 9 and n = 6 for pDC depletion experiments. Experiments were performed on 2 different days. Statistically significant differences are denoted as *P < 0.05, **P < 0.01, and ***P < 0.0001, as determined by one-way ANOVA and the Student t test.
The Mechanism by which pDCs Shift mDC Polarization from Th1 to Th2 Involves IFN-α
Recent studies indicated that C. pneumoniae may also signal through TLR9, in addition to TL2 and TLR4 (16). In pDCs, TLR9 signaling results in the robust expression of type I IFN (15) and results in a strong Th1 push. Hence, a possible mechanism for the suppression of the Th2 inflammatory response would occur via type I IFN expression by pDCs. Consistent with this possibility, we observed a higher expression of TLR9 in lung pDCs compared with lung mDCs (data not shown). We next sought to determine whether type I IFNs may play a role in the suppression of CSE-induced and/or C. pneumoniae–induced Th2 cytokine production. We used neutralizing Ab for IFN-α (2.5 ng/ml). The pretreatment of lung-derived CD11c+ cells with IFN-α–neutralizing Ab resulted in a significantly higher release of IL-5 after stimulation with CSE and/or C. pneumoniae (Figures 5C and 5D). These results are consistent with the interpretation that pDCs use IFN-α to suppress the activation of lung mDCs, leading to an alteration of polarization from predominantly Th1 toward predominantly Th2.
CSE and C. pneumoniae Induce IDO Expression in pDCs and Lead to Apoptosis
Recently, the tryptophan-catabolizing enzyme IDO was found to play a critical role in the suppression of various inflammatory responses (18, 19). Indoleamine-2,3-dioxygenase, which converts tryptophan into kynurinine, was implicated in various aspects of host defenses, including fungal and viral infections (18, 20), asthma (15, 18), and cancer (18). Moreover, pDCs express IDO, leading in turn to a suppressive state (20). Because IFN-α can induce the expression of IDO (19), we investigated the ability of CSE and C. pneumoniae to induce IFN-α expression in BMpDCs. In our experimental model, we observed that IFN-α was produced when BMpDCs were stimulated with both CSE and C. pneumoniae (Figure 6A). To assess the role of IDO in our model system, we investigated IDO expression levels directly in BMpDCs. We observed that CSE and/or C. pnuemoniae induced a significant increase in IDO expression, similar to the effect of IFN-γ used as a positive control stimulation (Figure 6B). The same effect was evident in the case of BMDCs, although the levels of IDO expression were much lower than those of BMpDCs (data not shown).
Figure 6.
The BMpDCs expressed higher levels of IFN-α and IDO in response to CSE and/or C. pneumoniae stimulation. (A) The BMpDCs were treated with CSE and/or C. pneumoniae, and concentrations of IFN-α mRNA were measured using real-time PCR. (B) Concentrations of IDO protein were evaluated by means of immunoblotting. Data are expressed as ratios between IFN-α (A) or IDO (B) compared with GAPDH expression. CpG oligodeoxynucleotides (1 μg/ml) was used as positive control for IFN-α mRNA expression. Quantitative data were obtained by analyzing optical density values with ImageJ software. Data represent mean ± SEM, n = 3 (A) and n = 3 (B). Statistically significant differences are denoted as *P < 0.05, and **P < 0.005, as determined by one-way ANOVA and the Student t test.
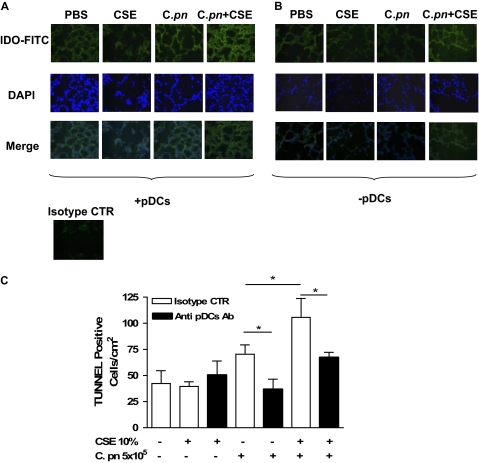
We next assessed levels of IDO expression in the lungs of mice that had received BMDCs pulsed with CSE and/or C. pneumoniae, using an immunofluorescence technique. The CSE or C. pneumoniae alone produced a slight increase in IDO expression, compared with PBS-treated mice (Figure 7A), but CSE and C. pneumoniae together noticeably increased IDO staining in lungs (Figure 7A). However, in the absence of pDCs, mice that received CSE-treated or C. pneumoniae–treated BMDCs presented a significantly diminished expression of IDO in the lungs (Figure 7B, compared with PBS-treated mice), indicating that pDCs were the major source of IDO. The isotype control (rat IgG2b) did not show any positive staining, and was used as negative control.
Figure 7.
The depletion of pDCs significantly decreased IDO and apoptotic cells in the lung. Mice were adoptively transferred with CSE-pulsed and/or C. pneumoniae–pulsed BMDCs, and lung sections were stained for IDO expression and TUNEL assay. The expression of IDO was determined by means of an immunofluorescence technique, using FITC either in the presence (A) or after the depletion (B) of pDCs. The isotype control (rat IgG2b) was used as negative control. (C) A TUNEL assay was performed, and positive cells were quantified. Data represent mean ± SEM, and each experiment was performed at least five times for each type of experimental condition. Statistically significant differences are denoted as *P < 0.05, as determined by one-way ANOVA and the Student t test.
Because one function of IDO is to induce cell death (18), we evaluated whether a higher expression of IDO was associated with increased apoptosis. Lung sections were analyzed using TUNEL assays. We observed that CSE enhanced C. pneumoniae–induced apoptosis in a more than additive manner compared with C. pneumoniae alone (Figure 7C). In contrast, in the absence of pDCs, CSE elicited reduced levels of apoptosis in mice that received intratracheal BMDCs pulsed with C. pneumoniae (Figure 7C). Finally, treatment with C. pneumoniae alone led to decreased cell death in the absence of pDCs (Figure 7C), but CSE treatment alone did not increase the TUNEL-positive cells in lungs (Figure 7C).
DISCUSSION
We investigated the role of DCs in instructing lung responses against CS and C. pneumoniae infection. We demonstrated that exposure to CSE modulates the functional activity of CD11c+ DCs in the lung, with and without concomitant C. pneumoniae infection. The BMDCs exposed to both CSE and C. pneumoniae in vitro resulted in a strong Th2 skew, the release of Th2 cytokines (IL-4 and IL-5), and a decrease in the Th1 cytokine IL-12. Although DCs are not generally considered to produce the Th2 cytokine, several studies documented their ability to secrete Th2 cytokine, and our data support those studies (21, 22). In addition, BMDCs alone exposed to C. pneumoniae or LPS were shown to drive Th2 sensitization and subsequent allergic airway inflammation (6, 23). The transfer of CSE-pulsed as well as C. pneumoniae–pulsed BMDCs into the lungs increased the influx of CD11c+ DCs (mDCs). Although BMDCs exposed to both CSE and C. pneumoniae resulted in a strong Th2 response in vitro, this response was not evident in vivo. Instead, when we adoptively transferred DCs pulsed ex vivo with both CSE and C. pneumoniae into the lungs of naive mice, we observed a decrease in the number of mDCs compared with either treatment alone, and more importantly, an influx of pDCs. The depletion of these pDCs resulted in a greatly increased Th2 response and subsequent inflammation, similar to the findings in our in vitro data. We also observed an increased expression of both IFN-α and IDO by BMDCs pulsed with both CSE and C. pneumoniae, both of which were reduced during pDC depletion, suggesting their potential involvement in the mechanism of altered polarization.
Exposure to CS can lead to increased hyperresponsiveness to infections, mainly because of the production of oxidants that can facilitate the nuclear translocation of NF-κB and activator protein-1 (9, 24). The activation of these transcription factors can mediate the production of proinflammatory cytokines and chemokines, influencing the influx of immune cells to the lung and the bacteria-mediated inflammatory process (9, 10). In this study, the adoptive transfer of BMDCs pulsed with CSE or C. pneumoniae alone increased the presence of macrophages and DCs in recipient mice, both of which can be activated by TLR signaling. In vitro studies revealed that CSE-induced cytokine release by BMDCs occurred in a TLR2-dependent and TLR4-dependent manner, a finding that is consistent with recent reports that TLR2-dependent and TLR4-dependent signaling is activated by oxidants and CSE in models of peritonitis and lung inflammation (9, 10). Collectively, our data are consistent with the interpretation that CS initiates inflammatory responses via TLR2/TLR4-dependent pathways, and this activation may modify host responses to a pathogen such as C. pneumoniae by altering the activity of DCs and thus the adaptive immunity polarization.
Our experimental model aimed to analyze the activity of CSE-pulsed and/or C. pneumoniae–pulsed DCs in the lung. Although this model is limited because the mice are not directly infected and do not receive first-hand smoke, it does allow us to investigate the specific role of mDCs in directing and instructing the immune response to these stimuli by the adaptive transfer of ex vivo pulsed cells intratracheally. The activation and transfer of these cells to the lung could induce the production of cytokines and chemokines capable of recruiting other immune cells to the lung. The CSE also increased the number of innate immune cells in the lungs of mice after an adoptive transfer of BMDCs pulsed with C. pneumoniae. Interestingly, beyond the recruitment of macrophages, neutrophils, and myeloid DCs, a large influx of pDCs into the lungs occurred after the transfer of BMDCs pulsed with both CSE and C. pneumoniae. D'Hulst and colleagues reported an increased infiltration of DCs in the airways and lung parenchyma of mice after chronic (24-wk) exposure to CS (3, 14). Our report describes the relevance of pDCs in lung inflammation induced by CSE and/or C. pneumoniae. The adoptive transfer of BMDCs pulsed with both CSE and C. pneumoniae led to higher numbers of pDCs in the lungs of mice compared with each stimulation alone, and pDCs attenuated CSE-induced and/or C. pneumoniae–induced lung inflammation by suppressing the Th2-like immune response of mDCs. The expression of IFN-α and IDO correlated with the suppression of Th2 inflammatory cells and cytokines associated with pDCs.
Previous studies showed that exposure to CS can exacerbate asthma. The increased incidence of asthma and airway hyperresponsiveness in smokers suggests that smoking alters the handling of inhaled antigens by the lungs, favoring the development of Th2-biased airway inflammation. In an ovalbumin-induced model of asthma, the exposure of mice to CS increased numbers of lung eosinophils, goblet cells, dendritic cells, and CD4-positive T cells (25). Moreover, immature human blood-derived DCs preincubated with CSE for 7 days showed a decreased release of typical Th1 cytokines such as IFN-γ and IL-12, and the concomitant augmented production of Th2 cytokines such as IL-4 and IL-10 (7), a pattern similar to that observed in our experimental model. Furthermore, IL-5 and IL-13, cytokines capable of inducing airway hyperresponsiveness, were increased in the lymph-node cell cultures of ovalbumin-exposed and CSE-exposed mice (25).
In this study, we demonstrate that the adoptive transfer of BMDCs pulsed ex vivo with both C. pneumoniae and CSE into naive mice increased numbers of pDCs in the lung, and that these pDCs appeared to suppress the ability of mDCs to skew the lung toward Th2-type inflammation. The depletion of pDCs resulted in acute inflammation of the lung and increased Th2 cytokines, in both in vivo and ex vivo experiments. The BMDCs stimulated in vitro with CSE and C. pneumoniae naturally skewed toward Th2 cytokine expression, but in vivo, the BMDCs directed a pDC response that could reverse the Th2 skew and prevent inflammation. One potential mechanism by which pDCs reduced the cellular influx to the lung would occur via the up-regulation of the immunosuppressive enzyme IDO. The absence of pDCs correlated with decreased levels of IDO, and the levels of apoptosis in the lung were also decreased. Beyond its metabolic activity, IDO can either promote the apoptosis of T cells or induce regulatory T cells (18). The activation of TLR9 signaling in pDCs promotes the expression of IFN-α, which in turn can promote the expression of IDO. Concentrations of IDO are also influenced by oxidant concentrations, which are necessary for IDO activity (21). The presence of both CSE and C. pneumoniae significantly increased the expression of IDO and the number of TUNEL-positive cells, suggesting that increased apoptosis may have been mediated by the higher expression of IDO in pDCs. It is interesting to speculate that the CSE-induced suppression of host DC function may also be relevant to the pathology of cancer, where the imbalance in IL-12/IL-10 may lead to a tolerogenic state (21). This possibility calls for further study. Indeed, the Th2 bias of mDCs and the increased presence of pDCs, which correlated with a higher expression of IDO, could lead to an immunosuppressive condition in a chronic inflammation model. That possibility would also be interesting to investigate in future studies.
In conclusion, our study demonstrates the ability of CSE to modulate the ability of DCs to direct the appropriate immune response to infection with C. pneumoniae. The increase in pDCs resulted in reduced numbers of mDCs, an increase in the expression of IDO, increased positive TUNEL staining in the lung, and the prevention of acute inflammation. On the other hand, the depletion of pDCs reversed this phenotype, and allowed an acute Th2-like inflammation to occur.
Acknowledgments
The authors are very thankful to Polly Sun, who has always been very kind and supportive to their group, and very helpful in this work.
This work was supported by National Institutes of Health grants AI 067995 and HL66436 (M.A.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0224OC on November 9, 2009
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Chalmers GW, MacLeod KJ, Thomson L, Little SA, McSharry C, Thomson NC. Smoking and airway inflammation in patients with mild asthma. Chest 2001;120:1917–1922. [DOI] [PubMed] [Google Scholar]
- 2.Hylkema MN, Sterk PJ, de Boer WI, Postma DS. Tobacco use in relation to COPD and asthma. Eur Respir J 2007;29:438–445. [DOI] [PubMed] [Google Scholar]
- 3.D'Hulst AI, Maes T, Bracke KR, Demedts IK, Tournoy KG, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary emphysema in scid-mice. Is the acquired immune system required? Respir Res 2005;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3α/CCL-20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 2003;28:648–654. [DOI] [PubMed] [Google Scholar]
- 5.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schröder NW, Crother TR, et al. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol 2008;181:7176–7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröder NW, Crother TR, Naiki Y, Chen S, Wong MH, Yilmaz A, Slepenkin A, Schulte D, Alsabeh R, Doherty TM, et al. Innate immune responses during respiratory tract infection with a bacterial pathogen induce allergic airway sensitization. J Allergy Clin Immunol 2008;122:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassallo R, Tamada K, Lau JS, Kroening PR, Chen L. Cigarette smoke extract suppresses human dendritic cell function leading to preferential induction of Th-2 priming. J Immunol 2005;175:2684–2691. [DOI] [PubMed] [Google Scholar]
- 8.Gaschler GJ, Zavitz CC, Bauer CM, Skrtic M, Lindahl M, Robbins CS, Chen B, Stämpfli MR. Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. Am J Respir Cell Mol Biol 2008;38:218–226. [DOI] [PubMed] [Google Scholar]
- 9.Paul-Clark MJ, McMaster SK, Sorrentino R, Sriskandan S, Bailey LK, Moreno L, Ryffel B, Quesniaux VF, Mitchell JA. Toll-like receptor 2 is essential for the sensing of oxidants during inflammation. Am J Respir Crit Care Med 2009;179:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul-Clark MJ, Sorrentino R, Bailey LK, Sriskandan S, Mitchell JA. Gram-positive and gram-negative bacteria synergize with oxidants to release CXCL8 from innate immune cells. Mol Med 2008;14:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naiki Y, Michelsen KS, Schröder NW, Alsabeh R, Slepenkin A, Zhang W, Chen S, Wei B, Bulut Y, Wong MH, et al. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J Biol Chem 2005;280:29242–29249. [DOI] [PubMed] [Google Scholar]
- 12.de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004;200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blasi F, Aliberti S, Allegra L, Piatti G, Tarsia P, Ossewaarde JM, Verweij V, Nijkamp FP, Folkerts G. Chlamydophila pneumoniae induces a sustained airway hyperresponsiveness and inflammation in mice. Respir Res 2007;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Puawels RA. Time course of cigarette smoke induced pulmonary inflammation in mice. Eur Respir J 2005;26:204–213. [DOI] [PubMed] [Google Scholar]
- 15.Romani L, Bistoni F, Montagnoli C, Gaziano R, Bozza S, Bonifazi P, Zelante T, Moretti S, Rasi G, Garaci E, et al. Thymosin alpha1: an endogenous regulator of inflammation, immunity, and tolerance. Ann N Y Acad Sci 2007;1112:326–338. [DOI] [PubMed] [Google Scholar]
- 16.Joyee AG, Yang X. Role of Toll-like receptors in immune responses to chlamydial infections. Curr Pharm Des 2008;14:593–600. [DOI] [PubMed] [Google Scholar]
- 17.Asselin-Paturel C, Brizard G, Pin JJ, Brière F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol 2003;171:6466–6477. [DOI] [PubMed] [Google Scholar]
- 18.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 2003;24:242–248. [DOI] [PubMed] [Google Scholar]
- 19.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol 2007;7:817–823. [DOI] [PubMed] [Google Scholar]
- 20.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, Shearer GM. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 2007;109:3351–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett 2008;121:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsubara S, Koya T, Takeda K, Joetham A, Miyahara N, Pine P, Masuda ES, Swasey CH, Gelfand EW. Syk activation in dendritic cells is essential for airway hyperresponsiveness and inflammation. Am J Respir Cell Mol Biol 2006;34:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walters MJ, Paul-Clark MJ, McMaster SK, Ito K, Adcock IM, Mitchell JA. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Mol Pharmacol 2005;68:1343–1353. [DOI] [PubMed] [Google Scholar]
- 25.Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF. Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res 2006;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]