Abstract
Reactive oxidants such as nitrogen dioxide (NO2) injure the pulmonary epithelium, causing airway damage and inflammation. We previously demonstrated that nuclear factor-κ B (NF-κB) activation within airway epithelial cells occurs in response to NO2 inhalation, and is critical for lipopolysaccharide-induced or antigen-induced inflammatory responses. Here, we investigated whether manipulation of NF-κB activity in lung epithelium affected severe lung injuries induced by NO2 inhalation. Wild-type C57BL/6J, CC10-IκBαSR transgenic mice with repressed airway epithelial NF-κB function, or transgenic mice expressing a doxycycline-inducible, constitutively active I κ B kinase β (CC10-rTet-CAIKKβ) with augmented NF-κB function in airway epithelium, were exposed to toxic levels of 25 ppm or 50 ppm NO2 for 6 hours a day for 1 or 3 days. In wild-type mice, NO2 caused the activation of NF-κB in airway epithelium after 6 hours, and after 3 days resulted in severe acute lung injury, characterized by neutrophilia, peribronchiolar lesions, and increased protein, lactate dehydrogenase, and inflammatory cytokines. Compared with wild-type mice, neutrophilic inflammation and elastase activity, lung injury, and several proinflammatory cytokines were significantly suppressed in CC10-IκBαSR mice exposed to 25 or 50 ppm NO2. Paradoxically, CC10-rTet-CAIKKβ mice that received doxycycline showed no further increase in NO2-induced lung injury compared with wild-type mice exposed to NO2, instead displaying significant reductions in histologic parameters of lung injury, despite elevations in several proinflammatory cytokines. These intriguing findings demonstrate distinct functions of airway epithelial NF-κB activities in oxidant-induced severe acute lung injury, and suggest that although airway epithelial NF-κB activities modulate NO2-induced pulmonary inflammation, additional NF-κB–regulated functions confer partial protection from lung injury.
Keywords: epithelium, NF-κB, inflammation, nitrogen dioxide, lung injury
CLINICAL RELEVANCE.
This research demonstrates that airway epithelial nuclear factor (NF)-κB activation in the absence of other overt stimuli causes an injury and an inflammatory response qualitatively similar to those induced by NO2 exposure, and the effects of NO2 inhalation are not augmented but are somewhat diminished by previous airway epithelial NF-κB activation. Therefore, the therapeutic modulation of NF-κB in lung disease needs to take into account the diverse functions of this potent transcription factor.
Acute lung injury (ALI) is induced by a variety of insults, including endotoxin, acid aspiration, complement activation, hyperoxia, and oxidant gases such as nitrogen dioxide (NO2) and ozone, and it is characterized by the increased presence of neutrophils, platelets, fibrin, edema, and epithelial and endothelial damage and cell death (1). Nitrogen dioxide, as a byproduct of combustion, is a toxic gas present in ambient air that can cause respiratory symptoms at low doses, and severe respiratory distress or death at higher concentrations (2). In addition to exogenous sources, NO2 can also be produced endogenously, as a byproduct of inflammatory cell activity (3). Inhaled NO2 is absorbed along the respiratory tract (4), and the stable endproduct of NO2 reactivity, nitrotyrosine, is present in the lungs of patients with asthma, chronic obstructive pulmonary disease (COPD), and other pulmonary diseases (5). Studies using lung epithelial cells demonstrated proinflammatory activities and cell death in response to NO2. For instance, human bronchial epithelial cells exposed to NO2 secrete increased levels of IL-8, IL-1β, TNF-α, granulocyte/macrophage colony–stimulating factor (GM-CSF), and nitric oxide (6, 7). Nitrogen dioxide also promotes the selective death of proliferating or migrating epithelial cells via a mechanism involving the Fas-dependent activation of c-Jun-N-terminal kinase (6, 8–10).
In recent years, the role of the airway epithelium in the functional response to diverse stimuli has become an area of substantial research, and airway epithelial cells are now appreciated to be among the primary responders to respiratory insults produced by bacteria, viruses, and oxidant stress (5, 6, 11, 12). Activation of the transcription factor nuclear factor-κ B (NF-κB) within the airway epithelium augments the production of proinflammatory cytokines, leading to an inflammatory response in the lung (11, 12). The activation of NF-κB also exerts antiapoptotic affects, because this transcription factor positively regulates the expression of prosurvival genes (13, 14). Notably, NF-κB–dependent survival factors, including growth arrest and DNA damage induced gene 45 (GADD45) and manganese superoxide dismutase (MnSOD) are important in down-regulating the activity of c-Jun-N-terminal kinase, and in protecting against oxidant-induced cell death (15).
Airway epithelial NF-κB participates in the control of processes that include both inflammation and protection from cell death. However, the outcome of modulation of NF-κB within airway epithelium in the pathophysiology of severe ALI is unclear. Protection from inflammation and enhanced cell death or damage are both plausible outcomes of inhibiting NF-κB activities in these cells in vivo. The goal of the present study was to determine whether positive or negative modulation of NF-κB activity in the airway epithelium affects the extent of NO2-induced lung injury.
MATERIALS AND METHODS
Mice
Transgenic mice (CC10-IκBαSR) with repressed NF-κB function in airway epithelium (11) were backcrossed with wild-type C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) for more than 10 generations. Bi-transgenic mice (CC10-rTet-CAIKKβ) (16) expressing CAIKKβ in the bronchiolar epithelium after the administration of 6 g/kg doxycycline (Dox) in chow (TestDiet, Richmond, IN) were backcrossed with C57BL/6J mice for at least five generations. Wild-type mice were age-matched and sex-matched transgene-negative littermates. Mice were maintained on a 12-hour light/dark cycle, and were provided food and water ad libitum. All animal studies were approved by the University of Vermont Institutional Animal Care and Use Committee.
Exposure to NO2
Mice were exposed to high efficiency particulate air (HEPA)-filtered air or to NO2 (17) at doses of 25 ppm or 50 ppm for 6 hours a day for 3 consecutive days, to induce severe ALI. Lower doses of NO2 or less exposure time induced only modest levels of lung injury and inflammatory response (5). As indicated, mice were provided Dox chow ad libitum for 3 days before the 3-day exposure to NO2, and were maintained on Dox chow until being killed on Day 7.
Bronchoalveolar Lavage and Lung Processing
Lungs were lavaged with a single instillation and recovery of 1 ml ice-cold PBS (Gibco, Carlsbad, CA). Lavage samples were centrifuged at 400 × g for 6 minutes, and the supernatant was separated from the cell pellet. Cells were resuspended in 200 μl PBS, counted, spun onto glass slides with a cytospin, and stained with hematoxylin and eosin for the differential counting of more than 200 cells/slide. Cell-free bronchoalveolar lavage (BAL) fluid was flash-frozen in liquid N2 for cytokine analysis. Left lung lobes were inflated with 4% paraformaldehyde in PBS, and immersed overnight in the same solution before being transferred to 70% ethanol for 24 hours. Lungs were then embedded in paraffin blocks, cut into 5-μm sections, mounted onto slides, and stained with hematoxylin and eosin. The right lung lobes were flash-frozen for RNA and protein analysis.
Protein, Cytokine, and Enzymatic Activity Profiling from BAL Fluid
Cytokine levels were assessed using a Bio-Plex 23-plex panel (Bio-Rad, Hercules, CA). Protein levels were measured using the Bradford assay (Bio-Rad). Lactate dehydrogenase activity was determined using the LDH Detection Assay Kit (Promega, Madison, WI). Neutrophil elastase activity was measured using the EnzCheck Elastase Assay kit (Molecular Probes, Eugene OR).
Assessment of Localization of Antibody Recognizing p65 in Lung Tissue
Mice were exposed to room air or 25 ppm NO2 for 6 hours once, and killed 1 hour later. Lungs were instilled with and placed into Tissue-Tek OCT Compound (Sakura Finetek, Inc., Torrance, CA), and frozen in liquid nitrogen–chilled isopentane, and 5-μm frozen sections were cut and placed onto glass slides. Slides were fixed for 30 minutes with 4% paraformaldehyde in PBS, washed, and permeabilized for 20 minutes with 1% Triton X-100 in PBS, and blocked with 1% bovine serum albumin in PBS for 1 hour. Slides were then incubated overnight at 4°C with antibody (Ab) recognizing p65 (RelA) (5 μg/ml, SC-372; Santa Cruz Biotechnology, Santa Cruz, CA) in 1% BSA/PBS. after three washes in 1% BSA/PBS, slides were incubated for 1 hour with goat anti-rabbit Alexa 568–labeled secondary Ab (Molecular Probes) in PBS, and counterstained with a 1:8,000 dilution of SYTOX Green (Molecular Probes) in PBS to label DNA. Slides were washed with PBS, rinsed with H2O, and coverslipped using Aqua PolyMount (Polysciences, Inc., Warrington, PA). Sections were scanned using a Bio-Rad MRC 1024 confocal scanning laser microscope system and a ×40 objective.
Histologic Scoring Method
To measure overall injury of lung sections, the total numbers of bronchioles with a length:diameter ratio of less than 2:1 were counted per slide and then assessed for percent injured, as defined by the appearance of disrupted airway wall or alveolar thickening. This was determined to be the “percent foci” per section. Subsequently, 10 airways per slide were scored by two independent researchers blinded to the identity of samples, and graded on a scale of 1–3 for intensity of injury, with 1 as the least injured, and 3 as the most severely injured. Parameters of airway injury included airway epithelial thickening, peribronchiolar lesions, and the presence of inflammatory cells.
Immunoblotting
Twenty micrograms of protein from lung homogenates prepared in 1× PBS were run on a 15% acrylamide gel, and transferred to a nitrocellulose membrane. Lysates were blotted for IKKβ (Cell Signaling Technology, Danvers, MA). Membranes were stripped and reprobed with β-actin as a loading control.
Statistical Analyses
All statistical analyses were performed using Excel software (Microsoft, Seattle, WA) with one-way ANOVA, followed by the Tukey test for multiple comparisons. Values of P < 0.05 were considered statistically significant. Data are presented as mean value ± SEM.
RESULTS
Nitrogen Dioxide–Induced RelA Nuclear Translocation in Airway Epithelium Is Attenuated in CC10-IκBαSR Mice
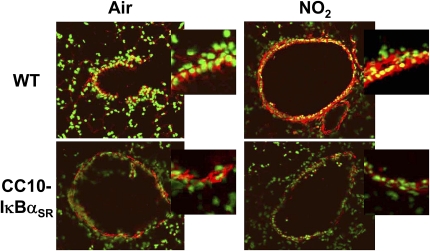
We previously reported that brief exposure of mice to 10 ppm NO2 induces airway epithelial NF-κB activation, as characterized by the presence of nuclear RelA accumulation (17). To determine whether higher doses and longer durations of inhaled NO2 induce airway epithelial NF-κB activation, lung sections were examined by RelA immunofluoresence after a single 6-hour, 25-ppm exposure to NO2. Neither wild-type nor transgenic mice expressing IκBαSR in airway epithelium displayed activation of NF-κB after exposure to control air (Figure 1). However, NO2 induced the nuclear translocation of RelA in epithelial cells lining the airways of wild-type mice. In contrast, CC10-IκBαSR mice demonstrated abrogated RelA nuclear translocation, confirming that the transgene inhibits the activation of NF-κB in response to NO2 inhalation. These data demonstrate that higher doses and longer durations of NO2 activate NF-κB in the airway epithelium, and that this NF-κB activation is inhibited in mice expressing IκBαSR in airway epithelium, thus suggesting a potential role for NF-κB in NO2-induced severe lung injury.
Figure 1.
Localization of NF-κB activation in lung sections from wild-type and CC10-IκBαSR transgenic mice exposed to NO2. The CC10-IκBαSR and wild-type (WT) littermate control animals were exposed to room air or 25 ppm NO2 for 6 hours, and killed 1 hour later. Lung sections were evaluated for nuclear translocation of RelA, using immunofluorescence and confocal laser scanning microscopy. Nuclei were visualized with Sytox (green), and RelA was visualized using a Cy3-conjugated secondary antibody (red). Nuclear localization is indicated by the overlap of fluorophores (yellow). Original magnification of large images is ×200, and is representative of patterns observed in at least four mice per group. For a better illustration of the differences in nuclear NF-κB localization, airway epithelial cells from the original images were magnified using a 2.5 optical zoom and are shown at upper right (insets).
Nitrogen Dioxide–Induced Airway Inflammation Requires Epithelial NF-κB Activation
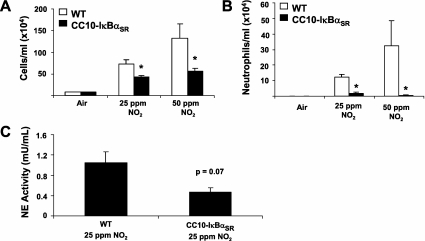
Exposure to NO2 (25 or 50 ppm) for 6 hours a day for 3 days induced a significant recruitment of inflammatory cells to the lavageable airspaces (Figure 2A). Although the influx was predominantly monocytic, there was a significant increase of neutrophils in response to either dose of NO2 (Figure 2B). The CC10-IκBαSR mice lacking airway epithelial NF-κB activation showed significantly decreased BAL neutrophil numbers in response to NO2 exposure, and monocytic cell accumulation was also attenuated. These data indicate that NF-κB in the airway epithelium is required for NO2-induced neutrophil and monocyte recruitment into the airways. We next evaluated the activity of neutrophil elastase, a 29-kD serine protease stored in neutrophil granules that can degrade extracellular matrix, plasma proteins, and protease inhibitors, and activate matrix metalloproteases (18). Moreover, NO2-induced neutrophil protease production was attenuated in CC10-IκBαSR mice exposed to 25 ppm NO2 (Figure 2C), consistent with the decreased neutrophil numbers.
Figure 2.
Assessment of airway inflammation and neutrophil elastase activity in wild-type (WT) and CC10-IκBαSR mice exposed to NO2. Total (A) and neutrophil (B) cell counts in BAL fluid from WT and CC10-IκBαSR mice are shown after 25 ppm and 50 ppm exposure to NO2 (6 hours per day for 3 days). (C) Neutrophil elastase (NE) activity in BAL of WT and CC10-IκBαSR mice exposed to 25 ppm NO2 for 6 hours a day for 3 days (*P < 0.05 compared with WT mice; air, n = 6 mice; 25 ppm NO2, n = 8 mice; 50 ppm NO2, n = 5 mice).
Nitrogen Dioxide–Induced Lung Injury Is Attenuated in CC10-IκBαSR Mice
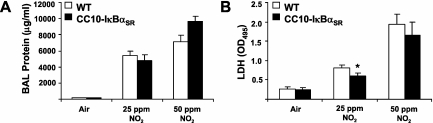
To quantitate the extent of lung injury in mice exposed to NO2, we assessed levels of total protein and lactate dehydrogenase activity in BAL fluid. Exposure to NO2 caused increases in protein levels to similar extents in wild-type and CC10-IκBαSR mice (Figure 3A). In contrast, increases in lactate dehydrogenase activity in BAL fluid in response to 25 ppm NO2 were somewhat attenuated in CC10-IκBαSR mice compared with wild-type mice. However, these differences were not evident in response to 50 ppm NO2 (Figure 3B).
Figure 3.
Evaluation of protein (A) and lactate dehydrogenase (LDH, B) in BAL from wild-type (WT) or CC10-IκBαSR mice exposed for 6 hours/day to 25 ppm NO2 for 3 days (*P < 0.05 compared with WT mice; air, n = 6 mice; 25 ppm NO2, n = 8 mice; 50 ppm NO2, n = 5 mice).
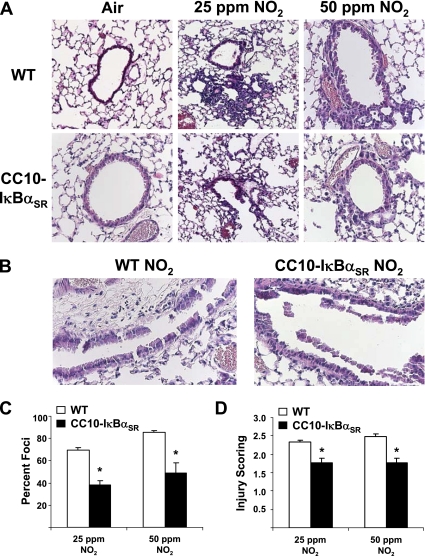
To further assess the extent of ALI, histologic analyses of lung sections from mice exposed to NO2 were performed. Wild-type mice displayed infiltrations of inflammatory cells, peribronchiolar lesions (Figure 4A), and shedding of the epithelial lining, which was extensive in response to 50 ppm NO2 (Figure 4B). The extent of NO2-induced injury was attenuated in CC10-IκBαSR mice compared with wild-type groups, and both the extent (Figure 4C) and severity (Figure 4D) of injury were attenuated in transgenic mice in response to both NO2 doses. However, in mice exposed to 50 ppm NO2, considerable epithelial shedding was apparent in the large airways, and this effect was not abrogated in CC10-IκBαSR mice (Figure 4B). Taken together, these results demonstrate a causal role for the activation of NF-κB in airway epithelium in the pathogenesis of NO2-induced severe ALI.
Figure 4.
Evaluation of histopathology in wild-type (WT) and CC10-IκBαSR mice exposed for 6 hours/day to 25 ppm or 50 ppm of NO2 for 3 days. (A) Hematoxylin-and-eosin staining of 5-μm sections of mouse lungs. (B) Hematoxylin-and-eosin staining of large airways from mice exposed to 50 ppm NO2. All magnifications are ×200, and are representative of patterns observed in each group. Scoring of lesions by assessment of the percentage of airways involved per section (C) and the intensity of foci (D) was performed as described in Materials and Methods (*P < 0.05 compared with WT mice; 25 ppm NO2, n = 8 mice/group; 50 ppm NO2, n = 5 mice/group).
Nitrogen Dioxide Exposure Induces Inflammatory Chemokines in an NF-κB–Dependent Manner
Nuclear factor–κB transcriptionally activates a myriad of chemokines and cytokines that participate in leukocyte recruitment and activation. Analyses of BAL fluid demonstrated that exposure to NO2 increased concentrations of IL-6, IL-9, IL-12 (p40), granulocyte colony–stimulating factor (G-CSF), keratinocyte-derive chemoattractant (KC), macrophage chemotactic protein (MCP)-1, and regulated upon activation normal T-cell expressed and presumably secreted (RANTES) RANTES in wild-type mice (Table 1). The expressions of RANTES, MIP-1β, macrophage inflammatory protein (MIP)-1α, MIP-2, and MCP-1 were also visualized at the RNA level, using RNase protection assays (as shown in Figure E1 in the online supplement), demonstrating elevated expression in response to NO2. As expected, CC10-IκBαSR mice demonstrated significantly abrogated concentrations of these chemokines and cytokines, compared with wild-type mice (Table 1).
TABLE 1.
CYTOKINE PROFILES IN BRONCHOALVEOLAR LAVAGE FLUID AFTER EXPOSURE FOR 6 HOURS/DAY TO 25 ppm NO2 FOR 3 DAYS
| Air |
NO2 |
|||
|---|---|---|---|---|
| WT | CC10-IκBαSR | WT | CC10-IκBαSR | |
| IL-6 | 0 | 0.2 ± 0.1 | 8.2 ± 2.9* | 0.9 ± 0.6† |
| IL-9 | 1.5 ± 0.9 | 4.9 ± 1.0 | 4.2 ± 0.8* | 2.1 ± 0.4† |
| IL-12 (p40) | 38.4 ± 12.7 | 22.8 ± 1.8 | 114.2 ± 21.5* | 86. 2 ± 22.1† |
| G-CSF | 0.7 ± 0.4 | 0.7 ± 0.2 | 14.7 ± 4.3* | 7.0 ± 2.4† |
| KC | 0.6 ± 0.1 | 0.2 ± 0.1 | 7.3 ± 1.2* | 1.2 ± 0.1† |
| MCP-1 | 0 | 0.5 ± 0.5 | 31.4 ± 8.2* | 14.5 ± 2.6† |
| RANTES | 0.1 ± 0.04 | 0.1 ± 0.05 | 0.5 ± 0.1* | 0.2 ± 0.1† |
Definition of abbreviations: G-CSF, granulocyte colony–stimulating factor; KC, keratinocyte-derived chemoattractant; MCP-1, macrophage chemotactic protein; RANTES, regulated upon activation normal T cell expressed and presumably secreted; WT, wild-type.
Concentrations of IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-10, IL-12 (p70), IL-13, IL-17, GM-CSF, MIP-1α, eotaxin, TNF-α, and IFN-γ were below the limits of detection in all samples. Concentrations of MIP-1β were within the limits of detection, but were not different between groups. Data represent cytokine levels in pg/ml, and are expressed as mean ± SEM from 4–6 mice/group.
P ≤ 0.05 compared with WT mice exposed to air.
P ≤ 0.05 compared with WT mice exposed to NO2.
Nuclear Factor–κB Activation Before Exposure to NO2 Modulates Neutrophil Accumulation in the Airways
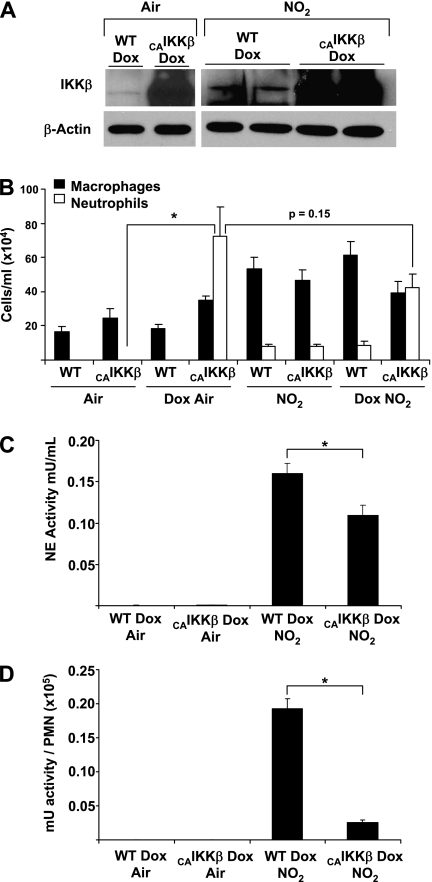
Based on our observations that inhibition of NF-κB in the airway epithelium decreases NO2-induced inflammation and lung injury, we postulated that enhanced activation of NF-κB in the epithelium before exposure to NO2 would worsen inflammation and injury. We reported that CC10-rTet-CAIKKβ bi-transgenic mice demonstrate an activation of airway epithelial NF-κB when receiving Dox chow, resulting in robust airway neutrophilia, increases in inflammatory cytokines, and airway hyperresponsiveness (16). In the present study, we confirmed the expression of IKKβ in lung homogenates from wild-type littermate controls, and augmented expression in Dox-fed CC10-CAIKKβ mice. The results depicted in Figure 5A demonstrate marked increases in the expression of IKKβ protein in CAIKKβ transgenic mice that received Dox compared with wild-type littermate controls, and that increases in IKKβ protein levels were not affected after exposure to NO2. Furthermore, CC10-rTet-CAIKKβ mice receiving doxycycline demonstrated an increase in neutrophils in BAL fluid, whereas wild-type littermates or CC10-rTet-CAIKKβ mice that received regular food had neutrophil concentrations in BAL fluid similar to those of control animals (Figure 5B). As shown in Figure 2, the inhalation of 25 ppm NO2 for 6 hours/day for 3 days also resulted in increases in airway neutrophilia. However, the extent of NO2-induced neutrophilia (10,000 cells/ml) was markedly less than for the airway neutrophilia observed in CAIKKβ-expressing mice (Figure 5B). Surprisingly, in mice expressing the CAIKKβ transgene, subsequent exposure to NO2 resulted in no significant differences (P = 0.15) in airway neutrophilia, compared with CAIKKβ-expressing mice that were maintained in room air (Figure 5B). Although CAIKKβ mice on doxycycline showed enhanced neutrophilia in response to NO2 compared with non-Dox control animals, the magnitude of neutrophilia was not additive between transgene expression and NO2 exposure.
Figure 5.
Assessment of inflammation and inflammatory mediators in BAL fluid of (WT) or CAIKKβ-expressing mice exposed to room air or 25 ppm of NO2 for 3 days. (A) CC10-CAIKKβ mice or WT littermates received Dox food for 7 days. The NO2 exposure groups received Dox food for 3 days before and during the exposure regimen of 6 hours per day of 25 ppm NO2 for 3 days. All mice were killed on Day 7, and whole-lung homogenates were prepared for evaluation of IKKβ content (top) and β-actin (bottom). Representative blots are shown. (B) Cell counts in BAL from WT and CAIKKβ-expressing mice exposed to air or 25 ppm NO2 exposure (*P < 0.05 compared with WT group; air, n = 5 mice/group; Dox air, n = 8 mice/group; NO2, n = 6 mice/group; Dox NO2, n = 8 mice/group). Expression of neutrophil elastase (NE) activity in BAL fluid (C) or presented as a ratio of milli-units (mU) per 100,000 polymorphonuclear cells (PMNs) (D), from WT and CAIKKβ-expressing mice receiving Dox and exposed to air or 25 ppm NO2.
Interestingly, although concentrations of neutrophil elastase activity in the BAL fluid of Dox-fed and air-exposed wild-type and CC10-CAIKKβ mice were undetectable, when the neutrophil elastase activity in the BAL fluid of wild-type and CAIKKβ mice receiving Dox and exposed to NO2 was measured, a significant reduction occurred in the amount of elastase activity in the CAIKKβ mice (Figure 5C). When this reduced activity is expressed relative to the number of neutrophils present in BAL fluid, although the CAIKKβ mice display enhanced neutrophilia, they exhibit severely decreased neutrophil elastase activity (Figure 5D). Therefore, the large numbers of neutrophils in CAIKKβ mice on Dox do not likely contribute to the injury, because they are not inherently active with respect to neutrophil elastase activity, especially in comparison to that induced by inhalation of NO2.
Effects of NF-κB Activation in Airway Epithelium on NO2-Induced Protein and Lactate Dehydrogenase in BAL
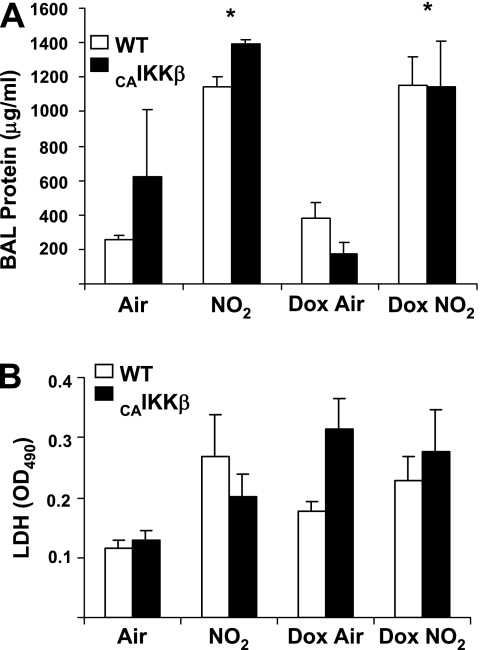
To determine whether enhanced NF-κB activity would affect the extent of lung injury in response to NO2, protein content and lactate dehydrogenase activity in BAL fluid were analyzed. As shown in Figure 6, increases in total BAL protein (Figure 6A) and lactate dehydrogenase (LDH) (Figure 6B) were not augmented by previous activation of NF-κB (CAIKKβ-expressing mice).
Figure 6.
Evaluation of protein (A) and lactate dehydrogenase (LDH, B) in BAL from wild-type (WT) or CC10-CAIKKβ transgenic mice exposed for 6 hours/day to 25 ppm NO2 for 3 days. * P < 0.05 compared with WT groups; n = 4 mice/group). Mice received regular or doxycycline food (Dox) for 3 days before beginning exposure to NO2. All groups were killed on Day 7 (*P < 0.05 compared with WT littermate groups; air, n = 8 mice/group; Dox, n = 8 mice/group; NO2, n = 6 mice/group; Dox NO2, n = 8 mice/group).
Nuclear Factor–κB Activation in Airway Epithelium Diminishes Lung Injury in Response to NO2
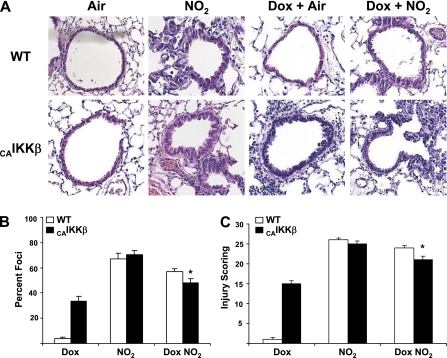
We next evaluated lung histopathology in CAIKKβ-expressing or wild-type mice exposed to room air or 25 ppm NO2 for 3 days In agreement with our previous observations, CAIKKβ mice receiving Dox exhibited robust leukocyte recruitment and evidence of thickening of the epithelial lining (16). In response to NO2, all groups demonstrated leukocyte infiltrates, epithelial wall thickening, and perivascular lesions (Figure 7A). The CAIKKβ mice that received Dox and were exposed to NO2 displayed somewhat attenuated lesions compared with NO2-exposed littermate controls or CAIKKβ mice receiving regular chow (Figure 7A). Blinded scoring of these lung sections revealed a small but statistically significant reduction in both the percentage of airways involved (Figure 7B) and the intensity of injury (Figure 7C) in CAIKKβ-expressing mice exposed to NO2, compared with wild-type control animals. Measurements of prosurvival and antioxidant genes known to be regulated by NF-κB showed no difference between transgenic and wild-type mice receiving Dox and exposed to NO2 (Figure E2). Furthermore, the abundance of the cleaved (active) form of the proapoptotic protein caspase-3 was not different in the lungs of wild-type or CC10-CAIKKβ mice exposed to NO2 (Figure E3).
Figure 7.
Evaluation of histopathology from wild-type (WT) and CC10-CAIKKβ transgenic mice exposed to room air or 6 hours/day of 25 ppm of NO2 for 3 days. Mice received regular or doxycycline food (Dox) for 3 days before beginning exposure to NO2. All groups were killed on Day 7. (A) Hematoxylin-and-eosin staining of 5-μm sections of mouse lungs. All magnifications are ×200, and are representative of patterns observed in each group. Scoring of lesions was performed by assessment of percentage of airways involved per section evaluated (B) and the intensity of foci (C), as described in Materials and Methods (*P < 0.05 compared with WT littermate groups; Dox, n = 8 mice/group; NO2, n = 6 mice/group; Dox NO2, n = 8 mice/group).
Nuclear Factor–κB Activation in Airway Epithelium Modulates Concentrations of Inflammatory Cytokines in Response to NO2
We next evaluated the impact of airway epithelial NF-κB activation on NO2-induced inflammatory mediators in BAL fluid. In line with the results described in Table 1, exposure to NO2 increased the levels of multiple proinflammatory mediators. Table 2 demonstrates robust increases in the levels of IL-5, IL-6, IL-9, IL-10, IL-12 (p40), G-CSF, KC, MCP-1, MIP-1β, and RANTES in BAL fluid from mice exposed to NO2. Activation of NF-κB in the airway epithelium alone caused significant expression of IL-12 (p40), G-CSF, KC, MCP-1, MIP-1β, and RANTES. In addition, concentrations of IL-12 (p40) and MCP-1 were significantly enhanced when NF-κB was activated in transgenic mice before exposure to NO2, compared with their respective control groups (Table 2). Furthermore, administration of Dox to wild-type or CAIKKβ mice attenuated the increases in IL-9 and IL-10 concentrations that occurred in response to NO2 exposure. The increases in proinflammatory mediators that follow the induction of CAIKKβ expression explain the recruitment of leukocytes, but are paradoxical in light of the diminished lung injury measured in these mice.
TABLE 2.
CYTOKINE PROFILES FROM BRONCHOALVEOLAR FLUID OF WILD-TYPE AND CC10-CAIKKβ MICE AFTER 1 WEEK OF NORMAL OR DOX CHOW AND AIR OR EXPOSURE TO 25 ppm NO2
| Air |
NO2 |
Dox Air |
Dox NO2 |
|||||
|---|---|---|---|---|---|---|---|---|
| WT | CAIKKβ | WT | CAIKKβ | WT | CAIKKβ | WT | CAIKKβ | |
| IL-5 | 0.3 ± 0.3 | 1.4 ± 0.07 | 18.2 ± 2.5* | 12.0 ± 2.5* | 3.4 ± 2.1 | 4.3 ± 1.8 | 12.9 ± 3.0 | 2.8 ± 0.7 |
| IL-6 | 6.7 ± 6.7 | 21.6 ± 1.8 | 29.9 ± 1.5* | 29.9 ± 0.6* | 16.3 ± 0.9 | 30.7 ± 4.2* | 25.5 ± 31 | 29.9 ± 1.5 |
| IL-9 | 21.9 ± 3.5 | 14.9 ± 5.9 | 148.4 ± 56.0* | 141.6 ± 43.6* | 32.0 ± 4.3 | 21.4 ± 2.6 | 60.6 ± 30.0 | 31.2 ± 8.3 |
| IL-10 | 15.3 ± 4.7 | 18.0 ± 7.4 | 102.8 ± 35.0* | 100.0 ± 23.2* | 23.5 ± 1.4 | 23.1 ± 5.0 | 51.6 ± 14.4 | 24.5 ± 5.0 |
| IL-12 (p40) | 33.5 ± 6.0 | 38.7 ± 3.8 | 98.1 ± 3.4* | 84.3 ± 9.4* | 38.1 ± 7.3 | 119.8 ± 24.5‡ | 79.8 ± 10.1 | 166.8 ± 21.8† |
| G-CSF | 3.2 ± 0.8 | 4.9 ± 1.5 | 28.8 ± 3.5* | 34.9 ± 0.9* | 4.5 ± 1.2 | 48.4 ± 23.7‡ | 26.2 ± 10.0 | 37.3 ± 4.0 |
| KC | 19.8 ± 3.1 | 26.1 ± 4.0 | 34.8 ± 5.4* | 40.7 ± 3.0* | 16.2 ± 2.6 | 64.8 ± 15.0‡ | 22.3 ± 3.9 | 55.4 ± 7.5 |
| MCP-1 | 1.1 ± 1.1 | 4.0 ± 2.0 | 32.7 ± 5.8* | 32.2 ± 2.6* | 14.2 ± 3.9 | 27.9 ± 6.5‡ | 15.4 ± 5.4 | 53.2 ± 11.0† |
| MIP-1β | 0.0 ± 0.0 | 0.0 ± 0.0 | 8.9 ± 1.0* | 15.2 ± 2.6* | 0.0 ± 0.0 | 11.8 ± 4.7‡ | 5.2 ± 0.2 | 10.8 ± 2.3 |
| RANTES | 9.0 ± 1.3 | 9.4 ± 1.4 | 21.6 ± 1.6* | 24.5 ± 1.7* | 11.3 ± 1.7 | 45.5 ± 16.0‡ | 20.2 ± 2.0 | 43.2 ± 4.6 |
| TNF-α | 8.1 ± 4.1 | 7.8 ± 3.9 | 14.2 ± 1.5 | 23.9 ± 1.0 | 9.5 ± 3.9 | 12.9 ± 1.8 | 6.6 ± 2.3 | 13.9 ± 2.1 |
Definition of abbreviations: G-CSF, granulocyte colony–stimulating factor; Dox, doxycycline; KC, keratinocyte-derived chemoattractant; MCP-1, macrophage chemotactic protein; RANTES, regulated upon activation normal T-cell expressed and presumably secreted; WT, wild-type.
Mice received regular or Dox-containing food (Dox) for 3 days before inhalation of 25 ppm NO2 for 6 hours/day for an additional 3 days. All groups were analyzed on Day 7. Concentrations of IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-12 (p70), IL-13, IL-17, GM-CSF, MIP-1α, eotaxin, and IFNγ were below the limits of detection in all samples. Data represent cytokine levels in pg/ml, and are expressed as mean ± SEM from 4–6 mice/group.
P ≤ 0.05 compared with air control groups.
P ≤ 0.05 compared with WT Dox NO2 group.
P ≤ 0.05 compared with WT Dox air group.
DISCUSSION
Because it functions as a regulator of both proinflammatory and prosurvival factors, as well as processes modulating proliferation and cell fate, establishing the role of NF-κB in severe ALI is complex. Nonetheless, the inhibition of NF-κB is considered a means of alleviating inflammation in diseases such allergic asthma (19) and other lung diseases, including ALI (20).
As previous studies demonstrated (5), high doses of NO2 exposure result in a robust inflammatory response that includes leukocyte influx, increased cytokine expression, and tissue injury characterized by airway epithelial thickening and perivascular lesions. We demonstrate here that this inflammation is mediated by the activities of airway epithelial NF-κB, because RelA is present in the nuclei of airway epithelia after the first 6 hours of NO2 exposure, and the inflammatory response to sustained NO2 exposure is abrogated in CC10-IκBαSR mice. The lack of canonical NF-κB activation in these mice also interferes with the induction of the NF-κB–regulated inflammatory chemokines KC, MCP-1, and IL-12 (p40), and subsequently decreases the number of inflammatory leukocytes. This correlation between the lack of neutrophils and the reduction in neutrophil elastase suggests that a causal role may exist for neutrophils in oxidant-induced, NF-κB–dependent airway injury.
The reduction in airway epithelial inflammation and injury in CC10-IκBαSR mice in vivo is consistent with previous studies. Patients with neutropenia demonstrated a reduced severity of ALI (21), and patients show an increased severity of ALI as neutrophil numbers recover (22). In addition, neutrophils were also implicated in airway remodeling, and this was suggested to occur via neutrophil production of matrix metalloproteases and elastase (18). Furthermore, normal human bronchial epithelial cells exposed to NO2 show an enhanced adhesion to neutrophils, and in turn an increased rate of epithelial cell death (23). Finally, the depletion of neutrophils in animal models reduces the activation of NF-κB observed in ALI (24). Consistent with these findings, our studies demonstrate reduced neutrophil numbers and subsequently lower neutrophil elastase activity in CC10-IκBαSR mice, corresponding with the evidence of decreased lung injury. While attacking pathogens, neutrophils activate a respiratory burst and undergo apoptosis, releasing a number of factors that are indiscriminate in their damaging effects. Therefore, apoptotic neutrophils must be cleared from the site of inflammation in a timely manner, and thus are rapidly scavenged by macrophages. Dysregulated neutrophil apoptosis was suggested to contribute to lingering inflammatory conditions (25). This concept is supported by the fact that BAL fluid from patients with ALI often contains antiapoptotic factors such as GM-CSF and G-CSF (26). Therefore, the expression of prosurvival factors downstream of airway epithelial NF-κB activation may plausibly prolong the survival of the neutrophils themselves, leading to an overaccumulation at the site of injury and subsequent chronic inflammation (27).
Intriguingly, in our study, airway epithelial CAIKKβ-expressing mice recruited an overwhelming number of neutrophils, yet showed significantly less severe injury than wild-type mice exposed to NO2, despite the augmented expression of some proinflammatory mediators. These findings raise the question of whether these recruited neutrophils are inactive in the absence of other stimuli. Previous studies in which mice expressed the neutrophil chemokines KC and MIP-2 in the lung demonstrated that the subsequent neutrophil influx does not cause injury or vascular leakage. These studies indicated that although KC and other CXC chemokines are potent recruiters of neutrophils, additional mediators are required for their full activation and degranulation (28). Indeed (as shown by our measurement of neutrophil elastase activity), despite the increased population of neutrophils seen in CAIKKβ mice receiving Dox and exposed to NO2, the lavageable airspaces appear to possess a marked reduction in elastase activity, suggesting that these cells are not as active as those from wild-type mice under the same conditions.
The influx of neutrophils and macrophages in response to NO2 is clearly explained by the host of well-known chemotactic factors present in BAL fluid, and particularly KC and MCP-1. In addition, mice exposed to NO2 show significant increases in concentrations of IL-12 (p40) in BAL fluid, which appears to be strongly NF-κB–driven, because it is enhanced in CAIKKβ mice after exposure to NO2. Interleukin-12 (p40) was found in the BAL fluid of patients with asthma (29) and patients with fibrosis associated with silica exposure (30), suggesting another link for airway epithelial NF-κB in asthma and airway remodeling beyond those we (11, 16, 31, 32) and others (12, 33–36) described using mouse models of disease.
The relationship between pulmonary inflammation and tissue damage is not uniformly direct. Leukocyte infiltration does not necessarily correlate with protein increase, and the influx of neutrophils and monocytes can occur independently of vascular leakage (37). Studies in intestinal epithelium showed that inhibiting caspases can prevent barrier dysfunction (38). However, the expression of prosurvival and antioxidant genes that may be enhanced by CAIKKβ mice receiving Dox was not significantly increased. Furthermore, the measurement of apoptosis (cleaved caspase-3; Figure E3) in NO2-exposed lungs showed no differences between wild-type and CAIKKβ mice, leading us to believe that, in this case, the CAIKKβ mice do not exert a prosurvival, antiapoptotic effect in the epithelium itself.
Although further studies are required to elucidate the signaling mechanism responsible for NO2-induced injury and the underlying mechanism of neutrophil activity modulation, the recruited activated neutrophils clearly play a role in damaging airway epithelium and lung tissue, and that this is, in part, NF-κB–dependent. Our work further indicates that although NF-κB activation is sufficient to recruit neutrophils to the lung, additional factors are required for their activation, degranulation, and subsequent tissue injury. Perhaps the most intriguing outcome in our study is the finding that airway epithelial NF-κB activation in the absence of other overt stimuli causes an injury and an inflammatory response qualitatively similar to that induced by NO2 exposure, and that the effects of NO2 inhalation are not augmented but are somewhat diminished by previous airway epithelial NF-κB activation. Collectively, these novel findings indicate that a therapeutic modulation of NF-κB in lung disease needs to take into account the diverse functions of this potent transcription factor.
Supplementary Material
This work was supported by National Institutes of Health grants R01HL089177 and P20RR015557 (M.E.P.), R01HL084200 (B.T.S.), and R01HL060014 (Y.M.W.J.-H.).
This article contains an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0416OC on November 9, 2009
Author Disclosure: J.F.A. received a sponsored grant from Centocor R&D, Inc., for $50,001–$100,000, from the National Institutes of Health (NIH) for $50,001–$100,000, from the American Thoracic Society for $50,001–$100,000, and from the Parker B. Francis Foundation for more than $100,001. J.L.A. received a sponsored grant from the Parker B. Francis Foundation for $50,001–$100,000 and the National Institutes of Health for >$100,001. A.L.B. received a sponsored grant from the NIH for $10,001–$50,000. A.S.G. received a sponsored grant from the NIH for $10,001–$50,000. Y.M.W.J.-H. received a sponsored grant from the NIH for >$100,001. B.T.S. received a sponsored grant from the NIH for more than $100,001. M.E.P. received lecture fees from Sepracor for $1,001–$5,000, and a sponsored grant from Sepracor for $50,001–$100,000 and from the NIH for more than $100,001.
References
- 1.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 2005;33:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen TM, Gokhale J, Shofer S, Kuschner WG. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci 2007;333:249–256. [DOI] [PubMed] [Google Scholar]
- 3.Byun J, Mueller DM, Fabjan JS, Heinecke JW. Nitrogen dioxide radical generated by the myeloperoxidase–hydrogen peroxide–nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett 1999;455:243–246. [DOI] [PubMed] [Google Scholar]
- 4.Velsor LW, Ballinger CA, Patel J, Postlethwait EM. Influence of epithelial lining fluid lipids on NO(2)-induced membrane oxidation and nitration. Free Radic Biol Med 2003;34:720–733. [DOI] [PubMed] [Google Scholar]
- 5.Poynter ME, Persinger RL, Irvin CG, Butnor KJ, van Hirtum H, Blay W, Heintz NH, Robbins J, Hemenway D, Taatjes DJ, et al. Nitrogen dioxide enhances allergic airway inflammation and hyperresponsiveness in the mouse. Am J Physiol Lung Cell Mol Physiol 2006;290:L144–L152. [DOI] [PubMed] [Google Scholar]
- 6.Ayyagari VN, Januszkiewicz A, Nath J. Effects of nitrogen dioxide on the expression of intercellular adhesion molecule-1, neutrophil adhesion, and cytotoxicity: studies in human bronchial epithelial cells. Inhal Toxicol 2007;19:181–194. [DOI] [PubMed] [Google Scholar]
- 7.Devalia JL, Campbell AM, Sapsford RJ, Rusznak C, Quint D, Godard P, Bousquet J, Davies RJ. Effect of nitrogen dioxide on synthesis of inflammatory cytokines expressed by human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol 1993;9:271–278. [DOI] [PubMed] [Google Scholar]
- 8.Janssen-Heininger YM, Persinger RL, Korn SH, Pantano C, McElhinney B, Reynaert NL, Langen RC, Ckless K, Shrivastava P, Poynter ME. Reactive nitrogen species and cell signaling: implications for death or survival of lung epithelium. Am J Respir Crit Care Med 2002;166:S9–S16. [DOI] [PubMed] [Google Scholar]
- 9.McElhinney B, Poynter ME, Shrivastava P, Hazen SL, Janssen-Heininger YM. Eosinophil peroxidase catalyzes JNK-mediated membrane blebbing in a rho kinase-dependent manner. J Leukoc Biol 2003;74:897–907. [DOI] [PubMed] [Google Scholar]
- 10.Shrivastava P, Pantano C, Watkin R, McElhinney B, Guala A, Poynter ML, Persinger RL, Budd R, Janssen-Heininger Y. Reactive nitrogen species-induced cell death requires Fas-dependent activation of c-jun N-terminal kinase. Mol Cell Biol 2004;24:6763–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol 2003;170:6257–6265. [DOI] [PubMed] [Google Scholar]
- 12.Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, Cheng DS, Yull FE, Christman JW, Blackwell TS. Targeted immunomodulation of the NF-kappaB pathway in airway epithelium impacts host defense against pseudomonas aeruginosa. J Immunol 2006;176:4923–4930. [DOI] [PubMed] [Google Scholar]
- 13.Pahl HL. Activators and target genes of rel/NF-kappaB transcription factors. Oncogene 1999;18:6853–6866. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Ahsan MH, Zhu L, Sambucetti LC, Purchio AF, West DB. Nf-kappaB and not the MAPK signaling pathway regulates GADD45beta expression during acute inflammation. J Biol Chem 2005;280:21400–21408. [DOI] [PubMed] [Google Scholar]
- 15.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest 2005;115:2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantano C, Ather JL, Alcorn JF, Poynter ME, Brown AL, Guala AS, Beuschel SL, Allen GB, Whittaker LA, Bevelander M, et al. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med 2008;177:959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bevelander M, Mayette J, Whittaker LA, Paveglio SA, Jones CC, Robbins J, Hemenway D, Akira S, Uematsu S, Poynter ME. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J Immunol 2007;179:3680–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty P, Rogan MP, Greene CM, Boxio RM, Poiriert T, O'Mahony M, Belaaouaj A, O'Neill SJ, Taggart CC, McElvaney NG. Neutrophil elastase up-regulates cathepsin b and matrix metalloprotease-2 expression. J Immunol 2007;178:5871–5878. [DOI] [PubMed] [Google Scholar]
- 19.Adcock IM, Ito K, Barnes PJ. Glucocorticoids: effects on gene transcription. Proc Am Thorac Soc 2004;1:247–254. [DOI] [PubMed] [Google Scholar]
- 20.Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med 2003;2:211–219. [DOI] [PubMed] [Google Scholar]
- 21.Ognibene FP, Martin SE, Parker MM, Schlesinger T, Roach P, Burch C, Shelhamer JH, Parrillo JE. Adult respiratory distress syndrome in patients with severe neutropenia. N Engl J Med 1986;315:547–551. [DOI] [PubMed] [Google Scholar]
- 22.Azoulay E, Darmon M, Delclaux C, Fieux F, Bornstain C, Moreau D, Attalah H, Le Gall JR, Schlemmer B. Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med 2002;30:781–786. [DOI] [PubMed] [Google Scholar]
- 23.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003;31(Suppl 4)S195–S199. [DOI] [PubMed] [Google Scholar]
- 24.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2000;279:L1137–L1145. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Harrington EO, Rounds S. Apoptosis and lung injury. Keio J Med 2005;54:184–189. [DOI] [PubMed] [Google Scholar]
- 26.Matute-Bello G, Liles WC, Radella F II, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Modulation of neutrophil apoptosis by granulocyte colony–stimulating factor and granulocyte/macrophage colony–stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med 2000;28:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Miskolci V, Rollins J, Vu HY, Ghosh CC, Davidson D, Vancurova I. NFkappaB is persistently activated in continuously stimulated human neutrophils. Mol Med 2007;13:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lira SA, Zalamea P, Heinrich JN, Fuentes ME, Carrasco D, Lewin AC, Barton DS, Durham S, Bravo R. Expression of the chemokine N51/KC in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J Exp Med 1994;180:2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J Exp Med 2001;193:339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huaux F, Arras M, Tomasi D, Barbarin V, Delos M, Coutelier JP, Vink A, Phan SH, Renauld JC, Lison D. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J Immunol 2002;169:2653–2661. [DOI] [PubMed] [Google Scholar]
- 31.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 2002;160:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen SM, Cheng DS, Williams BJ, Sherrill TP, Han W, Chont M, Saint-Jean L, Christman JW, Sadikot RT, Yull FE, et al. The nuclear factor kappa-B pathway in airway epithelium regulates neutrophil recruitment and host defence following pseudomonas aeruginosa infection. Clin Exp Immunol 2008;153:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Bakkouri K, Wullaert A, Haegman M, Heyninck K, Beyaert R. Adenoviral gene transfer of the NF-kappa B inhibitory protein Abin-1 decreases allergic airway inflammation in a murine asthma model. J Biol Chem 2005;280:17938–17944. [DOI] [PubMed] [Google Scholar]
- 35.Sadikot RT, Han W, Everhart MB, Zoia O, Peebles RS, Jansen ED, Yull FE, Christman JW, Blackwell TS. Selective I kappa B kinase expression in airway epithelium generates neutrophilic lung inflammation. J Immunol 2003;170:1091–1098. [DOI] [PubMed] [Google Scholar]
- 36.Xu S, Xu Y, Zhang Z, Ni W, Chen S. Effect of nuclear factor-kappaB on airway remodeling in asthmatic rats. J Huazhong Univ Sci Technolog Med Sci 2004;24:13–18. [DOI] [PubMed] [Google Scholar]
- 37.Huang AJ, Furie MB, Nicholson SC, Fischbarg J, Liebovitch LS, Silverstein SC. Effects of human neutrophil chemotaxis across human endothelial cell monolayers on the permeability of these monolayers to ions and macromolecules. J Cell Physiol 1988;135:355–366. [DOI] [PubMed] [Google Scholar]
- 38.Abreu MT, Palladino AA, Arnold ET, Kwon RS, McRoberts JA. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology 2000;119:1524–1536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.